Abstract
Postnatal mortality in isolated congenital diaphragmatic hernia (CDH) is mainly related to the associated pulmonary hypertension (PH) and to right-to-left shunting.
Endothelins (ETs) are potent vasoconstrictors and pro-mitogenic peptides. Strong evidences support their participation in CDH and in the etiology of PH via the activation of ETA receptors (ETA-Rs).
Evaluation of the effect of ABT-627, a selective non-peptidic ETA-R antagonist, given from −15 to 210 min post-delivery (1 mg kg−1 bolus +0.01 mg kg−1 h−1 infusion, i.v.), was conducted in the lamb model of CDH.
Severity of CDH was assessed in comparison to untreated controls (n=5). Untreated CDH lambs (n=7) had a higher mean pulmonary arterial pressure (MPAP; P<0.0001), lower mean blood pressure (MBP; P=0.0004), higher MPAP / MBP ratio (P<0.0001), lower arterial pH (P<0.0001), higher paCO2 (P<0.0001), lower paO2 (P<0.0001) and lower post-ductal pulsatile SaO2 (P<0.0001) than untreated controls.
Treated controls (n=7) showed a higher MPAP, lower MBP, higher MPAP/MBP ratio, lower arterial pH, higher paCO2, lower paO2, lower post-ductal pulsatile SaO2 and lower plasmatic ir-ET ratios compared to untreated controls (P<0.0001).
Treated CDH lambs (n=8) showed a higher MBP (P<0.0001), lower MPAP / MBP ratio (P<0.0001), higher arterial pH (P<0.0001), lower paCO2 (P<0.0001), higher paO2 (P=0.0228), higher post-ductal pulsatile SaO2 (P=0.0016) and lower plasmatic ir-ET ratios (P=0.0247) when compared to untreated CDH lambs.
These observations revealed that, although acute perinatal treatment with a selective non-peptidic ETA-R antagonist had some adverse effects in controls, it attenuated the progressive cardiopulmonary deterioration that occurred after birth in CDH lambs.
Keywords: Congenital diaphragmatic hernia, pulmonary hypertension, right-to-left shunting, endothelins, endothelin receptors, ABT-627, antagonist
Introduction
In congenital diaphragmatic hernia (CDH), there is a defect in the diaphragm during the embryonic life and part of intra-abdominal viscera develops into the chest cavity. Development of the both lungs is affected, revealing a more or less severe, non-uniform, pulmonary hypoplasia (Kitagawa et al., 1971; Kent et al., 1972). Severe lung vascular changes that include proliferation of smooth muscle cells have been found in small pulmonary arteries at post-mortem (Levin, 1978), which may contribute to the pulmonary hypertension (PH) found at birth (Olivet et al., 1978; Starrett & de Lorimier, 1975). After birth, further deterioration in the cardiopulmonary profile can still occur, either pre- or post-operatively, with the occurrence of a right-to-left shunt through the ductus arteriosus and the foramen ovale (Murdock et al., 1971; Dibbins & Wiener, 1974; Collins et al., 1977). These elements account for the morbidity and the mortality encountered in this disease (Thibeault & Haney, 1998; Beresford & Shaw, 2000).
Endothelins (ETs : ET-1, -2 and -3) are a family of 21-amino acid isopeptides (Yanagisawa et al., 1988) produced from distinct precursors named big endothelins (big ETs : big ET-1, -2 and -3; Inoue et al., 1989). The molecular conversion of these 38 – 41 amino acid precursors is essential for the full expression of their biological activities and dependent on proteases called endothelin-converting enzymes (ECEs : ECE-1a, -1b, -1c, -1d, -2a, -2b and -3; Turner et al., 1998). Once produced and released, ETs act directly on specific G-protein-coupled seven transmembrane-spanning ET receptors (ET-Rs : ETA and ETB) to elicit their effects on vascular, non-vascular smooth muscle and other types of cells (Sakurai et al., 1992). ETA-Rs are mainly located on vascular smooth muscle cells and mediate mostly vasoconstriction (White et al., 1993a) and smooth muscle proliferation (Zamora et al., 1993). ETB-Rs, present on both the vascular endothelium and smooth muscle cells, can elicit either vasodilatation (Takayanagi et al., 1991) or vasoconstriction (Clozel et al., 1992), respectively.
Strong evidences support the participation of ETs in the etiology of PH. It has been demonstrated that mRNA expression of both ET-1 and ETA-Rs is up-regulated (Stelzner et al., 1992; Li et al., 1994) whereas mRNA expression of ETB-Rs is down-regulated (Yorikane et al., 1993) in rat models of idiopathic and hypoxia-induced PH. Blockade of the ET system by ETA-R antagonists has been shown to attenuate the development of PH in monocrotaline- and hypoxic-rats (Bonvallet et al., 1994; Dupuis & Prie, 1999) and in hypoxic-pigs (Holm et al., 1998). It has also been demonstrated that chronic intrauterine PH caused by ductus arteriosus ligature in a foetal lamb led to abnormalities of the ET system, such as a decrease in ETB-Rs mRNA expression and in ETB-Rs-mediated vasodilatation, associated with an enhanced ETA-R-mediated vasoconstriction (Ivy et al., 1996; 1998). More recently, it has been shown that prolonged blockade of the ETB-Rs in the normal ovine foetal with BQ-788, a selective peptidic ETB-Rs antagonist, caused PH, right ventricular hypertrophy, elevated pulmonary vascular resistance (PVR) and hypermuscularization of small pulmonary arteries (Ivy et al., 2000). These evidences suggest a predominant role of ETA-Rs in the development of PH in various animal models.
Moreover, since 1993, a number of studies have shown the possible implication of ETs in human neonates with CDH (Rosenberg et al., 1993; Kobayashi & Puri, 1994), in nitrofen-induced CDH in newborn rats (Okazaki et al., 1998; Coppola et al., 1998; Shima et al., 2000; Kavanagh et al., 2000) and in surgically-induced diaphragmatic hernia in newborn lambs (Thébaud et al., 2000).
