Abstract
Isolated aortic rings (endothelium-intact and -denuded) from spontaneously hypertensive (SHR) and Wistar-Kyoto (WKY) rats were used in this study to examine the vasoactive effects of various adenosine analogues.
In phenylephrine contracted aortic rings, concentration-response curves were constructed by cumulative additions (10−11 – 10−5 M) of (2S)-N6-[2-endo-Norbornyl] adenosine (ENBA), N6-cyclopentyladenosine (CPA), R-N6-(2-phenylisopropyl) adenosine (R-PIA), 2-p-(-2-carboxyethyl) phenethylamino-5′-N-thylcarboxamido adenosine (CGS-21680).
A non-specific adenosine receptor agonist 2-chloroadenosine (CAD) resulted in biphasic response with a small contraction at lower concentrations (10−9 – 10−8 M) followed by a significant relaxation at higher concentration in endothelium-intact SHR tissues, suggesting presence of both A1 and A2 adenosine receptors in SHR aorta. However, only relaxation was observed in WKY.
Contractile response in SHR had the following rank order of potency: ENBA>CPA>R-PIA>CAD. The relaxation response in SHR and WKY had the following rank order of potency: CGS 21680>CAD>R-PIA>CPA>ENBA.
Removal of endothelium abolished the adenosine analogue induced contractions in SHR aorta and attenuated the vasorelaxation responses in the WKY and SHR.
The contractile response in SHR was abolished by A1 adenosine receptor antagonist N6-endonorbornan-2-yl-9-methyladenine (N-0861). A2 adenosine receptor antagonist, 3,7-dimethyl-1-proparglyxanthine (DMPX) did not affect the contraction response of adenosine analogues.
Endothelium-dependent contractions elicited by A1 receptor agonists were blocked by indomethacin and by free radical scavengers.
These data suggest that the contractile response to adenosine analogues in SHR aorta is probably mediated by free radicals which are generated through the increased cyclo-oxygenase activity occurring in the vascular endothelium of SHR but not the WKY rats.
Keywords: A1 adenosine receptor, A2 adenosine receptor, vascular endothelium, vascular smooth muscle, indomethacin, free radicals
Introduction
Adenosine is known for its protective role in myocardial ischemia by reducing myocardial oxygen requirement and increasing oxygen supply to the heart by vasodilation (Belardinelli et al., 1989; Bruns, 1991). The effects of adenosine are mediated through cell surface receptors subdivided into A1 coupled to adenylate cyclase in an inhibitory manner and A2 coupled to adenylate cyclase in a stimulatory manner (Bruns et al., 1980). The vasodilatory effects of adenosine and its analogues are mediated through adenosine A2 receptors (Cushing et al., 1991; Laurent et al., 1999; Lewis et al., 1994; Makujina et al., 1992; Mustafa & Askar, 1985; Prentice & Hourani, 1996). Vasorelaxant responses to adenosine are partly mediated through adenosine induced release of endothelium derived relaxing factor (EDRF) in some blood vessels (Fahim et al., 2001; Li & Bukoski, 1993; Lockette et al., 1986; Luscher et al., 1990). The negative inotropic and chronotropic actions of adenosine have been shown to be mediated via the A1 adenosine receptors (Ramagopal et al., 1993). Some of the reports have indicated the presence of A1 adenosine receptors in blood vessels of guinea-pig (Stoggall & Shaw, 1990) and dogfish shark (Evans, 1992). In isolated vascular smooth muscle from the ventral aorta of the dogfish shark A1 agonist ENBA produced significant increase in the tension only at higher concentration (Evans, 1992).
Contractile adenosine A1 receptors have been demonstrated in only a very few tissues, therefore the role of A1 adenosine receptor in the regulation of vascular tone remains unresolved. There are several reports showing diminished vasorelaxation response to adenosine in hypertension (Azevedo & Osswald, 1992; Biaggioni, 1992; Fahim et al., 2001; Illes et al., 1989), however there is no clear cut evidence of exagerated contractile response to adenosine A1 receptor in hypertension.
Therefore, in the present study we examined the effects of specific A1 (Evans, 1992) and A2 (Makujina et al., 1992) adenosine receptor agonists on the isolated aorta of normotensive (WKY) and spontaneously hypertensive (SHR) rats.
