Abstract
To characterize the prostanoid receptors (TP, FP, EP1 and/or EP3) involved in the vasoconstriction of human pulmonary veins, isolated venous preparations were challenged with different prostanoid-receptor agonists in the absence or presence of selective antagonists.
The stable thromboxane A2 mimetic, U46619, was a potent constrictor agonist on human pulmonary veins (pEC50=8.60±0.11 and Emax=4.61±0.46 g; n=15). The affinity values for two selective TP-antagonists (BAY u3405 and GR32191B) versus U46619 were BAY u3405: pA2=8.94±0.23 (n=3) and GR32191B: apparent pKB=8.25±0.34 (n=3), respectively. These results are consistent with the involvement of TP-receptor in the U46619 induced contractions.
The two EP1-/EP3- agonists (17-phenyl-PGE2 and sulprostone) induced contraction of human pumonary veins (pEC50=8.56±0.18; Emax=0.56±0.24 g; n=5 and pEC50=7.65±0.13; Emax=1.10±0.12 g; n=14, respectively). The potency ranking for these agonists: 17-phenyl-PGE2>sulprostone suggests the involvement of an EP1-receptor rather than EP3. In addition, the contractions induced by sulprostone, 17-phenyl-PGE2 and the IP-/EP1- agonist (iloprost) were blocked by the DP-/EP1-/EP2-receptor antagonist (AH6809) as well as by the EP1 antagonist (SC19220).
PGF2α induced small contractions which were blocked by AH6809 while fluprostenol was ineffective. These results indicate that FP-receptors are not implicated in the contraction of human pulmonary veins.
These data suggest that the contractions induced by prostanoids involved TP- and EP1-receptors in human pulmonary venous smooth muscle.
Keywords: AH6809, BAY u3405, contraction, human pulmonary veins, GR32191B, iloprost, prostaglandin, prostanoid receptors, SC19220, sulprostone, U46619
Introduction
Prostanoids may contract or relax smooth muscle by activating different prostanoid receptors (Coleman et al., 1994). Results derived from isolated tissues showed that prostanoid activation of TP-, FP-, EP1- or EP3-receptors produced smooth muscle contraction by increasing Ca2+ or reducing cyclic AMP intracellular levels (Negishi et al., 1995). The preferential receptor for thromboxane A2 (TP-receptor) has been extensively described in platelet aggregation and in smooth muscle contraction (Shen & Tai, 1998). In most of the human arteries, U46619 the TP selective agonist induced contraction (Maddox et al., 1985; Ohlstein et al., 1988; Uski et al., 1984; Baxter et al., 1995; Templeton et al., 1991), while studies on human veins have been rarely reported. However, the TP-receptor has been described in vasoconstriction of veins in the hand, the placenta and the leg (Arner et al., 1991; Boura et al., 1986; Mais et al., 1985). The involvement of FP-, EP1- or EP3-receptors in the contraction of numerous non-human smooth muscle preparations is frequently reported. The activation of FP-receptors by prostanoids induced contraction of cat iris sphincter, bovine ciliary muscle, rabbit uterus and ewe myometrium (Woodward et al., 1989; Krauss et al., 1997; Chen et al., 1998; Crankshaw & Gaspar, 1995). In mammals, activation of EP3-receptor induced contraction of smooth muscles present in ileum, colon, myometrium and corpus luteum (Botella et al., 1993; 1995; Crankshaw & Gaspar, 1995; Sharif et al., 1998). The EP1-receptor has been classically described using selective antagonist (SC19220) against PGE2-induced contractions in preparations derived from either the guinea-pig trachea or gastrointestinal tract (Kennedy et al., 1982; Coleman & Kennedy, 1985). However, few studies characterizing FP-, EP1- or EP3- receptors involved in the control of human vascular tone have been reported. The FP-receptor has been described in different non-vascular human smooth muscle: urinary bladder, and myometrium (Palea et al., 1998; Senior et al., 1992). Furthermore, EP1-receptors have not been associated with any human smooth muscle contraction and there is limited data on human pulmonary artery and myometrium contractions due to EP3-receptor activation (Qian et al., 1994; Senior et al., 1993).
The aim of the present study was to investigate not only the TP but also the FP-, EP1- or EP3-receptors associated with the contractions induced by prostanoids in human pulmonary veins.
Methods
Isolated preparations
Human lung tissues were obtained from patients (21 male and four female) who had undergone surgery for lung carcinoma. The mean age was 58±2 years. Pulmonary venous preparations were removed, dissected free from adjoining connective tissue and lung parenchyma, placed in Tyrode's solution (concentration mM): NaCl 139.2, KCl 2.7, CaCl2 1.8, MgCl2 0.49, NaHCO3 11.9, NaH2PO4 0.4 and glucose 5.5; pH 7.4 and maintained at 4°C. All preparations were used within 1 – 12 h postsurgery. Vascular preparations with intact endothelium were cut as rings (3 – 6 mm internal diameter, 3 – 5 mm in length). The rings were then set up in 10 ml organ baths containing Tyrode's solution, gassed with 95% O2/5% CO2 and maintained at 37°C. An optimal load (1.5 g), which ensured maximal physiological responses to the agonists, was applied to each ring.
Changes in force were recorded by isometric force displacement transducers (Narco F-60) and physiographs (Linseis). Subsequently, preparations were allowed to equilibrate for 90 min with bath fluid changes taking place every 10 min.
Experimental protocol
After the equilibration period, the venous preparations were incubated 30 min with indomethacin (1.7 μM) and 15 min with NG-nitro-L-arginine (L-NOARG; 0.1 mM). These agents were used to avoid any physiological effect induced by the release of endogenous prostanoids and nitric oxide. The preparations were then contracted with increasing concentrations of prostanoids or selective agonists applied in a cumulative fashion. In some experiments, during the incubation period, different antagonists: BAY u3405 (TP), GR32191B (TP), AH6809 (DP/EP1/EP2) or SC19220 (EP1) were added with indomethacin and L-NOARG. These antagonist were used to determine either their affinity values or to illustrate one response through activation of a single receptor subtype for the agonists which may act on different receptor subtypes. The maximal contraction of each preparation with norepinephrine (10 μM) was obtained at the end of each experimental protocol.
Data analysis
The changes in force were measured from isometric recordings in grams (g). The contractions produced with the different agonists were expressed either as grams or as per cent of the contraction induced with norepinephrine. The maximal contraction (Emax value) produced with an agonist and the half-maximum effective concentration value (EC50 value) were interpolated from the individual concentration-effect curves. The pEC50 values were calculated as the negative log of EC50 values. When the pEC50 values obtained in the absence and presence of antagonist were significantly different and the tentative assumption was made that the Schild equation held in our experiments, then the apparent pKB value was calculated as the negative log of the equilibrium dissociation constant for the antagonist (KB value). The KB value was determined using the Schild equation: KB=[B]/(DR-1), where [B] is the concentration of the antagonist and DR (dose ratio) is the ratio of EC50 values of agonist in the presence and absence of antagonist. In studies on veins with the contractile agonist U46619, different concentrations of BAY u3405 were used to determine the pA2 value according to the method of Arunlakshana & Schild (1959). For each lung sample, Schild plot analysis was performed, the slope and pA2 value were determined by least square fitting of a regression line to the points. All results are expressed as means±s.e.mean and were derived from different lung samples (n). Statistical analysis was performed using Student's paired or unpaired t-test and Mann-Whitney rank sum test with a confidence level of 95%.
