Abstract
We compared the antithrombotic efficacy of a potent factor Xa inhibitor, FXV673, to heparin and RPR109891, a GPIIb/IIIa antagonist, when used as adjunctive therapy in a canine model of rt-PA-induced coronary thrombolysis.
Thrombus formation was induced by electrolytic injury to stenosed coronary artery. After thrombotic occlusion, a 135 min infusion of saline (n=8), FXV673 (10, 30 or 100 μg kg−1+1, 3, or 10 μg kg−1 min−1, respectively; n=8 per dose), heparin (60 u kg−1+0.7 u kg−1 min−1, n=8), or RPR109891 (30 μg kg−1+0.45 μg kg−1 min−1, n=8), was initiated. Aspirin (5 mg kg−1, i.v.) was administered to all animals. Fifteen minutes after the start of drug infusion, rt-PA was administered (100 μg kg−1+20 μg kg−1 min−1 for 60 min).
The incidence of reperfusion in the high dose FXV673 (8/8, 100%) was significantly greater than that in the heparin group (4/8, 50%), with a trend to faster reperfusion (23±5 min for FXV673 versus 41±11 min for heparin). Only 2/8 (25%) of the vessels reoccluded in the high dose FXV673 group, compared to 4/4 (100%) and 5/5 (100%) vessels in the heparin and RPR109891 groups, respectively (P<0.05).
Throughout the protocol, blood flow was higher in the FXV673 treated group compared to other groups. FXV673 enhanced vessel patency in a dose-dependent manner.
Compared to vehicle and heparin groups, the thrombus mass was decreased by 60% in the high dose FXV673. FXV673, heparin and RPR109891 increased the bleeding time by 2.7, 1.7 and 4 fold, and APTT by 2.8, 2.7 and 1.2 fold, respectively.
In conclusion, FXV673 is more effective than heparin and at least as effective as RPR109891 when used as an adjunct during rt-PA-induced coronary thrombolysis.
Keywords: Coronary thrombolysis, reperfusion, FXV673, factor Xa inhibitor
Introduction
Current use of heparin as an adjunct to thrombolysis for acute myocardial infarction is less than adequate. Heparin therapy is fraught with several limitations such as neutralization by plasma cofactors (Eitzman et al., 1994), induction of thrombocytopenia (Bell, 1988), inability of heparin-ATIII complex to inhibit thrombus-bound IIa/Xa activity (Weitz et al., 1990) and difficulty in monitoring the therapy. Also, discontinuation of therapy results in rebound coagulation as a result of increased thrombin activity (Granger et al., 1995). Thrombin inhibitors such as argatroban, inogatran and hirudin have overcome some of these limitations. However, the GUSTO-IIb and TIMI-9B trials with hirudin failed to demonstrate superiority over heparin (GUSTO-IIb Investigators, 1996; Antman, 1996). Similarly, the MINT study found that the TIMI 3 flow rates with argatroban were similar to heparin (Jang et al., 1999). On the other hand, although GPIIb/IIIa receptor antagonists appear to be effective adjunct to thrombolysis (Antman et al., 1999), safety may be still an issue. For these reasons, antagonists of fXa have been proposed to provide potent inhibition of thrombin generation and the subsequent platelet activation during thrombolysis. Due to the penultimate position of fXa in the coagulation system with respect to thrombin, its inhibition is proposed to have minimal effects on haemostasis compared to heparin, direct thrombin inhibitors and GPIIb/IIIa receptor antagonists.
The structure-based design of fXa inhibitors, led to the bis-benzamidine series. First among these compounds was DX-9065a, which is a potent (Ki=41 nM) and selective fXa inhibitor, and is effective antithrombotic in animal models (Hara et al., 1994; Herbert et al., 1996). The bis- benzamidine series was expanded by the addition of sulphonamide groups that led to YM-60828 (Ki=1.3 nM), which has also proven to be an effective antithrombotic (Taniuchi et al., 1998). Further, replacing the strongly basic para-benzamidine group of the bis-benzamidine fXa inhibitor with a substituted biphenyl moiety improved inhibitory potency against fXa. For example, the monobasic isoxazoline fXa inhibitors such as SF303 (Ki=6.3 nM) and SK549 (Ki=0.52 nM) still retain the potency and selectivity (Wong et al., 2000). Based on de novo design approach and using the X-ray crystal structure of fXa, we synthesized β-aminoester series of fXa inhibitors. Among these, FXV673 was characterized as a reversible, highly potent (Ki=0.5 nM for fXa) and selective (Ki for thrombin=3956 nM, trypsin=301 nM, APC=18491 nM, plasmin=656 nM and t-PA=8681 nM) inhibitor (Chu et al., 2000).
In the initial studies, FXV673 was found to possess a short-biological half-life in rats, dogs and rhesus monkeys (0.27–0.33 h) and was a potent antithrombotic agent when administered as an intravenous infusion in rat and canine models of arterial thrombosis (unpublished observations). The pharmacokinetic and pharmacodynamic properties of FXV673 make it a promising adjunct to rt-PA-induced thrombolysis in acute coronary syndromes. Therefore, we examined the efficacy of FXV673 in a canine model of coronary artery thrombolysis. Since GPIIb/IIIa receptor antagonists have been proposed as adjuncts to coronary thrombolysis, we also tested RPR109891, a GPIIb/IIIa receptor antagonist, and heparin in our study for comparison.