Although the pathobiological development of CDH is not yet fully understood, we hypothesize that ETs may play a role, not only with regards to the PH found at birth, but also in the subsequent cardiopulmonary deterioration that can occur afterwards. Blockade of the ET system could prevent the return to foetal circulation that may occur in high-risk CDH and, consequently, constitute a novel approach of treatment. In the present study, we investigated the effect of a highly potent and selective non-peptidic ETA-R antagonist, ABT-627 (A-147627), in a lamb model of CDH.
Methods
The current protocol has been conducted according to the guidelines of the Canadian Council of Animal Care (CCAC) and authorized by the local Animal Care and Use Committee at Laval University (CPAUL; no. 98 – 150). Ewes were obtained from Thomas D. Morris Inc. (Reisterstown, MD, U.S.A.).
CDH animal model
Diaphragmatic hernia was induced surgically in foetal lambs at about 90 days of gestation. The surgical technique has been previously described by our team (de Luca et al., 1987). Briefly, the foetal hemithorax was identified by direct palpation and was marsupialized to the uterine wall. A left thoracotomy was performed on the foetus. An opening of approximately 1 cm was made into the diaphragm through which the stomach was pulled into the chest cavity. The thorax was closed and gestation was allowed to continue until near term. All ewes received ketoprofen (2 mg kg−1 day−1, s.c.×3 days), progesterone (50 mg ewe−1 day−1, i.m.×7 days) and trivetrin 40/200 (0.1 ml kg−1 day−1, i.m.×5 days) in pre- and post-surgery.
Perinatal experimental protocol
At around 138 days of gestation (full term 145 days), foetal lambs were delivered via caesarean section from ewes anaesthetized with isoflurane (1.5 – 2%). The womb was open and the head of the neonate lamb was exposed. To avoid spontaneous breathing, a rubber glove containing warm saline was immediately placed over the snout. Under local anaesthesia (xylocaine 2%), the left carotid artery, right and left jugular veins and trachea were cannulated. Fifteen minutes prior clamping of the umbilical cord, all foetal lambs received the vehicle (NaHCO3−, 2 mmol kg−1) or ABT-627 (1 mg kg−1, bolus i.v.) and a constant infusion (5 ml kg−1 h−1, i.v.) of dextrose (0.5 g kg−1 h−1), pancuronium bromide (0.1 mg kg−1 h−1), NaHCO3− (0.5 mmol kg−1 h−1) and ABT-627 (0.01 mg kg−1 min−1 in treated animals only). Bolus of pancuronium bromide (0.1 mg kg−1) and NaHCO3− (2 mmol kg−1) were given i.v. to the foetus just before delivery.
After clamping of the umbilical cord (t0), newborn lambs were rapidly weighed, connected to a Sechrist mechanical ventilator (Anaheim, CA, U.S.A.) and to a Hewlett Packard Omnicare monitoring apparatus (HP54S model; Sao Paulo, CA, U.S.A.) and wrapped in warming blankets with continuous monitoring of temperature. An elastic band was placed around the abdomen to avoid spontaneous reduction of CDH (Major et al., 1995). A 4F wedge flow-directed catheter was positioned into the pulmonary artery via the right jugular vein. The positioning of all catheters was later confirmed at autopsy. During the experimental protocol, respiratory parameters were adjusted according to arterial blood gases, to maximize gas exchanges and pulmonary mechanics throughout the experiment while minimizing pulmonary barotrauma (peak inspiratory pressure/positive end expiratory pressure (PIP/PEEP) ⩽30/⩽5 cm H2O, respiratory rate ⩽100 breaths min−1, FiO2=1.0). Also, NaHCO3− was administered as a bolus, when necessary, to correct the base deficit using the following formula: mmol needed=negative base excess (mmol l−1)×body weight (kg)×0.3. Newborn lambs receive ketamine (0.1 mg kg−1) and valium (5 mg) as required, according to physiological parameters.
All the newborn lambs were divided into four age-matched groups: untreated non-CDH (controls; C), untreated CDH (CDH), treated non-CDH (C+ABT-627) and treated CDH (CDH+ABT-627). The highly potent and selective non-peptidic selective ETA-R antagonist, ABT-627 (A-147627; [2R, 3R, 4S]-2-(4-methoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-[[N,N-dibutylamino)-carboxyl] methyl] pyrrolidine-3-carboxylic acid; IC50=0.034 nM for ETA-Rs and 63.3 nM for ETB-Rs; 1861 fold more selective for ETA-Rs; Winn et al., 1996; Chen et al., 1997; Liu et al., 1998), was synthesized at Abbott Laboratories Inc. (Abbott Park, IL, U.S.A.).
Experimental measurements
Physiological parameters were continuously monitored using a HP Omnicare apparatus. Cardiovascular parameters (systemic, wedge and pulmonary arterial blood pressures), respiratory functions (respiratory compliance (CR), ventilatory index (VI)), arterial and venous blood gases and post-ductal pulsatile saturation were recorded serially until death or sacrifice at 210 min post-delivery.
Measurement of physiological shunt was performed using arterial and venous blood gases (PCO2, PO2, SO2, Hb), expiratory volume and oxygen consumption measurements (Fournier & Major, 1981). CR was calculated by least mean square analysis of the tracheal pressure, airflow and volume data. Airflow was measured using a pneumotachometer (Fleisch no. 0) positioned between the ventilator and a 3.5F endotracheal tube. Respiratory pressure was recorded with a Validyne M-45 transducer (Validyne Engineering Corporation; Northridge, CA, U.S.A.). VI was calculated using the following equation: mean airway pressure (MAWP)×respiratory frequency, where MAWP=[(inspiratory time×PIP)+(expiratory time×PEEP)]/(inspiratory time+expiratory time) (Marinelli, 1981). At 60 and 120 min, cardiac output (CO) was obtained by the Fick method and PVR was calculated using standard equation (Mark et al., 2000). At the end of the experiment, lungs were weighed for the calculation of lung indexes (lungs' wet weight over body's weight×100).
