Abstract
Administration of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy') to mice produces acute hyperthermia and long-term degeneration of striatal dopamine nerve terminals. Attenuation of the hyperthermia decreases the neurodegeneration. We have investigated the mechanisms involved in producing the neurotoxic loss of striatal dopamine.
MDMA produced a dose-dependent loss in striatal dopamine concentration 7 days later with 3 doses of 25 mg kg−1 (3 h apart) producing a 70% loss.
Pretreatment 30 min before each MDMA dose with either of the N-methyl-D-aspartate antagonists AR-R15896AR (20, 5, 5 mg kg−1) or MK-801 (0.5 mg kg−1×3) failed to provide neuroprotection.
Pretreatment with clomethiazole (50 mg kg−1×3) was similarly ineffective in protecting against MDMA-induced dopamine loss.
The free radical trapping compound PBN (150 mg kg−1×3) was neuroprotective, but it proved impossible to separate neuroprotection from a hypothermic effect on body temperature.
Pretreatment with the nitric oxide synthase (NOS) inhibitor 7-NI (50 mg kg−1×3) produced neuroprotection, but also significant hypothermia. Two other NOS inhibitors, S-methyl-L-thiocitrulline (10 mg kg−1×3) and AR-R17477AR (5 mg kg−1×3), provided significant neuroprotection and had little effect on MDMA-induced hyperthermia.
MDMA (20 mg kg−1) increased 2,3-dihydroxybenzoic acid formation from salicylic acid perfused through a microdialysis tube implanted in the striatum, indicating increased free radical formation. This increase was prevented by AR-R17477AR administration. Since AR-R17477AR was also found to have no radical trapping activity this result suggests that MDMA-induced neurotoxicity results from MDMA or dopamine metabolites producing radicals that combine with NO to form tissue-damaging peroxynitrites.
Keywords: 3,4-methylenedioxymethamphetamine; MDMA; ecstasy; NMDA antagonists; nitric oxide synthase inhibitors; clomethiazole; PBN; free radicals; dopamine; neuroprotection
Introduction
Administration of 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) to rats produces a rapid and marked release of both 5-hydroxytryptamine (5-HT) (Schmidt et al., 1987; Gough et al., 1991; Gudelsky & Nash, 1996) and dopamine (Koch & Galloway, 1997; Sabol & Seiden, 1998; Colado et al., 1999) in the brain.
There is general agreement that MDMA also produces a substantial and sustained long term neurotoxic loss of 5-HT nerve terminals in several brain regions of rats, guinea-pigs and several species of non-human primates (Stone et al., 1986; Schmidt, 1987; Schmidt & Kehne, 1990; Steele et al., 1994; Green et al., 1995; White et al., 1996; Colado et al., 1997; 1999; Huether et al., 1997). There is also a general consensus that MDMA, given at a dose that produces a major neurotoxic loss of 5-HT, produces no long term neurotoxic loss of cerebral dopamine content in these species (Stone et al., 1986; Battaglia et al., 1987; Schmidt & Kehne, 1990; Lew et al., 1996; Sabol et al., 1996; Colado et al., 1997; 1999). In contrast, MDMA is generally accepted to behave as a relatively selective dopaminergic neurotoxin in mice, having little effect on 5-HT containing neurones, but producing a sustained loss in the concentration of dopamine and its metabolites in the striatum (Stone et al., 1987; Logan et al., 1988; O'callaghan & Miller, 1994; O'shea et al., 2001). The MDMA-induced loss in the striatal catechol content in mice presumably reflects a neurotoxic degeneration of dopamine nerve terminals similar to that seen following methamphetamine administration to both rats and mice (Koda & Gibb, 1973; Sonsalla et al., 1989; Green et al., 1992; Baldwin et al., 1993; Bowyer et al., 1994; Itzhak & Ali, 1996).
We recently reported on a study that examined the effect of monoamine uptake inhibitors on their ability to prevent MDMA-induced neurodegeneration in mouse striatum (O'shea et al., 2001). We have now extended these observations to try to obtain a greater understanding of the mechanisms involved in the neurodegenerative effect of MDMA on dopamine neurones in mouse brain by examining the effect of a range of putative neuroprotective agents. We have examined the effect of N-methyl-D-aspartate (NMDA) antagonists, the free radical trapping nitrone PBN and several nitric oxide synthase (NOS) inhibitors. The effect of clomethiazole, a GABAmimetic agent which has been shown to protect against MDMA-induced neurotoxicity to 5-HT neurones in rat brain (Colado et al., 1993; 1998), has also been investigated. In addition, the effect of MDMA on free radical formation in the striatum was examined by use of in vivo microdialysis and measurement of the conversion of salicylic acid to 2,3-dihydroxy benzoic acid (2,3-DHBA) as previously described (Colado et al., 1997). In all experiments rectal temperature of the mice was monitored as there is substantial evidence in both rats (Malberg et al., 1996; Farfel & Seiden, 1995a, 1995b; Colado et al., 1998) and mice (Miller & O'callaghan, 1994; Albers & Sonsalla, 1995) that preventing the normal MDMA-induced hyperthermia, or production of frank hypothermia, is an effective way of producing a neuroprotective effect.
Methods
Animals
Adult male NIH/Swiss mice (Harlan UK Ltd., Bicester, Oxon, U.K. and Harlan Iberica, Barcelona, Spain), weighing 25 – 30 g, were used in almost all studies. They were housed in groups of 5 – 7, at an ambient temperature of 20±2°C and a 12 h light/dark cycle (lights on: 0730 h). Food and water were freely provided.
In the study on the effect of 7-NI, adult male Swiss-Webster mice (CFW1, Harlan Iberica, Barcelona, Spain) weighing 30 – 35 g were used. They were housed in groups of 10, in conditions of constant temperature (21±2°C) and a 12 h light/dark cycle (lights on: 0700 h) and given free access to food and water.
Drugs and drug administration
Pretreatment compounds were administered 30 min prior to each dose of MDMA (25 mg kg−1 i.p.) or saline, which was repeated for a total of 3 times, at 3 h intervals. This dose regime for MDMA was used because it produces a significant loss of dopaminergic nerve terminals in mice (O'shea et al., 2001). In the studies on 7-NI and S-MTC and the microdialysis experiments a dose of MDMA of 20 mg kg−1 was used because the higher dose was causing some mortality. When this dose was used a hyperthermic response and striatal dopamine loss were produced similar to those seen at the higher dose of MDMA, suggesting that the main effects of the drug were similar to that seen in other parts of the study. Both control (saline injected) and MDMA-treated groups were always studied in each experiment to control for any variation in response.
Drugs were administered intraperitoneally (i.p.) unless otherwise specified. All drugs were administered in normal saline (0.9% NaCl−1) at a volume of 10 ml kg w v−1, unless otherwise stated and doses are quoted as the base weight.
Drugs were obtained from the following sources: (±)3,4-methylenedioxymethamphetamine HCl (MDMA) (Sigma-Aldrich Company Ltd., Poole, Dorset, U.K.; Dr P. Guiry, University College, Dublin and NIDA, Research Triangle Park, NC, U.S.A.), S-(+)-α-phenyl-2-pyridine ethanamide dihydrochloride (AR-R15896AR) and N-(4-(2-((3-chlorophenylmethyl) amino)-ethyl)phenyl) 2-thiophene carboxamidine (AR-R17477AR) (AstraZeneca R&D Wilmington, Wilmington, DE, U.S.A.), (+)-MK 801 maleate (Tocris Cookson Ltd., Avonmouth, Bristol, U.K.), α-phenyl-N-tert-butyl nitrone (PBN) and 7-nitroindazole (7-NI) (Sigma), S-methyl-L-thiocitrulline (S-MTC) (Calbiochem), clomethiazole edisilate (AstraZeneca R&D Södertälje, Södertälje, Sweden).
Measurement of rectal temperature
Temperature measurement was performed using an MC 8700 thermometer, with digital readout (EXACON A/S, Roskilde, Denmark) and a lubricated H-SF1 rectal temperature probe (Cranbrook Electronics, Ascot, Berkshire, U.K.). Each mouse was lightly restrained by hand, for approximately 10 s, whilst the probe was inserted approximately 2 cm into its rectum and a steady reading was obtained.
Effect of different doses of MDMA on striatal dopamine concentration
In order to assess the effect of different total doses of MDMA on striatal dopamine concentration, the protocol employed for all other experiments was modified. In this experiment, groups of mice (n=6 in each case) were administered either MDMA (25 mg kg−1) or saline 3 times at 3 h intervals (0, 3, 6 h). The first group were administered MDMA at each time point, the second group were administered MDMA at 0 and 3 h and saline at 6 h, the third group were administered MDMA at 0 h and saline at 3 and 6 h and the fourth group were administered saline at each of the three time-points.