Methods
Drugs and reagents
This study incorporates the use of FXV673 (Figure 1) and RPR109891, which have been characterized and described earlier (Chu et al., 1997; 2000). These two compounds were synthesized by the Department of Medicinal Chemistry, Aventis Pharmaceuticals (Collegeville, PA, U.S.A.). Recombinant tissue plasminogen activator (rt-PA; Alteplase®) was purchased from Genentech, Inc. (South San Francisco, CA, U.S.A.). All other reagents used in this study were obtained from commercial sources.
Figure 1.
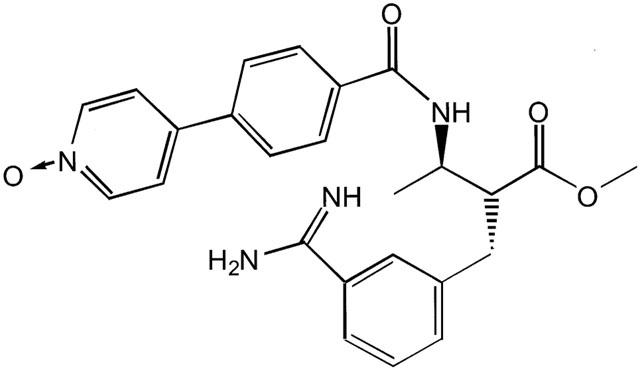
Chemical structure of FXV673 (MW=482.9): 2-(R)-(3-Carbamimidoylbenzyl)-3-(R)-[4-(1-oxypyridin-4-yl)benzoylamino]-butyric acid methyl ester.
Guidelines for animal research
All procedures in this study were performed in compliance with the Animal Welfare Act Regulations and with the Guide for the Care and Use of Laboratory Animals (International Standard Book Number 0-309-05377-3, 1986).
Model of coronary artery thrombolysis
Mongrel dogs of either sex (15–21 kg) were anaesthetized with sodium pentobarbitone (30 mg kg−1, i.v.), intubated, and ventilated (tidal volume=30 ml kg−1, rate=12 breaths min−1) using a respirator (Harvard Apparatus, S. Natick, MA, U.S.A.). All surgical procedures were performed with a cauterization and blunt dissection to minimize bleeding during the subsequent administration of thrombolytics. A tri-lumenal catheter (SAFEDWELplus Becton Dickinson) was placed in the right femoral vein for the administration of test agents and supplemental anaesthesia. The right femoral artery was cannulated for measurement of arterial blood pressure and for obtaining blood samples.
A left thoracotomy was performed at the fifth intercostal space and the heart was suspended in a pericardial cradle. The left circumflex coronary artery was isolated and dissected for a distance of 2 cm, tying side branches when necessary. A calibrated electromagnetic flow probe (Carolina Medical Electronics, 501D) was placed proximal to the first obtuse marginal branch to measure coronary blood flow. A snare ligature was placed on the distal portion of the vessel to validate zero flow and to elicit a reactive hyperaemic response by occluding the vessel for 10 s. A critical stenosis was achieved by the placement of a Goldblatt clamp (around the vessel proximal to the snare), which reduced the lumen area of the vessel by 80% thereby preventing the hyperaemic response, while minimally affecting basal flow. A stimulation electrode, consisting of a silver-coated, copper wire with a 25-gauge needle tip (4 mm) bent 90° was inserted distal to the flow probe into the artery and pulled back to ensure its contact with the intimal surface of the vessel. The electrode was connected in series with a 250 kΩ variable resistor to the anode (positive terminal) of a 9 V battery. The cathode (negative terminal) was secured to subcutaneous tissue.
Experimental protocol
Protocol for the study is outlined in Figure 2. The thrombus formation was initiated with passage of direct current (150 μA) through the intracoronary electrode until coronary blood flow was 0 ml min−1. The current remained on until 5 min after occlusion, then the thrombus was allowed to age for 60 min. After 60 min of a continuous no-flow state, dogs were randomly assigned to one of the six adjunctive treatment groups (Figure 2). All groups received aspirin (5 mg kg−1, i.v.). Compounds were diluted in saline and bolus injections were made using a volume of 5 ml and constant infusions (135 min) were made using a volume of 20 ml. A blinded experimental design was used so that investigator did not know which agents they were administering to the animals. All adjunctive therapy infusions were performed over 135 min. Fifteen minutes into the infusion of adjunctive agents, recombinant tissue plasminogen activator (rt-PA) was administered as a bolus of 100 μg kg−1 followed by an infusion at 20 μg kg−1 min−1 for 1 h. Lidocaine (2 mg kg−1, i.v.) was administered as necessary to control reperfusion arrhythmia. After administration of adjunctive agents was terminated, the animals were monitored for an additional 2 h. Throughout the protocol, continuous recordings of lead II ECG, blood pressure, heart rate, and mean and phasic coronary artery blood flow were made on a Gould model TA6000 Recorder (Gould Instrument Systems, Valley View, OH, U.S.A.). Serial blood samples were taken just prior to initiating the current (B1, control), 55 min into the ageing of the thrombus (B2), 15 min after the start of adjunctive therapy (B3), 55 min after the rt-PA infusion was begun (B4), 55 min after the rt-PA infusion was terminated (B5), and 45 and 90 min after termination of the adjunctive treatment (B6 and B7, respectively, Figure 2). Upon conclusion of the protocol, the vessel segment was isolated with vascular clamps and removed. A lengthwise incision was made, and any detectable thrombus mass was removed and weighed immediately.
Figure 2.