Biochemical analysis
Plasmas were obtained at t−15, t+30, t+60, t+90, t+120, t+150, t+180 and t+210 from neonatal lambs. Each ml of blood collected in EDTA Vacutainer (Becton Dickinson; Franklin Lakes, NJ, U.S.A.) was replaced by 3 ml of sterile lactate Ringer solution to avoid hypovolemia. Samples were then centrifuged (12,000×g for 6 min at 4°C) and stored immediately at −76°C until assayed for the measurements of immunoreactive-endothelins (ir-ETs).
Non-polar extraction of ir-ETs was obtained by applying 500 ul of plasma into a 500 mg C2 ethyl Sep-Pak cartridge (Amprep RPN 1913; Amersham Life Science, Arlington Heights, IL, U.S.A.). Columns were equilibrated with 2 ml of methanol followed by 2 ml of distilled water. Plasmas were applied on column and washed with 5 ml of 0.1% trifluoroacetic acid. The effluent was collected with 2 ml of 80% acetonitrile and dried using a centrifugal evaporator (speed-vac apparatus; Savant Instruments Inc., Holbrook, NY, U.S.A.). Plasmatic ir-ETs levels were determined by radioimmunoassay according to the guidelines of the company (Amerlex RPA 555; Amersham Life Science, Arlington Heights, IL, U.S.A.). Determination of radioactivity was performed using a gamma scintillation counter (60 s; Cobra II, Auto-Gamma counter, Packard Instument Corporation, Meriden, CT, U.S.A.). The detection limit of the assay was 0.5 fmol tube−1. ET-1 antiserum cross-reacted with ET-1 (100%), ET-2 (144%), ET-3 (52%), but not with big ET-1 (0.4%) or other peptides. The final amount of ir-ETs for each aliquot was expressed in pg ml−1. Plasmatic ir-ETs levels were expressed as a ratio: value at a given time/value at 15 min before birth (before starting treatment in all groups).
Statistical analyses
All results are expressed as mean±s.e.m. For most of the parameters, since multiple measurements were obtained on the same experiment, comparisons between groups were made using analyses of repeated measurements (SUDAAN software; Research Triangle Park, Durham, NC, U.S.A.). Models that were obtained are presented in Table 2 and in Figures 1, 2, 3 and 4. Satterwaite P-value was used for these statistical analyses. For lung index, CO and PVR, the non-parametric Wilcoxon statistical test comparing each group was used. Wilcoxon score tests were performed with SAS software and the exact two-tail P-value was used. Statistical significance was established at P<0.05.
Table 2.
Statistical equations comparing treated control animals and CDH newborn lambs

Figure 1.
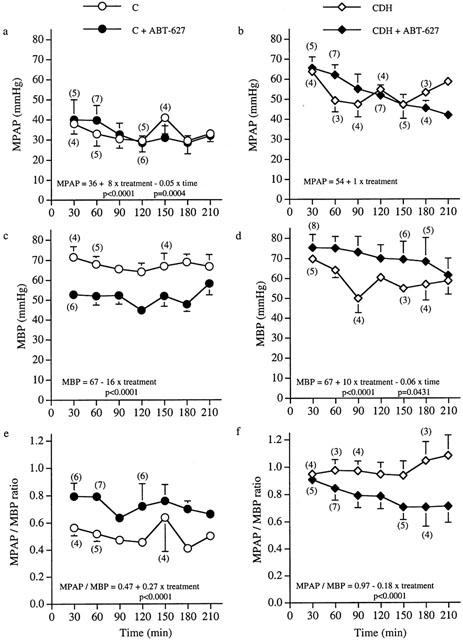
Postnatal evolution of mean pulmonary arterial pressure (MPAP), mean blood pressure (MBP) and MPAP/MBP ratio in all untreated and treated groups.
Figure 2.

Arterial blood gases profile analyses in all untreated and treated age-matched controls (C) and CDH newborn lambs.
Figure 3.

Measurement of post-ductal pulsatile SaO2 in all untreated and treated age-matched controls (C) and CDH newborn lambs.
Figure 4.

Relative immunoreactive endothelin (ir-ET) ratios in plasma from newborn age-matched controls (C) and CDH lambs, treated or not with an antagonist of ETA-Rs.
Results
Following in utero surgery, 15 CDH lambs were obtained. In seven other lambs, the surgically-induced hole in the diaphragm got blocked and no CDH was found at post-mortem examination. These lambs were pooled together with five untouched twins to constitute the non-CDH group (controls; C). Therefore, the 27 newborn lambs used in this experiment were divided as follows: C (n=5), CDH (n=7), C+ABT-627 (n=7) and CDH+ABT-627 (n=8).
All CDH lambs had huge hernia with most part of the rumen, the small bowel and the colon inside the thorax. Lungs were hypoplastics and much smaller than the chest cavity (Table 1). The communication between the thorax and the abdomen was unrestrained in all CDH lambs whereas the hernia seemed partially reduced in some of them.
Table 1.
Lung indexes and survival time in the four groups of newborn lambs

All untreated and treated control lambs survived the entire 210 min protocol, except one that was sacrificed at 90 min for technical reasons. In comparison, 4/7 (57%) of untreated CDH animals survived the entire experiment versus 5/8 (63%) for the treated CDH group. The demise in both groups was due to progressive cardiopulmonary deterioration. There was no difference in overall survival time between untreated and treated CDH animals (Table 1).
Statistical equations comparing untreated controls and CDH lambs for various parameters are reported in Table 2.
Cardiovascular parameters
Mean pulmonary arterial pressure (MPAP), mean blood pressure (MBP) and MPAP/MBP ratio (Figure 1a,f)
MPAP and MPAP/MBP ratio were both significantly higher in untreated CDH group than in untreated control animals (P<0.0001). At 30 min, untreated CDH lambs presented a similar MBP than untreated control lambs. Then, MBP decreased and remained significantly lower in untreated CDH compared to untreated control lambs until the end at 210 min (P=0.0004).