Compounds
U46619 (9,11-dideoxy-11α,9α-methanoepoxy PGF2α), PGE2, PGF2α, fluprostenol ((±) 16-m-trifluoromethylphenoxy tetranor PGF2α) and 17-phenyl-trinor-PGE2, were purchased from Cayman Chemical Company, Ann Arbor, MI, U.S.A. Iloprost (5-[(E)-(1S,5S,6R,7R)-7-hydroxy-6-[(E)-(3S,4RS)-3-hydroxy-4-methyl-1-octen-6-inyl]bicyclo[3.3.0]-octan-3-ylidene] pentanoic acid) and sulprostone (N-(methylsulphonyl)-9-oxo-11α,15R-dihydroxy-16-phenoxy-17,18,19,20-tetranor-prosta-5Z, 13E-dien-1-amide) were a gift from Schering AG, Berlin, Germany. AH6809 (6-isopropoxy-9-oxaxanthene-2-carboxylic acid) and GR32191B ([1R-[1α(z),2β,3β,5α]]-(+)-7-[5[[(1,1′-biphenyl)-4-yl] methoxy]-3-hydroxy-2-(1-piperidinyl) cyclopentyl]-4-heptenoic acid, hydrochloride) were a gift from Glaxo Wellcome, U.K. BAY u3405 (3(R)-3-(4-fluorophenylsulphonamido)-1,2,3,4-tetrahydro-9-carbazole propanoic acid) was a gift from Bayer, Stokes Poges, U.K. SC19220 (8-chlorodibenz [b,f][1,4] oxazepine-10(11H)-carboxy-(2-acetyl)hydrazide) was a gift from Searle Research and Development, Skokie, IL, U.S.A. Norepinephrine, L-NOARG (NG-nitro-L-arginine) and indomethacin were purchased from Sigma Chemical Co., St. Louis, MO, U.S.A.
Results
The cocktail of inhibitors (indomethacin, L-NOARG) and antagonists with which the venous preparations were incubated induced a small contraction on the resting tone of these preparations (0.07±0.06 g; n=18). At the end of the protocols, venous preparations were contracted with norepinephrine (10 μM: 3.10±0.44 g; n=25).
Concentration-dependent contractions of human pulmonary venous preparations produced by U46619 are shown Figure 1a and Table 1. U46619 was a potent constrictor with a maximal effect which was 50% greater than that induced by norepinephrine (10 μM). BAY u3405 (0.03; 0.1; 0.3 μM) caused a concentration-related rightward shift of the U46619 concentration-effect curves with a pA2 of 8.94±0.23 (n=3) and a Schild plot slope of 1.07±0.03, which was not significantly different from unity. These values were derived from Schild plots (Figure 1b). In additional lung samples, BAY u3405 and GR32191B (1 μM) reduced the Emax and/or the pEC50 values obtained from U46619 concentration-effect curves (Table 1). In presence of BAY u3405 (1 μM) the U46619 concentration-effect curves were difficult to restore and no plateau was reached even at the maximal concentration available for U46619 (10 μM). When possible, the apparent pKB values for each concentration of BAY u3405 and GR32191B were calculated and these values are presented Table 1.
Figure 1.
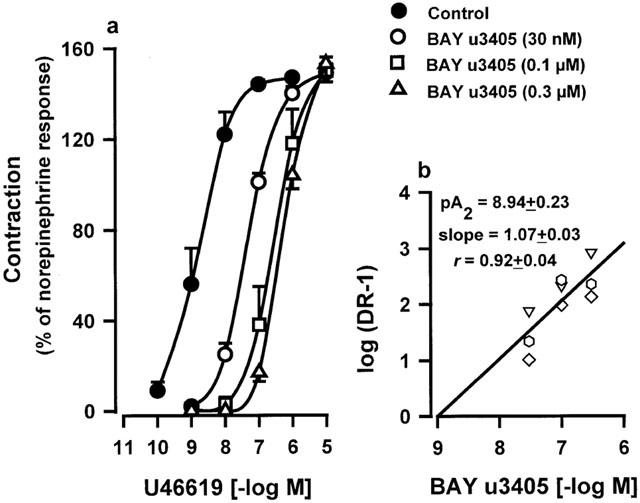
(a) Contraction of human isolated pulmonary veins induced by U46619. Responses were expressed as per cent of the norepinephrine (10 μM) contraction. Values are means±s.e.mean. (b) Schild-plot analysis of the antagonism by BAY u3405 of U46619-contractions in veins derived from three human lung samples (each lung sample is presented as a different symbol). Analysis was based on pEC50 values calculated in treated preparations which were significantly different from control values. The linear regression presented was performed using all data points. The calculated average of pA2 values, slopes and regression coefficients performed with data derived from each lung sample are indicated.
Table 1.
Effect of TP-, EP- and FP- receptor agonists or antagonists on human isolated pulmonary venous preparations
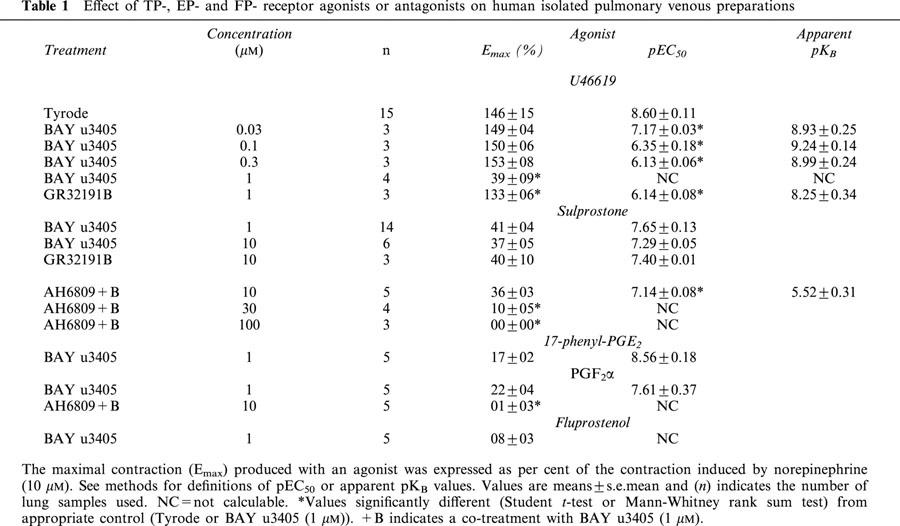
Concentration-effect curves induced by sulprostone on human pulmonary venous preparations in the absence of antagonist were biphasic, the average curve failed to reach a plateau and was not sigmoïdal (Figure 2). The contraction obtained with the highest available concentration of sulprostone was 54±09% (n=5) of norepinephrine contraction (Figure 2). Sigmoïdal curves were obtained with sulprostone, in the presence of either BAY u3405 (1; 10 μM) or GR32191B (10 μM) and the Emax did not exceed 50% of the norepinephrine contraction (Figure 2 and Table 1).
