Abstract
The present studies were aimed at testing the hypothesis that S-nitrosylated captopril (CapNO), a novel crystalline nitric oxide (NO) donor, readily permeates both in vitro and in vivo endothelial monolayers, resulting in its pharmacodynamic effects.
CapNO and Captopril (Cap) were added to apical side of endothelial monolayers formed on microporous membranes, and the permeated drugs were collected from basolateral side and detected by a HPLC method. The permeability coefficient (Papp; cm sec−1) of CapNO across the endothelial monolayers was 6.0×10−5, higher than that of Cap (3.13×10−5), indicating the enhancement effect of the attached NO group in CapNO on cellular permeability. The Papp of CapNO and Cap across Caco-2 cells were 3.15×10−5 and 1.53×10−5, respectively. The low Papp of CapNO to Caco-2 cells may be attributed to the high membrane resistance of Caco-2 cells.
A bolus injection of CapNO to epicardial coronary artery of chronically-instrumented awake dogs caused significant increases in coronary blood flow and coronary diameters dose-dependently without significant changes in aortic pressure. In contrast, the equimolar doses of Cap did not produce haemodynamic responses.
Intravenous CapNO caused an instant increase in the regional cerebral blood flow determined by H2-clearance, whereas the equimolar doses of Cap did not enhance the cerebral blood flow.
These results conclude that the NO group, an active component of CapNO, enhances both in vitro and in vivo endothelial permeability to the entire compound, resulting in instant increases in blood flow and vascular diameters. In contrast, the equimolar Cap does not have the instant vascular effects.
Keywords: S-nitrosylated captopril, captopril, nitric oxide, endothelial cells, coronary blood flow, cerebral blood flow, Caco-2 cells, permeability
Introduction
For discovering nitric oxide (NO) as a signalling molecule in the cardiovascular system, Furchgott and Ignarro received the 1998s Nobel Prize in Physiology and Medicine. They and others had noted that S-nitrosocysteine showed close physiological and pharmacological resemblance to NO (Furchgott et al., 1992; Ignarro et al., 1981, Myers et al., 1990; Feelisch et al., 1994). In the course of comparing the similarities between S-nitrosocysteine and NO as relaxants of vascular smooth muscle, Jia & Furchgott (1993) found that S-nitrosocysteine was a powerful relaxing agent being equal to or somewhat greater in potency than NO on rings of rabbit aorta in bioassay. This finding has generated an interest in linking NO to thiol-containing molecules to synergistically improve the efficacy of the parent thiol-containing molecules, and thus creating them dual capacities of both a NO donor and a parent molecule without the introduction of toxicity. Led by this hypothesis, we had synthesized both S-nitrosylated haemoglobin (Jia et al., 1996; Stamler et al., 1997) and S-nitrosylated vasoactive intestinal peptide (Jia & Stamler, 1999). Both molecules exhibited NO-like vascular relaxing activity without the loss of their original bioactivities. Noting that S-nitrosylated molecules (RS-NOs, where R can be any one of a large range of chemical entities) serve as the body's endogenous storage and transport forms for NO, many efforts have been made to convert the SH- group of captopril (Cap) into a S-NO group, and synthesize a powder form of S-nitrosylated captopril, i.e., S-nitrosocaptopril (1-[(2S)-3-nitrosomercapto-2-methyl-1-oxopropyl]-L-Proline; CapNO) (Loscalzo et al., 1989; Park, 1992; Amano et al., 1994). This compound can be used as both an NO-containing drug and an angiotensin converting enzyme inhibitor.
We are the first who synthesized red crystalline CapNO in a large scale (Lin et al., 1998; Jia & Blantz, 1998), systemically characterized the physicochemical properties of the novel crystals (Jia et al., 1999; 2000), and revealed the LD50 of oral CapNO at 2078±100 mg kg−1 without adverse effects after subchronic toxicity study (Jia et al., 2001). In isolated blood vessels, we found that CapNO at μM levels produced quick and sustained vasorelaxation (Lin et al., 1998), suggesting that the compound promptly permeates through the endothelial monolayers and acts on the underlying smooth muscle to produce vasorelaxation. To test the aforementioned hypothesis, we established both an in vitro endothelial model and in vivo dog and rat models. The human endothelial cells were cultured on microporous membranes (Transwell) that represent a more physiological environment to allow substances to transport across cellular monolayers. In addition, CapNO was also locally administered to animals to observe the corresponding pharmacodynamic responses triggered by CapNO after permeating through the endothelial luminal surface. The animal protocol was approved by the Institutional Animal Care and Use Committee. The present studies were performed in comparison of CapNO effects with that of Cap. The results of these studies are reported here.
