Abstract
Humanin is a very recently discovered 24 amino acid linear polypeptide, which protects against cell death induced by either familial Alzheimer's disease mutant of amyloid precursor protein, presenilin-1 or presenilin-2 in vitro. However, it has remained uncertain whether humanin is a useful drug for the animal model of learning and memory deficit. In this study, we evaluated the effects of [Gly14]-humanin, a more potent humanin analogue, on the scopolamine HBr (1 mg kg−1 s.c.)-induced impairment of spontaneous alternation behaviour in the Y-maze, an index of short-term memory in mice. [Gly14]-Humanin (1000 pmol 5 μl−1 i.c.v.) reversed the impairment without affecting the number of arm entries. These results suggest that (I) [Gly14]-humanin is a beneficial drug for the impairment of learning and memory and (II) it modulates the learning and memory function mediated via cholinergic systems in mice.
Keywords: Humanin, [Gly14]-humanin, Alzheimer's disease, learning and memory, Y-maze test
Introduction
Humanin (Met- Ala- Pro- Arg- Gly- Phe- Ser- Cys- Leu- Leu- Leu- Leu- Thr- Ser- Glu- Ile- Asp- Leu- Pro- Val- Lys- Arg- Arg- Ala; M.W.=2656.3), a new endogenous polypeptide has very recently been found by Hashimoto et al. (2001a, 2001b). This neuropeptide is potent to protect against cell death in vitro induced by either mutant of amyloid precursor protein, presenilin-1 or presenilin-2 mutants, which are believed to be responsible for the early-onset of familial Alzheimer's disease (FAD) (Hashimoto et al., 2001a, 2001b). Furthermore, the primary structural relationship is examined in detail, and this has a somewhat unusual single cysteine at position 8. When this cysteine is replaced by alanine, activity is lost. Surprisingly, when the amino acid at position 14 (Ser) is replaced by glycine ([Gly14]-humanin), there is an increase in activity of 1000 fold (Hashimoto et al., 2001a). While the exact mechanisms by which humanin exerts its protective actions are not known, these findings will provide a potentially exciting approach for treating AD and other memory impairments. Further studies will be required to validate these initial promising results and to determine if modifications of the fragile peptide into a more robust, drug-like molecule might offer new hope for AD therapy. In the process of developing humanin as a new drug, the effects of [Gly14]-humanin on the impaired animal model of learning and memory should be evaluated.
In this study, we used scopolamine (a muscarinic acetylcholine receptor antagonist)-induced impairment model of learning and memory, which is used widely to evaluate the effects of anti-amnesic drugs on learning ability in experimental animals (Bartus et al., 1982; Newhouse, 1990). Furthermore, recent studies have indicated that neuropeptides modulate learning and memory processes in rodents (see Kovacs & De Wied, 1994). Thus, we investigated the effects of [Gly14]-humanin, a more potent humanin analogue, on the scopolamine-induced impairment of spontaneous alternation behaviour in the Y-maze, an index of short-term memory in mice.
Methods
Male ddY mice (Nihon SLC Co., Shizuoka, Japan), 6 – 8 weeks of age, were used. The animals were housed in a controlled environment (23±1°C, 50±5% humidity) and were allowed food and water ad libitum. The room lights were on between 0800 and 2000 h. All experiments were performed in accordance with the Guidelines for Animal Experiments of the Meijo University and the Guiding Principles for the Care and Use of Laboratory Animals approved by the Japanese Pharmacological Society (1987). Scopolamine HBr (1 mg kg−1, s.c., Sigma) and [Gly14]-humanin (0.1 – 1000 pmol, i.c.v.; Peptide Institute, Osaka) were dissolved in saline. The i.c.v. injection was carried out under light ether anaesthesia as reported previously (Hiramatsu et al., 1998; Mamiya et al., 2000; Ukai et al., 2000). To verify the locus of the injection, a group of mice was injected i.c.v. with indian ink because of free hand injection. Then, we checked the ink was diffused throughout the cerebral ventricle in more than 90% of the animals examined. Scopolamine HBr (0.1 ml 10 g−1 body weight) and [Gly14]-humanin (5 μl mouse−1) were administered 30 and 15 min before the Y-maze test, respectively. The protocol was performed according to previous reports (Hiramatsu & Inoue, 1999; Ukai et al., 2000). Briefly, short-term memory performance was examined by monitoring spontaneous alternation behaviour in the Y-maze test. The maze was made of black painted wood; each arm was 40 cm long, 12 cm high, 3 cm wide at the bottom and 10 cm wide at the top. The arms converged at an equilateral triangular central area that was 4 cm at its longest axis. The apparatus placed on the floor of experimental room and was illuminated with a 100-W bulb from 200 cm above. Each mouse was placed at the end of one arm and allowed to move freely through the maze during an 8-min session and the series of arm entries was recorded visually. Alternation was defined as successive entry into the three arms, on overlapping triplet sets. Alternation behaviour (%) was calculated as the ratio of actual alternations to possible alternations (defined as the total number of arm entries minus two), multiplied by 100 (Mamiya et al., 2000; Ukai et al., 2000). After the experiments, we counted the number of arm entries and faeces. The results are expressed as the mean±s.e.mean. Statistical significance was determined by one-way ANOVA followed by the Dunnett multiple comparisons test. P<0.05 was taken as the significant level of difference.