Treatment of control lambs significantly increased MPAP, lowered MBP and increased MPAP / MBP ratio compared to untreated control animals (P<0.0001). Treated CDH animals had a significantly higher MBP and a significantly lower MPAP/MBP ratio than untreated CDH newborn lambs (P<0.0001). Although no statistical differences were observed between these two groups regarding MPAP, a continuous decrease in MPAP, that reached 36% at 210 min, was observed in treated CDH lambs throughout the experiment.
Physiological shunt
Untreated control newborn lambs presented a stable physiological shunt around 30% for all the protocol. In the untreated CDH group, physiological shunt was increased at 30 min and further increased from 180 min up to the end by 54%. Thus, physiological shunt remained higher in untreated CDH lambs than in untreated controls all over the experiment (P<0.0001; Table 2).
Treated control lambs presented a higher physiological shunt at 30 min, which stayed higher, compared to untreated controls for all the remaining of the experiment (44+21xC+ABT−627 − 0.11xtime; P<0.0001). When regarding untreated and treated CDH lambs, no statistical difference was observed in physiological shunt.
Cardiac output (CO) and pulmonary vascular resistance (PVR)
No statistical difference was observed when comparing CO at 60 and 120 min for all untreated and treated groups. Similarly, PVR at 60 and 120 min was not statistically different between all four groups (data not shown).
Respiratory functions
Respiratory compliance (CR)
In the untreated control group, CR increased steadily along time. Untreated CDH lambs showed a significantly lower CR compared to untreated controls all over the experiment (P<0.0001; Table 2).
Treatment significantly lowered CR in controls compared to untreated control lambs (0.41 – 0.10xC+ABT−627; P=0.0004). CR was not statistically different between treated and untreated CDH lambs for all the experiment.
Ventilatory index (VI)
In the untreated control group, a 1.9 fold decrease was observed for VI from birth to 210 min post-delivery. Untreated CDH lambs showed a significantly higher VI than untreated controls all over the experiment (P<0.0001; Table 2).
There was no statistical difference in VI between untreated and treated control lambs. VI was also not statistically different between untreated and treated CDH lambs all over the experiment.
Arterial blood gases
pH, paCO2 and paO2 (Figure 2a,f)
In untreated control group, pH and paCO2 remained stable for the entire 210 min whereas paO2 rose from 50 to 150 mmHg at the end of experiment. Untreated CDH animals presented a lower pH, higher paCO2 and lower paO2 than untreated controls over time (P<0.0001).
Treated control animals presented a lower pH, higher paCO2 and lower paO2 than untreated control lambs (P<0.0001). In CDH lambs, treatment caused a significant increase in pH (P<0.0001), decrease in paCO2 (P<0.0001) and increase in paO2 (P=0.0228) compared to untreated CDH animals over time.
Arterial post-ductal saturation
Post-ductal pulsatile SaO2 (Figure 3a,b)
In untreated control group, post-ductal pulsatile SaO2 stayed stable for the entire 210 min. Untreated CDH animals presented a steadily lower post-ductal pulsatile SaO2 than untreated controls over time (P<0.0001).
Treated control animals presented a lower post-ductal pulsatile SaO2 than untreated control lambs (P<0.0001). When regarding CDH lambs, treatment caused a significant increase in post-ductal pulsatile SaO2 (P=0.0016) compared to untreated CDH animals over time.
Biochemical analysis
Plasmatic immunoreactive endothelin ratios (ir-ET ratios; Figure 4a,b)
In the untreated control group, plasmatic ir-ET ratios remained stable during all the experiment. Plasmatic ir-ET ratios were stable during the first 90 min in untreated CDH, followed by a 2 fold increase at 210 min.
Treated control animals presented a lower plasmatic ir-ET ratios compared to untreated control lambs (P<0.0001). Treated CDH animals also showed a lower plasmatic ir-ET ratios compared to untreated CDH lambs (P=0.0247).
Discussion
When compared to untreated age-matched controls, untreated lambs with CDH presented the following pattern at 30 min: (1) higher MPAP and MPAP/MBP ratio; (2) higher physiological shunt; (3) lower CR; (4) lower arterial pH; (5) higher paCO2 and (6) lower post-ductal pulsatile SaO2. In spite of optimal resuscitation procedures, further deterioration occurred in untreated CDH lambs during the period of observation: (1) MBP decreased whereas MPAP and MPAP/MBP ratio increased; (2) physiological shunt increased; (3) post-ductal pulsatile SaO2 decreased and (4) plasmatic ir-ET ratios increased sharply.
Haemodynamic and biochemical disturbances observed at birth in untreated CDH animals can be explained by the severe pulmonary hypoplasia of the both lungs confirmed at post-mortem. Since the vascular cross-sectional area is reduced, PVR is increased (Poiseuille law). Decrease in MBP and increases in MPAP/MBP ratio and in plasmatic ETs ratios occurred over time. Although the drop in MBP remain unexplained, the elevated circulating ET levels may have been induced by shearing forces within the pulmonary vascular bed (Kuchan & Frangos, 1993), or may be attributed to reduced pulmonary clearance by hypoplastic lungs as observed in other diseases associated with pulmonary hypertensive states of various aetiologies (Dupuis et al., 1998). Under normal conditions, a balance between vasoconstrictors, such as ETs, and vasodilators regulates vascular homeostasis (Furchgott & Vanhoutte, 1989). However, an imbalance seems to appear in CDH lambs where ETs may act excessively on ETA-Rs, triggering the right-to-left shunt through the ductus arteriosus and/or the foramen ovale. Noticeably, ETA-Rs mRNA expression is up-regulated in nitrofen-induced CDH newborn rats (Okazaki et al., 1998; Shima et al., 2000).
The severity of the diaphragmatic hernia was similar in both untreated and treated CDH groups, as assessed by lung indexes (Table 1) and by cardiovascular parameters, pulmonary function and arterial blood gases values at birth (Table 2; Figures 1, 2 and 3). Treatment with the selective non-peptidic ETA-R antagonist improved the overall cardiopulmonary profile of CDH animals over time: (1) higher MBP and lower MPAP/MBP ratio; (2) higher arterial pH; (3) lower paCO2; (4) higher paO2; (5) higher post-ductal pulsatile SaO2 and (6) lower plasmatic ir-ET ratios.