Methods
Biostability studies of CapNO
To ensure that CapNO did not undergo biomatrix-catalyzed decomposition, we first determined the stability of CapNO in a serum-free Dulbecco's modified Eagle medium (DMEM) at 37°C for 6 h using a HPLC method (Jia et al., 1999) with minor modifications. CapNO crystals were dissolved in the nonbicarbonate-based DMEM medium to make a final concentration of 1 mM, which was then maintained at 37°C in a well-humidified incubator containing 5% CO2. An aliquot of the medium was withdrawn at various times and centrifuged at 14,000×g for 10 min. Forty microliters of the supernatant were directly injected into a reversed-phase C18 column (Luna 3 μm, 100×4.6 mm i.d., Phenomenex, Torrance, CA, U.S.A.) through a HPLC system (Model HP 1100, Agilent Technologies, CA, U.S.A.). The column was maintained at 25°C, and protected with a C18 octadecyl guard cartridge (4×3.0 mm i.d., Phenomenex). A mobile phase composed of phosphoric acid and HPLC-grade water (0.1%, v v−1) was pumped onto the column at the rate of 0.7 ml min−1 for 12 min. The u.v. absorbance of CapNO was monitored at 220 nm.
Cell culture and CapNO cellular permeability assay
The CapNO cellular permeability assay was implemented on endothelial monolayers and analysed by the aforementioned HPLC method. Both human umbilical endothelial cells and Caco-2 cells were obtained from American Type Culture Collection (Rockville, MD, U.S.A.). The endothelial cells were grown in Kaighn's F12K medium supplemented with 2 mM of L-glutamine, 1.5 mg ml−1 of sodium bicarbonate, 20% foetal bovine serum, 0.1 mg ml−1 of heparin, 0.3 mg ml−1 of endothelial cell growth supplement, 50 U ml−1 of penicillin and 50 μg ml−1 of streptomycin. The Caco-2 cells were cultured in DMEM plus 25 mM of glucose, 10% foetal bovine serum, 10 mM of HEPES, 10 μg ml−1 of transferrin, and the same concentrations of L-glutamine, penicillin, streptomycin, and sodium bicarbonate as used for the endothelial cells. The cells were incubated at 37°C and 5% CO2 in culture flasks with growth area of 75 cm2 and a density of 105 cells cm−2. The medium was replaced with fresh medium three times per week until the cells formed confluent monolayers. The cells were harvested from the flasks with a mixture solution of 0.05% trypsin and 0.53 mM of EDTA-4Na in Hank's balance salt solution when the cells reached confluence. The cells were then transferred and seeded onto clear Transwell inserts (Catalogue No. 3460; Corning Costar Corp., Cambridge, MA, U.S.A.). The inserts are featured as polyester membrane filters with a pore size of 0.4 μm and a diameter of 12 mm. Half of the Transwells acted as control wells without cells seeded, and the other half were plated with endothelial cells at a density of 105 cells per cm2. Cells were allowed to grow for 7 – 10 days until a monolayer was formed on the membrane. On the day of experiment, the medium was aspirated. With the inserts suspended in the wells, serum-free DMEM (pH 7.3) containing CapNO was added to the apical chamber to give a final concentration of 100 μM while the basolateral chamber was filled with prewarmed DMEM. An aliquot (50 μl) of the DMEM was removed from the basolateral chamber at 0.5, 1 and 2 h, and immediately stored in −80°C until the HPLC analysis.
The apparent permeability coefficient (Papp) of CapNO was determined according to Artursson (1990) equation:
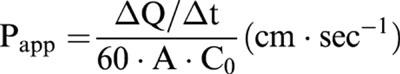 |
where ΔQ/Δt is the permeability rate (μM s−1) of the drug across the microporous membrane, which is calculated from the initial straight slopes. A is the surface area of the membrane (i.e., 1 cm2), and C0 is the initial CapNO concentration in the donor chamber at t=0 (μM). The results of experiments performed in six replicates are presented as mean±s.d.mean.
Preparation for awake dogs with chronically coronary artery catheterization
Mongrel dogs (21 – 27 kg) were anaesthetized with intravenous thiamylal sodium (60 – 80 mg kg−1) and subjected to left thoracotomy in the fourth intercostal space. The left circumflex coronary artery distal to the left atrial appendage was minimally dissected. A pair of 7-MHz piezoelectric crystals (1.5×2.5 mm, 15 – 20 mg) was attached to a Dacron backing and sutured to the adventitia on opposite surfaces of the dissected vessel segment with 6-0 prolene. Oscilloscope monitoring and on-line sonomicrometry were used to ensure proper crystal position. A pulse Doppler flow probe was implanted distal to the crystals. An inflatable balloon occluder was placed distal to the flow probe. All branches of the circumflex artery between the crystals and the occluder were ligated. Heparin sodium-filled polyvinyl catheters were inserted into the left ventricular cavity via the apex, into the left atrium via the atrial appendage, and into the ascending aorta via the left internal thoracic artery. The catheters, tubing, and wires were tunnelled to a subcutaneous pouch at the base of the neck. After a 10- to 15-day recovery period, the catheters and wires were exteriorized under general anaesthesia.