Measurement of monoamines and their metabolites in cerebral tissue
One week following MDMA, mice were killed by cervical dislocation and decapitation, the brains rapidly removed and the striatum dissected out on ice. Tissue was homogenized and dopamine, homovanillic acid (HVA) and 3,4-dihydroxyphenylacetic acid (DOPAC) were measured by high performance liquid chromatography (h.p.l.c.). Briefly, the mobile phase consisted of KH2PO4 (0.05 M), octanesulphonic acid (0.16 mM), EDTA (0.1 mM) and methanol (16%), and was adjusted to pH 3 with phosphoric acid, filtered and degassed. The flow rate was 1 ml min−1 and the working electrode potential was set at +800 mV.
The h.p.l.c. system consisted of a pump (Waters 510) linked to an automatic sample injector (Loop 200 μl, Waters 712 WISP), a stainless steel reversed-phase column (Spherisorb ODS2, 5 μm, 150×4.6 mm) with a precolumn and an amperometric detector (Waters M460). The current produced was monitored via an integrator (Waters M745).
Implantation of microdialysis probe in the striatum
Mice were anaesthetized with sodium pentobarbitone (Euta-Lender, 40 mg kg−1, i.p.) and secured in a Kopf stereotaxic frame (model 900) coupled to a Kopf mouse adaptor (model 921). The dialysis probe (2.5 mm×240 μm; Cuprophan) was implanted in the right striatum +0.7 mm anteroposterior, −2.0 mm mediolateral from bregma and −6 mm dorsoventral from the surface of the brain (Franklin & Paxinos, 1997). Probes were secured to the skull as described by Baldwin et al. (1994).
Measurement of free radical formation using in vivo microdialysis
Free radical formation in the brain was measured in vivo by the method described in detail by Colado et al. (1997) with some modifications. The method relies on the fact that hydroxyl free radicals react with salicylic acid to generate 2,3- and 2,5-dihydroxybenzoic acids (2,3-DHBA and 2,5-DHBA). This reaction is utilized by measuring the formation of 2,3- and 2,5-DHBA in the dialysate of a microdialysis probe implanted in the striatum (see above), which is being perfused with salicylic acid (see Chiueh et al., 1992; Giovanni et al., 1995). As 2,5-DHBA is also formed peripherally through salicylate hydroxylation by cytochrome P450, it has been considered that 2,3-DHBA concentration in the dialysate is the only reliable marker of hydroxyl radical formation in the striatum.
Twenty-four hours after implantation, probes were perfused with artificial cerebrospinal fluid (mM): KCl 2.5; NaCl 125; MgCl2.6H2O 1.18; CaCl2.2H2O 1.26; NaH2PO4.H2O 0.5; Na2HPO4.2H2O 5; pH=6.55 containing salicylic acid (2.5 mM) at a rate of 1 μl min−1 and samples collected from the freely moving animals at 30 min intervals. The first 60 min sample was discarded and the next two 30 min baseline samples collected. The mobile phase consisted of KH2PO4 (0.025 M) acetonitrile (20%) and methanol (10%) and was adjusted to pH 3.25 with phosphoric acid, filtered and degassed. The flow rate was 1 ml min−1.
The h.p.l.c. system consisted of a pump (Waters 510) linked to manual sample injector (Loop 20 μl Rheodyne), a stainless steel reversed-phase column (250×4.6 mm, 5 μm C8 Ultracarb, Phenomenex) with a precolumn (30×4.6 mm, 5 μm C8 Ultracarb, Phenomenex) and a coulometric detector (Coulochem 5100A) with a 5011 analytical cell. The working electrode potential was set at ±400 mV with 1 μA gain. The current produced was monitored via a computer data handling system (AXXIOM 747).
FeCl2+ascorbic acid-induced lipid peroxidation
Rat cortical synaptosomes (P2 fraction) were prepared as described by Lynch & Voss (1990) and resuspended in Tris buffer (50 mM; pH 7.4) containing NaCl (120 mM) and KCl (5 mM) to produce a protein concentration of approximately 400 μg ml−1. Membranes (400 μl) were added to 50 μl FeCl2 (30 μM), 50 μl ascorbic acid (1 mM) and 50 μl AR-R17477AR (1 – 100 μM final concentration) and incubated at 37°C for 30 min. The free radical scavenger butylated hydroxytoluene (BHT; 0.1 – 100 μM final concentration) was added to the incubation as a positive control. Lipid peroxidation was measured by a modification of the method described by Das & Ratty (1987), whereby the thiobarbituric acid-reacting substances (TBARS), predominantly malondialdehyde, produced as a secondary product were quantified by use of the 2-thiobarbituric acid colour reaction. Briefly, 150 μl HCl (0.5 N) and 300 μl trichloroacetic acid (40% v v−1) were added to the incubation mixture followed by 300 μl 2-thiobarbituric acid (2% w v−1). Samples were heated for 15 min at 90°C, then centrifuged at 1500×g at 4°C for 15 min. The pink colour resulting from the reaction was measured by recording the optical density at 532 nm and the malondialdehyde concentration was thus calculated by the use of a standard curve prepared with malondialdehyde tetrabutylammonium salt. The experiments were performed at least three times for each compound and assays were performed in triplicate.
Statistics
Comparison of MDMA-treated and saline-treated groups with respect to striatal monoamine concentrations was performed using an unpaired t-test. All other monoamine data were analysed using one-way analysis of variance (ANOVA) followed by the Tukey multiple comparison test when a significant F value was obtained. Statistical analyses of the temperature measurements and microdialysis studies were performed using the statistical computer package BMDP/386 Dynamic (BMDP Statistical Solutions, Cork, Eire). Data were analysed by ANOVA with repeated measures (program 2V) or, where missing values occurred, an unbalanced repeated measures model (program 5V) was used. Both used treatment as the between subjects factor and time as the repeated measure. ANOVA was performed on both pre-treatment and post-treatment data.
Results
Effect of repeated doses of MDMA on rectal temperature
The first dose of MDMA (25 mg kg−1 i.p.) produced a rapid rise in rectal temperature lasting over 2 h. The rectal temperature also increased rapidly following both the second and third doses of MDMA (25 mg kg−1), which were injected at 3 and 6 h after the first administration (see for example Figure 2b).
Figure 2.
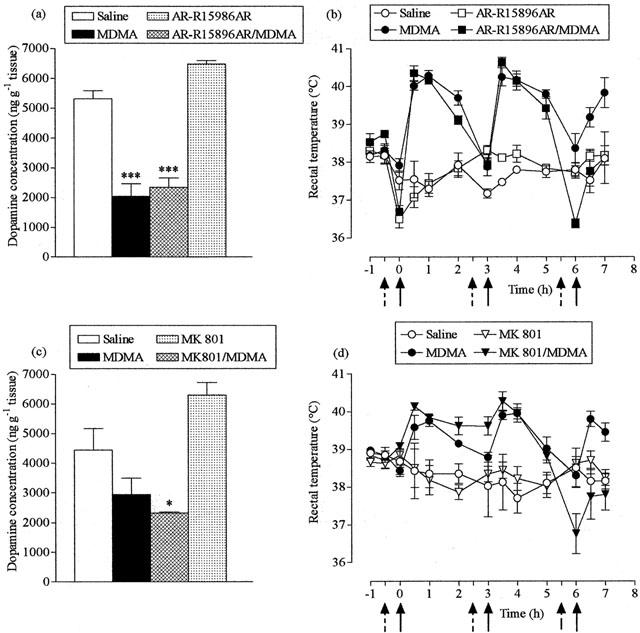
Effect of AR-R15896AR and MK-801 on MDMA-induced striatal dopamine loss (a,c) and acute hyperthermia (b,d). AR-R15896AR (20, 5, 5 mg kg−1, i.p.), MK-801 (0.5 mg kg−1, i.p.) or saline (broken arrows) were administered 30 min before MDMA (25 mg kg−1, i.p.) or saline (full arrows), three times at 3 h intervals. Mice were sacrificed 7 days later. Results shown as mean±s.e. mean (n=4 – 5 mice). (a) MDMA treatment resulted in a significant reduction in dopamine levels (***P<0.001) compared to saline-treated mice, while AR-R15896AR did not protect against this reduction (AR-R15896AR+MDMA different from saline, ***P<0.001). (b) MDMA produced a significant rise in rectal temperature (F(1,8)=196.83, P<0.001) compared with the saline injected group. AR-R15896AR attenuated the MDMA-induced hyperthermia after the second dose (F(1,10)=18.16, P<0.01), but did not alter temperature in saline-treated mice. (c) MDMA treatment resulted in a reduction in dopamine levels compared to saline-treated control animals, although this effect was non-significant, and MK-801 did not protect against this reduction (MK-801+MDMA different from saline, *P<0.05). (d) MDMA produced a significant rise in rectal temperature (F(1,8)=8.76, P<0.05) compared with the saline-injected group. MK-801 did not modify the hyperthermic response induced by MDMA and did not alter the rectal temperature of saline treated mice.
