Abstract
Hydrogen peroxide (H2O2) caused a transient contraction in endothelium-intact (E+) and -denuded (E−) mesenteric arteries (MA) from 8 – 10-month-old spontaneously hypertensive rats (SHR) and normotensive Wistar-Kyoto rats (WKY) in a concentration-dependent manner (10−5 M to 10−3 M).
The contraction to H2O2 in MA (E+ or E−) was greater in SHR than in WKY. Removal of endothelium potentiated the contraction to H2O2 in WKY but not in SHR. Tachyphylaxis to H2O2 was less prominent in SHR than in WKY.
The contraction of aorta to H2O2 (5×10−4 M), expressed as a percentage of 80 mM KCl-induced contraction, was approximately half of that found in the MA. A greater contraction was found in E+ but not E− SHR aortic rings.
The contraction of MA to H2O2 (5×10−4 M) was greatly inhibited by SQ 29548 and ICI 192605 (thromboxane A2 (TXA2)/prostaglandin H2 receptor antagonists), quinacrine (a phospholipase A2 (PLA2) inhibitor), indomethacin and diclofenac (cyclooxygenase (COX) inhibitors), and furegrelate (a TXA2 synthase inhibitor).
Production of thromboxane B2 induced by H2O2 (5×10−4 M) was greater in SHR MA than in WKY, and was inhibited by quinacrine, indomethacin and diclofenac, and furegrelate, but not by SQ 29584 and ICI 192605.
These results suggested (1) that SHR MA exhibits a higher contraction involving an increased smooth muscle reactivity and less tachyphylaxis to H2O2 than WKY; (2) that a greater production of TXA2 through activation of PLA2-COX-TXA2 synthase pathway appeared to be responsible for the enhanced contraction in SHR MA. The enhanced vascular response to H2O2 may be related to hypertension in SHR.
Keywords: Hydrogen peroxide, hypertension, mesenteric artery, reactive oxygen species, spontaneously hypertensive rats, thromboxane A2, Wistar-Kyoto rats
Introduction
Although hydrogen peroxide (H2O2) is a non-radical form of reactive oxygen species (ROS) and only possesses moderate oxidant reactivity, it is probably more harmful than superoxide anion (O2−.), because H2O2 can easily diffuse across plasma membrane and enter the inner compartments of a cell (Bergendi et al., 1999). O2−. can be produced by NAD(P)H oxidase (Griendling et al., 2000) and serves as the substrate for superoxide dismutase (SOD) to generate H2O2. Previous studies on ROS were mainly focused on their deleterious effects on the cells or organisms including oxidation of membrane lipids, inactivation and oxidization of certain enzymes and proteins, and damage to DNA by ROS (Machlin & Bendich, 1987; Oliver, 1987). Recent finding that some forms of ROS (e.g. H2O2) may serve as an important second messenger molecule in certain vital processes such as cell growth (Bass & Berk, 1995; Sundaresan et al., 1995) has turned our attention to other physiological roles that ROS may play.
Vascular tissues including endothelial cells, smooth muscle cells and fibroblasts are a rich source of ROS (Griendling & Alexander, 1997). The vasoactive effects of ROS have been shown in various arteries from normal animals including rat aorta (Rodriguez-Martinez et al., 1998; Hibino et al., 1999), canine basilar artery (Katusic et al., 1993), bovine pulmonary artery (Wolin et al., 1985), and human placental arteries (Omar et al., 1992). The mitogenic or apoptotic effects of ROS on vascular smooth muscle cells have also been reported (Bass & Berk, 1995; Li et al., 1997). Taken together, these results suggest that ROS may participate in physiological or pathological regulation of vascular function and structure.
In hypertension, the role of ROS is gaining increasing attention. Patients with uncontrolled hypertension showed a higher level of H2O2 and O2−., and these high levels had reverted to normal levels after the control of high blood pressure (Prabha et al., 1990). In animal models of hypertension, deoxycorticosterone acetate-salt hypertensive rats and Dahl hypertensive rats have been shown to exhibit enhanced vascular O2−. production (Swei et al., 1997; Somers et al., 2000). Hypertension produced by exogenously applied angiotensin II or by renovascular ligation also showed similar increase of ROS level (Rajagopalan et al., 1996; Harrison et al., 1999). In the spontaneously hypertensive rats (SHR), a higher level of ROS and lipid peroxidation in mesenteric arterioles and myocardium has been reported (Ito et al., 1992; Suzuki et al., 1995). In vascular reactivity studies with isolated vessels from SHR, higher contractile responses to exogenously applied H2O2 or O2−. or lipid peroxides have been shown (Auch-Schwelk et al., 1989; Rodriguez-Martinez et al., 1998; Hibino et al., 1999; Garcia-Cohen et al., 2000). However, these results were all derived from aorta, which is a large elastic conduit artery. It is not known if similar changes also occur in smaller arteries such as the mesenteric arteries (MA).
Although H2O2 is widely used in in vitro vascular reactivity studies to induce oxidative stress, the detailed pathway and the mediator responsible for H2O2-induced contraction are not fully understood. Activation of several key enzymes such as phospholipase A2 (PLA2) (Chakraborti et al., 1989), phospholipase C (Sheehan et al., 1993), tyrosine kinase (Jin & Rhoades, 1997), protein kinase C (Yang et al., 1998), cyclooxygenase (COX) (Rodriguez-Martinez et al., 1998) has been suggested in H2O2-induced vascular contraction. For the mediator of H2O2-induced contraction of SHR artery, as far as we know, there is only one study which suggested that thromboxane A2 (TXA2) / prostaglandin H2 (TP) may be the candidate in the aorta but the measurement of tissue level of TXA2 metabolite was not carried out (Rodriguez-Martinez et al., 1998). Therefore, information on the mediator(s) of H2O2-induced vascular contraction in hypertensive animals is still lacking.
In this study, we used a combined functional and biochemical approach to examine the reactivity of isolated SHR MA to H2O2 stimulation and measured the vascular production of TXA2. The possible pathway and the mediator involved in the contraction were studied and the responses to H2O2 in MA and aorta were compared.
Methods
Animals
Male SHR and WKY at the age of 8 – 10 months were obtained from the rat colonies maintained at the McMaster University Central Animal Facilities. These colonies originated from the Charles River strains, and we have maintained these inbred colonies at our institute for over 20 years. The care and the use of these animals were in accordance with the guidelines of the Canadian Council on Animal Care.