Methods
Spontaneously hypertensive (SHR) and Wistar Kyoto (WKY) male rats of same age (15 – 17 weeks) were purchased from Taconic (NY) and housed in plastic cages. Mean body weights of these rats were 282±4 g (n=18) and 345±5 g (n=20) for SHR and WKY, respectively. All animals were fed standard chow and water ad libitum. Twelve hour light/dark cycle was maintained. All experiments were approved by the School of Medicine, East Carolina University Institutional Animal Care and Use Committee and were carried out under the guidelines for the Care and Use of Experimental Animals. Each rat was weighed before use and blood pressure was measured in the conscious state by using tail cuff pump (model 20-NW), pulse amplifier (model 59) and HR-pressure meter (model 72) of IITC, Inc. Woodland Hills, CA, U.S.A., using a polygraph (model 7D, Grass Inst. Co., Quincy, MA, U.S.A.). Mean systolic blood pressures were 175±4 and 124±2 mm Hg in SHR and WKY, respectively.
The rats were sacrificed by decapitation. Aortae were dissected and cleaned of fat and connective tissue. Rings of approximately 4 mm in length were prepared and vertically mounted in 10 ml organ baths filled with Krebs – Henseleit solution and oxygenated with 95% O2+5% CO2 (pH 7.4, 37°C). The composition of Krebs – Henseleit buffer was (mM): NaCl, 118; KCl, 4.8; MgSO4, 1.2; KH2PO4 1.2; NaHCO3 25; CaCl2, 2.5 and glucose, 11. Changes in isometric tension were measured with force transducers (Grass FT .03) connected to Sensormedics dynographs (R 611). Rings were equilibrated for 1 h under an initial tension of 2 g (determined separately from the length – tension relationship); the buffer being changed every 15 min. Rings were challenged with 10−7 M (ED ∼50) phenylephrine (PE) until constant and reproducible contractions were achieved. ED ∼50 values for phenylephrine were calculated from phenylephrine concentration-response curves. The concentration of phenylephrine producing approximately 50% of the maximum tension developed in the aortic rings represents ED ∼50. After achieving sustained and reproducible contractions with 10−7 M PE, various agonists were added in a cumulative fashion to the bath to obtain concentration-response curves. In some of the aortic rings endothelium was denuded by rotating a forcep inside the rings. The integrity of endothelium was assessed by the ability of acetylcholine (10−6 M) to relax the PE-contracted vascular rings. In view of the possible biphasic response of adenosine analogues, the tissues were initially contracted with 10−7 M phenylephrine followed by concentration-response observations.
Concentration-response curves to ENBA, CAD, R-PIA, and CGS-21680 were carried out in endothelium-intact and-denuded rings from SHR and WKY aortae. Concentration-response curves to ENBA, CPA and R-PIA were also carried out following the treatment of endothelium-intact tissues with N-0861, an adenosine A1 receptor antagonist (Barrett et al., 1992), indomethacin (cyclo-oxygenase inhibitor), superoxide dismutase plus catalase and deferoxamine (free radical scavengers) and dilazep (an adenosine uptake inhibitor). Concentration-response curves to adenosine analogues were also carried out in endothelium-intact rings from SHR and WKY before and after treating the tissues with DMPX (10−5 M), an adenosine A2 receptor antagonist (Vangalen et al., 1992).
Drugs
Phenylephrine, acetylcholine, indomethacin, superoxide dismutase, catalase, defeorxamine and 2-chloroadenosine, were obtained from Sigma. N6-cyclopentyladenosine (CPA), R-N6-(2-phenylisopropyl)adenosine, (R-PIA), (2S)-N6-[2-endo-Norbornyl]adenosine (S-ENBA), 2-p-(2-carboxyethyl)-phenethylamine-5′-N-ethylcarboxamidoadenosine (CGS-21680), N6-endonorbornan-2-yl-9-methyladenine (N-0861), and 3,7-dimethyl-1-propargylxanthine (DMPX), NG monomethyl-L-arginine (L-NMMA) were purchased from Research Biochemicals, Inc. (MA). Dilazep was a gift from Asta Medica (Frankfurt, Germany) and N-0861 was a gift from Discovery Therapeutics Inc. (Richmond, VA, U.S.A.).
Statistical analysis
The responses to adenosine analogues were expressed as per cent contraction over that obtained with 10−7 M PE. All values are expressed as mean±s.e.mean. Statistical significance was determined using two-way analysis of variance (ANOVA). Differences from the control (tension produced by 10−7 M phenylephrine) were calculated using a paired, 2-tailed, Student's t-test. The P value for statistical significance was set at 0.05 level.