Effect of repeated doses of MDMA on the concentration of dopamine and 5-HT in the striatum 7 days later
A single dose of MDMA (25 mg kg−1, at t=0 h) produced a 3% non-significant decrease in striatal dopamine concentration, compared to saline-treated control animals (Figure 1a) 7 days later. Mice administered 2 doses of MDMA (25 mg kg−1, at t=0 and 3 h) had a 57% decrease in striatal dopamine concentration, while mice administered 3 doses of MDMA (25 mg kg−1, at t=0, 3 and 6 h) had a 71% decline in striatal dopamine levels (Figure 1a). Thus MDMA-induced dopamine loss was dose-dependent (Figure 1b). The concentration of the two dopamine metabolites HVA and DOPAC decreased in a similar way (Figure 1c,d).
Figure 1.
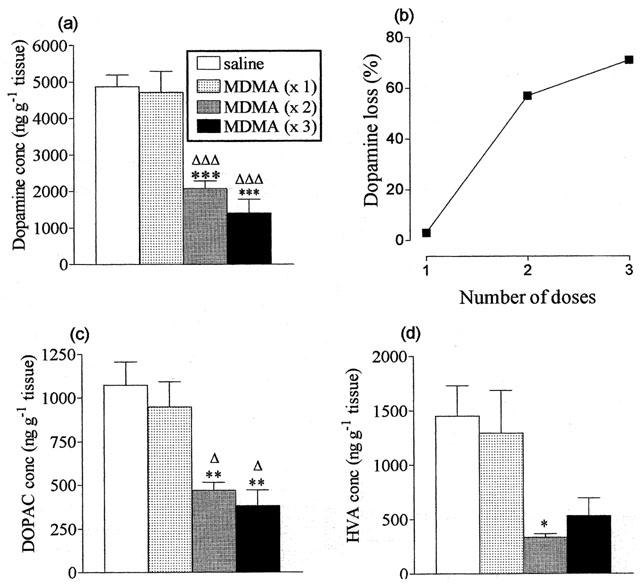
Effect of different doses of MDMA on striatal dopamine and dopamine metabolite concentrations 7 days later. Mice were administered MDMA (25 mg kg−1 i.p.) or saline three times at 3 h intervals. Results shown as mean±s.e. mean (n=5 – 6). (a) Striatal dopamine concentrations. MDMA treatment resulted in a significant reduction in dopamine levels in both MDMA (×3) and MDMA (×2) groups, compared to saline-treated control mice (***P<0.001) and compared to the MDMA (×1) group (ΔΔΔP<0.001), while there was no significant difference between MDMA (×3) and MDMA (×2) treatment groups. (b) Dopamine loss measured 1 week following administration of 1, 2 or 3 doses of MDMA, calculated as a % of control values. (c) Striatal DOPAC concentrations. MDMA treatment resulted in a significant reduction in DOPAC levels in both MDMA (×3) and MDMA (×2) groups, compared to saline-treated control mice (**P<0.01) and compared to the MDMA (×1) group (ΔP<0.05, (ΔΔP<0.01). There was no significant difference between MDMA (×3) and MDMA (×2) treatment groups. (d) Striatal HVA concentrations. MDMA treatment resulted in a significant reduction in HVA levels in the MDMA (×2) treatment group, compared to saline-treated control mice (*P<0.05), while there was no significant difference between MDMA (×3) and MDMA (×2) treatment groups.
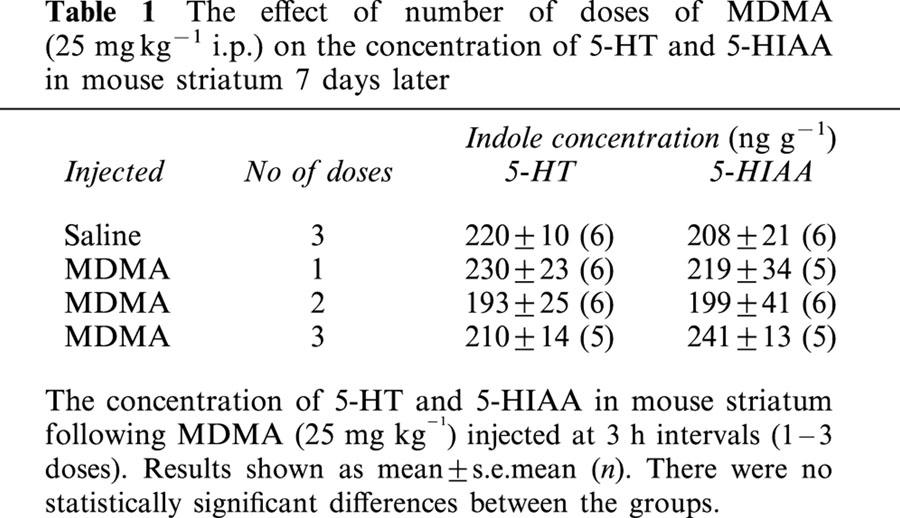
In contrast, administration of a single dose or repeated doses of MDMA (25 mg kg−1) was without effect on the striatal concentration of 5-HT or its major metabolite 5-HIAA (Table 1).
Table 1.
The effect of number of doses of MDMA (25 mg kg−1 i.p.) on the concentration of 5-HT and 5-HIAA in mouse striatum 7 days later
Effect of NMDA antagonists on MDMA-induced changes
Pretreatment with the non-competitive NMDA antagonist AR-R15896AR (20 mg kg−1 before the 1st dose and 5 mg kg−1 before the 2nd and 3rd doses) 30 min prior to each dose of MDMA (25 mg kg−1) did not protect against the MDMA-induced damage to the striatum as indicated by the loss in dopamine concentration (Figure 2a). This dose schedule for AR-R15896AR had no effect on the acute hyperthermic response following the first two injections of MDMA but abolished the rise seen following the third dose (Figure 2b).
Similar results were obtained when mice were pretreated with the high affinity non-competitive antagonist MK-801 (0.5 mg kg−1). The drug produced no neuroprotection against MDMA-induced striatal damage (Figure 2c) and had no marked effect on the temperature rise other than on the increase seen following the third MDMA administration (Figure 2d).
Effect of clomethiazole on MDMA-induced changes
Pretreatment with the GABAmimetic clomethiazole (50 mg kg−1) failed to protect against MDMA-induced striatal dopamine loss (Figure 3a). Clomethiazole pretreatment resulted in hypothermic responses prior to each MDMA injection. However, apart from after the third dose of clomethiazole where hypothermia was sustained, the peak hyperthermic response seen in clomethiazole-pretreated mice was equal to that of mice administered MDMA alone (Figure 3b). Clomethiazole also induced a hypothermic response, following each dose, in control mice.
Figure 3.
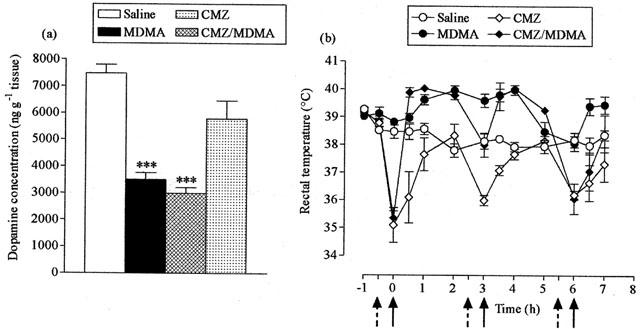
Effect of clomethiazole (CMZ) on MDMA-induced striatal dopamine loss (a) and acute hyperthermia (b). CMZ (50 mg kg−1, i.p.) or saline (broken arrows) were administered 30 min before MDMA (25 mg kg−1, i.p.) or saline (full arrows), three times at 3 h intervals. Mice were sacrificed 7 days later. Results shown as mean±s.e. mean (n=3 – 6). (a) MDMA treatment resulted in a significant reduction in dopamine levels (***P<0.001) compared to saline-treated control animals, while CMZ did not protect against this reduction (CMZ+MDMA different from saline, ***P<0.001). (b) MDMA produced a significant rise in rectal temperature (F(1,9)=20.93, P<0.01) compared with the saline injected group. CMZ attenuated the MDMA-induced hyperthermia (F(1,9)=7.46, P<0.05) and lowered the rectal temperature of saline-treated mice (F(1,10)=12.71, P<0.01).
Effects of PBN on MDMA-induced changes
The nitrone free radical trapping agent PBN at a dose of 120 mg kg−1 did not protect against MDMA-induced striatal dopamine loss (Figure 4a). A hypothermic response was observed in MDMA-treated mice following the first dose of PBN (Figure 4b) but, thereafter, there was no difference between the hyperthermic response of PBN-pretreated mice and animals administered MDMA alone. PBN induced a hypothermic response in control animals, temperature values remaining below those of saline-injected mice throughout the experiment. In contrast, following the higher dose of PBN (150 mg kg−1) the dopamine levels were the same in PBN+MDMA-treated mice and saline-treated mice (Figure 4c). However, PBN pretreatment resulted in a hypothermic response in both the MDMA-treated and control mice (Figure 4d).
Figure 4.