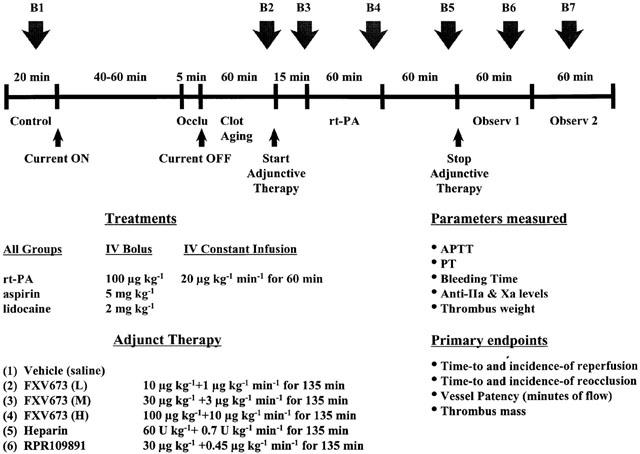
Representation of the experimental protocol to investigate the effect of FXV673, heparin and RPR109891 in a canine model of coronary artery thrombolysis.
Time-to-occlusion was defined as the time from initiation of current until blood flow decreased to 0 ml min−1. Reperfusion was defined as establishment of 50% of control blood flow for 5 min or any flow for 15 min after the initiation of rt-PA. Reocclusion was defined as any occurrence of zero flow after termination of rt-PA. Vessel patency is reported as minutes of flow during the 240 min period after initiation of rt-PA.
Determination of coagulation parameters
All blood samples (10 ml) were collected in 3.7% trisodium citrate (1 : 10 citrate/blood vol vol−1). Platelet rich plasma (PRP) was prepared by centrifugation of the whole blood at 150×g for 10 min, allowing the centrifuge to stop without braking. Platelet-poor plasma (PPP) was prepared by centrifugation of the remaining red cells by centrifugation at 1000×g for 10 min. Platelet count was determined using whole blood utilizing a cell counter (Biochem Immunosystems, Allentown, PA, U.S.A.). When necessary platelet count was adjusted to 3×108 platelets ml−1 using autologous PPP. PRP (250 μl) was incubated at 37°C while being stirred at 1200 r.p.m.. After preincubation with epinephrine (1 μM, Chrono-par 393, Chrono-log Corp., Havertown, PA, U.S.A.), platelet aggregation was induced by 10 μg/ml equine tendon collagen reagent (Chrono-par 385, Chrono-log Corp., Havertown, PA, U.S.A.), or 10 μM α-thrombin (ERI, South Bend, IN, U.S.A.) plus Gly-Pro-Arg-Pro (2 mM, Sigma Chemical Co., St. Louis, MO, U.S.A.), a fibrin polymerization inhibitor. Platelet aggregation was monitored spectrophotometrically with a PAP-4C platelet aggregometer (Bio Data Corp, Horsham, PA, U.S.A.). Results are expressed as a per cent inhibition of the extent of aggregation as compared to the pre-drug aggregation response.
Coagulation profiles were measured in all the animals. Both activated partial thromboplastin time (APTT) and prothrombin time (PT) were measured using a Coagulation Analyzer MCA210 (Bio Data Corp, Horsham, PA, U.S.A.) and Dade® reagents (Thromboplastin-C and Actin® FS Activated PTT reagent, Baxter Diagnostics, Inc., Deerfield, IL, U.S.A.). Gingival template bleeding times were measured with a Surgicutt® automated incision making device (ITC, Edision, NJ, U.S.A.). Uniform incisions were made on the mucous membrane of the inner upper lip. Blood was blotted with a blotting paper every 30 s, being careful not to touch the incision site. Bleeding times were timed from the moment of the incision until the blood no longer stained the blotting paper. Bleeding times of 10 min were taken to be maximal. The schedule for template bleeding times corresponded to the above mentioned blood sample times (Figure 2).
Analysis of anti-Xa and anti-IIa activity
Anti-Xa and anti-IIa activity were analysed by chromogenic methods using kits supplied by American Diagnostica (actichrome® Heparin anti-Xa and actichrome® Heparin anti-IIa, Greenwich, CT, U.S.A.), with minor modifications. The 4th International Standard for Unfractionated Heparin (National Institute for Biological Standards and Control, London, U.K.) was used to construct standard curve for measuring the ex vivo activity of heparin samples. For anti-IIa determinations, samples or standards (25 μl) were added to a 96-well microtiter plate. AT-III (75 μl), thrombin (50 μl) and Spectrozyme TH (50 μl) were added at 1 min intervals, mixing between the addition of each reagent. For anti-Xa measurements, samples or standards (20 μl) were added to a 96-well microtiter plate. AT-III (60 μl), bovine fXa (60 μl) and Spectrozyme Xa (60 μl) were added at 1 min intervals, mixing between the addition of each reagent. The plate was maintained at 37°C and then read kinetically at 405 nm using a SpectraMax 250, 96-well microplate spectrophotometer (Molecular Devices, CA, U.S.A.). Maximal velocity of the reaction in each well was obtained and analysed using Softmax Pro software (Molecular Devices, CA, U.S.A.). The inhibition of IIa or Xa was determined for each standard or sample by using the following equation: (1-Vmax sample/Vmax of control)×100. The standard curve was constructed as the concentration of above standard (IU/ml) versus per cent inhibition of IIa or Xa. If the samples yielded per cent inhibitions outside of the linear portion of the standard curve (i.e., high plasma concentrations), the samples were further diluted with control plasma and re-assayed. Plasma concentration in the samples was determined from the standard curve and corrected for the dilution of the plasma.
Ex vivo anti-fXa activity of FXV673 was determined by using the anti-Xa assay described above, except that assay buffer replaced AT-III.