Figure 2.
Effect of BAY u3405 (1; 10 μM) or GR32191B (10 μM) on the contraction of human isolated pulmonary veins induced by sulprostone. Control and treated preparations were incubated with indomethacin (1.7 μM) and L-NOARG (0.1 mM). Responses were expressed as per cent of the norepinephrine (10 μM) contraction. Values are means±s.e.mean, number of lung samples used are indicated in Table 1 and in the Results section.
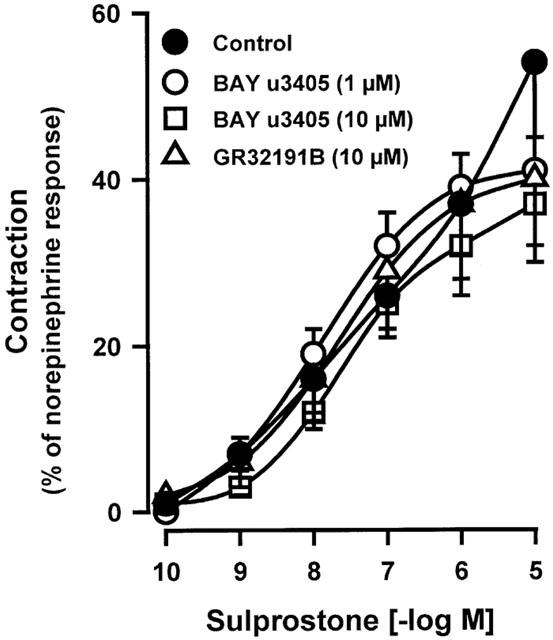
In presence of BAY u3405 (1 μM), the contractions induced by sulprostone on venous preparations were inhibited by AH6809 (10; 30 or 100 μM; Figure 3a and Table 1). AH6809 (30, 100 μM) had a non-competitive behaviour on sulprostone responses, the antagonism was not reversible. Under the same conditions (presence of BAY u3405, 1 μM), SC19220 (100 μM) significantly inhibited the vasoconstrictions induced by sulprostone (Figure 3a). In presence of this latter treatment, the contraction obtained with the highest available concentration of sulprostone was 32±09% (n=3) of norepinephrine contraction, the derived estimations of pEC50 and apparent pKB were (<6.97±0.41, >4.75±0.36), respectively. AH6809 (10 μM) significantly inhibited the vasoconstrictions induced by 17-phenyl-PGE2 in the human pulmonary veins treated with BAY u3405, 1 μM, (Figure 3b and Table 1). In this latter protocol, the contraction obtained with the highest available concentration of 17-phenyl-PGE2 was 15±05% (n=4) of norepinephrine contraction and the derived estimations of pEC50 and the apparent pKB were (<7.66±0.13, >5.88±0.20), respectively. Venous preparations derived from three human lung samples, treated with BAY u3405 (1 μM), contracted when challenged with iloprost (Figure 4) this response was abolished in the presence of AH6809 (10 μM). In the presence of BAY u3405 (1 μM), the PGE2 concentration-effect curves were quite variable, PGE2 either had no effect, or induced contraction or relaxation of human pulmonary venous preparations (Figure 5). These contractions induced by PGE2 were inhibited by AH6809 (10 μM, n=3; data not shown).
Figure 3.
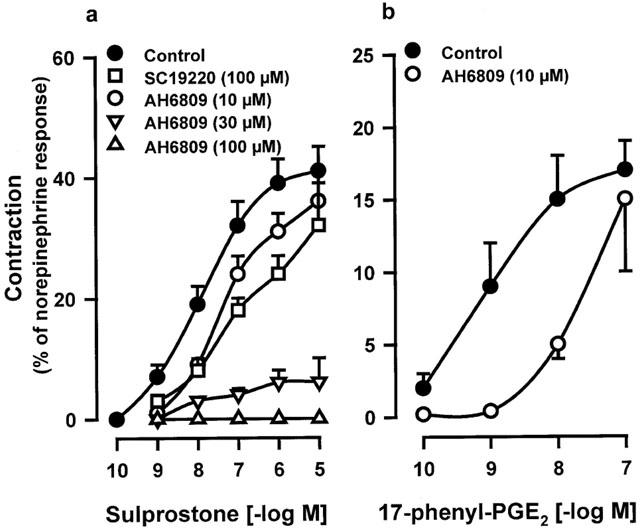
Effect of AH6809 (10; 30; 100 μM) or SC19220 (100 μM) on contraction of human isolated pulmonary veins induced by sulprostone (a) or 17-phenyl-PGE2 (b). Control and treated preparations were incubated with indomethacin (1.7 μM), L-NOARG (0.1 mM) and BAY u3405 (1 μM). Responses were expressed as per cent of the norepinephrine (10 μM) contraction. Values are means±s.e.mean, number of lung samples used are indicated in Table 1 and in the Results section.
Figure 4.
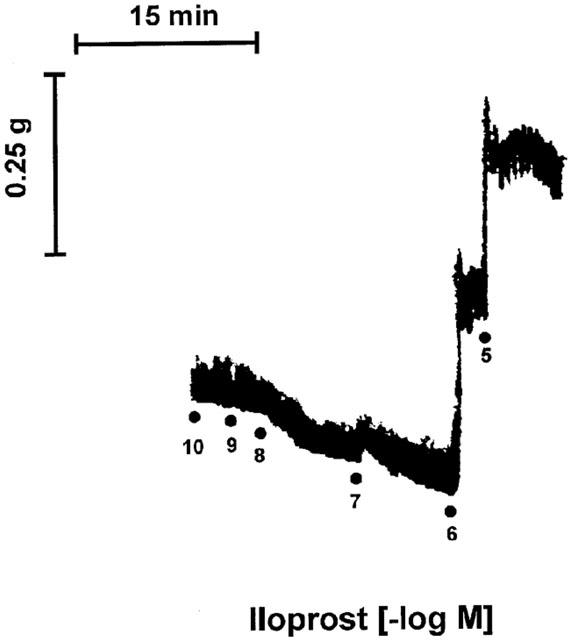
Representative tracing of the contraction induced by iloprost observed in isolated pulmonary veins derived from three human lung samples. Experiments were performed in presence of indomethacin (1.7 μM), L-NOARG (0.1 mM) and BAY u3405 (1 μM).