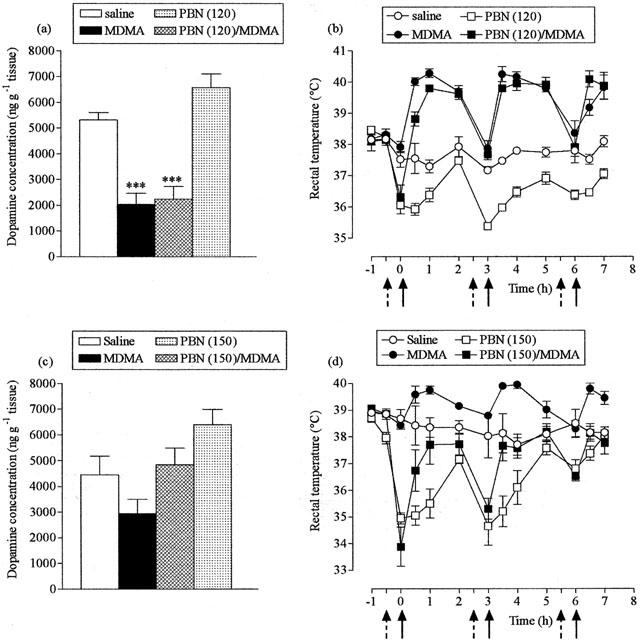
Effect of α-phenyl-N-tert-butyl nitrone (PBN) on MDMA-induced striatal dopamine loss (a,c) and acute hyperthermia (b,d). PBN (120 or 150 mg kg−1, i.p.) or saline (broken arrows) were administered 30 min before MDMA (25 mg kg−1, i.p.) or saline (full arrows), three times at 3 h intervals. Mice were sacrificed 7 days later. Results shown as mean±s.e. mean (n=3 – 6 mice). (a) MDMA treatment resulted in a significant reduction in dopamine levels (***P<0.001) compared to saline-treated animals, while PBN did not protect against this reduction (PBN+MDMA different from saline, ***P<0.001). (b) MDMA produced a significant rise in rectal temperature (F(1,8)=208.37, P<0.001) compared with the saline-injected group. PBN (120 mg kg−1) induced a hypothermic response in MDMA-treated mice, following the first dose (F(1,9)=15.28, P<0.01), and reduced body temperature in saline-treated mice F(1,7=77.39, P<0.001). (c) MDMA treatment resulted in a reduction in dopamine levels compared to saline-treated control animals, although this effect was non-significant, and there was no difference between PBN+MDMA-treated mice and saline-treated mice. (d) MDMA produced a significant rise in rectal temperature (F(1,8)=8.76, P<0.05) compared with the saline-injected group. PBN (150 mg kg−1) abolished the hyperthermic response induced by MDMA (F(1,9)=34.22, P<0.001) and produced hypothermia in saline treated mice (F(1,6)=14.69, P<0.01).
Effect of nNOS inhibitors on MDMA-induced changes
Administration of 7-NI (50 mg kg−1) before each dose of MDMA produced substantial neuroprotection (Figure 5a) but also induced a marked hypothermic response in both the control and MDMA-treated animals (Figure 5b).
Figure 5.
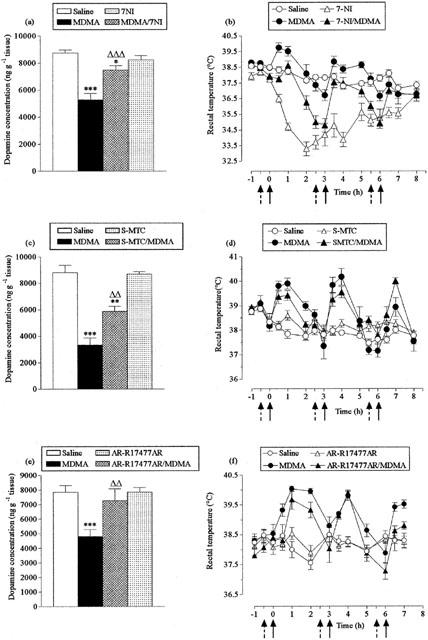
Effect of 7-nitroindazole (7-NI), S-methyl-L-thiocitrulline (S-MTC) and AR-R17477AR MDMA-induced striatal dopamine loss (a,c,e) and acute hyperthermia (b,d,f). 7-NI (50 mg kg−1, i.p.), S-MTC (10 mg kg−1, i.p.), AR-R17477AR (5 mg kg−1, s.c.), or saline (broken arrows) were administered 30 min before MDMA (20 mg kg−1, i.p.) or saline (full arrows), three times at 3 h intervals. Mice were sacrificed 7 days later. Results shown as mean±s.e.mean (n=6 10 mice). (a) MDMA treatment resulted in a significant reduction in dopamine levels (***P<0.001) compared to saline-treated mice. 7-NI pretreatment significantly, although not completely, protected against this reduction (7-NI+MDMA different from MDMA, ΔΔΔP<0.001; 7-NI+MDMA different from saline, *P<0.05). (b) MDMA produced a significant rise in body temperature (F(1,22)=7.55, P<0.01) compared with the saline-injected group. 7-NI abolished the MDMA-induced hyperthermia (F(1,25)=24.33, P<0.001) and induced a frank hypothermia in saline-treated mice (F(1,16)=169.02, P<0.001). (c) MDMA treatment resulted in a significant reduction in dopamine levels (***P<0.001) compared to saline-treated mice. S-MTC pretreatment significantly, although not completely, protected against this reduction (S-MTC+MDMA different from MDMA, ΔΔP<0.01; S-MTC+MDMA different from saline, **P<0.01). (d) MDMA produced a significant rise in body temperature (F(1,16)=17.23, P<0.001) compared with the saline injected group. S-MTC did not modify the hyperthermic response induced by MDMA and did not alter the body temperature of saline-treated mice. (e) MDMA treatment resulted in a significant reduction in dopamine levels (***P<0.001) compared to saline-treated control mice. AR-R17477AR provided complete protection against this loss (AR-R17477AR+MDMA different from MDMA, ΔΔP<0.01). (f) MDMA produced a significant rise in body temperature (F(1,8)=18.03, P<0.01) compared with the saline injected group. AR-R17477AR attenuated the MDMA-induced hyperthermic response following the second dose (F(1,7)=68.60, P<0.001), but did not modify the rectal temperature of saline-treated mice.
In contrast, S-MTC (10 mg kg−1) administration provided significant neuroprotection (Figure 5c) and did not modify MDMA-induced hyperthermia (Figure 5d).
The novel NOS inhibitor AR-R17477AR (5 mg kg−1 s.c.) provided complete protection against MDMA-induced long-term dopamine loss in the striatum (Figure 5e) and had little effect on MDMA-induced hyperthermia except following the final dose (Figure 5f).
Free radical production in mouse striatum following MDMA and effect of AR-R17477AR
Administration of MDMA (20 mg kg−1) resulted in an increase in the conversion of salicylate to 2,3-DHBA in the striatal dialysate. The increase was modest in the first 2 h but was magnified and sustained following the 2nd and 3rd injections of MDMA (Figure 6).
Figure 6.
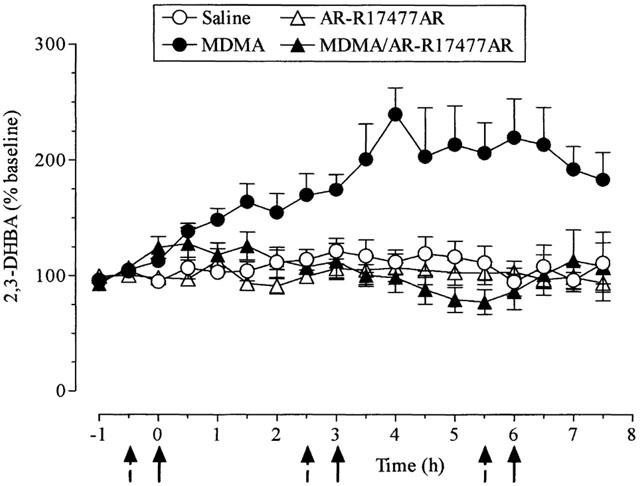
Changes in the level of 2,3-dihydroxybenzoic acid (DHBA) in the striatal dialysate of mice treated with AR-R17477AR and MDMA. AR-R17477AR (5 mg kg−1, s.c.) or saline (broken arrows) were administered 30 min before MDMA (20 mg kg−1, i.p.) or saline (full arrows), three times at 3 h intervals. Results shown as mean±s.e.mean (n=5 – 8 mice). MDMA increased the extracellular levels of 2,3-DHBA (F(1,11)=17.59, P<0.001). This effect was abolished by AR-R17477AR (F(1,14)=25.33, P<0.001). AR-R17477AR, when given to saline-treated mice, did not modify the extracellular levels of 2,3-DHBA.
Since AR-R17477AR (5 mg kg−1 s.c.) had been found to be strongly neuroprotective when injected before MDMA, this compound was used for further investigation of the possible role of NOS in the MDMA-induced production of free radicals. When AR-R17477 (5 mg kg−1) was injected before each dose of MDMA, the MDMA-induced increase in 2,3 DHBA formation was abolished (Figure 6). In confirmation of the previous study with AR-R17477AR, there was only a very modest attenuation of the MDMA-induced hyperthermia produced by this NOS inhibitor (data not shown).