Haemodynamic measurement in awake dogs after dosing
Dogs were trained to lie awake in the lateral recumbent position while loosely restrained. The laboratory was kept dimly illuminated and quiet. Aortic pressure, left ventricular end-diastolic pressure, dP/dt, external coronary diameter, and coronary flow were monitored continuously. CapNO and Cap were diluted in sterilized saline, and administered, respectively, to the dogs (100 and 500 μmol kg−1) via the left atrial catheter by a bolus injection. To verify potential effects of vehicle on vasculature, 0.1-ml saline were injected as vehicle control. Between injections, phasic coronary blood flow and coronary artery diameter were allowed to return to preinjection levels. Allowing a 15-min period between injections resulted in no modification of repeated dose injections. Epicardial coronary diameter, coronary blood flow, heart rate and aortic and left ventricular end-diastolic pressure were compared before and after each drug injection. To compare the time course of effects of CapNO and Cap on coronary vasodilation, the times of increases in epicardial coronary diameter and coronary blood flow were measured.
Rat surgery for measuring brain regional blood flow
Adult male Wistar rats (295 – 340 g) were anaesthetized with sodium pentobarbital (50 mg kg−1, i.p.), intubated and ventilated on room air with a small animal respirator at a rate and tidal volume sufficient to maintain normal values of PaCO2 (35 – 45 mmHg). The rats were catheterized with a PE50 tube in a femoral artery to monitor blood pressure and obtain blood samples, and in a femoral vein to administer supplemental anaesthetics and saline. Aliquots of arterial blood (200 μl) were withdrawn periodically to monitor blood gas tensions and pH using a blood gas/pH analyzer. The blood was replaced intravenously with sterilized saline. The rats were placed on a heating pad, and the rectal temperature was monitored and maintained at 37°C.
Measurement of cerebral blood flow of anaesthetized rats
H2 clearance was used in the present studies for multiple in situ determinations of cerebral blood flow from tissues in which a small electrode was implanted. The method was similar to that described previously (Stamler et al., 1997). The tissue PO2 was measured continuously with polarographic platinum microelectrodes (50 μm outer diameter, coated with a hydrophobic gas permeable Nafion membrane to protect the electrode from fouling) mounted on a micromanipulator. The rat's head was immobilized in a Kopf stereotaxic frame. The lambda and the bregma were first localized. The microelectrodes were implanted, through a small hole in the skull after puncture of the dura mater, into the parietal cortex, caudate putamen nucleus (AP, +0.8 mm; ML, −2.5 mm, DV, −5.2 mm), and substantia nigra (AP, −5.3 mm; ML, −2.4 mm, DV, −8.2 mm) for measurement of regional blood flow. The PO2 electrodes were polarized to −0.65 V against an Ag/AgCl reference located on the tail, and arterial PO2 was adjusted by changing the inspired O2 concentration and atmospheric pressure. The current flow was measured using a low-impedance nanoampere meter during and after the inhalation of gas mixture of 2.5% H2 for 1 min. The H2-clearance and O2 tracings were recorded by using PC WINDAQ version 4.1 software program. Cerebral blood flow was calculated with the initial slope method by converting coefficient K to blood flow per volume of tissue when the tissue/blood partition coefficient of H2 is known (Aukland et al., 1964). Regional blood flow was monitored for 30 min before and after administration of CapNO or Cap (both 1 and 10 μmol kg−1, i.v.).
Results
CapNO permeability across endothelial and Caco-2 monolayers
Biostability testing of CapNO incubated with DMEM at 37°C for 6 h revealed only 14.2% of CapNO decomposed (Figure 1). The decomposition rate of CapNO was dependent on temperature and initial concentrations of the drug (Jia et al., 1999). To maintain the decomposition of CapNO within the given degree, the following permeability experiment was conducted under the same conditions as the biostability.
Figure 1.
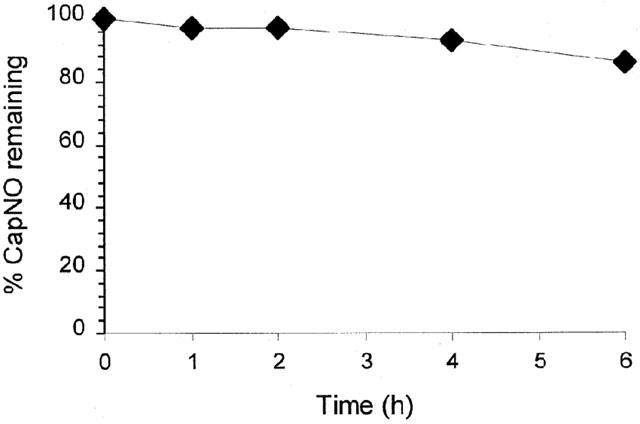
Biostability of CapNO (1 mM) incubated in DMEM culture medium at 37°C. The remaining amount of CapNO in the DMEM culture medium was determined by the HPLC method. Each point represents the mean±s.d. of three separate measurements.