Results
Concentration-response curves to adenosine receptor agonists
Phenylephrine (10−7 M) produced 750±66 mg tension in endothelium intact aortic rings of WKY, 895±87 mg in SHR, 790±71 mg and 882±79 mg in endothelium-denuded aortic rings of WKY and SHR respectively. The contractile responses to 10−7 M phenylephrine were not statistically different (P>0.05) between WKY and SHR. ENBA (10−10 – 10−6 M) produced significant (P<0.05) contractile response in endothelium-intact aortic rings from SHR (Figure 1A). It produced maximum contractile effect at a concentration of 10−8 M in SHR rings. Higher concentrations of ENBA caused reduction in contractile response in SHR and at 10−5 M concentration, contractile effect of ENBA had disappeared (Figure 1A). Endothelium-denuded rings from SHR and endothelium-intact or -denuded rings from WKY did not show any significant (P>0.05) contractile response to ENBA (Figure 1A).
Figure 1.
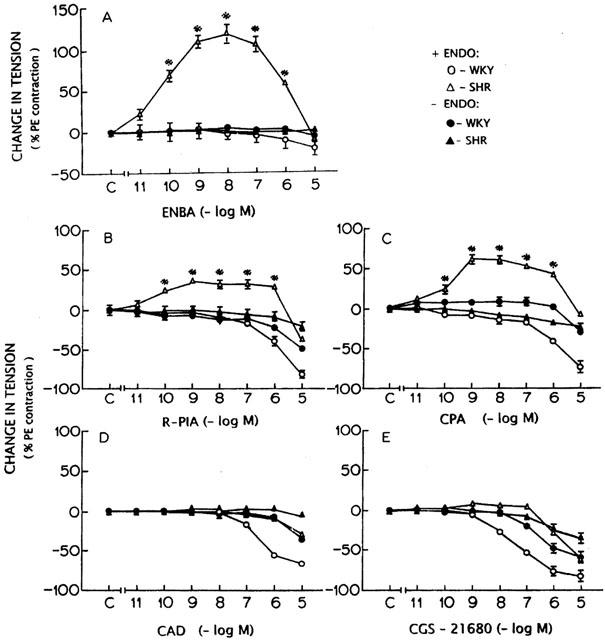
Concentration-response curves for adenosine analogues ENBA (A), R-PIA (B), CPA (C), CAD (D) and CGS 21680 (E) in endothelium-intact and -denuded aortic rings from WKY and SHR. *Significantly different from the control (C on X-axis); P<0.05. The points represent the mean and the vertical lines the s.e.mean.
Endothelium-intact rings from SHR showed contractile response to R-PIA and CPA at concentrations ranging from 10−10 – 10−6 M which were diminished at 10−5 M (Figure 1B,C). Endothelium-denuded rings from SHR did not show any significant contractile response to R-PIA or CPA (Figure 1B,C). R-PIA or CPA did not produce any contractile effect in WKY tissues with or without endothelium (Figure 1B,C). At higher concentrations (106 – 10−5 M) of R-PIA or CPA, a vasorelaxant effect was observed which was attenuated in endothelium-denuded rings from WKY (Figure 1B,C).
CAD and CGS-21680 at concentrations ranging from 10−9 – 10−8 M showed a small contractile response in endothelium-intact SHR tissues which was not found to be statistically significant (P>0.05) (Figure 1D,E). CAD and CGS-21680 did not produce any contraction in WKY tissues (Figure 1D,E). CAD (107 – 10−5 M) and CGS-21680 (10−8 – 10−5 M) produced significant relaxations in endothelium-intact aortic rings which were attenuated in the absence of endothelium in rings from SHR (Figure 1E).
Effect of N-0861 on the responses to adenosine analogues
N-0861 (10−5 M), a relatively A1 selective antagonist (Barrett et al., 1992) did not produce any significant (P>0.05) effect on the contractile response to phenylephrine (10−7 M) in WKY and SHR tissues and abolished the contractions elicited by ENBA (Figure 2A), R-PIA and CPA. R-PIA produced a concentration-dependent relaxant response in endothelium-intact aortic rings from SHR (Figure 3A,C). However, the relaxation response in WKY was not affected by N-0861.
Figure 2.