Statistical analyses
All data are expressed as mean±s.e.mean. Data obtained for haemodynamics, coagulation parameters and platelet aggregation were subjected to two-way analysis of variance (ANOVA) (repeated measures). Occlusion parameters were first analysed by one-way ANOVA. LSD (least significant difference) post-hoc test was used to determine significance at P<0.05. The incidence of reperfusion and reocclusion between groups was compared using Fisher's Exact Test.
Results
Coronary artery blood flow and patency status
Before the induction of electrolytic injury, the coronary artery blood flow in the vehicle, FXV673 (L), FXV673 (M), FXV673 (H), heparin and RPR109891 groups was 21±3, 19±2, 23±2, 19±1, 23±2 and 21±2 ml min−1, respectively (P=0.6). Electrolytic injury produced progressive decline in flow and subsequent thrombotic occlusion of the coronary artery. Infusion of rt-PA restored blood flow to only 5–6% of the preocclusion value (Figure 3). Co-administration of heparin and rt-PA restored the blood flow to 21% at 60 min, but there was abrupt closure of the recanalized arteries after the termination of infusion. Similarly, co-administration of RPR109891 and rt-PA resulted in maximal reperfusion of 28% at 60 min, however the flow decreased thereafter. Administration of low and medium dose FXV673 increased the extent of reperfusion such that the blood flow was 25–42% during 40–60 min of rt-PA infusion. Infusion of the high dose FXV673 resulted in earlier reperfusion such that after 20 min of rt-PA, 28% of the flow was restored. This was further increased to 47–48% at 40–60 min. Compared to the vehicle, heparin and RPR109891 groups, blood flow maintenance was significantly greater in the high dose FXV673 at each time-point throughout the protocol. In addition, the flow at the end of protocol (240 min after rt-PA) was 22% in the high dose FXV673 group compared to only 4% in the heparin group and 2% in the RPR109891 group (P<0.05).
Figure 3.
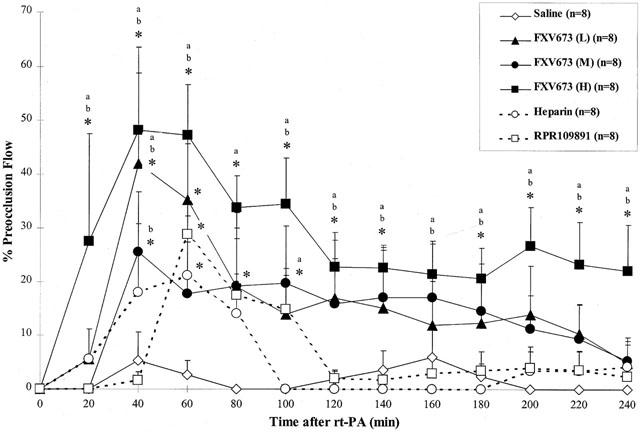
Coronary artery blood flow during the thrombolysis protocol. Blood flow is represented as the percentage of preocclusion flow. Compared to the heparin and RPR109891 group, the FXV673 group had a significant effect on sustaining coronary blood flow during and after the infusion. Values are expressed as mean±s.e.mean. *P<0.05 compared to saline; aP<0.05 compared to heparin; bP<0.05 compared to RPR109891.
Measuring the actual time for which the recanalized artery maintained blood flow (Figure 4) also quantitated adjunctive benefit of various treatment groups. The total time for which vehicle treated vessels maintained flow was ∼20 min, and this was increased to 36 min by co-administration of heparin. There was a dose-dependent prolongation of vessel patency after FXV673 infusion (78±29, 93±31 and 185±21 min for low, medium and high dose, respectively). The low and medium dose FXV673 were statistically similar to RPR109891 (77±27 min), indicating an equivalent effect. The high dose FXV673 maintained flow for 185±21 min, an effect that was significantly greater than the vehicle, heparin (36±13 min) and RPR109891 groups.
Figure 4.
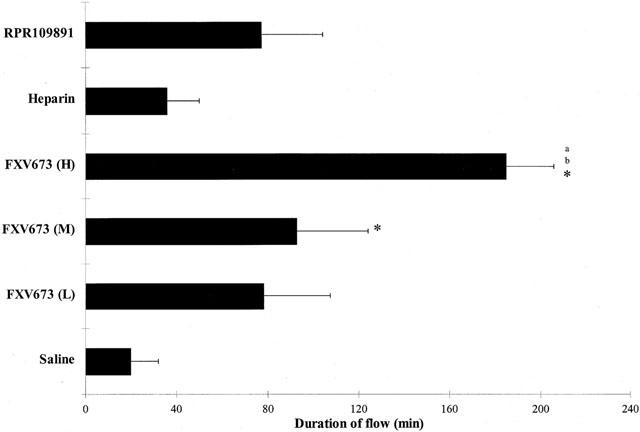
Patency status of coronary artery during the thrombolysis protocol. Vessel patency is represented as minutes of flow in each treatment group. Values are expressed as mean±s.e.mean *P<0.05 compared to saline; aP<0.05 compared to heparin; bP<0.05 compared to RPR109891.