The effect of AR-R17477AR on lipid peroxidation
We examined whether the inhibition of MDMA-induced free radical formation by AR-R17477AR was due to this compound having intrinsic radical trapping activity by investigating its effect on lipid peroxidation in vitro.
The incubation of synaptosomes with FeCl2+ascorbate resulted in an approximate 400% increase in malondialdehyde formation (data not shown). The free radical inhibitor BHT inhibited the peroxidation-induced increase in a concentration-dependent manner (Figure 7) with an IC50 value of less than 1 μM. In contrast, AR-R17477AR produced negligible inhibition of this reaction, even at a concentration of 1 mM (Figure 7).
Figure 7.
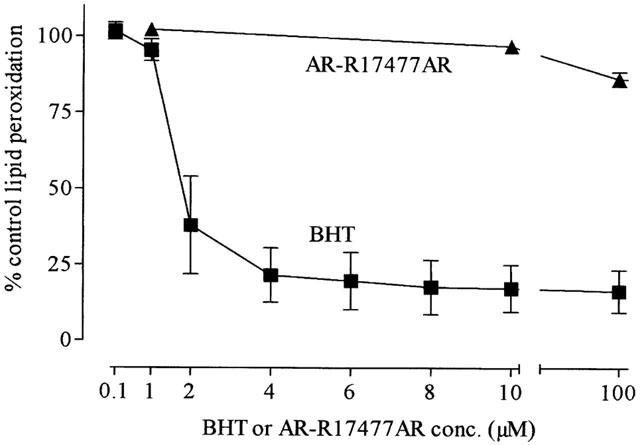
The effect of increasing concentrations of BHT or AR-R17477AR on FeCl2 (3 μM) plus ascorbic acid (0.1 mM) on lipid peroxidation in mice brain synaptosomes. Results shown as mean±s.e.mean of 3 – 4 mice.
Discussion
The current study has emphasized both the similarities and differences in the long-term neurotoxic effect of MDMA in rats and mice. As reviewed in the introduction the key difference is that, in mice, this amphetamine derivative damages dopaminergic rather than serotonergic nerve endings. In addition we have now shown that the profile of activity of putative neuroprotective compounds also differs.
Early studies demonstrated the efficacy of MK-801 in protecting against the neurodegenerative action of methamphetamine in both mice (Sonsalla et al., 1989; Green et al., 1992) and rats (Green et al., 1992) and also against MDMA-induced damage to 5-HT nerve endings in rats (Colado et al., 1993). However, it was subsequently shown that this protective action resulted from MK-801 lowering body temperature in both species thereby producing neuroprotection (Albers & Sonsalla, 1995; Malberg et al., 1996; Farfel & Seiden, 1995a; 1995b; Colado et al., 1998). Evidence suggests that merely preventing amphetamine-induced hyperthermia, rather than producing frank hypothermia, is enough to produce significant neuroprotection (Colado et al., 1998; 1999).
In the current study we investigated whether NMDA antagonists protected against MDMA-induced damage to striatal dopamine in mice. Neither MK-801 nor AR-R15896AR produced any neuroprotection, even though both were given at doses previously demonstrated to be neuroprotective in animal models of stroke (Cregan et al., 1997; Gill et al., 1987). The doses chosen had little effect on MDMA-induced hyperthermia until the third dose of MDMA. However, even the hypothermia seen following the last dose failed to provide any evidence of neuroprotection.
Clomethiazole is an effective neuroprotective agent in various animal models of stroke (Green, 1998; Green et al., 2000) and protects against MDMA-induced damage to 5-HT nerve endings in rats (Colado et al., 1998). In mice, however, a dose of clomethiazole shown to be protective in rats against MDMA-induced damage to 5-HT neurones in several brain regions (Colado et al., 1998) was totally without neuroprotective action against damage to striatal dopaminergic neurones in mice.
Administration of PBN has previously been demonstrated to protect the rat brain from the neurotoxic consequences of MDMA administration (Colado & Green, 1995; Colado et al., 1997; Yeh, 1999). Compelling evidence has been presented to support the contention that this free radical trapping compound is neuroprotective because it prevents the MDMA-induced increase in free radical formation in the brain (Colado et al., 1998). While we were able to demonstrate that the higher dose of PBN used in the current study (150 mg kg−1) was neuroprotective we were unable to show that this effect was separable from the hypothermic action of this compound. However, the study using in vivo microdialysis of salicylic acid and measurement of 2,3-DHBA did suggest strongly that MDMA does increase free radical formation in the mouse striatum.
It is interesting to note that the first injection of MDMA produced only a modest increase in 2,3-DHBA formation and that it was the second and third injections that resulted in the marked and sustained increase in free radical formation. This contrasts with our studies in the DA rat where a single injection of MDMA produces a rapid and sustained (over 6 h) increase in 2,3-DHBA production (Colado et al., 1997; 1999). It is noteworthy that a single injection of MDMA is sufficient to produce neurodegeneration of 5-HT pathways in the DA rat whereas several doses are necessary for this compound to produce neurotoxic damage to striatal dopamine in the mouse. This may be further exemplified by the results in the current study, where one, two or three doses of MDMA were administered and the loss of striatal dopamine measured 1 week later. A significant reduction in dopamine levels was observed in mice administered both two and three doses of MDMA, whereas a single dose had no effect on striatal dopamine concentration, values remaining the same as saline-treated control mice. The requirement of more than one dose of MDMA to produce a major increase in free radical production may, therefore, underlie the difference between rats and mice.
This evidence for a role of free radicals in the production of MDMA-induced neurotoxic damage in mice supports the study of Jayanthi et al. (1999) which found that oxidative stress produced by MDMA administration was attenuated in transgenic mice overexpressing human copper/zinc superoxide dismutase.
Nitric oxide has been implicated in cerebral neurotoxicity following drug abuse (Cerratic et al., 1995; Gunasekar et al., 1995) and inhibition of NOS with Nω-nitro-L-arginine (L-NOARG) has been reported to protect against MDMA-induced neurotoxicity in rats (Zheng & Laverty, 1998). However, L-NOARG has poor selectivity for neuronal NOS (nNOS) versus endothelial NOS (eNOS) and studies in experimental stroke provided evidence that, while nNOS activity was directly involved in ischaemia-induced neurodegeneration, eNOS inhibition produced neuroprotection by an action on cerebral blood flow (Huang et al., 1994).
The role of NOS inhibitors as neuroprotective agents against methamphetamine-induced damage in mice remains controversial. Both di monte et al. (1996) and Itzhak & Ali (1996) reported that 7-NI did not alter methamphetamine-induced hyperthermia and was neuroprotective. In contrast, Callaghan & Ricaurte (1998) suggested that this compound was protective primarily because of its action on body temperature. Our current studies on the effect of 7-NI on MDMA-induced damage confirmed that this agent has a marked effect on body temperature. Therefore, like Callaghan & Ricaurte (1998) we were unable to demonstrate any neuroprotective action that was separable from a hypothermic action. Taraska & Finnegan (1997) were similarly unable to separate the neuroprotective action of the NOS inhibitor L-NAME from its hypothermic action, concluding that nitric oxide played little role in MDMA-induced neurodegeneration.
However, in our studies, administration of S-MTC, given at a dose previously shown to protect against MPTP-induced damage to dopamine neurones (Matthews et al., 1997), had no effect on the MDMA-induced hyperthermia and provided a significant neuroprotective effect. Similarly, AR-R17477AR, when given at a dose that has no overt behavioural effects or actions on blood pressure (Johansson et al., 1999), had little effect on the hyperthermia following the first two doses of MDMA but was strongly neuroprotective. It is acknowledged that it did produce hypothermia following the third MDMA dose, but our study with the NMDA antagonists indicated that this action alone is not enough to produce neuroprotection.
The data, therefore, do suggest a role for NOS inhibitors in producing neuroprotection against MDMA-induced damage to dopamine nerve endings in mouse striatum and demonstrate that this effect is not primarily involved with any action on body temperature. The question that follows, however, concerns the selectivity of the inhibitors used. L-NOARG and 7-NI show little selectivity for nNOS versus eNOS (Dawson, 1995; O'neill et al., 2000). In contrast, both S-MTC and AR-R17477AR have been reported to display good selectivity towards nNOS using in vitro studies (Furfine et al., 1994; O'neill et al., 2000). While the selective action of AR-R17477AR is supported by evidence that this compound had no effect on blood pressure or heart rate when given to rats at twice the dose used in the present investigation (Johansson et al., 1999) it has to be pointed out that selectivity against rodent NOS isoforms was not examined by O'neill et al. (2000) so caution should be exercised in proposing that it is nNOS that is involved in the mechanisms whereby NOS inhibition protects against MDMA-induced neurotoxicity.