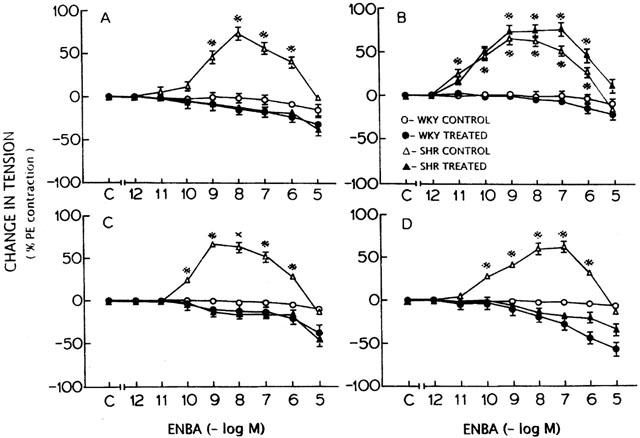
Effect of N-0861 (A), dilazep (B), indomethacin (C) and free radical scavenger (superoxide dismutase+catalase+deferoxamine) (D) treatment on the concentration-response curve for ENBA in WKY and SHR before and after the treatment. *Significantly different from the control (C on X-axis); P<0.05. The points represent the mean and vertical lines the s.e.mean.
Figure 3.
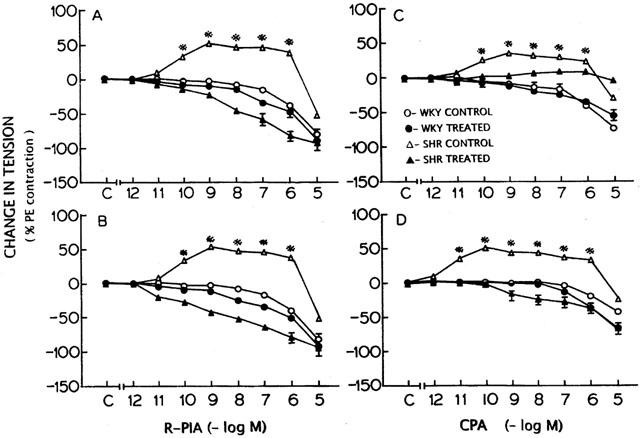
Effect of N-0861 (A, C) and free radical scavengers (superoxide dismutase+catalase+deferoxamine) treatment on the concentration-response curves for R-PIA (B) and CPA (D) in WKY and SHR before and after the treatment. *Significantly different from the control (C on X-axis); P<0.05. The points represent the mean and vertical lines the s.e.mean.
Effect of dilazep and indomethacin on responses to ENBA
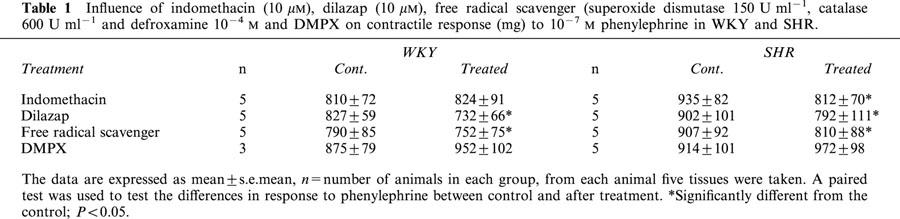
Treatment of aortic rings from WKY and SHR with dilazep (10−5 M) caused a significant fall in the contractile response to phenylephrine (10−7 M) (Table 1). The contractile response to higher concentrations of ENBA in SHR was more in the rings treated with dilazep than the control (Figure 2B). Indomethacin produced a significant fall in the contractile response to phenylephrine (10−7 M) only in SHR and not in WKY (Table 1). In the presence of Indomethacin (10−5 M) ENBA produced significant relaxations in endothelium-intact aortic rings from WKY and SHR (Figure 2C).
Table 1.
Influence of indomethacin (10 μM), dilazap (10 μM), free radical scavenger (superoxide dismutase 150 U ml−1, catalase 600 U ml−1 and defroxamine 10−4 M and DMPX on contractile response (mg) to 10−7 M phenylephrine in WKY and SHR.
Effect of free radical scavengers on the responses to adenosine analogues
Incubation of tissues with free radical scavengers produced a significant fall in contractile response to phenylephrine (10−7 M) in SHR (Table 1). Pretreatment of endothelium-intact tissues with superoxide dismutase (150 U ml−1), catalase (600 U ml−1) and deferoxamine (10−4 M) abolished the contractile response to ENBA in SHR (Figure 2D) and produced an increased relaxation in both SHR and WKY tissues. Similar responses were observed with R-PIA and CPA in both SHR and WKY tissues treated with free radical scavengers (Figure 3B,D).