Reactivity experiments
The rats were anaesthetized with sodium pentobarbitone (50 mg kg−1 i.p.), and then exanguinated by bleeding from the abdominal aorta. The mesentery and the thoracic aorta were isolated by dissection and placed in oxygenated Krebs solution at 4°C. The superior mesenteric artery and the aorta were carefully dissected out, cleaned of connective tissues and cut into 4 mm segments under a dissecting microscope. Each mesenteric arterial ring was mounted in a 3 ml organ bath with a resting tension of 1.5 g, and each aortic ring in 5 ml organ bath with 4 g resting tension, which we had determined to be the optimal resting tensions, and isometric contractile response was recorded. The composition of Krebs solution was (in mM): NaCl, 118; KCl, 4.7; CaCl2, 2.5; MgSO4, 1.18; KH2PO4, 1.08; NaHCO3, 25; Glucose, 11; HEPES, 8. The organ chamber was continuously bubbled with 95% O2 and 5% CO2, and maintained at 37°C. The solution in the organ bath was changed every 15 – 20 min. After 1 h of equilibration, the rings were challenged with 80 mM KCl until a stable contraction was achieved. In some segments, the endothelium was removed by rubbing the internal surface of the ring with a fine wooden stick. Successful removal of endothelium was indicated by the absence of relaxation response to carbamycholine chloride (3×10−6 M) in rings precontracted with phenylephrine (10−6 M).
Experimental protocol
To assess the contractile response to H2O2, arterial rings with and without endothelium was exposed to different concentrations of H2O2 for 5 min, followed by a thorough wash with Krebs solution. In experiments to assess the effects of catalase, SOD, and dimethylsulphoxide (DMSO), the rings with endothelium were incubated with one of these agents for 5 – 10 min before exposure to H2O2. In most cases, one ring was exposed to only one concentration of H2O2 because of the tachyphylaxis to H2O2 we had noticed in our preliminary experiments. In experiments to quantify the tachyphylaxis phenomenon, the same concentration of H2O2 was added at an interval of 30 min after the tension had returned to the basal level. The concentration-response curves for H2O2 were constructed using different rings. In assessing the effects of various enzyme inhibitors and receptor antagonists, endothelium-denuded rings were used and 25 min of incubation was allowed before exposure to H2O2. Generally, 5 – 6 rings were obtained from the middle part of the superior mesenteric arcade from each rat. We randomly took one ring as control, and the other rings to test various agents. The contraction to H2O2 was normalized with the contraction to 80 mM KCl. The contraction of the aorta and the mesenteric arteries to 80 mM KCl was similar between SHR and WKY (in grams, aorta: 3.2±0.22 for SHR, 2.9±0.17 for WKY; mesenteric arteries: 0.6±0.12 for SHR, 0.63±0.11 for WKY), and removal of the endothelium also did not affect the response of these vessels to 80 mM KCl (in grams, aorta 3.17±0.18 for SHR, 3.03±0.27 for WKY; mesenteric arteries: 0.62±0.14 for SHR, and 0.66±0.12 for WKY) (n=6 – 9). The inhibitory effects of enzyme inhibitor or receptor antagonist were expressed as per cent contraction of the control. All these inhibitors and receptor antagonists at the concentrations used did not affect the basal tension or the contractions to 80 mM KCl. The only exception was that quinacrine (10−4 M) slightly slowed down the onset of KCl-induced contraction. The concentration-response curve for U46619 was made in endothelium-denuded mesenteric arterial rings, and was expressed as per cent of 80 mM KCl-induced contraction. Concentration for 50% maximal response (EC50) was estimated by fitting each concentration-response curve. At the end of each experiment, the viability of the artery reactivity was tested by exposing the arteries to 80 mM KCl, and the wet weight of the artery was measured.
Measurement of thromboxane B2 (TXB2)
Samples of the bathing solution in the organ bath were taken before and 5 min after adding H2O2, and stored at −80°C. The incubation time was set to 5 min because the contraction to H2O2 was transient and had returned to the baseline within 5 min in most cases. The concentration of TXB2 was measured using a TXB2 enzyme-linked immunosorbent assay kit (Neogen, U.S.A.). The content of TXB2 was expressed as pg mg−1 wet weight of the arterial rings.
Chemicals
The following chemicals were used: H2O2 and DMSO (BDH Inc. Toronto, Canada); carbamycholine chloride, catalase, furegrelate sodium salt, indomethacin, quinacrine dihydrochloride, SOD, U46619 (Sigma, U.S.A.); diclofenac sodium, SQ 29548 (RBI, Sigma, U.S.A.); ICI 192605 (Tocris, U.S.A.). Indomethacin was dissolved in 0.5% sodium carbonate, diluted in deionized water, and freshly prepared before use; SQ 29548 and U46619 were dissolved in absolute ethanol and diluted in 50% ethanol; ICI 192605 was dissolved in DMSO and diluted in deionized water. All other agents were dissolved in deionized water and prepared fresh daily.
Statistical analysis
Contractile response to H2O2 was assessed by the tension developed. The results were expressed as mean±s.e.mean, where n represents the number of rats. Statistical analysis was performed by one-way ANOVA or unpaired Student's t-test. The differences were considered significant when P<0.05.
Results
At 8 – 10 months of age, body weight of SHR (387±20 g, n=18) was lighter than WKY (436±18 g, n=15, P<0.05). Systolic blood pressure measured using the tail-cuff compression method was significantly higher in the SHR (196±9 mmHg) than WKY (133±6 mmHg, P<0.01).
The contractile response of MA to a single concentration of H2O2 was composed of a quick onset and a transient component, and in most cases the tension developed had returned to the baseline within 5 min (Figure 1A). H2O2 at concentrations of 10−5 M, 10−4 M, and 10−3 M produced concentration-dependent contractile responses in MA. Removal of endothelium increased the contraction to H2O2 at 10−5 M, and 10−3 M in WKY but not in SHR. A higher level of contraction was found in endothelium-intact (E+) MA of SHR at the three concentrations of H2O2 tested and in endothelium-denuded (E−) MA of SHR at the two higher concentrations (10−4 M, and 10−3 M) as compared with WKY (Figure 1B).
Figure 1.