Noticeably, contrary to what was observed in untreated CDH lambs, MBP remained stable in the treated group. The MPAP was no longer equal nor higher than MBP but rather slowly decreased even though it did not reach statistical significance. This resulted in a significantly lower MPAP/MBP ratio, suggesting that the right-to-left shunting was less important. The ventilation-perfusion was then improved, which explains that even though no direct beneficial effect was measured on PVR, blood gases exchanges were improved in treated CDH lambs. These effects of the treatment may be direct, by blocking the vasoconstriction effect of ETs, but also indirect since it has been reported that ETA-R blockade improves nitric oxide (NO) mediated vasodilation in rats with monocrotaline-induced PH (Prie et al., 1998).
However, the treatment did not completely reverse the elevated MPAP nor reduced the MPAP/MBP ratio back to values observed in untreated controls. We tested one dose in a very complex model involving numerous variables. First, one may consider that a higher dose may be more effective over this short period of time or a long-term treatment may prove to be more beneficial (pre- or postnatal). Secondly, one may also suggest the participation of ETB-Rs in the pathophysiological response. It is established that both ET-R subtypes are involved in mediating vascular smooth muscle cells contractions (White et al., 1993a; Cardell et al., 1992). However, there is no evidence that suggest the presence of vasocontractile ETB-Rs in the newborn lamb pulmonary vasculature. In the adult lambs, like in the human pulmonary artery, ETA-R subtypes are dominant in this vascular bed (Toga et al., 1991; Fukuroda et al., 1994). Therefore, we believe that treatment with a selective ETB-R antagonist would prove to be ineffective unless this population of receptors would be located on smooth muscle cells and up-regulated in CDH. Even then, ETB-R antagonism may rather enhance pulmonary artery contraction and PVR by reducing the release of endothelial-derived vasodilators. To that effect, prolonged ETB-R blockade was shown to elevate PVR and cause PH in the ovine foetus (Ivy et al., 2000).
The stability in circulating levels of ir-ETs that we observed in treated CDH animals might be attributed to the effect of circulating ETs on endothelial ETB-Rs which are associated to the release of NO and prostacyclin, two vasodilators (Tsukahara et al., 1994; White et al., 1993b). Both mediators have been reported to subsequently down-regulate the expression, production and response of ET isopeptides (Kourembanas et al., 1993; Prins et al., 1994) and therefore explain the unvaried plasmatic ir-ETs ratios in treated CDH lambs.
Treatment with the selective non-peptidic ETA-R antagonist seemed to adversely affect the cardiopulmonary profile of control newborn lambs. When compared to untreated controls, treated control lambs have shown: (1) higher MPAP, lower MBP and higher MPAP/MBP ratio; (2) higher physiological shunt; (3) lower CR; (4) lower arterial pH; (5) higher paCO2; (6) lower paO2; (7) lower post-ductal pulsatile SaO2 and (8) lower plasmatic ir-ET ratios.
Treatment affected the normotensive homeostasis of control newborn lambs. These observations may also be attributed to the premature opening of the normal pulmonary vascular bed, thus causing a harmful imbalance in healthy non-CDH newborn lambs. It has been reported that ET-1 is a very potent contractile agent on isolated ductus arteriosus preparations from mature foetal lambs, suggesting a role in the closure at birth (Coceani et al., 1989). By treating control lambs, we may interfere with the natural process of closure and observed side-effects of the drug in normotensive animals at birth. Moreover, transition to extra-uterine air breathing and regulation of the pulmonary vascular tone is regulated by a complex interactive group of mechanisms (Heymann, 1999).
In summary, short-term acute treatment with a selective non-peptidic ETA-R antagonist statistically improved MPAP/ MBP ratio and blood gas exchanges in acute experiments on unreduced CDH lambs. Such experimental observations, even though preliminary, may bear clinical relevance and open the way to new investigations. A prophylactic treatment with this ETA-R antagonist given in utero during the last trimester of gestation, aimed at attenuating the pulmonary vascular remodelling, could lead to clinically relevant applications.
Acknowledgments
This work has been supported by MRCC/PMAC (Abbott Laboratories Inc.) grant no. 36624, Fondation du CHUQ/Fondation des employés de la banque de Montréal, Fonds de la Recherche en Santé du Québec (FRSQ) and Fondation de la Recherche sur les Maladies Infantiles (FRMI). The authors would like to thank Gaspard Montandon for the realization of the respiratory functions apparatus. The authors are also very grateful to Jim Gourdon, DMV, Mario Mercier, TSA, and Évelyne Vachon, TSA, for their technical support.