Effect of DMPX on the responses to adenosine analogues
Treatment of tissues with A2 adenosine receptor antagonist DMPX produced larger contractile response to 10−7 M phenylephrine in both WKY and SHR (Table 1). Concentration-response curves to adenosine analogues were carried out in the absence and presence of A2 adenosine receptor antagonist DMPX (10−5 M) (Vangalen et al., 1992). Blocking of A2 adenosine receptors, which are known to be involved in vasorelaxation elicited by adenosine, did not alter the contractile response of adenosine analogues R-PIA and CPA in SHR tissues while blocking the relaxation response (data not shown). Also, the inhibition of NO production with NG-monomethyl-L-arginine (L-NMMA) did not abolish the contractile response in SHR tissues to adenosine analogues R-PIA and CPA (data not shown).
Discussion
Our results clearly demonstrate a contractile response to several adenosine analogues (ENBA, CPA and R-PIA) in endothelium-intact aortic rings from SHR but not in WKY. These contractions are known to be directed by activation of A1 adenosine receptors (Linden, 1991). The contractions by these adenosine analogues in SHR tissues were blocked by N-0861, an antagonist for A1 adenosine receptor. DMPX, an A2 adenosine receptor antagonist (Vangalen et al., 1992) did not affect the contractile response to the adenosine analogues with a preference to A1 receptor in SHR suggesting that the nature of A2 antagonism by DMPX was competitive.
Previous studies have shown that the vasorelaxation response to adenosine and its analogues is attenuated in certain pathological conditions affecting the blood vessels, e.g., hypertension (Fahim et al., 1993; Li & Bukoski, 1993; Lockette et al., 1986; Luscher et al., 1987) and diabetes (Tesfamariam & Cohen, 1992). Furthermore, the use of a more specific A1 adenosine receptor agonist ENBA, which has an A1/A2 potency ratio of 4700 in rat brain membranes (Trivedi et al., 1989) produced a significant contraction response in comparison to other A1 adenosine analogues (R-PIA and CPA) (Linden, 1991). Vascular smooth muscle from WKY did not show contractile response to any A1 adenosine receptor agonist, therefore, it is unlikely that under normal conditions there is counteraction of A1 receptor mediated contractions by A2 receptor-mediated relaxations as firstly, A1-selective agonists did not evoke contractions in WKY, and secondly, antagonism of A2 receptors did not reveal contractions. However, in our study, neither DMPX, an A2 receptor antagonist (Vangalen et al., 1992) nor inhibition of NO synthesis by L-NMMA could influence the contractile response to adenosine analogues in SHR aorta. Removal of the endothelium abolished the contractile response to ENBA, R-PIA and CPA, while response to CGS-21680, and CAD was significantly reduced. CGS-21680 produced the maximum relaxation in WKY in comparison to other analogues, suggesting its high specificity for A2 receptor mediation. In vascular tissues from WKY rats, CAD and CGS-21680 did not produce any contraction and caused stronger relaxations as compared to SHR. The difference in the vasorelaxant responses to CAD and CGS-21680 between SHR and WKY vascular tissues were reduced in endothelium-denuded rings, suggesting that the attenuation in vasorelaxation to CAD and CGS-21680 in SHR tissues is due to endothelium malfunction. The biphasic response to several adenosine analogues in endothelium-intact tissues of SHR suggests the presence of both constrictor (A1) and dilator (A2) adenosine receptors. The presence of A1 and A2 adenosine receptors has been reported in guinea-pig aorta (Stoggall & Shaw, 1990) and ventral aorta of the dogfish shark (Evans, 1992). Since DMPX or L-NMMA did not produce any significant effect on contractile response of ENBA, R-PIA, and CPA, therefore, it is unlikely that inhibition of A2 receptor mediated relaxation or release of EDRF would have any role in the adenosine A1 receptor induced vasoconstrictor response in SHR. The chronic exposure of rats to A1 adenosine receptor agonist R-PIA decreases A1 receptor number along with an increase in basal and agonist stimulated adenylate cyclase activity in adipocytes and it is suggested that the regulation of both G2 and G1 proteins had a major role in the desensitization process of A1 receptor-adenylate cyclase system (Parsons & Stiles, 1987). Therefore, the lack of contraction in aortae from SHR at higher concentrations of the agonists during cumulative concentration-response desensitization of the A1 receptor.