It, therefore, seems reasonable to propose that NOS is involved in the neurodegeneration of dopamine nerve endings that follows administration of MDMA to mice. The question that remains, however, concerns the specific mechanisms involved. It is generally accepted in ischaemic stroke research that ischaemia-induced glutamate release produces excessive stimulation of NMDA receptors leading to an increase in intracellular calcium, activation of NOS and excess production of NO• (Garthwaite et al., 1989; Moncada et al., 1991). In turn NO• interacts with the superoxide anion, O2− and forms the highly toxic peroxynitrite ion (ONOO−) (Beckman et al., 1990). While this cascade of events has previously been proposed to explain methamphetamine-induced neurotoxicity in mice (for example, Itzhak et al., 1998), such a mechanism cannot explain the current observations. In the case of MDMA-induced neurotoxic degeneration our data provide clear evidence that an increase in excitatory amino acid neurotransmission does not underlie the neurotoxic effect of MDMA in mice since neither of the two NMDA antagonists examined (MK-801 and AR-R15896AR) provided any protection against MDMA-induced damage to dopamine neurones in the striatum. Nevertheless, administration of NOS inhibitors did result in substantial neuroprotection and mechanisms not involving raised extracellular glutamate must be evoked to explain the involvement of NOS in the neurodegenerative cascade.
It was recently shown that MDMA administration decreased cytochrome oxidase complex IV of the electron transport chain in dopamine rich areas (Burrows et al., 2000). It may be that the MDMA-induced release of dopamine compromised mitochondrial function because of auto-oxidation of dopamine metabolites to form quinones and hydroxyl and reactive oxygen species (Graham et al., 1978). Quinones and reactive oxygen species have been shown to inhibit mitochondrial enzymes (Zhang et al., 1990; Ben-Schachar et al., 1995). Since there is also evidence that MDMA is metabolized to catechol and quinone compounds, another possibility is that further metabolism of these compounds also leads to free radical formation (Colado et al., 1995; 1997; 1999; Sprague & Nichols, 1995; Yeh, 1999) and disruption of mitochondrial function. This would lead to an increase in intracellular calcium levels (Khodorov et al., 1999) and activation of both nNOS and eNOS (Stuehr & Griffith, 1992).
Of particular interest is the observation that administration of the NOS inhibitor AR-R17477AR prevented the MDMA-induced rise in free radical formation. Since we were able to demonstrate that AR-R17477AR is not a radical trapping agent, by its lack of effect on FeCl2/ascorbate-induced lipid peroxidation, these data indicate that nitric oxide is probably interacting in some way with MDMA and/or dopamine metabolite auto-oxidation products to produce a radical that can be detected by the salicylate/2,3-DHBA reaction pathway. The most likely candidate is the peroxynitrite ion, a radical which has already been implicated in the neurotoxic damage to dopamine neurones that follows methamphetamine administration (Imam & Ali, 2000; Imam et al., 1999; 2001) and is detected by the salicylate/2,3-DHBA assay (Narayan et al., 1997; Halliwell & Kaur, 1997).
In turn, this proposal goes some way to explaining why dopamine neurones are damaged following MDMA administration to mice, whereas 5-HT neurones are damaged following MDMA administration to rats. In our previous studies we have been unable to demonstrate that administration of NOS inhibitors prevented MDMA-induced neurodegeneration in rats (Colado et al., unpublished observations). Based on the results of the current study, therefore, it appears that the free radical entity produced in rats is not peroxynitrite. Thus, the neurotoxic damage produced in different monoaminergic systems in rats and mice may relate to the particular free radical ion being produced in these two species. This may, in turn, be related to a different pattern of metabolites being formed following administration of MDMA to rats and mice.
In conclusion, our data do demonstrate that free radicals are involved in MDMA-induced damage in mice, as they are in rats (Colado et al., 1997; 1999; Yeh, 1999). It seems probable that in mice MDMA metabolites produce specific radicals which in turn react with nitrite ions. By inhibiting NOS, this interaction is decreased sufficiently to abolish or attenuate the formation of the highly toxic peroxynitrite ions. This proposal is supported by our study using in vivo microdialysis.
Acknowledgments
M.I. Colado thanks Plan Nacional sobre Drogas (Ministerio del Interior), CICYT (SAF98-0074) and AstraZeneca R&D Södertälje for financial support.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- ANOVA
analysis of variance
- AR-R15896AR
S-(+)-α-phenyl-2-pyridine ethanamide dihydrochloride
- AR-R17477AR
N-(4-(2-((3-chlorophenylmethyl) amino)-ethyl)phenyl) 2-thiophene carboxamidine hydrochloride
- BHT
butylated hydroxytoluene
- 2,3-DHBA
2,3-dihydroxybenzoic acid
- DOPAC
3,4-dihydroxyphenylacetic acid
- GABA
γ-aminobutyric acid
- h.p.l.c.
high performance liquid chromatography
- 5-HT
5-hydroxytryptamine HVA, homovanillic acid
- L-NAME
NG-nitro-L-arginine methyl ester
- L-NOARG
Nω-nitro-L-arginine
- MDMA
(±) 3,4-methylenedioxymethamphetamine HCl
- MK-801
dizocilpine
- 7-NI
7-nitroindazole
- NMDA
N-methyl-D-aspartate
- NOS
nitric oxide synthase
- eNOS
endothelial nitric oxide synthase
- nNOS neuronal nitric oxide synthase; PBN
α-phenyl-N-tert-butyl nitrone
- S-MTC
S-methyl-L-thiocitrulline
References
- ALBERS D.S., SONSALLA P.K. Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacological profile of protective and nonprotective agents. J. Pharmacol. Exp. Ther. 1995;275:1104–1114. [PubMed] [Google Scholar]
- BALDWIN H.A., COLADO M.I., MURRAY T.K., DE SOUZA R.J., GREEN A.R. Striatal dopamine release in vivo following neurotoxic doses of methamphetamine and effect of neuroprotective drugs, chlormethiazole and dizocilpine. Br. J. Pharmacol. 1993;108:590–596. doi: 10.1111/j.1476-5381.1993.tb12847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALDWIN H.A., WILLIAMS J.L., SNARES M., FERREIRA T., CROSS A.J., GREEN A.R. Attenuation by chlormethiazole administration of the rise in extracellular amino acids following focal ischaemia in the cerebral cortex of the rat. Br. J. Pharmacol. 1994;112:188–194. doi: 10.1111/j.1476-5381.1994.tb13050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATTAGLIA G., YEH S.Y., O'HEARN E., MOLLIVER M.E., KUHAR M.J., DE SOUZA E.B. 3,4-Methylenedioxyamphetamine destroy serotonin terminals in rat brain: quantification of neurodegeneration by measurement of [3H]paroxetine-labeled serotonin uptake sites. J. Pharmacol. Exp. Ther. 1987;242:911–916. [PubMed] [Google Scholar]
- BECKMAN J.S., BECKMAN T.W., CHEN J., MARSHALL P.M., FREEMAN B.A. Apparent hydroxy radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1621–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEN-SCHACHAR D., ZUK R., GLINKA Y. Dopamine neurotoxicity: inhibition of mitochondrial respiration. J. Neurochem. 1995;64:718–723. doi: 10.1046/j.1471-4159.1995.64020718.x. [DOI] [PubMed] [Google Scholar]
- BOWYER J.F., DAVIES D.L., SCHMUED L., BROENING H.W., NEWPORT G.D., SLIKKER W., JR, HOLSON R.R. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J. Pharmacol. Exp. Ther. 1994;268:1571–1580. [PubMed] [Google Scholar]
- BURROWS K.B., GUDELSKY G., YAMAMOTO B.K. Rapid and transient inhibition of mitochondrial function following methamphetamine or 3,4-methylenedioxymethamphetamine administration. Eur. J. Pharmacol. 2000;398:11–13. doi: 10.1016/s0014-2999(00)00264-8. [DOI] [PubMed] [Google Scholar]