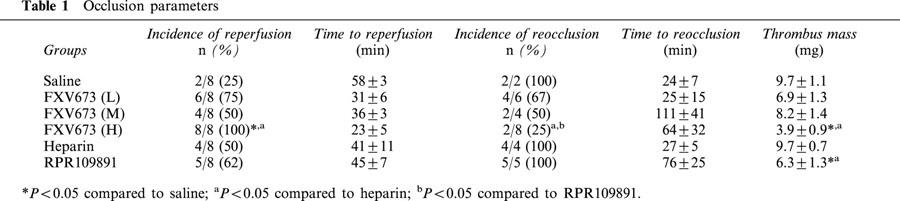
Occlusion parameters
Infusion of rt-PA, 60 min after persistent occlusion, resulted in lysis of the thrombus in two of eight arteries (25%) with a time to reperfusion of 58±3 min (Table 1). Infusion of heparin was associated with reperfusion in four of eight arteries (50%) with time to reperfusion of 41±11 min. RPR109891 had similar effect on reperfusion as heparin. Administration of the high dose FXV673 reperfused eight of eight arteries (100%), an effect that was significantly greater than in the vehicle and heparin treated groups. There was a trend to faster reperfusion (23±5 min in high dose FXV673 group versus 58±3 min in vehicle group and 41±11 min in heparin group) in the high dose FXV673 group. However, this effect did not reach statistical significance due to lower incidence of reperfusion in other groups. In the vehicle group, two of two arteries (100%) that reperfused initially, reoccluded 24±7 min after the termination of rt-PA infusion. Similarly, all arteries (100%) in the heparin and RPR109891 groups reoccluded after the end of infusions. The incidence of reocclusion decreased dose-dependently such that in the high dose FXV673 group the incidence of reocclusion was 25%, an effect that was significantly different compared to the heparin and RPR109891 groups. This resulted in longer times to reocclusion in the FXV673 treatment groups. Also, prolonged reperfusion in the injured coronary artery resulted in lower thrombus mass. The thrombus mass was significantly lower (60% reduction) than that in the vehicle and heparin treated groups.
Table 1.
Occlusion parameters
Ex vivo coagulation parameters
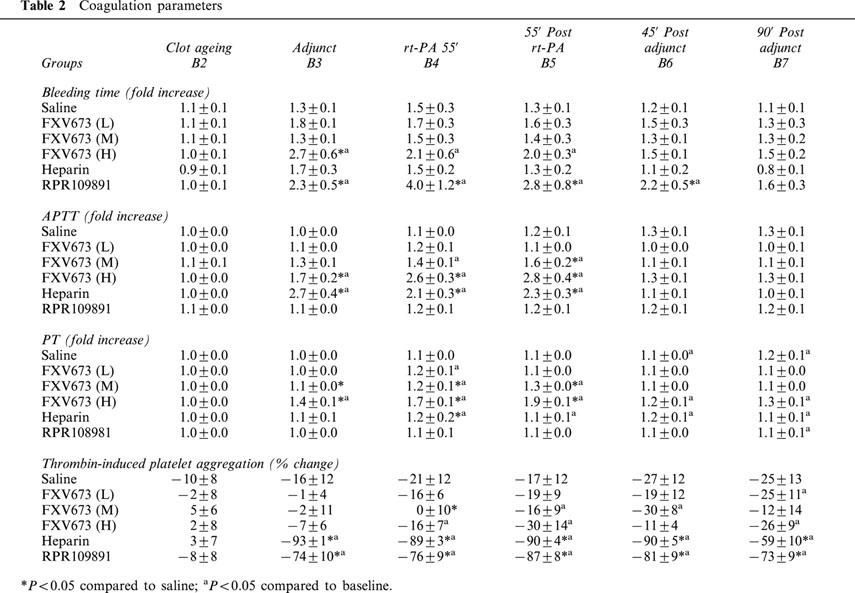
Infusion of FXV673 was associated with a dose-dependent increase in APTT (Table 2). The maximum prolongation induced by the low, medium and high doses were 1.2, 1.6 and 2.8 fold, respectively. PT was prolonged to 1.9 fold only by the high dose FXV673. The selected dose of heparin achieved a 2.7 and 1.2 fold increase in APTT and PT, respectively. These changes in APTT and PT returned to control values after cessation of the infusion. RPR109891 did not alter the APTT or PT due its specificity for GPIIb/IIIa receptors. Template bleeding time was performed to assess the effect on haemostasis. Heparin elicited a 1.7 fold increase, whereas the high dose FXV673 produced a 2.8 fold increase that returned to baseline value after discontinuation of the infusion. Owing to its direct inhibitory action on the platelets, RPR109891 resulted in maximal 4 fold prolongation of bleeding time.
Table 2.
Coagulation parameters
Platelet aggregation
Inhibition of ex vivo platelet aggregation was studied using thrombin and collagen as agonists. In all groups, collagen-induced aggregation was inhibited (data not shown). This effect was due to presence of aspirin in all groups. As compared with the vehicle group, none of the three doses of FXV673 significantly inhibited thrombin-induced platelet aggregation (Table 2). Consistent with its anti-IIa activity, heparin markedly inhibited thrombin-induced platelet aggregation by 93%. Similarly, antagonism of GPIIb/IIIa receptors by RPR109891 resulted in 87% inhibition of thrombin-induced platelet aggregation.
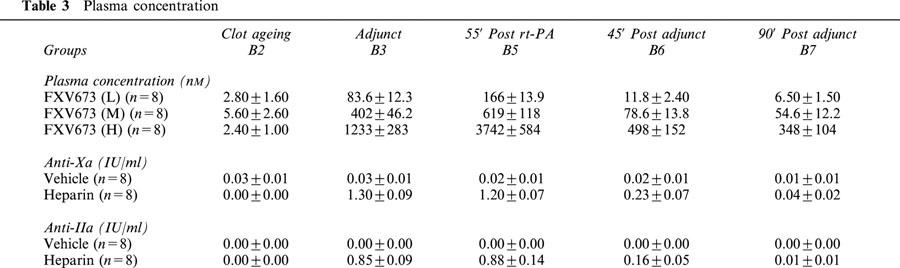
Plasma concentrations
The anti-Xa and anti-IIa levels were estimated using chromogenic assays (Table 3). The maximum anti-Xa and anti-IIa levels achieved by the infusion of heparin were 1.3 and 0.88 IU/ml respectively. These levels decreased rapidly after the end of infusion such that at 90 min they were similar to those in vehicle treated groups. Increasing doses of FXV673 produced dose-dependent rises in plasma FXV673 concentration. At 55 min into the infusion, the plasma concentrations were 166, 619 and 3742 nM in the low, medium and high dose groups, respectively. After the end of infusion, the drug levels declined rapidly, indicating a short biological half-life of FXV673.