Figure 5.
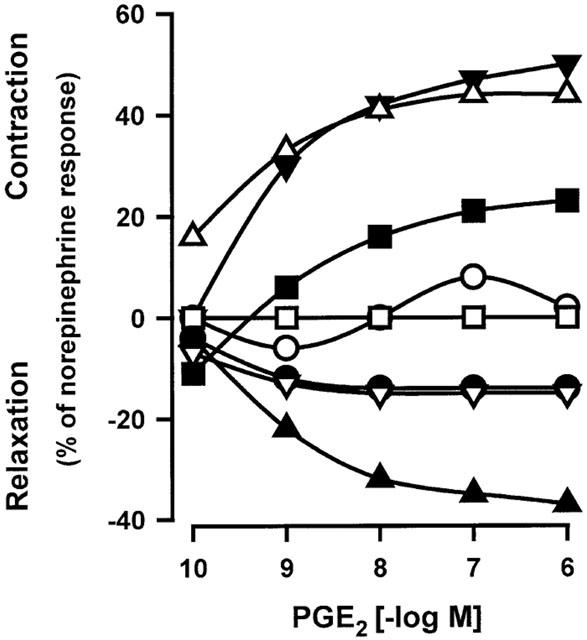
Variable effects induced by PGE2 in human isolated pulmonary veins. Experiments were performed in presence of indomethacin (1.7 μM), L-NOARG (0.1 mM) and BAY u3405 (1 μM). Responses were expressed as per cent of the norepinephrine (10 μM) contraction. Each symbol indicates data obtained with one lung sample.
In human pulmonary veins pre-treated with BAY u3405 (1 μM), the concentration-effect curves produced with PGF2α were small while fluprostenol failed to contract these tissues (Table 1). The PGF2α induced curves were abolished in the presence of BAY u3405 (1 μM) and AH6809 (10 μM; Table 1).
Discussion
The present report suggests the involvement of TP- and EP1- receptors in the prostanoid induced contraction of human pulmonary venous preparations.
The contractions observed with U46619 (stable thromboxane A2 mimetic) and their inhibition by the selective TP-antagonists (GR32191B, Lumley et al., 1989 and BAY u3405, McKenniff et al., 1991) suggest the presence of a TP-receptor in human pulmonary veins. These results have not previously been reported although the TP-receptor has been frequently described in human pulmonary arteries (Maddox et al., 1985; Sjoberg & Steen, 1989; Lumley et al., 1989; Norel et al., 1991; Ellis & Muller-Schweinitzer, 1991; Qian et al., 1994; Jino et al., 1996). Pharmacological studies as well as investigations using molecular biology have suggested heterogeneity among thromboxane receptors (Lumley et al., 1989; Tymkewycz et al., 1991; Furci et al., 1991; Pierce & Regan, 1998). Actually, the affinity values calculated in human pulmonary veins for GR32191B (8.25±0.34) and BAY u3405 (8.94±0.23) are comparable to those found in human pulmonary arteries: GR32191B (8.18 – 8.3; Lumley et al., 1989; Qian et al., 1994) and BAY u3405 (9.25; Norel et al., 1991), respectively. These data suggest that the TP-receptor present in both human pulmonary arteries and veins may be the same. In addition, the apparent pKB values obtained with GR32191B (present study) were also in accordance with those derived from pharmacological studies performed on human umbilical artery (8.04; Templeton et al., 1991), uterine artery (8.5; Baxter et al., 1995) or human platelet (8.2 – 8.8; Lumley et al., 1989).
The TP-receptor in human pulmonary veins was also activated by the high concentration (>1 μM) of sulprostone and the TP antagonists eliminated this response. Sulprostone is classically described as a selective agonist for EP1- and EP3- receptors (Coleman et al., 1987a, 1987b; 1988; Reeves et al., 1988), however high concentrations (30 μM) have been reported to induce contractions of the human bronchial preparations and isolated uterine artery where TP-receptors are the only excitatory prostanoid-receptors (Coleman & Sheldrick, 1989; Baxter et al., 1995). The findings (present study) are in agreement with this previous observation, high concentrations of sulprostone may act on the TP-receptor and for this reason all the experiments involving this compound were carried out in the presence of BAY u3405.
In human pulmonary veins, sulprostone and 17-phenyl-PGE2 (in presence of BAY u3405) induced smaller contractions than the response induced by U46619. The contractions of human pulmonary veins induced by sulprostone or 17-phenyl-PGE2, suggest the involvement of EP1- or EP3- receptors. The sensitivities (pEC50 value) of the venous preparations to sulprostone and 17-phenyl-PGE2 were comparable to those determined in standard functional assays for EP1-receptor, namely, the contractions of guinea-pig fundus (Coleman et al., 1987a) and trachea (Lawrence et al., 1992). In the present study, the potency ranking for these agonist, 17-phenyl-PGE2>sulprostone (equi-effective molar ratio=8 and 1, respectively), was similar to the one observed in the previous standard EP1-tissues. In these later tissues or in binding studies with cloned EP1-receptor (rat, Boie et al., 1997; mouse, Watabe et al., 1993; mouse, Kiriyama et al., 1997), 17-phenyl-PGE2 was 1.5 – 4 fold more potent than sulprostone. A greater potency, 10 fold, was found for 17-phenyl-PGE2 when compared with sulprostone to increase intracellular Ca2+ in rabbit cortical collecting duct via the activation of EP1-receptor (Guan et al., 1998). In contrast, the rank order of potency for these agonists was reversed in studies where the effects were mediated by the activation of EP3-receptors. Sulprostone was a more potent contractile agonist, 30 – 45 fold greater than 17-phenyl-PGE2 either in human pulmonary artery (Qian et al., 1994) or when inhibiting the twitch contraction of guinea-pig vas deferens (Lawrence et al., 1992). These results (present study) support the presence of an EP1-receptor associated with contraction in human pulmonary veins rather than an EP3-receptor. In addition, iloprost, a selective agonist for IP and EP1- receptors (Schrör et al., 1981; Dong & Jones, 1982; Dong et al., 1986) induced contraction of these preparations even though IP-receptors responsible for relaxation are present (Walch et al., 1999).