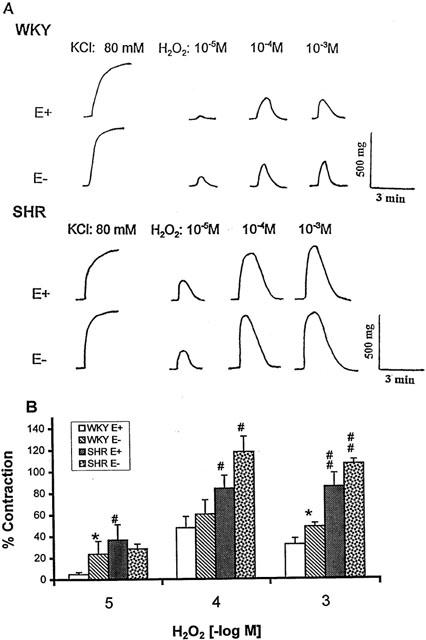
(A) Typical tracings showing the contractile effects of hydrogen peroxide (H2O2, 10−5 M, 10−4 M, and 10−3 M) in WKY and SHR mesenteric arteries with intact (E+) or denuded (E−) endothelium. (B) Concentration-response relation of H2O2. Results (mean±s.e.mean) were from 4 – 6 rats and expressed as the percentage of the contraction elicited by 80 mM KCl. *P<0.05, compared with respective E+ arteries; #P<0.05, ##P<0.01 between SHR and respective WKY arteries with or without endothelium.
Based on the concentration-response results, we had selected 5×10−4 M of H2O2 for further investigation on contraction mechanisms, because the tension developed at this concentration was higher in both E+ and E− MA from SHR than WKY, and endothelium removal only increased the contraction to H2O2 in WKY (Figure 2A). Contractile response to H2O2 (5×10−4 M) in MA was nearly abolished by catalase (1000 u/ml) but not affected by SOD (150 u/ml) or DMSO (5 mM) in E+ MA (Figure 2B).
Figure 2.
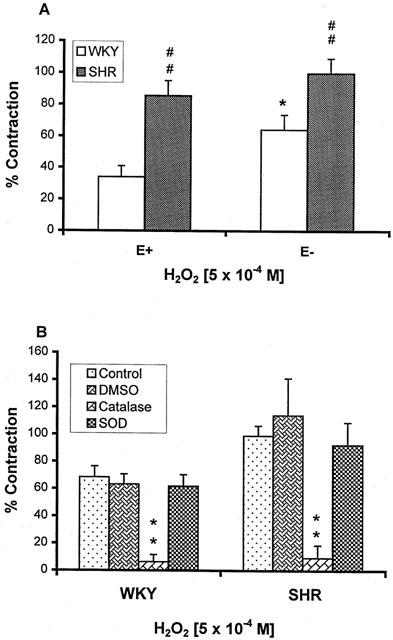
(A) Contractile effects of H2O2 (5×10−4 M) on the mesenteric artery with (E+) or without (E−) endothelium from SHR and WKY. *P<0.05 compared with E+ arteries; #P<0.05, ##P<0.01, compared with WKY arteries. (B) Effects of dimethylsulphoxide (DMSO, 5 mM), catalase (1000 u/ml), and superoxide dismutase (SOD, 150 u/ml) on the contraction induced by H2O2 (5×10−4 M) in the E+ mesenteric artery of 4 – 6 rats each from WKY and SHR. **P<0.01 compared with their respective control.
The contraction of MA to H2O2 (5×10−4 M) was compared with that of the aorta. H2O2 also caused a transient contractile response in aorta with or without endothelium, but the magnitude of contractions, expressed as per cent of contraction induced by 80 mM KCl, was only approximately half of that found in the MA (Figure 3A). Removal of endothelium increased the contraction in WKY but not in SHR. A higher level of contraction was found in SHR as compared with WKY in aortic rings with intact endothelium, but not in the rings where endothelium was removed (Figure 3B).
Figure 3.
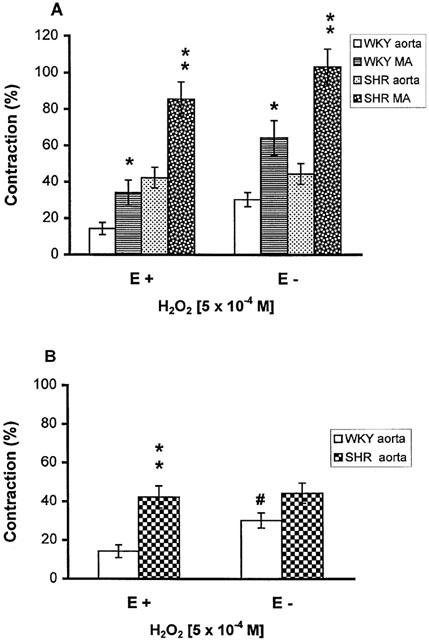
(A) Comparison of the contractile effects of H2O2 (5×10−4 M) on the mesenteric artery (MA) and the aorta, with (E+) or without (E−) endothelium, from SHR and WKY. *P<0.05, **P<0.01 compared with aorta; (B) Contraction to H2O2 (5×10−4 M) in E+ and E− aorta from SHR and WKY. **P<0.01, compared with WKY aorta, #P<0.05 compared with E+ rings. Results (mean±s.e.mean) were from 6 – 11 rats and expressed as the percentage of the contraction elicited by 80 mM KCl.
Upon repeated exposure to the same concentration of H2O2 (5×10−4 M), the contraction of the MA became smaller after the first challenge. In E− MA, the reduction in the amplitude of response was less in SHR than in WKY: the contraction in SHR arteries remained around 40% of KCl-induced contraction after the 4th challenge but only around 10% in WKY (Figure 4).
Figure 4.
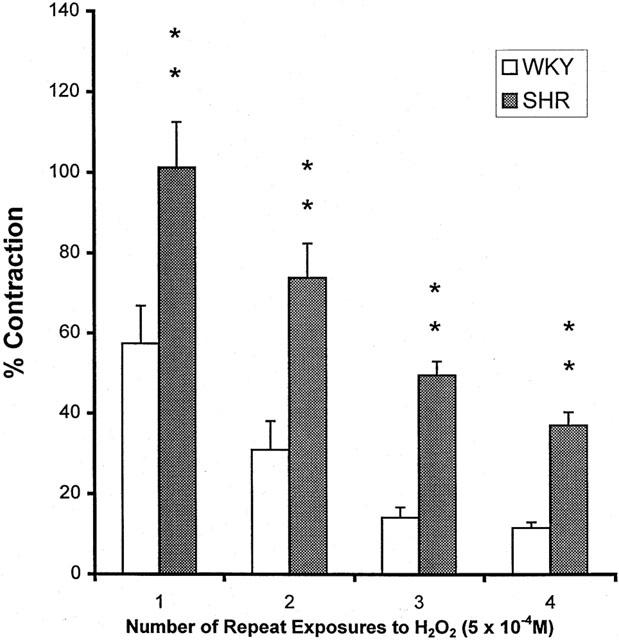
Tachyphylaxis of the contraction by H2O2 (5×10−4 M) in endothelium-denuded mesenteric artery from WKY and SHR. Results (mean±s.e.mean) were from four each of SHR or WKY and expressed as the percentage of the contraction elicited by 80 mM KCl. **P<0.01 compared with respective WKY arteries.