Table 3.
Plasma concentration
Discussion
Previous studies have demonstrated that FXV673 has a short biological half-life in rats, dogs and monkeys (0.27–0.33 h), and it is an effective antithrombotic agent after intravenous infusion in rat and canine carotid artery thrombosis models (unpublished observations). Since the main goal for developing FXV673 was for acute coronary syndromes, we tested its efficacy in a canine model of coronary artery thrombolysis. Compared to heparin and a GPIIb/IIIa receptor antagonist, RPR109891, infusion of FXV673 reperfused 50 and 38% more arteries with a trend for faster reperfusion. Also, the incidence of reocclusion was significantly reduced by 75% with FXV673 infusion. These effects translated into lower final thrombus mass in the injured artery. Similar effect was also observed in maintenance of blood flow during thrombolysis. The flow profile obtained for various groups indicates that the flow restoration by high dose FXV673 was significantly higher compared to vehicle, heparin and RPR109891 groups. Most importantly, the antithrombotic effects were sustained for an additional 2 h despite discontinuing the infusion. This was in contrast to the heparin and RPR109891 groups, wherein all vessels reoccluded upon termination of infusion. The actual minutes of flow (vessel patency) demonstrated a dose-dependent prolongation of flow during thrombolysis. Furthermore, the duration of flow obtained by high dose FXV673 was significantly longer than that observed for heparin and RPR109891.
The beneficial and superior effects of FXV673 during thrombolysis were observed without derangement of the haemostatic system. Infusion of FXV673 at a maximally effective dose in the canine thrombolysis model was associated with 2.8 fold increase in APTT and 2 fold increase in PT. Another index of haemostasis, the template bleeding time, was prolonged by 2.7 fold during the concomitant infusion of rt-PA and FXV673. Heparin produced clinically relevant changes in the haemostatic parameters. The dose of RPR109891 selected increased the bleeding time by 4 fold, and resulted in 80–90% inhibition of platelet aggregation, which is the target for many clinically used GPIIb/IIIa receptor antagonists. Despite these changes, heparin and RPR109891 were not as effective as FXV673 in maintaining the blood flow during thrombolysis. The changes in coagulation parameters induced by FXV673 were concentration dependent such that discontinuation of the infusion normalized these parameters. The latter effect confirms the short biological half-life of FXV673.
The superiority of FXV673 over heparin can be explained considering the known limitations of the latter. Unlike heparin, FXV673 is a specific and direct inhibitor, whether fXa is in the free form or in the prothrombinase complex. FXV673 inhibited fluid-phase and prothrombinase-bound fXa similarly, with IC50s of 1.52±0.06 and 1.38±0.09 nM, respectively (Chu et al., 2000). Heparin is an indirect, AT-III-dependent inhibitor of Xa/IIa activity. During thrombolysis there is heightened thrombin activity at the thrombus surface (Eisenberg et al., 1986; 1992), which is protected from fluid-phase natural inhibitors and standard heparin. The limited efficacy of heparin observed in preclinical and clinical studies reflects the lack of inhibition of thrombus-bound fXa and thrombin by the heparin-ATIII complex (Weitz, 1990) and neutralization by PF4 (Eitzman et al., 1994). Also, prothrombinase activity of whole blood clots is resistant to inhibition by ATIII-dependent inhibitor (Eisenberg et al., 1993). FXa binds to the clot and remains enzymatically active, and despite the presence of physiologic concentrations of antiproteases, clot-bound fXa is capable of cleaving prothrombin in vitro. Under these circumstances, clot-bound fXa could be equally inhibited by direct fXa inhibitors like FXV673 as demonstrated for DX 9065A (Herault et al., 1997). The efficacy data with FXV673 is in agreement with that obtained for another direct fXa inhibitor, ZK-807834 (K1=0.11 nM) in a similar model in dogs. However, a dose of 4 mg kg−1+4 mg kg−1 h−1 was required to demonstrate superiority of ZK-807834 over heparin+aspirin (Baum et al., 1999). At this dose, ZK-807834 resulted in 5, 4 and 2.5 fold increase in APTT, PT and bleeding time, respectively.