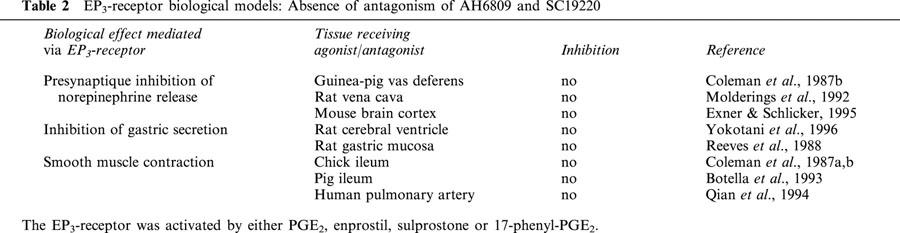
The effects of either the DP-/EP1-/EP2- receptor antagonist (AH6809; Coleman et al., 1985; Keery & Lumley, 1988; Woodward et al., 1995) or the EP1-receptor antagonist (SC19220; Sanner, 1969; Kennedy et al., 1982; Coleman et al., 1987a, 1987b) against the concentration-effect curves (present study) produced by both sulprostone and 17-phenyl-PGE2 also suggest the involvement of the EP1-receptor rather than EP3. However, the affinity values calculated or estimated for AH6809 in preparations derived from human lung (apparent pKB of 5.52 and >5.88) are lower than those obtained in similar physiological experiments performed in guinea-pig or dog EP1-preparations. Lawrence et al. (1992) using sulprostone or 17-phenyl-PGE2 as the contractile agonist, found the AH6809 affinity values ranking from 6.1 – 7.35 in either guinea-pig ileum or trachea. In other studies using PGE2 with the EP1-preparations (guinea-pig fundus, ileum and dog fundus), the affinity values for AH6809 ranked from 6.6 – 7.4 (Eglen & Whiting, 1988; Coleman et al., 1985). Similarly, the estimated affinity value found for SC19220 in the present study (apparent pKB>4.75) was lower than the pA2 of 5.6 calculated in experiments performed with the previous EP1-preparations (Coleman et al., 1985; Coleman & Kennedy, 1985). The reason for this discrepancy remains to be established. However, when EP1-receptors were assessed in either physiological or binding studies, the affinity values for AH6809 were always one order of magnitude greater than those for SC19220 (present study; Coleman et al., 1985; Boie et al., 1997; Funk et al., 1993). Since high concentrations of AH6809 (5 – 100 μM) or SC19220 (100 – 300 μM) did not block the EP3 mediated effects in many of the EP3 biological models (Table 2), the inhibitory effect of these antagonists in human pulmonary vein would suggest that the EP3-receptor is not involved in contractions.
Table 2.
EP3-receptor biological models: Absence of antagonism of AH6809 and SC19220
The variable effects induced by PGE2 in the human pulmonary venous preparations in presence of the TP-antagonist may be explained by two opposing effects of this prostaglandin in these preparations. A contraction via the EP1-receptor and a relaxation via another EP-receptor subtype as has been suggested by Walch et al. (1999). A similar paradoxical effect was observed in guinea-pig trachea as well as in human bronchial preparations where PGE2 may act on EP1- and/or TP- receptors to induce contraction while the activation of the EP2-receptor provokes the relaxation (Gardiner, 1975; Coleman & Kennedy, 1980; McKenniff et al., 1988).
The results obtained in this report with the EP- agonists or antagonists suggest a role for EP1- and not EP3- receptor in the contraction of human pulmonary vein. The involvement of EP1- or EP3- receptors in the control of vascular tone has been principally investigated in the ocular vascular bed. The EP- agonists decrease the intraocular pressure in various animal models of glaucoma (Woodward et al., 1993; 1994; Bhattacherjee et al., 1999; Waterbury et al., 1990) and contract the pig retinal vessels (Abran et al., 1994). However, the EP3-receptor is involved in vasoconstriction of guinea-pig aorta (Jones et al., 1998), rat renal afferent arteriole (Tang et al., 2000) and human pulmonary artery (Qian et al., 1994). Arner & Högestatt (1991) showed that iloprost contracted the human hand vein. One could associate this response with activation of an EP1-receptor. The data (present study) demonstrated a similar response to that of the hand veins whereas human arterial preparations did not exhibit a contractile response to this agonist (Arner & Högestatt, 1991; Qian et al., 1994). Therefore, the presence of EP1-receptors may be found only in human venous preparations.
The low potency of PGF2α or fluprostenol, the selective agonist for FP-receptor, suggests that the FP-receptor is not involved in the contraction of human pulmonary veins. In addition, the small contractions induced by PGF2α were inhibited by AH6809 suggesting that PGF2α activates an EP1- rather than FP- receptor.
In summary, the findings in the present study are consistent with the presence of TP- and EP1- receptors mediating constriction of human pulmonary veins. These findings may be relevant to the pulmonary circulation. Sulprostone is used in obstetrics and gynaecology and one of the clinical side effects observed with this compound is pulmonary oedema (Stock et al., 1995; Levy et al., 1994; Puura et al., 1995). The present study and the work of Qian et al. (1994) suggest that this side effect may involve vasospasm of the whole pulmonary vasculature. Such EP-agonist may activate at the same time TP-, EP3- receptors in the arteries and TP-, EP1- receptors in the veins.
Acknowledgments
The authors would like to thank Yvette Le Treut and Ginette Brille for excellent technical assistance.