The integrity of each batch of cell monolayers was first verified by microscopy, and then tested by measuring the leakage of [14C]D-mannitol in representative cell monolayers. D-mannitol is a molecule known to traverse cellular sheet exclusively via the paracellular route through tight junctions (zonula occludents) (Mullin et al., 1986). The apical to basolateral flux for this paracellular marker did not exceed values of 0.5% penetrated per hour. Both the morphological microscopic evaluation and mannitol leakage test indicated the integrity of the cell monolayers. The permeability rate of CapNO across endothelial monolayers seeded on Transwell was determined by the HPLC method, and shown in Figure 2. When applied to the apical side (donor chamber) of endothelial monolayers, CapNO (100 and 250 μM) was detectable on the basolateral side (collect chamber) at time point of 30 min. However, attempts to use 50 μM of CapNO for the study ran into difficulties related to the u.v. detection limit of HPLC, particularly, at early time points of 0.5 and 1 h. The time course for CapNO to appear in the collect chamber of control Transwell (cell-free) was close to that of Transwell seeded with cells, suggesting the relatively high permeability rate of CapNO across the endothelial cells. Under the well-controlled conditions, we found that the Papp (cm s−1) of CapNO (100 μM) across endothelial monolayers was 6.0×10−5, which was significantly higher than those of drugs with comparable molecular weight, such as Cap (3.13×10−5), Taxol, and carbendazim (unpublished observation) tested in this laboratory. Since the experiment was conducted by using the serum-free DMEM, the effects of the drugs bound to serum protein on the permeability rate could be avoided. Therefore, the permeability rate of individual drugs is comparable to each other without interference from plasma protein. Figure 2 also shows permeation of CapNO and Cap through Caco-2 cells, a model system of intestinal epithelial permeability. Comparing the results obtained from endothelial monolayers, it was seen that Caco-2 cells possessed more resistance against CapNO than endothelial cells. The Papp of CapNO and Cap through the Caco-2 cells were 3.15×10−5 and 1.53×10−5 cm s−1, respectively. Measurements of transcellular resistance in culture medium using a Millipore Millicell-ERS system (Millipore Corporation, Bedford, MA, U.S.A.) revealed that the transcellular resistance of Caco-2 and endothelial cells was about 420 – 500 and 15 – 25 Ω· cm2, respectively. The differences in resistance between the two cell lines appear to affect the corresponding cellular permeability to the drugs.
Figure 2.
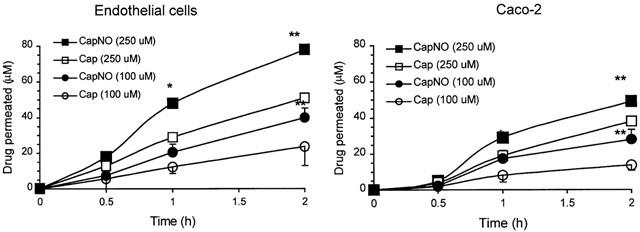
Cellular permeability of CapNO and Cap across human umbilical endothelial monolayers and Caco-2 monolayers. CapNO and Cap were added to the apical chambers of Transwell at final concentrations of 100 and 250 μM. The permeated CapNO and Cap were determined from the basolateral chambers by the HPLC method. The data represent the mean±s.d. (n=6), and evaluated for statistically significant differences by one way analysis of variance (ANOVA). *P<0.05 and **P<0.01, compared with CapNO. For clarity, only representative error bars were shown.
Comparison of effects of CapNO and Cap on hemodynamics of awake dogs
Bolus injections of CapNO (100 and 500 nmol kg−1) into the left atrium of the awake dogs produced immediate haemodynamic changes in a dose-dependent manner, including epicardial vasodilation, an increase in coronary diameter and blood flow, a transient decrease in aortic pressure, and a transient increase in heart rate and left ventricular dP/dt. Figure 3 illustrates a direct tracing of the hemodynamic responses to a bolus injection of CapNO (500 nmol kg−1). The increase in coronary blood flow and the concomitant decrease in aortic pressure represent a net decrease produced by CapNO in the resistance of vessels that regulates flow. Compared with the blood flow of regulatory resistance vessels (represented by blood flow), the coronary conductance vessels (represented by circumflex dimensions) (Zhang et al., 1993) were more sensitive to the effects of CapNO. The effects of CapNO on coronary dimension were sustained, lasting about 36 min (100 nmol kg−1) and 60 min (500 nmol kg−1) after aortic pressure, heart rate and left ventricular end-diastolic pressure had returned to control levels. In contrast, the changes in coronary blood flow were brief, lasting about 1 min, indicating marked differential effects of CapNO on the duration of vasodilation of proximal and distal coronary vessels. There were no significant differences in the changes in dimension with and without constant blood flow, indicating that the responses of the conductance vessels to CapNO resulted from a direct effect. At equimolar doses the epicardial vasodilation responses to Cap (100 and 500 nmol kg−1) were not significant. Basically, Cap did not cause appreciable changes in coronary diameter, blood flow, mean aortic pressure and left ventricular end-diastolic pressure. Table 1 tabulates haemodynamic changes before and after bolus injections of CapNO or Cap into left atrium of the awake dogs. Peak effects were measured. The table showed that CapNO (500 nmol kg−1) caused a ∼23% increase in coronary flow, and ∼11% increase in vessel dimensions that represented a >20% increase in cross-sectional area. Whereas, Cap (500 nmol kg−1) did not show any significant effects on haemodynamic parameters of the chronically-instrumented awake dogs (Table 1)
Figure 3.