The observation that FXV673 was superior to RPR109891 is difficult to explain. It may be argued that the dose of RPR109891 was not optimal in the present study. However, the dose selected resulted in a clinically relevant 80–90% inhibition of platelets ex vivo. Any further increase in dose could have increased the bleeding time by >4 fold. It is known that thrombolytic therapy gives rise to plasmin-mediated platelet activation (Fitzgerald et al., 1989; Montrucchio et al., 1993a, 1993b), and therefore a GPIIb/IIIa receptor antagonist should have negated the participation of platelets in reocclusion. However, it is becoming increasingly clear that in addition to platelet-dependent mechanisms, there are procoagulant factor-dependent pathways that can contribute to acute thrombosis (Eisenberg & Ghigliotti, 1999). Clinical trials with eptifibatide and rt-PA in acute MI also indicate that the markers of thrombin activity such as thrombin-antithrombin complex, fibrinopeptide A and prothrombin fragment 1.2 remain unchanged during therapy despite inhibition of platelet aggregation (Kleiman et al., 2000), indicating that inhibition of thrombin activity may be required even when GPIIb/IIIa receptor antagonists are used. Endothelial injury, such as that in the present model, can expose tissue factor (TF), resulting in the formation of TF-VIIa complex. The latter via IX activation leads to Xa generation, which in turn through the Xa/Va complex, leads to prothrombin activation. The resultant thrombin can promote platelet deposition and also further activate Xa production through V and VIII-dependent mechanisms. These procoagulant actions mediated by activation of factor X can be effectively inhibited by FXV673 but not by RPR109891. Moreover, discontinuation of RPR109891 infusion may have reversed the inhibition of platelets at the site of injury and activated the process of rethrombosis. There was flow reduction after the discontinuation of FXV673 infusion, but the extent was lower than that with RPR109891 because of 100% incidence of reocclusion in the latter group.
In summary, the results indicate that inhibition of fXa by FXV673 during rt-PA-induced thrombolysis can lead to enhanced reperfusion compared to conventional therapy. Specifically, the combination of aspirin and FXV673 appears to be superior to the combination of aspirin and heparin or GPIIb/IIIa receptor antagonist in maintaining blood flow in the recanalized coronary artery. These results suggest that FXV673 may prove to be an effective antithrombotic adjunct in patients undergoing thrombolysis for acute myocardial infarction.
Abbreviations
- APTT
activated partial thromboplastin time
- fXa
factor Xa
- LCX
left circumflex coronary artery
- PT
prothrombin time
- rt-PA
recombinant tissue plasminogen activator
References
- ANTMAN E.M. Hirudin in acute myocardial infarction. Thrombolysis and Thrombin Inhibition in Myocardial Infarction (TIMI) 9B trial. Circulation. 1996;94:911–921. doi: 10.1161/01.cir.94.5.911. [DOI] [PubMed] [Google Scholar]
- ANTMAN E.M., GIUGLIANO R.P., GIBSON C.M., MCCABE C.H., COUSSEMENT P., KLEIMAN N.S., VAHANIAN A., ADGEY A.A., MENOWN I., RUPPRECHT H.J., VAN DER WIEKEN R., DUCAS J., SCHERER J., ANDERSON K., VAN DE WERF F., BRAUNWALD E. Abciximab facilitates the rate and extent of thrombolysis: results of the thrombolysis in myocardial infarction (TIMI) 14 trial. The TIMI 14 Investigators. Circulation. 1999;99:2720–2732. doi: 10.1161/01.cir.99.21.2720. [DOI] [PubMed] [Google Scholar]
- BAUM P., LIGHT D., VERHALLEN P., EISENBERG P., ABENDSCHEIN D. A novel inhibitor of factor Xa accelerates coronary fibrinolysis and improves long-term patency in dogs. Thromb Haemost. 1999;82 Suppl:90. [Google Scholar]
- BELL W.R. Heparin-associated thrombocytopenia and thrombosis. J. Lab. Clin. Med. 1988;111:600–605. [PubMed] [Google Scholar]
- CHU V., BROWN K., COLUSSI D., GAO J., GUERTIN K., ZULLI A., PAULS H., SPADA A., PERRONE M.H., DUNWIDDIE C., LEADLEY R. In vitro characterization of a novel factor Xa inhibitor, RPR130673. Blood. 2000;96 Suppl:A236. doi: 10.1016/s0049-3848(00)00227-9. [DOI] [PubMed] [Google Scholar]
- CHU V., SABATINO R., BROWN K., KLEIN S., CZEKAJ M., GARDNER C., LEADLEY R., BOSTWICK J., KASIEWSKI C., O'CONNOR E., PERRONE M., DUNWIDDIE C. In vitro characterization of a novel fibrinogen receptor antagonist RPR109891 and its active metabolite RPR109039. Blood. 1997;90 Suppl.1:3214. [Google Scholar]
- EISENBERG P.R., GHIGLIOTTI G. Platelet-dependent and procoagulant mechanisms in arterial thrombosis. Int. J. Cardiol. 1999;68 Suppl.1:S3–S10. doi: 10.1016/s0167-5273(98)00284-8. [DOI] [PubMed] [Google Scholar]
- EISENBERG P.R., SHERMAN L., RICH M., SCHWARTZ D., SCHECHTMAN K., GLETMAN E.M., SOBEL B.E., JAFFE A.S. Importance of continued activation of thrombin reflected by fibrinopeptide A to the efficacy of thrombolysis. J. Am. Coll. Cardiol. 1986;7:1255–1262. doi: 10.1016/s0735-1097(86)80144-9. [DOI] [PubMed] [Google Scholar]