The mechanisms responsible for the contractile response to H2O2 were investigated in E− MA. SQ 29548 and ICI 192605, both are thromboxane A2/prostaglandin H2 (TP) receptor antagonists, significantly inhibited the contractile response of arteries to H2O2 in both SHR and WKY at concentrations of 1 and 3×10−8 M (Figure 5A), and nearly abolished the contraction at the concentration of 10−6 M (data not shown). The contractile response to U46619, a TP receptor agonist, in E− MA was similar between SHR and WKY as shown in Figure 5B (EC50: 17.9±0.3 nM for WKY, n=5; 17.5±0.7 nM for SHR, n=4).
Figure 5.
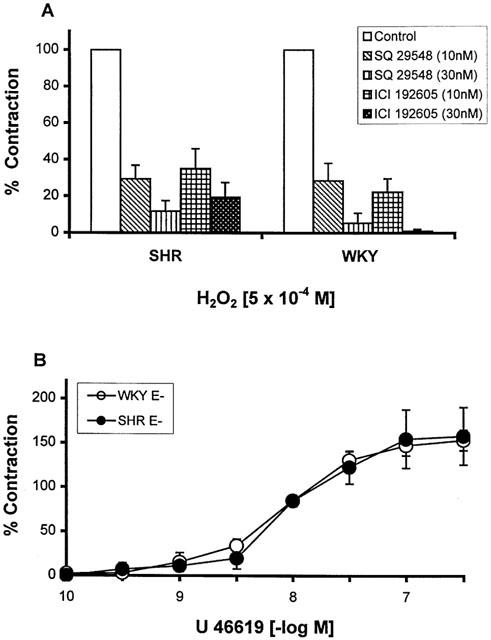
(A) Inhibitory effects of SQ 29548 and ICI 192605 on H2O2 (5×10−4 M) induced contraction in the endothelium-denuded (E−) mesenteric artery from WKY and SHR. Results (mean±s.e.mean) were from four each of SHR or WKY and expressed as the percentage of the contraction of the control arteries. (B) Contraction by U46619 in the E− mesenteric artery from WKY and SHR. Results (mean±s.e.mean) were from four each of SHR or WKY and expressed as the percentage of the contraction elicited by 80 mM KCl.
Incubation with quinacrine (a PLA2 inhibitor, 10−4 M), indomethacin and diclofenac (both are non-selective COX inhibitors, 1 and 3×10−6 M), and furegrelate (a TXA2 synthase inhibitor, 10−4 M) significantly inhibited the contractile response to H2O2 in both SHR and WKY MA (Figure 6).
Figure 6.
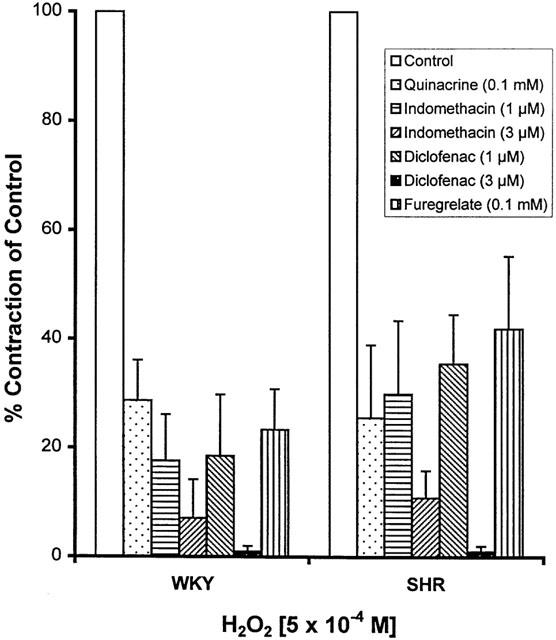
Inhibitory effects of quinacirne, indomethacin, diclofenac, and furegrelate on the contraction induced by H2O2 (5×10−4 M) in E− mesenteric artery from WKY and SHR. Results (mean±s.e.mean) were from four each of SHR or WKY and expressed as the percentage of the contraction in the control arteries.
The concentration of TXB2, a stable metabolite of TXA2, was measured in the bathing solutions from the contractility studies with E− MA. The basal levels of TXB2 were not different between SHR and WKY, but H2O2 (5×10−4 M) induced a greater increase of TXB2 in SHR than WKY. Incubation with quinacrine (10−4 M), indomethacin and diclofenac (10−6 M), and furegrelate (10−4 M) significantly reduced the production of TXB2 in response to H2O2, while SQ 29548 (10−8 M) and ICI 192605 (10−8 M) did not affect TXB2 production (Figure 7).
Figure 7.
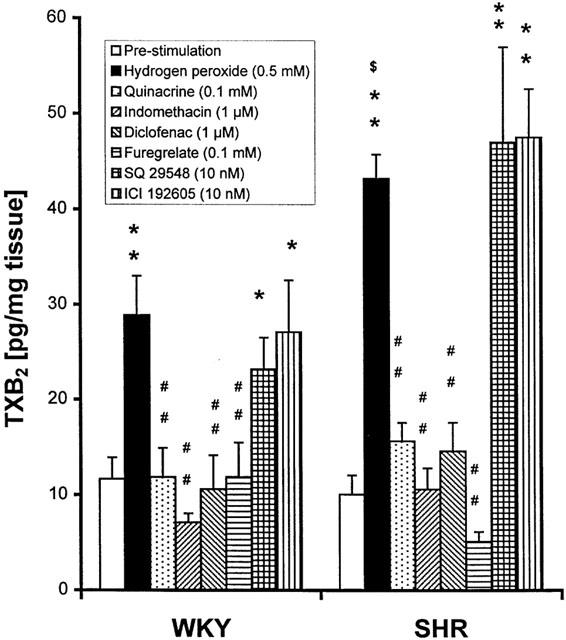
Thromboxane B2 (TXB2) production (pg/mg wet tissue), stimulated by H2O2 (5×10−4 M), and the effects of enzyme inhibitors (quinacirne, indomethacin, diclofenac, and furegrelate) and of thromboxane A2 and/or prostaglandin H2 receptor antagonists (SQ 29548 and ICI 192605) in E− mesenteric artery from WKY and SHR. Results (mean±s.e.mean) were from 4 – 6 rats each from SHR and WKY. *P<0.05, **P<0.01 compared with respective pre-stimulation level; ##P<0.01, compared with H2O2; $P<0.05 compared with respective WKY.