- CALLAGHAN B.T., RICAURTE G.A. Effects of 7- nitroindazole on body temperature and methamphetamine-induced dopamine toxicity. NeuroReport. 1998;9:2691–2695. doi: 10.1097/00001756-199808240-00001. [DOI] [PubMed] [Google Scholar]
- CERRATIC C., SHENG P., LADENHEIM B., EPSTEIN C.J., CADET J-L. Involvement of oxidative and L-arginine-NO pathways in the neurotoxicity of drugs of abuse in vitro. Clin. Exp. Pharmacol. Physiol. 1995;22:381–382. doi: 10.1111/j.1440-1681.1995.tb02025.x. [DOI] [PubMed] [Google Scholar]
- CHIUEH C.C., KRISHNA G., TULSI P., OBATA T., LANG K., HUANG S.J., MURPHY D.L. Role of dopamine autoxidation, hydroxyl radical generation and calcium overload in underlying mechanisms involved in MPTP-induced Parkinsonism. Adv. Neurol. 1992;60:251–258. [PubMed] [Google Scholar]
- COLADO M.I., GRANADOS R., O'SHEA E., ESTEBAN B., GREEN A.R. Role of hyperthermia in the protective action of clomethiazole against MDMA (‘ecstasy')-induced neurodegeneration, comparison with the novel NMDA channel blocker AR- R15896. Br. J. Pharmacol. 1998;124:479–484. doi: 10.1038/sj.bjp.0701859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLADO M.I., GREEN A.R. The spin trap reagent α-phenyl-N-tert-butyl nitrone prevents neurodegeneration following administration of ‘ecstasy'. Eur. J. Pharmacol. 1995;280:343–346. doi: 10.1016/0014-2999(95)00298-y. [DOI] [PubMed] [Google Scholar]
- COLADO M.I., MURRAY T.K., GREEN A.R. 5-HT loss in rat brain following 3,4-methylenedioxymethamphetamine (MDMA), p-chloroamphetamine and fenfluramine administration and effects of chlormethiazole and dizocilpine. Br. J. Pharmacol. 1993;108:583–589. doi: 10.1111/j.1476-5381.1993.tb12846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLADO M.I., O'SHEA E., GRANADOS R., ESTEBAN B., MARTIN A.B., GREEN A.R. Studies on the role of dopamine in the degeneration of 5-HT nerve endings in the brain of Dark Agouti rats following 3,4-methylenedioxymethamphetamine (MDMA or ‘ecstasy') administration. Br. J. Pharmacol. 1999;126:911–924. doi: 10.1038/sj.bjp.0702373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLADO M.I., O'SHEA E., GRANADOS R., MURRAY T.K., GREEN A.R. in vivo evidence for free radical involvement in the degeneration of rat brain 5-HT following administration of MDMA (‘ecstasy') and p-chloroamphetamine but not the degeneration following fenfluramine. Br. J. Pharmacol. 1997;121:889–900. doi: 10.1038/sj.bjp.0701213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLADO M.I., WILLIAMS J.L., GREEN A.R. The hyperthermic and neurotoxic effects of ‘Ecstasy' (MDMA) and 3,4-methylenedioxyamphetamine (MDA) in the Dark Agouti (DA) rat, a model of the CYP2D6 poor metaboliser phenotype. Br. J. Pharmacol. 1995;115:1281–1289. doi: 10.1111/j.1476-5381.1995.tb15037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREGAN E.F., PEELING J., CORBETT D., BUCHAN A.M., SAUNDERS J., AUER R.N., GAO M., MCCARTHY D.J., EISMAN M.S., CAMPBELL T.M., MURRAY R.J., STIGNITTO M.L., PALMER G.C. (S)-α-phenyl-2-pyridine-ethanamine dihydrochloride – a low affinity uncompetitive N-methyl-D-aspartic acid antagonist is effective in rodent models of global and focal ischemia. J. Pharmacol. Exp. Ther. 1997;283:1412–1424. [PubMed] [Google Scholar]
- DAS N.P., RATTY A.K. Studies on the effects of the narcotic alkaloids, cocaine, morphine and codeine on nonenzymatic lipid peroxidation in rat brain mitochondria. Biochem. Med. Metab. Biol. 1987;37:256–264. doi: 10.1016/0885-4505(87)90035-1. [DOI] [PubMed] [Google Scholar]
- DAWSON D.A. Nitric oxide, focal cerebral ischemia: multiplicity of actions and diverse outcomes. Cerebrovasc. Brain Metab. Rev. 1995;6:299–324. [PubMed] [Google Scholar]
- DI MONTE D.A., ROYLAND J.E., JAKOWEC M.W., LANGSTON J.W. Role of nitric oxide in methamphetamine neurotoxicity: protection by nitric oxide synthase. J. Neurochem. 1996;67:2443–2450. doi: 10.1046/j.1471-4159.1996.67062443.x. [DOI] [PubMed] [Google Scholar]
- FARFEL G.M., SEIDEN L.S. Role of hypothermia in the mechanism of protection against serotonergic toxicity. I. Experiments with 3,4-methylenedioxymethamphetamine, dizocilpine, CGS 19755 and NBQX. J. Pharmacol. Exp. Ther. 1995a;272:860–867. [PubMed] [Google Scholar]
- FARFEL G.M., SEIDEN L.S. Role of hypothermia in the mechanism of protection against serotonergic toxicity. II. Effects with methamphetamine, p-chloroamphetamine, fenfluramine, dizocilpine and dextromethorphan. J. Pharmacol. Exp. Ther. 1995b;272:868–875. [PubMed] [Google Scholar]
- FRANKLIN K.B.J., PAXINOS G. The mouse brain in stereotaxic coordinates. San Diego, California, Academic Press Inc; 1997. [Google Scholar]
- FURFINE E.S., HARMON M.F., PAITH J.E., KNOWLES R.G., SALTER M., KIFF R.J., DUFFY C., HAZLEWOOD R., OPLINGER J.A., GARVEY E.P. Potent and selective inhibition of human nitric oxide synthases. Selective inhibition of neuronal nitric oxide synthase by S-methyl-L-thiocitrulline and S-ethyl-L-thiocitrulline. J. Biol. Chem. 1994;269:26677–26683. [PubMed] [Google Scholar]
- GARTHWAITE J., GARTHWAITE G., PALMER R.M.J., MONCADA S. NMDA receptor activation induces nitric oxide synthesis from arginine in rat brain slices. Eur. J. Pharmacol. 1989;172:413–416. doi: 10.1016/0922-4106(89)90023-0. [DOI] [PubMed] [Google Scholar]
- GILL R., FOSTER A.C., WOODRUFF G.N. Systemic administration of MK-801 protects against ischemia-induced hippocampal degeneration in the gerbil. J. Neurosci. 1987;7:3343–3349. doi: 10.1523/JNEUROSCI.07-10-03343.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIOVANNI A., LIANG L.P., HASTINGS T.G., ZIGMOND M.J. Estimating hydroxyl radical content in rat brain using systemic and intraventricular salicylate; impact of methamphetamine. J. Neurochem. 1995;64:1819–1825. doi: 10.1046/j.1471-4159.1995.64041819.x. [DOI] [PubMed] [Google Scholar]
- GOUGH B., ALI S.F., SLIKKER W., JR, HOLSON R.R. Acute effects of 3,4-methylenedioxymethamphetamine (MDMA) on monoamines in rat caudate. Pharmacol. Biochem. Behav. 1991;39:619–623. doi: 10.1016/0091-3057(91)90137-q. [DOI] [PubMed] [Google Scholar]
- GRAHAM D.G., TIFFANY S.M., BELL W.R., JR, GUKNECHT J. Auto-oxidation versus covalent binding of quinones as the mechanism of toxicity of dopamine, 6-hydroxydopamine and related compounds towards C1300 neuroblastoma cells in vitro. Mol. Pharmacol. 1978;14:644–653. [PubMed] [Google Scholar]
- GREEN A.R. Clomethiazole (Zendra) in acute ischemic stroke: basic pharmacology and biochemistry and clinical efficacy. Pharmacol. Ther. 1998;80:123–147. doi: 10.1016/s0163-7258(98)00024-2. [DOI] [PubMed] [Google Scholar]
- GREEN A.R., CROSS A.J., GOODWIN G.M. Review of the pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA or ‘ecstasy') Psychopharmacology. 1995;119:247–260. doi: 10.1007/BF02246288. [DOI] [PubMed] [Google Scholar]
- GREEN A.R., DE SOUZA R.J., WILLIAMS J.L., MURRAY T.K., CROSS A.J. The neurotoxic effects of methamphetamine on 5-hydroxytryptamine and dopamine in brain: evidence for the protective effect of chlormethiazole. Neuropharmacology. 1992;31:315–321. doi: 10.1016/0028-3908(92)90062-t. [DOI] [PubMed] [Google Scholar]
- GREEN A.R., HAINSWORTH A.H., JACKSON D.M. GABA potentiation: a logical pharmacological approach for the treatment of acute ischaemic stroke. Neuropharmacology. 2000;39:1483–1493. doi: 10.1016/s0028-3908(99)00233-6. [DOI] [PubMed] [Google Scholar]
- GUDELSKY G.A., NASH J.F. Carrier-mediated release of serotonin by 3,4-methylenedioxymethamphetamine: Implications for serotonin-dopamine interactions. J. Neurochem. 1996;66:243–249. doi: 10.1046/j.1471-4159.1996.66010243.x. [DOI] [PubMed] [Google Scholar]