Abbreviations
- Emax
maximal contraction
- KB values
equilibrium dissociation constant for the antagonist
- L-NOARG
NG-nitro-L-arginine
- NC
not calculable
- PG
prostaglandin
References
- ABRAN D., VARMA D.R., LI D.Y., CHEMTOB S. Reduced responses of retinal vessels of the newborn pig to prostaglandins but not to thromboxane. Can. J. Physiol. Pharmacol. 1994;72:168–173. doi: 10.1139/y94-026. [DOI] [PubMed] [Google Scholar]
- ARNER M., HÖGESTATT E.D. Endothelium-dependent relaxation and effects of prostacyclin, endothelin and platelet-activating factor in human hand veins and arteries. Acta Physiol. Scand. 1991;142:165–172. doi: 10.1111/j.1748-1716.1991.tb09144.x. [DOI] [PubMed] [Google Scholar]
- ARNER M., HÖGESTATT E.D., USKI T.K. Characterization of contraction-mediating prostanoid receptors in human hand veins: effects of the thromboxane receptor antagonists BM13,505 and AH23848. Acta Physiol. Scand. 1991;141:79–86. doi: 10.1111/j.1748-1716.1991.tb09047.x. [DOI] [PubMed] [Google Scholar]
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAXTER G.S., CLAYTON J.K., COLEMAN R.A., MARSHALL K., SANGHA R., SENIOR J. Characterization of the prostanoid receptors mediating constriction and relaxation of human isolated uterine artery. Br. J. Pharmacol. 1995;116:1692–1696. doi: 10.1111/j.1476-5381.1995.tb16393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHATTACHERJEE P., WILLIAMS B.S., PATERSON C.A. Responses of intraocular pressure and the pupil of feline eyes to prostaglandin EP1 and FP receptor agonists. Invest. Ophthalmol. Vis. Sci. 1999;40:3047–3053. [PubMed] [Google Scholar]
- BOIE Y., STOCCO R., SAWYER N., SLIPETZ D.M., UNGRIN M.D., NEUSCHAFER-RUBE F., PUSCHEL G.P., METTERS K.M., ABRAMOVITZ M. Molecular cloning and characterization of the four rat prostaglandin E2 prostanoid receptor subtypes. Eur. J. Pharmacol. 1997;340:227–241. doi: 10.1016/s0014-2999(97)01383-6. [DOI] [PubMed] [Google Scholar]
- BOTELLA A., DELVAUX M., FIORAMONTI J., FREXINOS J., BUENO L. Receptor subtypes involved in dual effects induced by prostaglandin E2 in circular smooth muscle from dog colon. J. Pharmacol. Exp. Ther. 1995;273:1008–1014. [PubMed] [Google Scholar]
- BOTELLA A., DELVAUX M., FIORAMONTI J., FREXINOS J., BUENO L. Stimulatory (EP1 and EP3) and inhibitory (EP2) prostaglandin E2 receptors in isolated ileal smooth muscle cells. Eur. J. Pharmacol. 1993;237:131–137. doi: 10.1016/0014-2999(93)90102-n. [DOI] [PubMed] [Google Scholar]
- BOURA A.L., GUDE N.M., KING R.G., MAK K.K., WALTERS W.A. Characterization of thromboxane A2 receptors in the human fetal placental vessels and umbilical vein. Clin. Exp. Pharmacol. Physiol. 1986;13:83–86. doi: 10.1111/j.1440-1681.1986.tb00319.x. [DOI] [PubMed] [Google Scholar]
- CHEN J., WOODWARD D.F., YUAN Y.D., MARSHALL K., SENIOR J. Prostanoid-induced contraction of the rabbit isolated uterus is mediated by FP receptors. Prostaglandins Other Lipid Mediat. 1998;55:387–394. doi: 10.1016/s0090-6980(98)00034-3. [DOI] [PubMed] [Google Scholar]
- COLEMAN R.A., KENNEDY I. Contractile and relaxant actions of prostaglandins on guinea-pig isolated trachea. Br. J. Pharmacol. 1980;68:533–539. doi: 10.1111/j.1476-5381.1980.tb14569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLEMAN R.A., KENNEDY I. Characterisation of the prostanoid receptors mediating contraction of guinea-pig isolated trachea. Prostaglandins. 1985;29:363–375. doi: 10.1016/0090-6980(85)90096-6. [DOI] [PubMed] [Google Scholar]
- COLEMAN R.A., DENYER L.H., SHELDRICK R.L.G. AH6809, a prostanoid EP1 receptor bloking drug. Br. J. Pharmacol. 1985;85:273P. [Google Scholar]
- COLEMAN R.A., KENNEDY I., SHELDRICK R.L.G. Evidence for the existence of three subtypes of PGE2 sensitive (EP) receptors in smooth muscle. Br. J. Pharmacol. 1987a;91:323P. [Google Scholar]
- COLEMAN R.A., KENNEDY I., SHELDRICK R.L.G., TOLOWINSKA I.Y. Further evidence for the existence of three subtypes of PGE2- sensitive (EP-) receptors in smooth muscle. Br. J. Pharmacol. 1987b;91:407P. [Google Scholar]
- COLEMAN R.A., HUMPHRAY J.M., SHELDRICK R.L.G., WHITE B.P. Gastric antisecretory prostanoids: actions at different prostanoid receptors. Br. J. Pharmacol. 1988;95:724P. [Google Scholar]
- COLEMAN R.A., SHELDRICK R.L.G. Prostanoid-induced contraction of human bronchial smooth muscle is mediated by TP-receptors. Br. J. Pharmacol. 1989;96:688–692. doi: 10.1111/j.1476-5381.1989.tb11869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLEMAN R.A., SMITH W.L., NARUMIYA S. International union of pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol. Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- CRANKSHAW D.J., GASPAR V. Pharmacological characterization in vitro of prostanoid receptors in the myometrium of nonpregnant ewes. J. Reprod. Fertil. 1995;103:55–61. doi: 10.1530/jrf.0.1030055. [DOI] [PubMed] [Google Scholar]
- DONG Y.J., JONES R.L. Effects of prostaglandins and thromboxane analogues on bullock and dog iris sphincter preparations. Br. J. Pharmacol. 1982;76:149–155. doi: 10.1111/j.1476-5381.1982.tb09200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONG Y.J., JONES R.L., WILSON N.H. Prostaglandin E receptor subtypes in smooth muscle: agonist activities of stable protacyclin analogues. Br. J. Pharmacol. 1986;87:97–107. doi: 10.1111/j.1476-5381.1986.tb10161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGLEN R.M., WHITING R.L. The action of prostanoid receptor agonists and antagonists on smooth muscle and platelets. Br. J. Pharmacol. 1988;94:591–601. doi: 10.1111/j.1476-5381.1988.tb11565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIS P., MULLER-SCHWEINITZER E. Maintenance of functional activity of human pulmonary arteries after cryopreservation. Br. J. Pharmacol. 1991;103:1377–1380. doi: 10.1111/j.1476-5381.1991.tb09797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EXNER H.J., SCHLICKER E. Prostanoid receptors of the EP3 subtype mediate the inhibitory effect of prostaglandin E2 on noradrenaline release in the mouse brain cortex. Naunyn Schmiedebergs Arch. Pharmacol. 1995;351:46–52. doi: 10.1007/BF00169063. [DOI] [PubMed] [Google Scholar]