Discussion
The purpose of the present study was to compare the contractile response to exogenously applied H2O2 in the isolated MA from SHR and WKY, and to investigate the mechanisms underlying H2O2-induced contraction. The contractile responses to H2O2 in MA and in the aorta were also compared. The main findings of this study were (1) that MA from SHR exhibited a higher contraction and less tachyphylaxis to H2O2 as compared with MA from WKY; (2) that an increase in smooth muscle reactivity was involved in the enhanced contraction to H2O2 in SHR MA; (3) that a greater production of TXA2 through activation of PLA2-COX-TXA2 synthase pathway appeared to be responsible for the enhanced contraction in SHR. We also found that MA contracted more to H2O2 than the aorta and the higher contraction presented in SHR aorta was an event associated with reduced negative modulation of endothelium on the response to H2O2.
Among ROS, hydroxyl radical (OH.) is regarded as the most potent oxidant. The OH. can be generated when H2O2 comes into contact with copper ion (Cu2+) or iron ion (Fe2+) by Fenton reaction (Fenton, 1984). In bovine pulmonary artery smooth muscle cells, H2O2 markedly stimulated OH. formation (Roychoudhury et al., 1996). It has been shown that OH. can contract rat aorta (Shen et al., 2000a). O2−. can interact with H2O2 by Haber-Weiss reaction to form OH. (Cheeseman & Slater, 1993) in addition to its own vasoactive action (Somers et al., 2000). Therefore it is necessary to clarify if the contraction to H2O2 is caused by H2O2 itself, or by other related ROS. Our results showed that the contraction induced by H2O2 in rat MA was not through OH. or O2−, because catalase, the decomposing enzyme of H2O2, nearly abolished H2O2-induced contraction, while SOD, a O2− scavenger, or DMSO, a OH. scavenger, did not affect H2O2-induced contraction.
H2O2 can cause either vasoconstriction or relaxation depending on the contractile state of the vessels. Vasoconstriction was reported in vessels without pre-contraction. In arteries precontracted with phenylephrine (Zembowicz et al., 1993), norepinephrine (Fujimoto et al., 2001), or prostaglandin F2α (Beny & Von der Weid, 1991), H2O2 produced a dose-dependent relaxation response. The relaxation involves endothelium-dependent or -independent mechanisms, which could be mediated by nitric oxide (Zembowicz et al., 1993), cyclic GMP (Iesaki et al., 1996), cytochrome P450 products (Yang et al., 1999), or membrane hyperpolarization (Beny & Von der Weid, 1991). A recent report by Matoba et al. (2000) suggested that H2O2 is an endothelium-derived hyperpolarizing factor in acetylcholine-induced relaxation of mouse small MA, while another report by Hamilton et al. (2001) did not support this notion in the study on the relaxation response of human radial arteries to carbachol. The difference in animal species and vessel types may contribute to the difference in findings in these two reports.
Previous studies on the contractile response of MA to H2O2 include those by Hubel et al. (1993) who reported that there was no observable response to H2O2 in quiescent mesenteric arteries, and by Pelaez et al. (2000) who found that H2O2 induced contraction in MA from Sprague-Dawley rats through a calcium-independent mechanism. Our study is probably the first to show that H2O2 can induce prominent contraction of MA from SHR and WKY in a concentration-dependent manner. In order to find out whether MA is more reactive to H2O2 than aorta, we compared the contractile response to H2O2 in these vessels. Aorta is probably the most commonly used rodent vessel in in vitro vascular functional studies, but it is a large elastic conduit vessel and contributes little if any to the regulation of blood flow or peripheral resistance. Results generated using the aorta may not be applicable to smaller artery because their responses may differ qualitatively or quantitatively. For example, norbormide relaxes rat aorta by calcium entry blocker activity but contracts MA by stimulating phospholipase C-protein kinase C pathway (Bova et al., 2000). Another study showed that serotonin contracted rat aorta and MA with different EC50s (Adegunloye & Sofola, 1997). In this study, we found that MA from both SHR and WKY generated twice as much tension as those generated by the aorta. This showed that the MA was more sensitive to H2O2 than the aorta. It is possible that H2O2 may affect local blood flow by its vasomotor action on reactive vessels such as MA. Future studies on small resistance vessels will be helpful in understanding the role of H2O2 in blood pressure regulation.
Existing reports suggested that endothelium may participate in the vascular contractile response to H2O2 in certain arteries. In quiescent rat aorta, contraction to H2O2 was augmented by endothelium removal (Rodriguez-Martinez et al., 1998), but in human placental artery, removal of endothelium did not affect H2O2-induced contraction (Omar et al., 1992). It is well known that endothelium is an important regulator of vascular tone, and endothelial dysfunction may exist in SHR (Bauersachs et al., 1998). In the current study, we found that removal of endothelium potentiated H2O2-induced contractions of MA and aorta in normotensive rats WKY but not in SHR. This indicated that the negative modulatory role of endothelium in H2O2-induced contraction was impaired in SHR vessels.