The other possible mechanism(s) involved in contractile response to adenosine analogues in SHR could be the release of EDCF and/or cyclo-oxygenase products from the vascular endothelium. Inhibition of endothelium-dependent vasorelaxation to agonists in blood vessels of hypertensive (Li & Bukoski, 1993; Luscher et al., 1990) and diabetic (Tesfamariam & Cohen, 1992) animals has been suggested to be due to vasoconstrictor cyclo-oxygenase products. The release of a cyclo-oxygenase dependent EDCF (Auch-Schwelk & Vanhoutte, 1992; Taddei & Vanhoutte, 1993) in SHR vessels also indicated the involvement of cyclo-oxygenase products in the vasoconstriction response. In our study, contractile response of adenosine analogues in SHR aorta was blocked by indomethacin suggesting that the contractions elicited by adenosine analogues in SHR could partly be mediated through the release of certain cyclo-oxygenase products (EDCF) from the endothelial cells of SHR aorta. Earlier studies have indicated the release of a cyclo-oxygenase dependent EDCF in SHR blood vessels (Auch-Schwelk & Vanhoutte, 1992; Taddei & Vanhoutte, 1993). Cyclo-oxygenase mediated contractile response to A1 adenosine receptor stimulation has been reported in other tissues also. In cat lung and guinea-pig uterus and trachea, responses mediated by A1 receptors are markedly attenuated by inhibitor of cyclo-oxygenase (Caparrotta et al., 1984; Neely et al., 1991; Schiemann et al., 1991). These workers suggested that smooth muscle contraction in these tissues may be secondary to the synthesis of prostaglandin from arachidonic acid indicating the involvement of cyclo-oxygenase products in the contractile response to A1 receptor stimulation.
Oxygen-derived free radicals are known to inhibit endothelium-dependent vasorelaxation (Todoki et al., 1992; Tesfamariam & Cohen, 1992) and cause endothelium-dependent contraction (Katusic et al., 1993) through cyclo-oxygenase pathway. In this study, free radical scavengers (superoxide dismutase+catalase+deferoxamine) blocked the contractile response to A1 adenosine receptor agonists in SHR aorta indicating a role of oxygen-derived free radical generation from the endothelium.
Indomethacin or free radical scavenger per se caused significant relaxation of the aortic rings from WKY and SHR. Abolition of contractile response of ENBA following indomethacin or free radical scavenger treatment could not be due to the fall in tension of PE contracted aortic rings because a similar fall in tension by dilazep, an adenosine uptake inhibitor, did not block ENBA induced contractile response of SHR aorta. Treatment of tissues with a higher concentration of PE (5×10−7 M) producing same magnitude of contraction as before incubation of tissue with indomethacin or free radical scavengers did not produce any contractile response to ENBA. Dilazep treated tissues showed a small increase in the contraction of SHR tissues by ENBA probably due to the fall in tension by dilazep.
In conclusion, these data indicate that in SHR aorta, the endothelium-dependent contractile response to adenosine analogues appeared to be A1 adenosine receptor mediated and the relaxant response in WKY and SHR aortae appeared to be through A2 adenosine receptors. The contractions evoked by A1 adenosine receptor agonists in SHR aorta are likely to be mediated by free radicals possibly generated through the increased release of cyclo-oxygenase products from the endothelium of SHR aorta.
Acknowledgments
This work was supported by NIH grant HL 27339. The authors wish to thank Ms Pam Wynne and Ms Maninder for typing the manuscript.
Abbreviations
- CAD
2-chloroadenosine
- CGS-21680
2-p-(-2-carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine
- CPA
N6-cyclopentyladenosine
- DMPX
3,7-dimethyl-1-propargylxanthine
- ED∼50
the concentration of phenylephrine producing approximately 50% of the maximum tension developed
- EDCF