Abbreviations
- CDH
congenital diaphragmatic hernia
- CO
cardiac output
- CR
respiratory compliance
- ET-Rs
endothelin receptors
- ETs
endothelins (ET-1, -2 and -3)
- ir-ETs
immunoreactive-endothelins
- MBP
mean blood pressure
- MPAP
mean pulmonary arterial pressure
- PH
pulmonary hypertension
- PVR
pulmonary vascular resistance
- VI
ventilatory index
References
- BERESFORD M.W., SHAW N.J. Outcome of congenital diaphragmatic hernia. Pediatr. Pulmonol. 2000;30:249–256. doi: 10.1002/1099-0496(200009)30:3<249::aid-ppul9>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- BONVALLET S.T., ZAMORA M.R., HASUNUMA K., SATO K., HANASATO N., ANDERSON D., SATO K., STELZNER T.J. BQ123, an ETA-receptor antagonist, attenuates hypoxic pulmonary hypertension in rats. Am. J. Physiol. 1994;266:H1327–H1331. doi: 10.1152/ajpheart.1994.266.4.H1327. [DOI] [PubMed] [Google Scholar]
- CARDELL L.O., UDDMAN R., EDVINSSON L. Evidence for multiple endothelin receptors in the guinea-pig pulmonary artery and trachea. Br. J. Pharmacol. 1992;105:376–380. doi: 10.1111/j.1476-5381.1992.tb14261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN S.J., CHEN Y.F., OPGENORTH T.J., WESSALE J.L., MENG Q.C., DURAND J., DICARLO V.S., OPARIL S. The orally active nonpeptide endothelin A-receptor antagonist A-127722 prevents and reverses hypoxia-induced pulmonary hypertension and pulmonary vascular remodeling in Sprague-Dawley rats. J. Cardiovasc. Pharmacol. 1997;29:713–725. doi: 10.1097/00005344-199706000-00003. [DOI] [PubMed] [Google Scholar]
- CLOZEL M., GRAY G.A., BREU V., LOFFLER B.M., OSTERWALDER R. The endothelin ETB receptor mediates both vasodilation and vasoconstriction in vivo. Biochem. Biophys. Res. Commun. 1992;186:867–873. doi: 10.1016/0006-291x(92)90826-7. [DOI] [PubMed] [Google Scholar]
- COCEANI F., ARMSTRONG C., KELSEY L. Endothelin is a potent constrictor of the lamb ductus arteriosus. Can. J. Physiol. Pharmacol. 1989;67:902–904. doi: 10.1139/y89-141. [DOI] [PubMed] [Google Scholar]
- COLLINS D.L., POMERANCE J.J., TRAVIS K.W., TURNER S.W., PAPPELBAUM S.J. A new approach to congenital posterolateral diaphragmatic hernia. J. Ped. Surg. 1977;12:149–156. doi: 10.1016/s0022-3468(77)80001-8. [DOI] [PubMed] [Google Scholar]
- COPPOLA C.P., AU-FLIEGNER M., GOSCHE J.R. Endothelin-1 pulmonary vasoconstriction in rats with diaphragmatic hernia. J. Surg. Res. 1998;76:74–78. doi: 10.1006/jsre.1997.5293. [DOI] [PubMed] [Google Scholar]
- DE LUCA U., CLOUTIER R., LABERGE J.M., FOURNIER L., PRENDT H., MAJOR D., EDGELL D., ROY P.E., ROBERGE S., GUTTMAN F.M. Pulmonary barotrauma in congenital diaphragmatic hernia: experimental study in lambs. J. Pediatr. Surg. 1987;22:311–316. doi: 10.1016/s0022-3468(87)80231-2. [DOI] [PubMed] [Google Scholar]
- DIBBINS A.W., WIENER E.S. Mortality from neonatal diaphragmatic hernia. J. Ped. Surg. 1974;9:653–662. doi: 10.1016/0022-3468(74)90102-x. [DOI] [PubMed] [Google Scholar]
- DUPUIS J., PRIE S. The ET(A) receptor antagonist LU 135252 prevents the progression of established pulmonary hypertension induced by monocrotaline in rats. J. Cardiovasc. Pharmacol. Ther. 1999;4:33–39. doi: 10.1177/107424849900400106. [DOI] [PubMed] [Google Scholar]
- DUPUIS J., ROULEAU J.L., CERNACEK P. Reduced pulmonary clearance of endothelin-1 contributes to the increase of circulating levels in heart failure secondary to myocardial infarction. Circulation. 1998;98:1684–1687. doi: 10.1161/01.cir.98.16.1684. [DOI] [PubMed] [Google Scholar]
- FOURNIER L., MAJOR D. Comparison between a clinical short-cut method and a precise laboratory estimation of intrapulmonary shunt and A-aDo2. Can. Anaesth. Soc. J. 1981;28:263–267. doi: 10.1007/BF03005512. [DOI] [PubMed] [Google Scholar]
- FUKURODA T., KOBAYASHI M., OZAKI S., YANO M., MIYAUCHI T., ONIZUKA M., SUGISHITA Y., GOTO K., NISHIKIBE M. Endothelin receptor subtypes in human versus rabbit pulmonary arteries. J. Appl. Physiol. 1994;76:1976–1982. doi: 10.1152/jappl.1994.76.5.1976. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R.F., VANHOUTTE P.M. Endothelium-derived relaxing and contracting factors. FASEB. J. 1989;3:2007–2018. [PubMed] [Google Scholar]
- HEYMANN M.A. Control of the pulmonary circulation in the fetus and during the transitional period to air breathing. Eur. J. Obstet. Gynecol. Reprod. Biol. 1999;84:127–132. doi: 10.1016/s0301-2115(98)00321-2. [DOI] [PubMed] [Google Scholar]
- HOLM P., LISKA J., FRANCO-CERECEDA A. The ETA receptor antagonist, BMS-182874, reduces acute hypoxic pulmonary hypertension in pigs in vivo. Cardiovasc. Res. 1998;37:765–771. doi: 10.1016/s0008-6363(97)00291-5. [DOI] [PubMed] [Google Scholar]
- INOUE A., YANAGISAWA M., KIMURA S., KASUYA Y., MIYAUCHI T., GOTO K., MASAKI T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc. Natl. Acad. Sci. U.S.A. 1989;86:2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVY D.D., PARKER T.A., ABMAN S.H. Prolonged endothelin B receptor blockade causes pulmonary hypertension in the ovine fetus. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2000;279:L758–L765. doi: 10.1152/ajplung.2000.279.4.L758. [DOI] [PubMed] [Google Scholar]