Comparing to WKY, an enhanced contractile response to higher concentrations of H2O2 (10−4 M, and 10−3 M) was found in MA from SHR without intact endothelium, suggesting that the enhanced response to these concentrations of H2O2 was mediated by an increased reactivity of smooth muscles to H2O2. This is different from what was found in the aorta. In the aorta from SHR, the enhanced contraction to H2O2 was found in E+ aortic rings but removal of the endothelium eliminated the difference between SHR and WKY, indicating that endothelial dysfunction which resulted in a reduced modulating effect on smooth muscle contraction was responsible for the enhanced response to H2O2 in SHR aorta. However, in the MA endothelium removal only blunted the difference in the response to H2O2 found in E+ MA of SHR and WKY at low concentration (10−5 M). Taken together, in contrast to the results found in SHR and WKY aorta where difference in H2O2 response was related to endothelial function, in SHR MA an increased smooth muscle reactivity was involved in the enhanced response to exogenously applied H2O2. Furthermore, upon repeated exposure to the same concentration of H2O2, MA from SHR showed less tachyphylaxis than WKY vessels, suggesting that the hyperreactivity to H2O2 was well maintained in SHR even in the case of repeated stimulation by H2O2. This may be another mechanism through which an enhanced contractile response to H2O2 is maintained in the arteries from SHR as compared with those from WKY, which will affect the regulation of local blood flow and resistance.
Experiments were done in E− MA to explore the possible mechanisms responsible for the contractile response to H2O2. It has been reported that H2O2 can activate PLA2 in bovine pulmonary endothelial cells (Chakraborti et al., 1989) and rabbit pulmonary smooth muscle cells (Heinle, 1982; Chakraborti & Michael, 1993). In this study, we found that incubation with quinacrine, a PLA2 inhibitor, significantly reduced the contractile response, suggesting the involvement of PLA2 in H2O2-induced contraction of MA. It is still not known how H2O2 activates PLA2. Involvement of protein kinase C (Chakraborti & Michael, 1993) or mediation by P2-purinoceptors in H2O2-induced contraction (Shen et al., 2000b) has been suggested as possible mechanisms.
A number of reports have shown that COX is involved in ROS-induced contraction (Rodriguez-Martinez et al., 1998; Hibino et al., 1999; Garcia-Cohen et al., 2000). In this study, we found that indomethacin and diclofenac, two non-selective COX inhibitors, significantly inhibited the contraction to H2O2, confirming the participation of COX metabolite-related signal pathway in H2O2-induced vessel contraction. Furthermore, TP receptor antagonists (SQ 29548 and ICI 192605) significantly inhibited the contractile response to H2O2 at lower concentrations (1 and 3×10−8 M), and nearly abolished the response at a higher concentration (10−6 M). We also found that furegrelate (10−4 M), a TXA2 synthase inhibitor, significantly reduced the contraction to H2O2. These results strongly suggest that TXA2 generated from PLA2-COX-TXA2 synthase pathway may serve as the mediator of H2O2-induced contraction. There has been a report showing that TXA2 may act as the mediator in O2−.-stimulated contraction of SHR aorta (Hibino et al., 1999), but for H2O2-induced contraction of SHR artery, as far as we know, there is only one functional study with aorta suggesting that COX metabolites may be the mediator(s) but the measurement of tissue production of TXA2 was not carried out (Rodriguez-Martinez et al., 1998). Our results on the measurement of TXB2 (a stable TXA2 metabolite) clearly showed that H2O2 stimulated the production of TXB2 in the MA, and this production was greater in the arteries from SHR than from WKY. Incubation with enzyme inhibitors including quinacrine, indomethacin, diclofenac, and furegrelate inhibited H2O2-stimulated TXB2 production along with the inhibition of contraction. Treatment with TP receptor antagonists SQ 29584 or ICI 192605 did not inhibit H2O2-stimulated TXB2 production, but markedly inhibited H2O2-induced contraction because of the blockade of TP receptors. These are in agreement with our findings in the functional studies, and indicate that TXA2 is involved in H2O2-induced contraction.
The reactivity to U46619, a TP receptor agonist, was compared in the MA of SHR and WKY, because the greater contractile responses induced by H2O2 could be due to an enhanced reactivity of the SHR arteries to TXA2 as compared with WKY arteries. However, our results which showed that the contractile response of MA to U46619 was similar between SHR and WKY (either in maximal response or EC50), indicated that the enhanced contraction response to H2O2 in SHR was not due to the altered vascular reactivity to TXA2.
In summary, SHR MA exhibits a higher contractile response to H2O2 than MA from WKY, which involves an increased smooth muscle reactivity and less tachyphylaxis to H2O2. A greater production of TXA2 through activation of PLA2-COX-TXA2 synthase pathway appeared to be responsible for the enhanced contraction in SHR MA.
Acknowledgments
We thank Dr E.E. Daniel for the use of his equipment, Dr C.Y. Kwan and Mr Z.J. Shen for their discussion, and Mr Mathew Butler, Miss Angela Demeter and Nermin Attia for their technical assistance. Dr Y.J. Gao is a Research Fellow supported by the Joint Canadian Hypertension Society/Medical Research Council of Canada Special Initiative Program. This study was supported by the Heart and Stroke Foundation of Ontario.
Abbreviations
- COX
cyclooxygenase
- DMSO
dimethyl sulphoxide
- E+
endothelium-intact
- E−
endothelium-denuded
- EC50
concentration for 50% maximal response
- H2O2
hydrogen peroxide
- MA
mesenteric artery
- O2−.
superoxide
- OH.