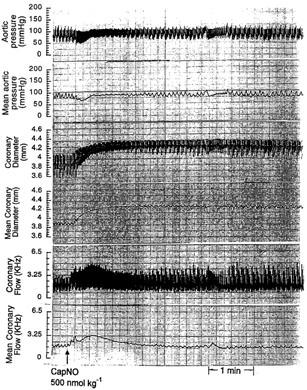
Representative haemodynamic responses of a chronically-instrumented awake mongrel dog to a bolus injection of CapNO (500 nmol kg−1) into left atrium. The arrow indicates the injection time. The haemodynamic parameters represent four individual experiments. Note the immediate and sustained increases in canine coronary diameter caused by CapNO.
Table 1.
Effects of CapNO and Cap on haemodynamic parameters of chronically-instrumented awake mongrel dogs
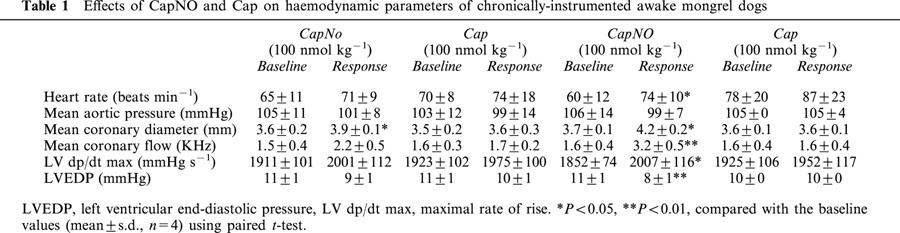
Comparison of effects of CapNO and Cap on rat cerebral blood flow
The basic H2-clearance in the parietal cortex, caudate nucleus, and substantia nigra of the rats was determined three times during and after inhalation of 2.5% H2 for 1 min. Inhalation of the 2.5% H2 gas did not change the H2 content in the three regions. Immediately after the third basic determination, the rats were intravenously given CapNO or Cap (1 and 10 μmol kg−1), and H2-clearance was determined at 3, 10 and 20 min post dose. Figure 4 dynamically shows the time course of increases in blood flow caused by CapNO in the regions of parietal cortex, substantia nigra and caudate nucleus. Figure 5 displays the dose-dependent peak effects of CapNO on cerebral blood flow. Concomitant with the increase in brain regional blood flow, CapNO produced a significant decrease in the mean blood pressure. For instance, the mean blood pressure of the rats (n=6) was 121±7 mmHg prior to dosing. Intravenous administrations of 1 and 10 μmol kg−1 of CapNO decreased blood pressure to 107±8 mmHg (P<0.01) and 92±7 mmHg (P<0.001), respectively. Attempt to use even higher dose of CapNO (50 μmol kg−1) failed because this intravenous dose of CapNO caused almost 50% drop in blood pressure. The maximum decrease in blood pressure was observed by 5 min after CapNO injections. In contrast to CapNO, intravenous Cap at equimolar doses (1 and 10 μmol kg−1) did not cause significant changes in the cerebral blood flow and mean blood pressure (Figure 4).
Figure 4.
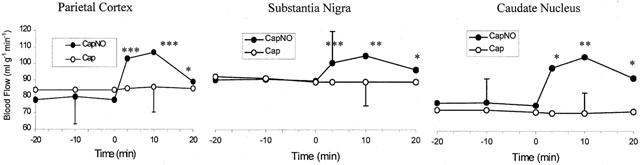
Comparison of effects of intravenous injections of CapNO (1 μmol kg−1, n=6) and Cap (1 μmol kg−1, n=6) on cerebral blood flow in rat parietal cortex, substantia nigra and caudate nucleus. The drugs were administered at 0 min. Each point represents the mean. Only one standard deviation bar is shown for clarity. Statistically significant differences compared with responses to the rats administered with Cap by one-way ANOVA: *P<0.05; **P<0.01; and ***P<0.001.
Figure 5.
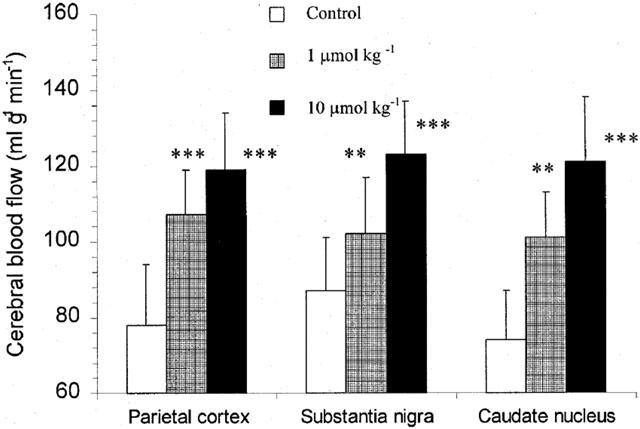
Peak effects of intravenous CapNO on cerebral blood flow of rat parietal cortex, substantia nigra and caudate nucleus. The effects were obtained at 10 min after dosing. The control represents blood flow before CapNO administration. The data represent the mean±s.d. (n=6), and evaluated for statistically significant differences by t-test: **P<0.01; and ***P<0.001.