- EISENBERG P.R., SIEGEL J.E., ABENDSCHEIN D.R., MILETICH J.P. Importance of factor Xa in determining the procoagulant activity of whole-blood clots. J. Clin. Invest. 1993;91:1877–1883. doi: 10.1172/JCI116404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISENBERG P.R., SOBEL B.E., JAFFE A.S. Activation of prothrombin accompanying thrombolysis with recombinant tissue-type plasminogen activator. J. Am. Coll. Cardiol. 1992;9:1065–1069. doi: 10.1016/0735-1097(92)90296-y. [DOI] [PubMed] [Google Scholar]
- EITZMAN D.T., CHI L., SAGGIN R.S., LUCCHESI B.R., FAY W.P. Heparin neutralization by platelet-rich thrombi. Role of platelet factor 4. Circulation. 1994;89:1523–1529. doi: 10.1161/01.cir.89.4.1523. [DOI] [PubMed] [Google Scholar]
- FITZGERALD D.J., WRIGHT F., FITZGERALD G.A. Increased thromboxane biosynthesis during coronary thrombolysis: Evidence that platelet activation and thromboxane A2 modulate the response to tissue-plasminogen activator in vivo. Circ. Res. 1989;65:83–94. doi: 10.1161/01.res.65.1.83. [DOI] [PubMed] [Google Scholar]
- GRANGER C.B., MILLER J.M., BOVILL E.G., GRUBER A., TRACY R.P., KRUCOFF M.W., GREEN C., BERRIOS E., HARRINGTON R.A., OHMAN E.M. Rebound increase in thrombin generation and activity after cessation of intravenous heparin in patients with acute coronary syndromes. Circulation. 1995;91:1929–1935. doi: 10.1161/01.cir.91.7.1929. [DOI] [PubMed] [Google Scholar]
- GUSTO IIb Investigators A comparison of recombinant hirudin with heparin for the treatment of acute coronary syndromes. The Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) IIb investigators. N. Engl. J. Med. 1996;335:775–782. doi: 10.1056/NEJM199609123351103. [DOI] [PubMed] [Google Scholar]
- HARA T., YOKOYAMA A., ISHIHARA H., YOKOYAMA Y., NAGAHARA T., IWAMOTO M. DX-9065a, a new synthetic, potent anticoagulant and selective inhibitor for factor Xa. Thromb. Haemost. 1994;71:314–319. [PubMed] [Google Scholar]
- HERAULT J.P., BERNAT A., PFLIEGER A.M., LORMWAU J.C., HERBERT J.M. Comparative effects of two direct and indirect factor Xa inhibitors on free and clot-bound prothrombinase. J. Pharmacol. Exp. Ther. 1997;283:16–22. [PubMed] [Google Scholar]
- HERBERT J.M., BERNAT A., DOL F., HERAULT J.P., CREPON B., LORMEAU J.C. DX 9065A a novel, synthetic, selective and orally active inhibitor of factor Xa: in vitro and in vivo studies. J. Pharmacol. Exp. Ther. 1996;276:1030–1038. [PubMed] [Google Scholar]
- JANG I.K., BROWN D.F., GIUGLIANO R.P., ANDERSON H.V., LOSORDO D., NICOLAU J.C., DUTRA O.P., BAZZINO O., VIAMONTE V.M., NORBADY R., LIPRANDI A.S., MASSEY T.J., DINSMORE R., SCHWARZ R.P., JR A multicenter, randomized study of argatroban versus heparin as adjunct to tissue plasminogen activator (TPA) in acute myocardial infarction: myocardial infarction with novastan and TPA (MINT) study. J. Am. Coll. Cardiol. 1999;33:1879–1885. doi: 10.1016/s0735-1097(99)00107-2. [DOI] [PubMed] [Google Scholar]
- KLEIMAN N.S., TRACY R.P., TALLEY J.D., SIGMON K., JOSEPH D., TOPOL E.J., CALIFF R.M., KITT M., OHMAN E.M. Inhibition of platelet aggregation with a glycoprotein IIb-IIIa antagonist does not prevent thrombin generation in patients undergoing thrombolysis for acute myocardial infarction. J. Thromb. Thrombolysis. 2000;9:5–12. doi: 10.1023/a:1018650123272. [DOI] [PubMed] [Google Scholar]
- MONTRUCCHIO G., ALLOATTI G., MARIANO F., LUPIA E., LUCCHINA P.G., MUSSO E., EMANUELLI G., CAMUSSI G. Role of platelet-activating factor in hypotension and platelet activation induced by infusion of thrombolytic agents in rabbits. Circ. Res. 1993b;72:658–670. doi: 10.1161/01.res.72.3.658. [DOI] [PubMed] [Google Scholar]
- MONTRUCCHIO G., BERGERONE S., ALLOATTI G., SILVESTRO L., LUPIA E., CRAVETTO A., LEO M., EMANUELLI G., CAMUSSI G. Streptokinase induces intravascular release of platelet activating factor in patients with acute myocardial infarction and stimulates its synthesis by cultured human endothelial cells. Circulation. 1993a;88:1476–1483. doi: 10.1161/01.cir.88.4.1476. [DOI] [PubMed] [Google Scholar]
- TANIUCHI Y., SAKAI Y., HISAMICHI N., KAYAMA M., MANO Y., SATO K., HIRAYAMA F., KOSHIO H., MATSUMOTO Y., KAWASAKI T. Biochemical and pharmacological characterization of YM-60828, a newly synthesized and orally active inhibitor of human factor Xa. Thromb. Haemostasis. 1998;79:543–548. [PubMed] [Google Scholar]
- WEITZ J.I., HUDOBA M., MASSEL D., MARAGANORE J., HIRSH J. Clot-bound thrombin is protected from inhibition by heparin-antithrombin III but is susceptible to inactivation by antithrombin III-independent inhibitors. J. Clin. Invest. 1990;86:385–391. doi: 10.1172/JCI114723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WONG P.C., QUAN M.L., CRAIN E.J., WATSON C.A., WEXLER R.R., KNABB R.M. Nonpeptide Factor Xa Inhibitors: I. Studies with SF303 and SK549, a New Class of Potent Antithrombotics. J. Pharmacol. Exp. Ther. 2000;292:351–357. [PubMed] [Google Scholar]