endothelium derived contracting factor
- EDRF
endothelium derived relaxing factor
- ENBA
(2S)-N6-[2-endo-Norbornyl]adenosine
- L-NMMA
NG-monomethyl-L-arginine
- N-0861
N6-endonorbornan-2-yl-9-methyladenine
- NO
nitric oxide
- PE
phenylephrine
- R-PIA
R-N6-(2-phenylisopropyl) adenosine
- SHR
spontaneously hypertensive rats
- U ml−1
units per ml
- WKY
Wistar-Kyoto
References
- AUCH-SCHWELK W., VANHOUTTE P.M. Contractions due to endothelium in normotensive and spontaneously hypertensive rats: role of endothelium and prostaglandins. Blood Pressure. 1992;1:45–49. doi: 10.3109/08037059209065123. [DOI] [PubMed] [Google Scholar]
- AZEVEDO I., OSSWALD W. Does adenosine malfunction play a role in hypertension. Pharmacol. Res. 1992;25:227–236. doi: 10.1016/s1043-6618(05)80071-4. [DOI] [PubMed] [Google Scholar]
- BARRETT R.J., DROPPLEMAN D.A., WRIGHT K.F. N-0861 selectively antagonizes adenosine A1 receptors in vivo. Eur. J. Pharmacol. 1992;216:9–16. doi: 10.1016/0014-2999(92)90202-f. [DOI] [PubMed] [Google Scholar]
- BELARDINELLI J.L., LINDEN J., BERNE R.M. The cardiac effects of adenosine. Prog. Cardiovasc. Dis. 1989;32:73–97. doi: 10.1016/0033-0620(89)90015-7. [DOI] [PubMed] [Google Scholar]
- BIAGGIONI I. Contrasting excitatory and inhibitory effects of adenosine in blood pressure regulation. Hypertension. 1992;20:457–465. doi: 10.1161/01.hyp.20.4.457. [DOI] [PubMed] [Google Scholar]
- BRUNS R.F. Role of adenosine in energy supply/demand balance. Nucleosides and Nucleotides. 1991;10:931–943. [Google Scholar]
- BRUNS R.F., DALY J.W., SNYDER S.H. Adenosine receptors in brain membranes: Binding of cyclohexyl [3H] adenosine and 1,3-diethyl-8[3H] phenylxanthine. Proc. Natl. Acad. Sci. USA. 1980;77:5547. doi: 10.1073/pnas.77.9.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAPARROTTA L., CILLO F., FASSIAN G., GAION R.M. Dual effect of (−)-N6-phenylisopropyladenosine on guinea-pig trachea. Br. J. Pharmacol. 1984;83:23–29. doi: 10.1111/j.1476-5381.1984.tb10115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUSHING D.J., BROWN G.L., SABOUNI M.H., MUSTAFA S.J.Adenosine receptor-mediated coronary artery relaxation and cyclic nucleotide production Am. J. Physiol. 1991261H343–H348.(Heart Cir Physiology 3B) [DOI] [PubMed] [Google Scholar]
- EVANS D.H. Evidence for the presence of A1 and A2 adenosine receptors in the ventral aorta of the dogfish shark, Squalus acanthias. J. Comp. Physiol. B. 1992;162:179–183. doi: 10.1007/BF00398345. [DOI] [PubMed] [Google Scholar]
- FAHIM M., HUSSAIN T., MUSTAFA S.J. Relaxation of rat aorta by adenosine in diabetes with and without hypertension; role of endothelium. Eur. J. Pharmacol. 2001;412:51–59. doi: 10.1016/s0014-2999(00)00869-4. [DOI] [PubMed] [Google Scholar]
- ILLES P., RICKMANN H., BROD I., BUCHER I., STOCLET J.C. Subsensitivity of presynaptic adenosine A1-receptors in caudal arteries of spontaneous hypertensive rats. Eur. J. Pharmacol. 1989;174:237–251. doi: 10.1016/0014-2999(89)90316-6. [DOI] [PubMed] [Google Scholar]
- KATUSIC Z.C., SCHUGEL J., COSENTINO F., VANHOUTTE P.M.Endothelium-dependent contractions to oxygen derived free radicals in the canine basilar artery Am. J. Physiol. 1993264H859–H864.(Heart Circ. Physiol. 33) [DOI] [PubMed] [Google Scholar]
- LAURENT M., HILLARIE-BUYS D., CHAPAL J., DIETZ S., PORTET K., GROS G., MICHEL A. Contrasting effects of streptozotocin-induced diabetes on the in vitro relaxant properties of adenosine in rat pancreatic vascular bed and thoracic aorta. Naunyn-Schmiedebergs Arch. Pharmacol. 1999;360 3:309–316. doi: 10.1007/s002109900061. [DOI] [PubMed] [Google Scholar]
- LEWIS C.D., HOURANI S.M., LONG C.J., COLLIS M.G. Characterization of adenosine receptors in the rat aorta. General Physiology. 1994;25:1381–1387. doi: 10.1016/0306-3623(94)90162-7. [DOI] [PubMed] [Google Scholar]
- LI J., BUKOSKI R.D. Endothelium-dependent relaxation of hypertensive resistance arteries is not impaired under all conditions. Circ. Res. 1993;72:290–296. doi: 10.1161/01.res.72.2.290. [DOI] [PubMed] [Google Scholar]