Discussion
The endothelium is a single layer of epithelioid cells that lines, as one cell thick, on the luminal surface of all blood vessels and lymphatics. In a normal adult, endothelial cells weigh in excess of 100 g and occupy a surface area of more than 1000 m2. Due to its key location, its sensitivity to changes in mechanical (haemodynamic) forces and in chemical and neurohumoral environment, and its ability to transmit these changes into compensatory adjustments of vascular smooth muscle tone, endothelium plays a vital role in cardiovascular homeostasis. The endothelium also serves as a permeability barrier and determines what substances penetrate the vessel wall. Commercial availability of the microporous membrane (Transwell) has enhanced reproducibility of drug cellular permeation studies. These tissue culture-treated microporous membranes represent a more physiological environment, which allows the exchange of substances across both the apical and basolateral membranes rather than just across the apical membrane as is the case with cells grown on plastic dishes. In conducting transport studies with cells cultured on microporous membranes, it is essential that control experiments be conducted using the same Transwell as shown in the present studies, and thus the results of these experiments will assure that the solute is freely permeable through the microporous membrane and the supporting matrix, and that the diffusion barrier is provided by the cell monolayers only. Under the well-controlled conditions that included using serum-free medium to avoid a protein-binding effect and monitoring CapNO decomposition during the period of incubation in DMEM, we demonstrated that CapNO permeates both endothelial cells and Caco-2 cells at a rate relatively faster than Cap (Figure 2), Taxol, and carbendazim tested in this laboratory. With a diffusible NO group attached to the compound, CapNO is conferred upon the high permeability through the cells. That may be a rational explanation why CapNO permeates through the cellular membrane faster than its parent Cap. We have reported that CapNO possesses the oral bioavailability of 22 – 25% after administration to rats (Jia et al., 1999). The results seem to be consistent with the present study of CapNO permeation through Caco-2 cells (Figure 2), a functional and anatomic feature characteristic of absorptive small intestinal enterocytes (Hidalgo et al., 1989). The NO donor showed a certain degree of in vitro and in vivo permeation through intestinal barrier. The differences in cellular permeability to CapNO between Caco-2 and endothelial cells may result from the difference in transmembrane resistance between the two cell lines: Caco-2 cells show higher transmembrane resistance, and hence lower permeation to CapNO. In addition, Caco-2 cells contain P-glycoprotein, an apically polarized transmembrane protein excluding drugs from cells (Gao et al., 2001). Whereas, endothelial cells may not contain P-glycoprotein. The discrepancy in P-glycoprotein content between the two cell lines may be attributed to the permeability variation.
To produce its characteristic effects (vasodilation), CapNO must be present in appropriate concentrations at its sites of action (vascular smooth muscle). Although obviously a function of the amount of CapNO administered, the concentrations attained also depend upon the extent and rate of CapNO passage across endothelium into smooth muscle. In general, drugs cross membranes either by passive diffusion along a concentration gradient or by mechanisms involving the active participation of components of the membrane. It seems probable that the observed increase in cellular permeability to CapNO in the present experiment was due to the passive diffusion mechanism because we have demonstrated that the absorption amount of oral CapNO into systemic circulation is proportional to the amount of CapNO dosed (Jia et al., 1999), although the precise permeability mechanism is not presently clear. Using 5 mM S-nitroso-N-acetylpenicillamine (one of RS-NO analogues), Salzman et al. (1995) have reported that NO reduced cellular ATP levels and reversibly increases the permeability of tight junctions in cultured Caco-2 cells. Although the concentrations used in their studies were too high, the possibility that NO increases cellular permeability can not be excluded.
The bolus injection technique provided in vivo assessment of the direct effect of CapNO on conductance and blood flow regulatory vessels (Figure 3), as well as cerebral blood flow (Figures 4 and 5) while minimizing the effects of pressure and heart rate. The present study was conducted by administering drugs via the left atrial catheter into awake dogs, differently from the experiment done by Shaffer et al. (1990), in which CapNO was injected via the femoral vein of anaesthetized dogs. CapNO caused increases in vascular dimension and blood flow in vivo (Figures 3, 4 and 5). The facts suggest that CapNO could somehow permeate through the local tunica intima consisting of a lining of endothelial cells, and directly produce vasorelaxation of smooth muscle. It is possible that the bolus local injection causes rapid vasodilation that briefly exceeds the local autoregulatory forces which rapidly return blood flow to control values (Figure 3). In contrast, epicardial vessels are not exposed to the same autoregulatory forces, and consequently, unopposed vasodilation of epicardial vessels is sustained. Myogenic constriction of small vessels is an additional factor that may have influenced brief duration of dilation of flow regulatory vessels by CapNO because blood flow regulatory vessels are under continuous modulation and regulation by the adjacent contracting myocardium. It may be hypothesized that close coupling of blood flow to myocardial metabolic needs would tend to attenuate and/or abbreviate response to a direct vasodilator stimulus that occurs in the resistance vasculature.