- FUNK C.D., FURCI L., FITZGERALD G.A., GRYGORCZYK R., ROCHETTE C., BAYNE M.A., ABRAMOVITZ M., ADAM M., METTERS K.M. Cloning and expression of a cDNA for the human prostaglandin E receptor EP1 subtype. J. Biol. Chem. 1993;268:26767–26772. [PubMed] [Google Scholar]
- FURCI L., FITZGERALD D.J., FITZGERALD G.A. Heterogeneity of prostaglandin H2/thromboxane A2 receptors: distinct subtypes mediate vascular smooth muscle contraction and platelet aggregation. J. Pharmacol. Exp. Ther. 1991;258:74–81. [PubMed] [Google Scholar]
- GARDINER P.J. The effects of some natural prostaglandins on isolated human circular bronchial muscle. Prostaglandins. 1975;10:607–616. doi: 10.1016/s0090-6980(75)80007-4. [DOI] [PubMed] [Google Scholar]
- GUAN Y., ZHANG Y., BREYER R.M., FOWLER B., DAVIS L., HEBERT R.L., BREYER M.D. Prostaglandin E2 inhibits renal collecting duct Na+absorption by activating the EP1 receptor. J. Clin. Invest. 1998;102:194–201. doi: 10.1172/JCI2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JINO H., KURAHASHI K., USUI H., NAKATA Y., SHIMIZU Y., TEMMA S. Pharmacological nature of TP receptor mediated contraction in human intrapulmonary artery. Life Sci. 1996;59:2059–2065. doi: 10.1016/s0024-3205(96)00559-0. [DOI] [PubMed] [Google Scholar]
- JONES R.L., QIAN Y.M., CHAN K.M., YIM A.P. Characterization of a prostanoid EP3-receptor in guinea-pig aorta: partial agonist action of the non-prostanoid ONO-AP-324. Br. J. Pharmacol. 1998;125:1288–1296. doi: 10.1038/sj.bjp.0702189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEERY R.J., LUMLEY P. AH6809, a prostaglandin DP-receptor blocking drug on human platelets. Br. J. Pharmacol. 1988;94:745–754. doi: 10.1111/j.1476-5381.1988.tb11584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEDY I., COLEMAN R.A., HUMPHREY P.P.A., LEVY G.P., LUMLEY P. Studies on the characterisation of prostanoid receptors: a proposed classification. Prostaglandins. 1982;24:667–689. doi: 10.1016/0090-6980(82)90036-3. [DOI] [PubMed] [Google Scholar]
- KIRIYAMA M., USHIKUBI F., KOBAYASHI T., HIRATA M., SUGIMOTO Y., NARUMIYA S. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br. J. Pharmacol. 1997;122:217–224. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAUSS A.H., WIEDERHOLT M., STURM A., WOODWARD D.F. Prostaglandin effects on the contractility of bovine trabecular meshwork and ciliary muscle. Exp. Eye Res. 1997;64:447–453. doi: 10.1006/exer.1996.0224. [DOI] [PubMed] [Google Scholar]
- LAWRENCE R.A., JONES R.L., WILSON N.H. Characterization of receptors involved in the direct and indirect actions of prostaglandins E and I on the guinea-pig ileum. Br. J. Pharmacol. 1992;105:271–278. doi: 10.1111/j.1476-5381.1992.tb14245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVY D.M., HINSHAW K., KNOX F.M., CAMPBELL D.M., SUTHERLAND H.W. Cardiogenic pulmonary oedema: presentation of pre-eclampsia exacerbated by prostaglandin abortifacients. Br. J. Obstet. Gynaecol. 1994;101:263–265. doi: 10.1111/j.1471-0528.1994.tb13127.x. [DOI] [PubMed] [Google Scholar]
- LUMLEY P., WHITE B.P., HUMPHREY P.P.A. GR32191, a highly potent and specific thromboxane A2 receptor blocking drug on platelets and vascular and airways smooth muscle in vitro. Br. J. Pharmacol. 1989;97:783–794. doi: 10.1111/j.1476-5381.1989.tb12017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADDOX Y., CUNARD C.M., SHAPIRO R., KAWAGUCHI A., GOLDMAN M., LOWER R.R., RAMWELL P.W. Prostaglandins, Leukotrienes and Lipoxins. Elsevier, Martin Bailey-J; 1985. A comparison of the contractile responses of rodent and human pulmonary vascular segments to eicosanoids; pp. 267–272. [Google Scholar]
- MAIS D.E., SAUSSY D.L., JR, CHAIKHOUNI A., KOCHEL P.J., KNAPP D.R., HAMANAKA N., HALUSHKA P.V. Pharmacologic characterization of human and canine thromboxane A2/prostaglandin H2 receptors in platelets and blood vessels: evidence for different receptors. J. Pharmacol. Exp. Ther. 1985;233:418–424. [PubMed] [Google Scholar]
- MCKENNIFF M., RODGER I.W., NORMAN P., GARDINER P.J. Characterisation of receptors mediating the contractile effects of prostanoids in guinea-pig and human airways. Eur. J. Pharmacol. 1988;153:149–159. doi: 10.1016/0014-2999(88)90601-2. [DOI] [PubMed] [Google Scholar]
- MCKENNIFF M.G., NORMAN P., CUTHBERT N.J., GARDINER P.J. BAY u3405, a potent and selective thromboxane A2 receptor antagonist on airway smooth muscle in vitro. Br. J. Pharmacol. 1991;104:585–590. doi: 10.1111/j.1476-5381.1991.tb12473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLDERINGS G., MALINOWSKA B., SCHLICKER E. Inhibition of noradrenaline release in the rat vena cava via prostanoid receptors of the EP3-subtype. Br. J. Pharmacol. 1992;107:352–355. doi: 10.1111/j.1476-5381.1992.tb12750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEGISHI M., SUGIMOTO Y., ICHIKAWA A. Molecular mechanisms of diverse actions of prostanoid receptors. Biochim. Biophys. Acta. 1995;1259:109–120. doi: 10.1016/0005-2760(95)00146-4. [DOI] [PubMed] [Google Scholar]
- NOREL X., LABAT C., GARDINER P.J., BRINK C. Inhibitory effects of BAY u3405 on prostanoid-induced contractions in human isolated bronchial and pulmonary arterial muscle preparations. Br. J. Pharmacol. 1991;104:591–595. doi: 10.1111/j.1476-5381.1991.tb12474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHLSTEIN E.H., KOPIA G.A., ZEID R.L., VALOCIK R.W., HOROHONICH S., HIEBLE J.P., WASSERMAN M.A. Effects of the thromboxane receptor antagonist SK&F 88046 in the canine, monkey and human coronary vasculature. Prostaglandins. 1988;36:69–84. doi: 10.1016/0090-6980(88)90103-7. [DOI] [PubMed] [Google Scholar]
- PALEA S., TOSON G., PIETRA C., TRIST D.G., ARTIBANI W., ROMANO O., CORSI M. Pharmacological characterization of thromboxane and prostanoid receptors in human isolated urinary bladder. Br. J. Pharmacol. 1998;124:865–872. doi: 10.1038/sj.bjp.0701903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIERCE K.L., REGAN J.W. Prostanoid receptor heterogeneity through alternative mRNA splicing. Life Sci. 1998;62:1479–1483. doi: 10.1016/s0024-3205(98)00093-9. [DOI] [PubMed] [Google Scholar]