Results
Effects of [Gly14]-humanin on scopolamine-induced impairment of spontaneous alternation behaviour and number of arm entries:
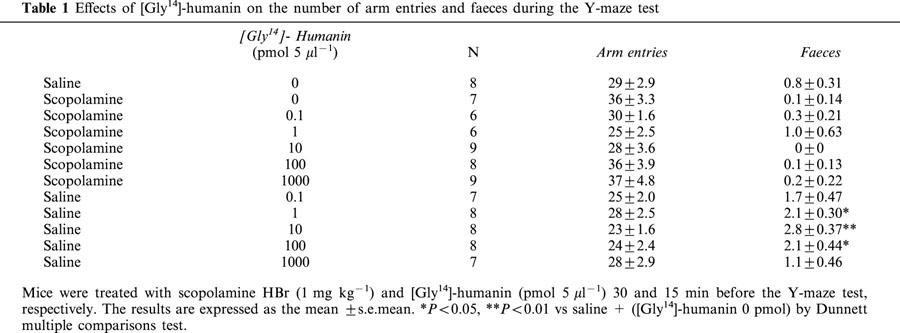
Scopolamine HBr (1 mg kg−1) markedly impaired spontaneous alternation behaviour and had a tendency to increase in the number of arm entries (Figure 1 and Table 1). [Gly14]-Humanin (1000 pmol 5 μl−1) significantly attenuated the impairment of spontaneous alternation behaviour induced by scopolamine (F(11,79)=4.0128, P<0.05), although [Gly14]-humanin alone had no significant effects on spontaneous alternation behaviour or number of arm entries (F(11,79)=2.4925, P>0.05). Furthermore, [Gly14]-humanin itself (1, 10 and 1000 pmol 5 μl−1) significantly increased the defecation during the Y-maze test (F(11,79)=8.4493, P<0.05), although its combination with scopolamine failed to affect the defecation (Table 1).
Figure 1.
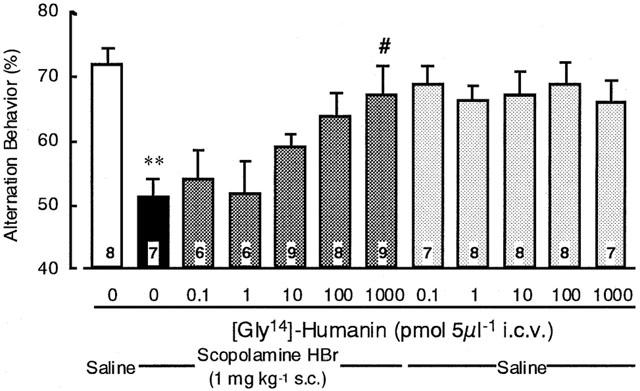
Effects of [Gly14]-humanin on scopolamine-induced impairment of spontaneous alternation behaviour in the Y-maze test. Mice were treated with scopolamine HBr (1 mg kg−1 s.c.) and [Gly14]-humanin (pmol 5 μl−1 i.c.v.) 30 and 15 min before the Y-maze test, respectively. The results are expressed as the mean±s.e.mean. The number of mice used is indicated in each column. **P<0.01 vs saline+([Gly14]-humanin 0 pmol), #P<0.05 vs saline +([Gly14]-humanin 0 pmol) by Dunnett multiple comparisons test.
Table 1.
Effects of [Gly14]-humanin on the number of arm entries and faeces during the Y-maze test
Discussion
Humanin is a novel candidate for anti-amnesic drug, although the first reports by Hashimoto et al. (2001a, 2001b) could not clarify the exact mechanism that it rescues the cell death induced by some mutants. Here, we examined the anti-amnesic effects of [Gly14]-humanin on learning and memory deficits in mice. Scopolamine, a muscarinic acetylcholine receptor antagonist which induces the impairment of learning and memory, is widely used. It is well known that cholinergic neuronal systems play an important role in the cognitive deficits associated with AD, ageing and neurodegenerative diseases (Bartus et al., 1982; Newhouse, 1990). Also, the spontaneous alternation behaviour in the Y-maze, as an index of short-term memory is a highly useful method to screen reagents against amnesic rodent model (Sarter et al., 1988; Hiramatsu & Inoue, 1999; Ukai et al., 1998; 2000). We evaluated [Gly14]-humanin at the range of 0.1 – 1000 pmol 5 μl−1 whether it is potent against the learning and memory deficits, because in our previous studies most of the neuropeptides have anti-amnesic effects within the range of pmol doses in mice (Hiramatsu et al., 1998; Hiramatsu & Inoue, 1999; Ukai et al., 1998; 2000). This neuropeptide (1000 pmol 5 μl−1) reversed the learning and memory impairment induced by scopolamine in mice, although it alone failed to affect the number of arm entries. Furthermore, it had no marked effects on general behaviour, i.e., righting reflex, muscle tone or feeding. These results indicate that [Gly14]-humanin is effective not only against the cell death in vitro but also the impairment of learning and memory in vivo.