hydroxyl radical
- PLA2
phospholipase A2
- ROS
reactive oxygen species
- SHR
spontaneously hypertensive rats
- SOD
superoxide dismutase
- TP
thromboxane A2/prostaglandin H2
- TXA2
thromboxane A2
- TXB2
thromboxane B2
- WKY
Wistar- Kyoto rats
References
- ADEGUNLOYE B.I., SOFOLA O.A. Differential responses of rat aorta and mesenteric artery to norepinephrine and serotonin in vitro. Pharmacology. 1997;55:25–31. doi: 10.1159/000139509. [DOI] [PubMed] [Google Scholar]
- AUCH-SCHWELK W., KATUSIC Z.S., VANHOUTTE P.M. Contractions to oxygen-derived free radicals are augmented in aorta of the spontaneously hypertensive rat. Hypertension. 1989;13:859–864. doi: 10.1161/01.hyp.13.6.859. [DOI] [PubMed] [Google Scholar]
- BASS A.S., BERK B.C. Differential activation of mitogen-activated protein kinases by H2O2 and O2− in vascular smooth muscle cells. Circ. Res. 1995;77:29–36. doi: 10.1161/01.res.77.1.29. [DOI] [PubMed] [Google Scholar]
- BAUERSACHS J., BOULOUMIÉ A., MÜLSCH A., WIEMER G., FLEMING I., BUSSE R. Vasodilator dysfunction in aged spontaneously hypertensive rats: changes in NO synthase III and soluble guanylyl cyclase expression, and in superoxide anion production. Cardiovasc. Res. 1998;37:772–779. doi: 10.1016/s0008-6363(97)00250-2. [DOI] [PubMed] [Google Scholar]
- BENY J.L., VON DER WEID P.Y. Hydrogen peroxide: an endogenous smooth muscle cell hyperpolarizing factor. Biochem. Biophys. Res. Commun. 1991;176:378–384. doi: 10.1016/0006-291x(91)90935-z. [DOI] [PubMed] [Google Scholar]
- BERGENDI L., BENES L., DURACKOVA Z., FERENCIK M. Chemistry, physiology and pathology of free radicals. Life Sci. 1999;65:1865–1874. doi: 10.1016/s0024-3205(99)00439-7. [DOI] [PubMed] [Google Scholar]
- BOVA S., TREVISI L., CIMA L., LUCIANI S., GOLOVINA V., CARGNELLI G. Signaling mechanisms for the selective vasoconstrictior effect of norbormide on the rat small arteries. J. Pharmacol. Exp. Ther. 2000;296:458–463. [PubMed] [Google Scholar]
- CHAKRABORTI S., GURTNER G.H., MICHAEL J.R. Oxidant-mediated activation of phospholipase A2 in pulmonary endothelium. Am. J. Physiol. 1989;257:L430–L437. doi: 10.1152/ajplung.1989.257.6.L430. [DOI] [PubMed] [Google Scholar]
- CHAKRABORTI S., MICHAEL J.R. Role of protein kinase C in oxidant-mediated activation of phospholipase A2 in rabbit pulmonary arterial smooth muscle cells. Mol. Cell. Biochem. 1993;122:9–15. doi: 10.1007/BF00925732. [DOI] [PubMed] [Google Scholar]
- CHEESEMAN K.H., SLATER T.F. An introduction to free radical biochemistry. Br. Med. Bull. 1993;49:481–493. doi: 10.1093/oxfordjournals.bmb.a072625. [DOI] [PubMed] [Google Scholar]
- FENTON H.J.H. Oxidation of tartaric acid in the presence of iron. J. Chem. Soc. 1984;65:899–910. [Google Scholar]
- FUJIMOTO S., ASANO T., SAKAI M., SAKURAI K., TAKAGI D., YOSHIMOTO N., ITOH T. Mechanisms of hydrogen peroxide-induced relaxation in rabbit mesenteric small artery. Eur. J. Pharmacol. 2001;412:291–300. doi: 10.1016/s0014-2999(00)00940-7. [DOI] [PubMed] [Google Scholar]
- GARCIA-COHEN E.C., MARIN J., DIEZ-PICAZO L.D., BAENA A.B., SALAICES M., RODRIGUEZ-MARTINEZ M.A. Oxidative stress induced by tert-butyl hydroperoxide causes vasoconstriction in the aorta from hypertensive and aged rats: role of cyclooxygenase-2 isoform. J. Pharmacol. Exp. Ther. 2000;293:75–81. [PubMed] [Google Scholar]
- GRIENDLING K.K., ALEXANDER R.W. Oxidative stress and cardiovascular disease. Circulation. 1997;96:3264–3265. [PubMed] [Google Scholar]
- GRIENDLING K.K., SORESCU D., USHIO-FUKAI M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ. Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- HAMILTON C.A., MCPHADEN A.R., BERG G., PATHI V., DOMINICZAK A.F. Is hydrogen peroxide an EDHF in human radial arteries. Am. J. Physiol. 2001;280:H2451–H2455. doi: 10.1152/ajpheart.2001.280.6.H2451. [DOI] [PubMed] [Google Scholar]
- HARRISON D., SCHIAVI P., FALGUI B. Renovascular hypertension, aortic superoxide production: effect of perindopril and indapamide. Am. J. Hypertens. 1999;12:50A. [Google Scholar]
- HEINLE H. Peroxide induced activation of glycogen phosphorylase A activity in vascular smooth muscle. Biochem. Biophys. Res. Commun. 1982;107:597–601. doi: 10.1016/0006-291x(82)91533-9. [DOI] [PubMed] [Google Scholar]
- HIBINO M., OKUMURA K., IWAMA Y., MOKUNO S., OSANAI H., MATSUI H., TOKI Y., ITO T. Oxygen-derived free radical-induced vasoconstriction by thromboxane A2 in aorta of the spontaneously hypertensive rat. J. Cardiovasc. Pharmacol. 1999;33:605–610. doi: 10.1097/00005344-199904000-00013. [DOI] [PubMed] [Google Scholar]
- HUBEL C.A., DAVIDGE S.T., MCLAUGHLIN M.K. Lipid hydroperoxides potentiate mesenteric artery vasoconstrictor responses. Free Radic. Biol. Med. 1993;14:397–407. doi: 10.1016/0891-5849(93)90089-d. [DOI] [PubMed] [Google Scholar]
- IESAKI T., OKADA T., SHIMADA I., YAMAGUCHI H., OCHI R. Decrease in CA2+ sensitivity as a mechanism of hydrogen peroxide-induced relaxation of rabbit aorta. Cardiovasc. Res. 1996;31:820–825. doi: 10.1016/0008-6363(96)00022-3. [DOI] [PubMed] [Google Scholar]
- ITO H., TORII M., SUZUKI T. A comparative study on defense systems for lipid peroxidation by free radicals in spontaneously hypertensive and normotensive rat myocardium. Comp. Biochem. Physiol. [B] 1992;103:37–40. doi: 10.1016/0305-0491(92)90410-s. [DOI] [PubMed] [Google Scholar]
- JIN N., RHOADES R.A. Activation of tyrosine kinases in H2O2-induced contraction in pulmonary artery. Am. J. Physiol. 1997;272:H2686–H2692. doi: 10.1152/ajpheart.1997.272.6.H2686. [DOI] [PubMed] [Google Scholar]