Differently from other endothelial cells, the brain capillary endothelium lacks aqueous pores (Pardridge et al., 1990). Drugs and solutes enter the brain based on their molecular size and shape, degree of ionization, and relative lipid solubility of their ionized and nonionized forms. Intravenous injections of CapNO produced a significant increase in cerebral blood flow, whereas the parent Cap did not. Again, the difference in pharmacodynamic effects between the two compounds can only be attributed to the NO group presented in CapNO.
In general, NO donors categorize into different groups, and each of them behaves differently. Some are NO donors and permeable to cells, and the others are NO sinks (Simon et al., 1996; Feelisch & Stamler, 1996). The biochemical differentiations among them rely on not only the mechanisms by which NO is released, but also their transmembrane permeability and tissue selectivity. N-nitroso compounds are viewed as chemical carcinogens. This group of compounds may be divided into two classes: the nitrosamines, which are N-nitroso derivatives of secondary amines, and nitrosamides, which are N-nitroso derivatives of substituted ureas, amides, carbamates, guanidines, and similar compounds (Mirvish, 1975). In contrast, RS-NOs are generally not considered as chemical carcinogens. It has been proven that CapNO was decomposed to yield NO and the corresponding disulphide Cap (Jia et al., 1999), which could then be reduced to Cap by either an intracellular spontaneous recovery mechanism or a general protein disulphide reductase. Therefore, CapNO is among the most advantageous of the known NO donor drugs from the therapeutic and toxicological point of view (Jia et al., 2001)
Acknowledgments
Research for this study was carried out by Dr L. Jia at the Institute for Drug Development, Texas, U.S.A. and the Department of Clinical Pharmacology, East Hospital, Fuzhou, China.
Abbreviations
- Cap
Captopril
- CapNO
S-nitrosylated captopril
- HPLC
high performance liquid chromatography
- NO
nitric oxide
- Papp
permeability coefficient
- RS-NOs
S-nitrosylated molecules
References
- AMANO M., TAKAHASHI M., KOSAKA T., KINOSHITA M. Differential inhibition of platelet aggregation and calcium mobilization by nitroglycerin and stabilized nitric oxide. J. Cardiovasc. Pharmacol. 1994;24:860–866. doi: 10.1097/00005344-199424060-00002. [DOI] [PubMed] [Google Scholar]
- AUKLAND K., BOWER B.F., BERLINER R.W. Measurement of local blood flow with hydrogen gas. Circ. Res. 1964;14:164–187. doi: 10.1161/01.res.14.2.164. [DOI] [PubMed] [Google Scholar]
- ARTURSSON P. Epithelial transport of drugs in cell culture. I: a model for studying the passive diffusion of drugs over intestinal absorptive (Caco-2) cells. J. Pharm. Sci. 1990;79:476–482. doi: 10.1002/jps.2600790604. [DOI] [PubMed] [Google Scholar]
- FEELISCH M., POEL M., ZAMORA R., DEUSSEN A., MONCADA S. Understanding the controversy over the identity of EDRF. Nature. 1994;368:62–65. doi: 10.1038/368062a0. [DOI] [PubMed] [Google Scholar]
- FEELISCH M., STAMLER J.S.Donors of nitric oxide Methods in Nitric Oxide Research 1996New York: Wiley and Sons; 71–115.ed. Feelisch, M. & Stamler, J.S [Google Scholar]
- FURCHGOTT R.F., JOTHIANANDAN D., KHAN M.T. Comparison of nitric oxide, S-nitrosocysteine and EDRF as relaxants of rabbit aorta. Jpn. J. Pharmacol. 2001;58 suppl 2:185P–191P. [PubMed] [Google Scholar]
- GAO J., MURASE O., SCHOWEN R.L., AUBE J., BORCHARDT R.T. A functional assay for quantitation of the apparent affinities of affinities of ligands of P-glycoprotein in Caco-2 cells. Pharm. Res. 1992;18:231–239. doi: 10.1023/a:1011076217118. [DOI] [PubMed] [Google Scholar]
- HIDALGO I.J., RAUB T.J., BORCHARDT R.T. Characterization of human colonic carcinoma cell line (Caco-2) as a model system of intestinal epithelial permeability. Gastroenterology. 1989;96:736–749. [PubMed] [Google Scholar]
- IGNARRO L.J., LIPPTON H., EDWARDS J.C., BARICOS W.H., HYMAN A.L., KADOWITZ P.J., GRUETTER C.A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J. Pharmacol. Exp. Ther. 1981;218:739–749. [PubMed] [Google Scholar]