- LINDEN J. Structures and function of A1 adenosine receptors. FASEB J. 1991;5:2668–2676. doi: 10.1096/fasebj.5.12.1916091. [DOI] [PubMed] [Google Scholar]
- LOCKETTE W., OSAKA Y., CARRETERO O. The loss of endothelium-dependent vascular relaxation in hypertension. Hypertension. 1986;8:II61–II66. doi: 10.1161/01.hyp.8.6_pt_2.ii61. [DOI] [PubMed] [Google Scholar]
- LUSCHER T.F., AARCHUS L.L., VANHOUTTE P.M. Indomethacin improves the impaired endothelium-dependent relaxations in small mesenteric arteries of the spontaneously hypertensive rats. Am. J. Hypertension. 1990;3:55–58. doi: 10.1093/ajh/3.1.55. [DOI] [PubMed] [Google Scholar]
- LUSCHER T.F., VANHOUTTE P.M., RAIJ L. Antihypertensive treatment normalizes decreased endothelium-dependent relaxations in rats with salt-induced hypertension. Hypertension. 1987;9:III193–III197. doi: 10.1161/01.hyp.9.6_pt_2.iii193. [DOI] [PubMed] [Google Scholar]
- MAKUJINA S.R., SABOUNI M.H., BHATIA S., MUSTAFA S.J. Vasodilatory effects of adenosine A2 receptor agonists CGS 21680 and CSG 22492 in human vasculature. Eur. J. Pharmacol. 1992;222:243–247. doi: 10.1016/0014-2999(92)90708-c. [DOI] [PubMed] [Google Scholar]
- MUSTAFA S.J., ASKAR A.O. Evidence suggesting a Ra-type adenosine receptor in bovine coronary arteries. J. Pharmacol. Exp. Ther. 1985;232:49–56. [PubMed] [Google Scholar]
- NEELY C.F., HAILE D.M., CAHILL B.E., KADOWITZ P.J. Adenosine and ATP produce vasoconstriction in the feline pulmonary vascular bed by different mechanisms. J. Pharmacol. Exp. Ther. 1991;258:753–761. [PubMed] [Google Scholar]
- PARSONS W.J., STILES G.L. Heterologous desensitization of the inhibitory A1 adenosine receptor-adenylate cyclase system in rat adipocytes. J. Biol. Chem. 1987;262:841–847. [PubMed] [Google Scholar]
- PRENTICE D.J., HOURANI S.M. Activation of multiple sites by adenosine analogues in the rat isolated aorta. Br. J. Pharmacol. 1996;118:1509–1517. doi: 10.1111/j.1476-5381.1996.tb15567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAGOPAL V.M., MONTAMAT S.C., BRUNS R.F., VESTAL R.E.Cardiac functional responses to adenosine by P, D, 81, 723, an allosteric enhancer of the adenosine A1 receptor Am. J. Physiol. 1993264H1017–H1022.(Heart Circ. Physiol 33) [DOI] [PubMed] [Google Scholar]
- SCHIEMANN W.P., DOGGWILER K.O., BUXTON I.L.O. Action of adenosine in estrogen-primed nonpregnant guinea-pig myometrium: Characterization of the smooth muscle receptor and coupling to phosphoinositide metabolism. J. Pharmacol. Exp. Ther. 1991;258:429–437. [PubMed] [Google Scholar]
- STOGGALL S.M., SHAW J.S. The co-existence of adenosine A1 and A2 receptors in guinea-pig aorta. Eur. J. Pharmacol. 1990;190:329–335. doi: 10.1016/0014-2999(90)94197-6. [DOI] [PubMed] [Google Scholar]
- TADDEI S., VANHOUTTE P.M. Role of endothelium in endothelium-evoked contractions in the rat aorta. Hypertension. 1993;21:9–15. doi: 10.1161/01.hyp.21.1.9. [DOI] [PubMed] [Google Scholar]
- TESFAMARIAM B., COHEN R.A.Free radicals mediated endothelial cell dysfunction caused by elevated glucose Am. J. Physiol. 1992263H321–H326.(Heart Circ Physiol 32) [DOI] [PubMed] [Google Scholar]
- TODOKI K., OKABE E., KIYOSE T., SEKISHITA T., ITO H.Oxygen free radical-mediated selective endothelial dysfunction in isolated coronary artery Am. J. Physiol. 1992263H806–H812.(Heart Circ Physiol 32) [DOI] [PubMed] [Google Scholar]
- TRIVEDI B.K., BRIDGES J.A., PATT W.C., PRIEBE S.R., BRUNS R.F. N6-bicycloakyladenosines with high potency and selectivity for the adenosine A1 receptor. J. Med. Chem. 1989;32:8–11. doi: 10.1021/jm00121a002. [DOI] [PubMed] [Google Scholar]
- VANGALEN P.J.M., STILES G.L., MICHAELS G., JACOBSON K.A. Adenosine A1 and A2 receptors: Structure-function relationships. Med. Res. Rev. 1992;12:423–471. doi: 10.1002/med.2610120502. [DOI] [PMC free article] [PubMed] [Google Scholar]