- KATUSIC Z.S., SCHUGEL J., COSENTINO F., VANHOUTTE P.M. Endothelium-dependent contractions to oxygen-derived free radicals in the canine basilar artery. Am. J. Physiol. 1993;264:H859–H864. doi: 10.1152/ajpheart.1993.264.3.H859. [DOI] [PubMed] [Google Scholar]
- LI P.F., DIETZ R., VON HARSDORF R. Reactive oxygen species induce apoptosis of vascular smooth muscle cell. FEBS Lett. 1997;404:249–252. doi: 10.1016/s0014-5793(97)00093-8. [DOI] [PubMed] [Google Scholar]
- MACHLIN L.J., BENDICH A. Free radical tissue damage: protective role of antioxidant nutrients. FASEB. 1987;1:441–445. [PubMed] [Google Scholar]
- MATOBA T., SHIMOKAWA H., NAKASHIMA M., HIRAKAWA Y., MUKAI Y., HIRANO K., KANAIDE H., TAKESHITA A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J. Clin. Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLIVER C.N. Inactivation of enzymes and oxidative modification of proteins by stimulated neutrophils. Arch. Biochem. Biophys. 1987;253:62–72. doi: 10.1016/0003-9861(87)90637-0. [DOI] [PubMed] [Google Scholar]
- OMAR H.A., FIGUEROA R., OMAR R.A., TEJANI N., WOLIN M.S. Hydrogen peroxide and reoxygenation cause prostaglandin-mediated contraction of human placental arteries and veins. Am. J. Obstet. Gynecol. 1992;167:201–207. doi: 10.1016/s0002-9378(11)91658-5. [DOI] [PubMed] [Google Scholar]
- PELAEZ N.J., BRAUN T.R., PAUL R.J., MEISS R.A., PACKER C.S. H2O2 mediates CA2+ - and MLC20 phosphorylation-independent contraction in intact and permeabilized vascular muscle. Am. J. Physiol. 2000;279:H1185–H1193. doi: 10.1152/ajpheart.2000.279.3.H1185. [DOI] [PubMed] [Google Scholar]
- PRABHA P.S., DAS U.N., KORATKAR R., SAGAR P.S., RAMESH G. Free radical generation, lipid peroxidation and essential fatty acids in uncontrolled essential hypertension. Prostaglandins Leukot. Essent. Fatty Acids. 1990;41:27–33. doi: 10.1016/0952-3278(90)90127-7. [DOI] [PubMed] [Google Scholar]
- RAJAGOPALAN S., KURZ S., MUNZEL T., TARPEY M., FREEMAN B.A., GRIENDLING K.K., HARRISON D.G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin.Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODRIGUEZ-MARTINEZ M.A., GARCIA-COHEN E.C., BAENA A.B., GONZALEZ R., SALAICES M., MARIN J. Contractile responses elicited by hydrogen peroxide in aorta from normotensive and hypertensive rats. Endothelial modulation and mechanism involved. Br. J. Pharmacol. 1998;125:1329–1335. doi: 10.1038/sj.bjp.0702200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROYCHOUDHURY S., GHOSH S.K., CHAKRABORTI T., CHAKRABORTI S. Role of hydroxyl radical in the oxidant H2O2-mediated CA2+ release from pulmonary smooth muscle mitochondria. Mol. Cell. Biochem. 1996;159:95–103. doi: 10.1007/BF00420911. [DOI] [PubMed] [Google Scholar]
- SHEEHAN D.W., GIESE E.C., GUGINO S.F., RUSSELL J.A. Characterization and mechanisms of H2O2-induced contractions of pulmonary arteries. Am. J. Physiol. 1993;264:H1542–H1547. doi: 10.1152/ajpheart.1993.264.5.H1542. [DOI] [PubMed] [Google Scholar]
- SHEN J.Z., ZHENG X.F., KWAN C.Y. Differential contractile actions of reactive oxygen species on rat aorta: selective activation of ATP receptor by H2O2. Life Sci. 2000a;66:PL 291–PL 296. doi: 10.1016/s0024-3205(00)00539-7. [DOI] [PubMed] [Google Scholar]
- SHEN J.Z., ZHENG X.F., KWAN C.Y. Evidence for P(2)-purinoceptors contribution in H2O2-induced contraction of rat aorta in the absence of endothelium. Cardiovas. Res. 2000b;47:574–585. doi: 10.1016/s0008-6363(00)00123-1. [DOI] [PubMed] [Google Scholar]
- SOMERS M.J., MAVROMATIS K., GALIS Z.S., HARRISON D.G. Vascular superoxide production and vasomotor function in hypertension induced by deoxycorticosterone acetate-salt. Circulation. 2000;101:1722–1728. doi: 10.1161/01.cir.101.14.1722. [DOI] [PubMed] [Google Scholar]
- SUNDARESAN M., YU Z.X., FERRANS V.J., IRANI K., FINKEL T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- SUZUKI H., SWEI A., ZWEIFACH B.W., SCHMID-SCHONBEIN G.W. In vivo evidence for microvascular oxidative stress in spontaneously hypertensive rats. Hydroethidine microfluorography. Hypertension. 1995;25:1083–1089. doi: 10.1161/01.hyp.25.5.1083. [DOI] [PubMed] [Google Scholar]
- SWEI A., LACY F., DELANO F.A., SCHMID-SCHONBEIN G.W. Oxidative stress in the Dahl hypertensive rat. Hypertension. 1997;30:1628–1633. doi: 10.1161/01.hyp.30.6.1628. [DOI] [PubMed] [Google Scholar]
- WOLIN M.S., RODRIGUES A.M., YU J.M. Peroxides cause dose-dependent relaxant and contractor responses in isolated bovine intrapulmonary arterial and venous rings. Fed. Prol. Am. Soc. Exp. Biol. 1985;44:821–826. [Google Scholar]
- YANG Z., ZHANG A., ALTURA B.T., ALTURA B.M. Hydrogen peroxide-induced endothelium-dependent relaxation of rat aorta. Involvement of CA2+ and other cellular metabolites. Gen. Pharmacol. 1999;33:325–336. doi: 10.1016/s0306-3623(99)00019-1. [DOI] [PubMed] [Google Scholar]
- YANG Z.W., ZHENG T., ZHANG A., ALTURA B.T., ALTURA B.M. Mechanisms of hydrogen peroxide-induced contraction of rat aorta. Eur. J. Pharmacol. 1998;344:169–181. doi: 10.1016/s0014-2999(97)01576-8. [DOI] [PubMed] [Google Scholar]
- ZEMBOWICZ A., HATCHETTR R.J., JAKUBOWSKI A.M., GRYGLEWSKI R.J. Involvement of nitric oxide in the endothelium-dependent relaxation induced by hydrogen peroxide in the rabbit aorta. Br. J. Pharmacol. 1993;110:151–158. doi: 10.1111/j.1476-5381.1993.tb13785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