- IVY D.D., LE CRAS T.D., HORAN M.P., ABMAN S.H. Increased lung preproET-1 and decreased ETB-receptor gene expression in fetal pulmonary hypertension. Am. J. Physiol. Lung. Cell. Mol. Physiol. 1998;274:L535–L541. doi: 10.1152/ajplung.1998.274.4.L535. [DOI] [PubMed] [Google Scholar]
- IVY D.D., ZIEGLER J.W., DUBUS M.F., FOX J.J., KINSELLA J.P., ABMAN S.H. Chronic intrauterine pulmonary hypertension alters endothelin receptor activity in the ovine fetal lung. Pediatr. Res. 1996;39:435–442. doi: 10.1203/00006450-199603000-00010. [DOI] [PubMed] [Google Scholar]
- KAVANAGH M., BATTISTINI B., KLUTH D., JEAN S., FOURNIER L., JENG A.Y., MAJOR D., CLOUTIER R. Effect of CGS 26303, an endothelin-converting enzyme/neutral endopeptidase inhibitor, on nitrofen-induced congenital diaphragmatic hernia in the rat. J. Pediatr. Surg. 2000;35:780–784. doi: 10.1053/jpsu.2000.6068. [DOI] [PubMed] [Google Scholar]
- KENT G.M., OLLEY P.M., CREIGHTON R.E., DOBBINSON T., BRYAN M.H., SYMCHYCH P., ZINGG W., CUMMINGS J.N. Hemodynamic and pulmonary changes following surgical creation of a diaphragmatic hernia in fetal lambs. Surg. 1972;72:427–433. [PubMed] [Google Scholar]
- KITAGAWA M., HISLOP A., BOYDEN E.A., REID L. Lung hypoplasia in congenital diaphragmatic hernia. A quantitative study of airway, artery, and alveolar development. Br. J. Surg. 1971;58:342–346. doi: 10.1002/bjs.1800580507. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI H., PURI P. Plasma endothelin levels in congenital diaphragmatic hernia. J. Pediatr. Surg. 1994;29:1258–1261. doi: 10.1016/0022-3468(94)90818-4. [DOI] [PubMed] [Google Scholar]
- KOUREMBANAS S., MCQUILLAN L.P., LEUNG G.K., FALLER D.V. Nitric oxide regulates the expression of vasoconstrictors and growth factors by vascular endothelium under both normoxia and hypoxia. J. Clin. Invest. 1993;92:99–104. doi: 10.1172/JCI116604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUCHAN M.J., FRANGOS J.A. Shear stress regulates endothelin-1 release via protein kinase C and cGMP in cultured endothelial cells. Am. J. Physiol. 1993;264:H150–H156. doi: 10.1152/ajpheart.1993.264.1.H150. [DOI] [PubMed] [Google Scholar]
- LEVIN D.L. Morphologic analysis of the pulmonary vascular bed in congenital leftsided diaphragmatic hernia. J. Pediatr. 1978;92:805–809. doi: 10.1016/s0022-3476(78)80162-0. [DOI] [PubMed] [Google Scholar]
- LI H., CHEN S.J., CHEN Y.F., MENG Q.C., DURAND J., OPARIL S., ELTON T.S. Enhanced endothelin-1 and endothelin receptor gene expression in chronic hypoxia. J. Appl. Physiol. 1994;77:1451–1459. doi: 10.1152/jappl.1994.77.3.1451. [DOI] [PubMed] [Google Scholar]
- LIU G., HENRY K.J., JR, SZCZEPANKIEWICZ B.G., WINN M., KOZMINA N.S., BOYD S.A., WASICAK J., VON GELDERN T.W., WU-WONG J.R., CHIOU W.J., DIXON D.B., NGUYEN B., MARSH K.C., OPGENORTH T.J. Pyrrolidine-3-carboxylic acids as endothelin antagonists. 3. Discovery of a potent, 2-nonaryl, highly selective ETA antagonist (A-216546) J. Med. Chem. 1998;41:3261–3275. doi: 10.1021/jm980217s. [DOI] [PubMed] [Google Scholar]
- MAJOR D., CADENAS M., CLOUTIER R., FOURNIER L., WOLFSON M.R., SHAFFER T.H. Combined gas ventilation and perfluorochemical tracheal instillation as an alternative treatment for lethal congenital diaphragmatic hernia in lambs. J. Pediatr. Surg. 1995;30:1178–1182. doi: 10.1016/0022-3468(95)90016-0. [DOI] [PubMed] [Google Scholar]
- MARINELLI P. Mean airway pressure calculation (letter) J. Pediatr. 1981;99:168–169. doi: 10.1016/s0022-3476(81)80996-1. [DOI] [PubMed] [Google Scholar]
- MARK J.B., SLAUGHTER T.F., REVES J.G.Cardiovascular monitoring Anaesthesia 2000USA: Churchill-Levingstone; 1117–1206.Miller, R.D., ed. 5th ed. Vol. 1 [Google Scholar]
- MURDOCK A.I., BURRINGTON J.B., SWYER P.R. Alveolar to arterial oxygen tension difference and venous admixture in newly born infants with congenital diaphragmatic herniation through the foramen of Bochdalek. Biol. Neonate. 1971;17:161–172. doi: 10.1159/000240311. [DOI] [PubMed] [Google Scholar]
- OKAZAKI T., SHARMA H.S., MCCUNE S.K., TIBBOEL D. Pulmonary vascular balance in congenital diaphragmatic hernia enhanced endothelin-1 gene expression as a possible cause of pulmonary vasoconstriction. J. Pediatr. Surg. 1998;33:81–84. doi: 10.1016/s0022-3468(98)90367-0. [DOI] [PubMed] [Google Scholar]
- OLIVET R.T., RUPP W.M., TELANDER R.L., KAYE M.P. Hemodynamics of congenital diaphragmatic hernia in lambs. J. Pediatr. Surg. 1978;13:231–235. doi: 10.1016/s0022-3468(78)80392-3. [DOI] [PubMed] [Google Scholar]
- PRIE S., STEWART D.J., DUPUIS J. EndothelinA receptor blockade improves nitric oxide-mediated vasodilation in monocrotaline-induced pulmonary hypertension. Circulation. 1998;97:2169–2174. doi: 10.1161/01.cir.97.21.2169. [DOI] [PubMed] [Google Scholar]