- JIA L., BLANTZ R.C. The effects of S-nitrosocaptopril on renal filtration and blood pressure in rats. Eur. J. Pharmacol. 1998;354:33–41. doi: 10.1016/s0014-2999(98)00424-5. [DOI] [PubMed] [Google Scholar]
- JIA L., BONAVENTURA C., BONAVENTURA J., STAMLER J.S. S-nitrosoheamoglubin: A dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- JIA L., FURCHGOTT R.F. Inhibition by sulfhydryl compounds of vascular relaxation induced by nitric oxide and endothelium-derived relaxing factor. J. Pharmacol. Exp. Ther. 1993;267:371–378. [PubMed] [Google Scholar]
- JIA L., PEI R., LIN M., YANG X. Acute and subacute toxicity and efficacy of S-nitrosylated captopril, an ACE inhibitor possessing nitric oxide activities. Food Chem. Toxi. 2001;39:1135–1143. doi: 10.1016/s0278-6915(01)00079-5. [DOI] [PubMed] [Google Scholar]
- JIA L., STAMLER J.S. Dual actions of S-nitrosylated derivative of vasoactive intestinal peptide as a vasoactive intestinal peptide-like mediator and a nitric oxide carrier. Eur. J. Pharmacol. 1999;366:69–78. doi: 10.1016/s0014-2999(98)00921-2. [DOI] [PubMed] [Google Scholar]
- JIA L., WU C., YOUNG X. Anti-angiogenetic effects of S-nitrosocaptopril crystals as a nitric oxide donor. Eur. J. Pharmacol. 2000;391:137–144. doi: 10.1016/s0014-2999(99)00794-3. [DOI] [PubMed] [Google Scholar]
- JIA L., YANG X., GUO W. Physicochemistry, pharmacokinetics and pharmacodynamics of S-nitrosocaptopril crystals, a new nitric oxide donor. J. Pharm. Sci. 1999;88:981–986. doi: 10.1021/js990108g. [DOI] [PubMed] [Google Scholar]
- LIN M.J., YANG X.P., WANG J., JIA B.J., JIA L. Inhibitory effects of S-nitrosocaptopril on vasomotor tone. Acta Pharmacol. Sin. 1998;19:321–327. [PubMed] [Google Scholar]
- LOSCALZO J., SMICK D., ANDON N., COOKE J. S-nitrosocaptopril: I. Molecular characterization and effects on the vasculature and on platelets. J. Pharmacol. Exp. Ther. 1989;249:726–729. [PubMed] [Google Scholar]
- MIRVISH S.S. Formation of N-nitroso compounds: Chemistry kinetics, and in vivo occurrence. Toxicol. Appl. Pharmacol. 1975;31:325–335. doi: 10.1016/0041-008x(75)90255-0. [DOI] [PubMed] [Google Scholar]
- MULLIN J.M., FLUK L., KLEINZELLER A. Basal-lateral transport and transcellular flux of methyl α-D-glucoside across LLC-PK1 renal epithelial cells. Biochim. Biophys. Acta. 1986;885:233–239. doi: 10.1016/0167-4889(86)90237-5. [DOI] [PubMed] [Google Scholar]
- MYERS P.R., MINOR R.L., JR, GUERRA R., JR, BATES J.N., HARRISON D.G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990;345:161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- PARDRIDGE W.M., TRIGUERO D., YANG J., CANCILLA P.A. Comparison of in vitro and in vico models of drug trnascytosis through the blood-brain barrier. J. Pharmacol. Exp. Ther. 1990;253:884–891. [PubMed] [Google Scholar]
- PARK J.W. Dual role of S-nitrosocaptopril as an inhibitor of angiotensin-converting enzyme and a nitroso group carrier. Biochem. Biophys. Res. Commun. 1992;189:206–210. doi: 10.1016/0006-291x(92)91545-2. [DOI] [PubMed] [Google Scholar]
- SALZMAN L.A., MENCONI M.J., UNNO N., EZZELL R.M., CASEY D.M., GONZALEZ P.K., FINK M.P. Nitric oxide dilates tight junctions and depletes ATP in cultured Caco-2Bbe intestinal epithelial monolayers. Am. J. Physiol. 1995;268:G361–G373. doi: 10.1152/ajpgi.1995.268.2.G361. [DOI] [PubMed] [Google Scholar]
- SHAFFER J., LEE F., THOMSON S., HAN B., COOKE J., LOSCALZO J. The hemodynamic effects of S-nitrosocaptopril in anesthetized dogs. J. Pharmacol. Exp. Ther. 1990;256:704–709. [PubMed] [Google Scholar]
- SIMON D.I., MULLINS M.E., JIA L., GASTON B., SINGEL D., STAMLER J.S. Polynitrosylated proteins: characterization, bioactivity and functional consequences. Proc. Natl. Acad. Sci. U.S.A. 1996;93:4736–4741. doi: 10.1073/pnas.93.10.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAMLER J.S., JIA L., EU J.P., MCMAHON T.J., DEMCHENKO I.T., BONAVENTURA J., PIANTADOSI C.A. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- ZHANG J., SOMERS M., COBB Frederick. Heterogeneous effects of nitroglycerin on the conductance and resistance coronary arterial vasculature. Am. J. Physiol. 1993;264:H1960–H1968. doi: 10.1152/ajpheart.1993.264.6.H1960. [DOI] [PubMed] [Google Scholar]