Additionally, this finding is at least a clue for new drugs to treat memory disorders, although it is accepted that it may be difficult to discover and develop a CNS-penetrant, small molecule, humanin-like agonist; as has proved the case with agonists of various other polypeptide ligands.
Interestingly, [Gly14]-humanin (1, 10 and 100 pmol 5 μl−1) increased defecation during the Y-maze test, suggesting that humanin may possibly induce emotional changes, such as anxiety or fear. The effective dose range of [Gly14]-humanin itself on the emotion appeared to be bell-shaped, as usually observed for cognitive enhancers (Maurice & Privat, 1997; Mamiya et al., 2000), but the exact mechanism should be clarified in the next investigation.
In conclusion, although further studies are required to examine the toxicity and stability of humanin in vivo, this is the first report that [Gly14]-humanin will be a highly useful drug for memory disturbance like AD.
Acknowledgments
This study was supported in part by Grants-in-Aid for Scientific Research and High-Tech Research Center Project from Ministry of Education, Culture, Sports, Science and Technology, Japan.
Abbreviations
- AD
Alzheimer's disease
- CNS
central nervous system
- FAD
familial Alzheimer's disease
- i.c.v.
intracerebroventicular
- s.c.
subcutaneous
References
- BARTUS R.T., DEAN R.L., BEER B., LIPPA A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–417. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO Y., NIKURA T., TAJIMA H., YASUKAWA T., SUDO H., ITO Y., KITA Y., KAWASUMI M., KOUYAMA K., DOYU M., SOBUE G., KOIDE T., TSUJI S., LANG J., KUROKAWA K., NISHIMOTO I. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and Aβ. Proc. Natl. Acad. Sci. U.S.A. 2001a;98:6336–6341. doi: 10.1073/pnas.101133498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHIMOTO Y., ITO Y., NIKURA T., SHAO Z., HATA M., OYAMA F., NISHIMOTO I. Mechanisms of neuroprotection by a novel rescue factor humanin from Swedish mutant amyloid precursor protein. Biochem. Biophys. Res. Commun. 2001b;283:460–468. doi: 10.1006/bbrc.2001.4765. [DOI] [PubMed] [Google Scholar]
- HIRAMATSU M., INOUE K. Nociceptin/orphanin FQ and nocistatin on learning and memory impairment induced by scopolamine in mice. Br. J. Pharmacol. 1999;127:655–660. doi: 10.1038/sj.bjp.0702595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRAMATSU M., MURASAWA H., MORI H., KAMEYAMA T. Reversion of muscarinic autoreceptor agonist-induced acetylcholine decrease and learning impairment by dynorphin A (1-13), an endogenous kappa-opioid receptor agonist. Br. J. Pharmacol. 1998;123:920–926. doi: 10.1038/sj.bjp.0701671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOVACS G.L., DE WIED D. Peptidergic modulation of learning and memory processes. Pharmacol. Rev. 1994;46:269–291. [PubMed] [Google Scholar]
- MAMIYA T., NODA Y., NODA A., HIRAMATSU M., KARASAWA K., KAMEYAMA T., FURUKAWA S., YAMADA K., NABESHIMA T. Effects of sigma receptor agonists on the impairment of spontaneous alternation behavior and decrease of cyclic GMP level induced by nitric oxide synthase inhibitors in mice. Neuropharmacology. 2000;39:2391–2398. doi: 10.1016/s0028-3908(00)00078-2. [DOI] [PubMed] [Google Scholar]
- MAURICE T., PRIVAT A. SA4503, a novel cognitive enhancer with sigma1 receptor agonist properties, facilitates NMDA receptor-dependent learning in mice. Eur. J. Pharmacol. 1997;328:9–18. doi: 10.1016/s0014-2999(97)83020-8. [DOI] [PubMed] [Google Scholar]
- NEWHOUSE A. Cholinergic drug studies in dementia and depression. Adv. Exp. Med. Biol. 1990;282:65–76. doi: 10.1007/978-1-4613-0665-8_6. [DOI] [PubMed] [Google Scholar]
- SARTER M., BODEWITZ G., STEPHENS D.N. Attenuation of scopolamine-induced impairment of spontaneous alternation behavior by antagonist but not inverse agonist and antagonist β-carboline. Psychopharmacology. 1988;94:491–495. doi: 10.1007/BF00212843. [DOI] [PubMed] [Google Scholar]
- UKAI M., SHINKAI N., KAMEYAMA T. Involvement of dopamine receptors in beneficial effects of tachykinins on scopolamine-induced impairment of alternation performance in mice. Eur. J. Pharmacol. 1998;350:39–45. doi: 10.1016/s0014-2999(98)00231-3. [DOI] [PubMed] [Google Scholar]
- UKAI M., WATANABE Y., KAMEYAMA T. Effects of endomorphins-1 and -2, endogenous μ-opioid receptor agonists, on spontaneous alternation performance in mice. Eur. J. Pharmacol. 2000;395:211–215. doi: 10.1016/s0014-2999(00)00179-5. [DOI] [PubMed] [Google Scholar]