- PRINS B.A., HU R.M., NAZARIO B., PEDRAM A., FRANK H.J., WEBER M.A., LEVIN E.R. Prostaglandin E2 and prostacyclin inhibit the production and secretion of endothelin from endothelial cells. J. Biol. Chem. 1994;269:11938–11944. [PubMed] [Google Scholar]
- ROSENBERG A.A., KENNAUGH J., KOPPENHAFER S.L., LOOMIS M., CHATFIELD B.A., ABMAN S.H. Elevated immunoreactive endothelin-1 levels in newborn infants with persistent pulmonary hypertension. J. Pediatr. 1993;123:109–114. doi: 10.1016/s0022-3476(05)81552-5. [DOI] [PubMed] [Google Scholar]
- SAKURAI T., YANAGISAWA M., MASAKI T. Molecular characterization of endothelin receptors. Trends Pharmacol. Sci. 1992;13:103–108. doi: 10.1016/0165-6147(92)90038-8. [DOI] [PubMed] [Google Scholar]
- SHIMA H., OUE T., TAIRA Y., MIYAZAKI E., PURI P. Antenatal dexamethasone enhances endothelin receptor B expression in hypoplastic lung in nitrofen-induced diaphragmatic hernia in rats. J. Pediatr. Surg. 2000;35:203–207. doi: 10.1016/s0022-3468(00)90010-1. [DOI] [PubMed] [Google Scholar]
- STARRETT R.W., DE LORIMIER A.A. Congenital diaphragmatic hernia in lambs: hemodynamic and ventilatory changes with breathing. J. Ped. Surg. 1975;10:575–582. doi: 10.1016/0022-3468(75)90359-0. [DOI] [PubMed] [Google Scholar]
- STELZNER T.J., O'BRIEN R.F., YANAGISAWA M., SAKURAI T., SATO K., WEBB S., ZAMORA M., MCMURTRY I.F., FISHER J.H. Increased lung endothelin-1 production in rats with idiopathic pulmonary hypertension. Am. J. Physiol. 1992;262:L614–L620. doi: 10.1152/ajplung.1992.262.5.L614. [DOI] [PubMed] [Google Scholar]
- TAKAYANAGI R., KITAZUMI K., TAKASAKI C., OHNAKA K., AIMOTO S., TASAKA K., OHASHI M., NAWATA H. Presence of non-selective type of endothelin receptor on vascular endothelium and its linkage to vasodilation. FEBS Lett. 1991;282:103–106. doi: 10.1016/0014-5793(91)80454-b. [DOI] [PubMed] [Google Scholar]
- THÉBAUD B., DE LAGAUSIE P., FORGUES D., AIGRAIN Y., MERCIER J.C., DINHXUAN A.T. ET(A)-receptor blockade and ET(B)-receptor stimulation in experimental congenital diaphragmatic hernia. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2000;278:L923–L932. doi: 10.1152/ajplung.2000.278.5.L923. [DOI] [PubMed] [Google Scholar]
- THIBEAULT D.W., HANEY B. Lung volume, pulmonary vasculature, and factors affecting survival in congenital diaphragmatic hernia. Pediatrics. 1998;101:289–295. doi: 10.1542/peds.101.2.289. [DOI] [PubMed] [Google Scholar]
- TOGA H., USHA RAJ J., HILLYARD R., KU B., ANDERSON J. Endothelin effects in isolated, perfused lamb lungs: role of cyclooxygenase inhibition and vasomotor tone. Am. J. Physiol. 1991;261:H443–H450. doi: 10.1152/ajpheart.1991.261.2.H443. [DOI] [PubMed] [Google Scholar]
- TSUKAHARA H., ENDE H., MAGAZINE H.I., BAHOU W.F., GOLIGORSKY M.S. Molecular and functional characterization of the non-isopeptide-selective ETB receptor in endothelial cells. Receptor coupling to nitric oxide synthase. J. Biol. Chem. 1994;269:21778–21785. [PubMed] [Google Scholar]
- TURNER A.J., BARNES K., SCHWEIZER A., VALDENAIRE O. Isoforms of endothelin-converting enzyme: why and where. Trends Pharmacol. Sci. 1998;19:483–486. doi: 10.1016/s0165-6147(98)01251-6. [DOI] [PubMed] [Google Scholar]
- WHITE D.G., CANNON T.R., GARRATT H., MUNDIN J.W., SUMNER M.J., WATTS I.S. Endothelin ETA and ETB receptors mediate vascular smooth-muscle contraction. J. Cardiovasc. Pharmacol. 1993a;22:S144–S148. doi: 10.1097/00005344-199322008-00039. [DOI] [PubMed] [Google Scholar]
- WHITE D.G., MUNDIN J.W., SUMNER M.J., WATTS I.S. The effect of endothelins on nitric oxide and prostacyclin production from human umbilical vein, porcine aorta and bovine carotid artery endothelial cells in culture. Br. J. Pharmacol. 1993b;109:1128–1132. doi: 10.1111/j.1476-5381.1993.tb13739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINN M., VON GELDERN T.W., OPGENORTH T.J., JAE H.S., TASKER A.S., BOYD S.A., KESTER J.A., MANTEI R.A., BAL R., SORENSEN B.K., WU-WONG J.R., CHIOU W.J., DIXON D.B., NOVOSAD E.I., HERNANDEZ L., MARSH K.C. 2,4-Diarylpyrrolidine-3-carboxylic acids-potent ETA selective endothelin receptor antagonists. 1. Discovery of A-127722. J. Med. Chem. 1996;39:1039–1048. doi: 10.1021/jm9505369. [DOI] [PubMed] [Google Scholar]
- YANAGISAWA M., KURIHARA H., KIMURA S., TOMOBE Y., KOBAYASHI M., MITSUI Y., YAZAKI Y., GOTO K., MASAKI T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- YORIKANE R., MIYAUCHI T., SAKAI S., SAKURAI T., YAMAGUCHI I., SUGISHITA Y., GOTO K. Altered expression of ETB-receptor mRNA in the lung of rats with pulmonary hypertension. J. Cardiovasc. Pharmacol. 1993;22:S336–S338. doi: 10.1097/00005344-199322008-00088. [DOI] [PubMed] [Google Scholar]
- ZAMORA M.A., DEMPSEY E.C., WALCHAK S.J., STELZNER T.J. BQ123, an ETA receptor antagonist, inhibits endothelin-1-mediated proliferation of human pulmonary artery smooth muscle cells. Am. J. Respir. Cell. Mol. Biol. 1993;9:429–433. doi: 10.1165/ajrcmb/9.4.429. [DOI] [PubMed] [Google Scholar]