- PUURA A., SCHAVIKIN L., YLI-KESTI O., YLA-OUTINEN A., VIRTANEN V., KAUKINEN S. [Critical pulmonary edema following cesarean section] Duodecim. 1995;111:249–252. [PubMed] [Google Scholar]
- QIAN Y.M., JONES R.L., CHAN K.M., STOCK A.I., HO J.K.S. Potent contractile actions of prostanoid EP3-receptor agonists on human isolated pulmonary artery. Br. J. Pharmacol. 1994;113:369–374. doi: 10.1111/j.1476-5381.1994.tb16997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REEVES J.J., BUNCE K.T., SHELDRICK R.L.G., STABLES R. Evidence for the PGE receptor subtype mediating inhibition of acid secretion in the rat. Br. J. Pharmacol. 1988;95:805P. [Google Scholar]
- SANNER J.H. Antagonism of prostaglandin E2 by 1-acetyl-2-(8-chloro-10,11-dihydrodibenz (b,f) (1,4) oxazepine-10-carbonyl) hydrazine (SC-19220) Arch. Int. Pharmacodyn. Ther. 1969;180:46–56. [PubMed] [Google Scholar]
- SCHRÖR K., DARIUS H., MATZKY R., OHLENDORF R. The antiplatelet and cardiovascular actions of a new carbacyclin derivative (ZK36374) equi-potent to PGI2in vitro. Naunyn-Schmiedebergs Arch. Pharmacol. 1981;316:252–255. doi: 10.1007/BF00505658. [DOI] [PubMed] [Google Scholar]
- SENIOR J., MARSHALL K., SANGHA R., CLAYTON J.K. In vitro characterization of prostanoid receptors on human myometrium at term pregnancy. Br. J. Pharmacol. 1993;108:501–506. doi: 10.1111/j.1476-5381.1993.tb12832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SENIOR J., SANGHA R., BAXTER G.S., MARSHALL K., CLAYTON J.K. In vitro characterization of prostanoid FP-, DP-, IP- and TP-receptors on the non-pregnant human myometrium. Br. J. Pharmacol. 1992;107:215–221. doi: 10.1111/j.1476-5381.1992.tb14489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARIF N.A., XU S.X., WILLIAMS G.W., CRIDER J.Y., GRIFFIN B.W., DAVIS T.L. Pharmacology of [3H]prostaglandin E1/[3H]prostaglandin E2 and [3H]prostaglandin F2alpha binding to EP3 and FP prostaglandin receptor binding sites in bovine corpus luteum: characterization and correlation with functional data. J. Pharmacol. Exp. Ther. 1998;286:1094–1102. [PubMed] [Google Scholar]
- SHEN R.F., TAI H.H. Thromboxanes: synthase and receptors. J. Biomed. Sci. 1998;5:153–172. doi: 10.1007/BF02253465. [DOI] [PubMed] [Google Scholar]
- SJOBERG T., STEEN S. The strong contractile effect of the thromboxane receptor agonist U-46619 in isolated human pulmonary arteries and its competitive antagonism by BM-13.505. Acta. Physiol. Scand. 1989;136:161–165. doi: 10.1111/j.1748-1716.1989.tb08648.x. [DOI] [PubMed] [Google Scholar]
- STOCK A., JONES R., CHUNG T., FUNG H.Y. Pulmonary edema in association with an intravenous infusion of sulprostone. Acta Obstet. Gynecol. Scand. 1995;74:156–158. doi: 10.3109/00016349509008927. [DOI] [PubMed] [Google Scholar]
- TANG L., LOUTZENHISER K., LOUTZENHISER R. Biphasic actions of prostaglandin E(2) on the renal afferent arteriole : role of EP(3) and EP(4) receptors. Circ. Res. 2000;86:663–670. doi: 10.1161/01.res.86.6.663. [DOI] [PubMed] [Google Scholar]
- TEMPLETON A.G., MCGRATH J.C., WHITTLE M.J. The role of endogenous thromboxane in contractions to U46619, oxygen, 5-HT and 5-CT in the human isolated umbilical artery. Br. J. Pharmacol. 1991;103:1079–1084. doi: 10.1111/j.1476-5381.1991.tb12303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TYMKEWYCZ P.M., JONES R.L., WILSON N.H., MARR C.G. Heterogeneity of thromboxane A2 (TP-) receptors: evidence from antagonist but not agonist potency measurements. Br. J. Pharmacol. 1991;102:607–614. doi: 10.1111/j.1476-5381.1991.tb12220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USKI T.K., ANDERSSON K.E., BRANDT L., LJUNGGREN B. Characterization of the prostanoid receptors and of the contractile effects of prostaglandin F2 alpha in human pial arteries. Acta Physiol. Scand. 1984;121:369–378. doi: 10.1111/j.1748-1716.1984.tb07468.x. [DOI] [PubMed] [Google Scholar]
- WALCH L., LABAT C., GASCARD J.P., DE MONTPREVILLE V., BRINK C., NOREL X. Prostanoid receptors involved in the relaxation of human pulmonary vessels. Br. J. Pharmacol. 1999;126:859–866. doi: 10.1038/sj.bjp.0702393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATABE A., SUGIMOTO Y., HONDA A., IRIE A., NAMBA T., NEGISHI M., ITO S., NARUMIYA S., ICHIKAWA A. Cloning and expression of cDNA for a mouse EP1 subtype of prostaglandin E receptor. J. Biol. Chem. 1993;268:20175–20178. [PubMed] [Google Scholar]
- WATERBURY L.D., EGLEN R.M., FAUROT G.F., COOPER G.F. EP3, but not EP2, FP, or TP prostanoid-receptor stimulation may reduce intraocular pressure. Invest Ophthalmol. Vis. Sci. 1990;31:2560–2567. [PubMed] [Google Scholar]
- WOODWARD D.F., BURKE J.A., WILLIAMS L.S., PALMER B.P., WHEELER L.A., WOLDEMUSSIE E., RUIZ G., CHEN J. Prostaglandin F2 alpha effects on intraocular pressure negatively correlate with FP-receptor stimulation. Invest. Ophthalmol. Vis. Sci. 1989;30:1838–1842. [PubMed] [Google Scholar]
- WOODWARD D.F., CHAN M.F., BURKE J.A., CHENG-BENNETT A., CHEN G., FAIRBAIRN C.E., GAC T., GARST M.E., GLUCHOWSKI C., KAPLAN L.J., LAWRENCE R.A., ROOF M., SACHS G., SHAN T., WHEELER L.A., WILLIAMS L.S. Studies on the ocular hypotensive effects of prostaglandin F2 alpha ester prodrugs and receptor selective prostaglandin analogs. J. Ocul. Pharmacol. 1994;10:177–193. doi: 10.1089/jop.1994.10.177. [DOI] [PubMed] [Google Scholar]
- WOODWARD D.F., LAWRENCE R.A., FAIRBAIRN C.E., SHAN T., WILLIAMS L.S. Intraocular pressure effects of selective prostanoid receptor agonists involve different receptor subtypes according to radioligand binding studies. J. Lipid Mediat. 1993;6:545–553. [PubMed] [Google Scholar]
- WOODWARD D.F., PEPPERL D.J., BURKEY T.H., REGAN J.W. 6-isopropoxy-9-oxoxanthene-2-carboxylic acid (AH6809) a human EP2 receptor antagonist. Biochem. Pharmacol. 1995;50:1731–1733. doi: 10.1016/0006-2952(95)02035-7. [DOI] [PubMed] [Google Scholar]
- YOKOTANI K., OKUMA Y., OSUMI Y. Inhibition of vagally mediated gastric acid secretion by activation of central prostanoid EP3 receptors in urethane-anaesthetized rats. Br. J. Pharmacol. 1996;117:653–656. doi: 10.1111/j.1476-5381.1996.tb15240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