- GUNASEKAR P.G., KANTHASAMY A.G., BOROWITZ J.L., ISOM G.E. NMDA receptor activation produces concurrent generation of nitric oxide and reactive oxygen species: implication for cell death. J. Neurochem. 1995;65:2016–2021. doi: 10.1046/j.1471-4159.1995.65052016.x. [DOI] [PubMed] [Google Scholar]
- HALLIWELL B., KAUR H. Hydroxylation of salicylate and phenylalanine as assays for hydroxyl radicals: a cautionary note visited for the third time. Free Rad. Res. 1997;27:239–244. doi: 10.3109/10715769709065762. [DOI] [PubMed] [Google Scholar]
- HUANG Z., HUANG P.L., PANAHIAN N., DALKARA T., FISHMAN M.C., MOSKOWITZ M.A. Effects of cerebral ischemia in mice deficient in neuronal nitre oxide synthase. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- HUETHER G., ZHOU D., RUTHER E. Causes and consequences of the loss of serotonergic presynapses elicited by the consumption of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy') and its congeners. J. Neural. Transm. 1997;104:771–791. doi: 10.1007/BF01285547. [DOI] [PubMed] [Google Scholar]
- IMAM S.Z., ALI S.F. Selenium, an antioxidant, attenuates methamphetamine-induced dopaminergic-neurotoxicity and peroxynitrite generation. Brain Res. 2000;855:186–191. doi: 10.1016/s0006-8993(99)02249-0. [DOI] [PubMed] [Google Scholar]
- IMAM S.Z., CROW J.P., NEWPORT G.D., ISLAM F., SLIKKER W., JR, ALI S.F. Methamphetamine generates peroxynitrite and produces dopaminergic neurotoxicity in mice: protective effects of peroxynitrite decomposition catalyst. Brain Res. 1999;837:15–21. doi: 10.1016/s0006-8993(99)01663-7. [DOI] [PubMed] [Google Scholar]
- IMAM S.Z., NEWPORT G.D., ITZHAK Y., CADET J.L., ISLAM F., SLIKKER W., JR, ALI S.F. Peroxynitrite plays a role in methamphetamine-induced dopaminergic neurotoxicity: evidence from mice lacking neuronal nitric oxide synthase gene or overexpressing copper-zinc superoxide dismutase. J. Neurochem. 2001;76:745–749. doi: 10.1046/j.1471-4159.2001.00029.x. [DOI] [PubMed] [Google Scholar]
- ITZHAK Y., ALI S.F. The neuronal nitric oxide synthase inhibitor, 7-nitroindazole, protects against methamphetamine-induced neurotoxicity in vivo. J. Neurochem. 1996;67:1770–1773. doi: 10.1046/j.1471-4159.1996.67041770.x. [DOI] [PubMed] [Google Scholar]
- ITZHAK Y., GANDIA C., HUANG P.L., ALI S.F. Resistance of neuronal oxide synthase-deficient mice to methamphetamine-induced dopaminergic neurotoxicity. J. Pharmacol. Exp. Ther. 1998;284:1040–1047. [PubMed] [Google Scholar]
- JAYANTHI S., LADENHEIM B., ANDREWS A.M., CADET J.L. Overexpression of human copper/zinc superoxide dismutase in transgenic mice attenuates oxidative stress caused by methylenedioxymethamphetamine (ecstasy) Neuroscience. 1999;91:1379–1387. doi: 10.1016/s0306-4522(98)00698-8. [DOI] [PubMed] [Google Scholar]
- JOHANSSON C., DEVENEY A.M., REIF D., JACKSON D.M. The neuronal selective nitric oxide inhibitor AR-R 17477, blocks some effects of phencyclidine, while having no observable behavioural effects when given alone. Pharmacol. Toxicol. 1999;84:226–233. doi: 10.1111/j.1600-0773.1999.tb01487.x. [DOI] [PubMed] [Google Scholar]
- KHODOROV B., PINELIS V., STOROZHEVYKH T., YURAVICHUS A., KHASPEKHOV L. Blockade of mitochondrial Ca2+ uptake by mitochondrial inhibitors amplifies the glutamate-induced calcium response in cultured cerebellar granule cells. FEBS Letts. 1999;458:162–166. doi: 10.1016/s0014-5793(99)01130-8. [DOI] [PubMed] [Google Scholar]
- KOCH S., GALLOWAY M.P. MDMA induced dopamine release in vivo: role of endogenous serotonin. J. Neural. Transm. 1997;104:135–146. doi: 10.1007/BF01273176. [DOI] [PubMed] [Google Scholar]
- KODA L.Y., GIBB J.W. Adrenal and striatal tyrosine hydroxylase activity after methamphetamine. J. Pharmacol. Exp. Ther. 1973;185:42–47. [PubMed] [Google Scholar]
- LEW R., SABOL K.E., CHOU C., VOSMER G.L., RICHARDS J.B., SEIDEN L.S. Methylenedioxymethamphetamine-induced serotonin deficits are followed by partial recovery over a 52-week period. Part II: Radioligand binding and autoradiography studies. J. Pharmacol. Exp. Ther. 1996;276:855–865. [PubMed] [Google Scholar]
- LOGAN B.J., LAVERTY R., SANDERSON W.D., YEE Y.B. Differences between rats and mice in MDMA (methylenedioxymethylamphetamine) neurotoxicity. Eur. J. Pharmacol. 1988;152:227–234. doi: 10.1016/0014-2999(88)90717-0. [DOI] [PubMed] [Google Scholar]
- LYNCH M.A., VOSS K.L. Arachidonic acid increases inositol phospholipid metabolism and glutamate release in synaptosomes prepared from hippocampal tissue. J. Neurochem. 1990;55:215–221. doi: 10.1111/j.1471-4159.1990.tb08841.x. [DOI] [PubMed] [Google Scholar]
- MALBERG J.E., SABOL K.E., SEIDEN L.S. Co-administration of MDMA with drugs that protect against MDMA neurotoxicity produces different effects on body temperature in the rat. J. Pharmacol. Exp. Ther. 1996;278:258–267. [PubMed] [Google Scholar]
- MATTHEWS R.T., YANG L., BEAL M.F. S-Methylthiocitrulline, a neuronal nitric oxide synthase inhibitor, protects against malonate and MPTP neurotoxicity. Exp. Neurol. 1997;143:282–286. doi: 10.1006/exnr.1996.6406. [DOI] [PubMed] [Google Scholar]
- MILLER D.B., O'CALLAGHAN J.P. Environment-, drug- and stress-induced alterations in body temperature affect the neurotoxicity of substituted amphetamines in the C57BL/6J mouse. J. Pharmacol. Exp. Ther. 1994;270:752–760. [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M.J., HIGGS E.A. Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol. Rev. 1991;42:109–142. [PubMed] [Google Scholar]
- NARAYAN M., BERLINER L.J., MEROLA A.J., DIAZ P.T., CLANTON T.L. Biological reactions of peroxynitrite: evidence for an alternative pathway of salicylate hydroxylation. Free Rad. Res. 1997;27:63–72. doi: 10.3109/10715769709097839. [DOI] [PubMed] [Google Scholar]
- O'CALLAGHAN J.P., MILLER D.B. Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J. Pharmacol. Exp. Ther. 1994;270:741–751. [PubMed] [Google Scholar]
- O'NEILL M.J., MURRAY T.K., MCCARTY D.R., HICKS C.A., DELL C.P., PATRICK K.E., WARD M.A., OSBORNE D.J., WIERNSKI T.R., ROMAN C.R., LODGE D., FLEISCH J.H., SINGH J.P. ARL17477, a selective nitric oxide synthase inhibitor, with neuroprotective effects in animal models of global and focal cerebral ischaemia. Brain Res. 2000;871:234–244. doi: 10.1016/s0006-8993(00)02471-9. [DOI] [PubMed] [Google Scholar]
- O'SHEA E., ESTEBAN B., CAMARERO J., GREEN A.R., COLADO M.I. Effect of GBR 12909 and fluoxetine on the acute and long term changes induced by MDMA (‘ecstasy') on the 5-HT and dopamine concentrations in mouse brain. Neuropharmacology. 2001;40:65–74. doi: 10.1016/s0028-3908(00)00106-4. [DOI] [PubMed] [Google Scholar]
- SABOL K.E., LEW R., RICHARDS J.B., VOSMER G.L., SEIDEN L.S. Methylenedioxymethamphetamine-induced serotonin deficits are followed by partial recovery over a 52 week period: Part 1: synaptosomal uptake and tissue concentrations. J. Pharmacol. Exp. Ther. 1996;276:846–854. [PubMed] [Google Scholar]
- SABOL K.E., SEIDEN L.S. Reserpine attenuates D-amphetamine and MDMA-induced transmitter release in vivo: a consideration of dose, core temperature and dopamine synthesis. Brain Res. 1998;806:69–78. doi: 10.1016/s0006-8993(98)00720-3. [DOI] [PubMed] [Google Scholar]
- SCHMIDT C.J. Neurotoxicity of the psychedelic amphetamine, methylenedioxymethamphetamine. J. Pharmacol. Exp. Ther. 1987;240:1–7. [PubMed] [Google Scholar]
- SCHMIDT C.J., KEHNE J.H. Neurotoxicity of MDMA: neurochemical effects. Ann. N.Y. Acad. Sci. 1990;600:665–680. doi: 10.1111/j.1749-6632.1990.tb16917.x. [DOI] [PubMed] [Google Scholar]
- SCHMIDT C.J., LEVIN J.A., LOVENBERG W. in vitro and in vivo neurochemical effects of methylenedioxymethamphetamine on striatal monoaminergic systems in the rat brain. Biochem. Pharmacol. 1987;36:747–755. doi: 10.1016/0006-2952(87)90729-5. [DOI] [PubMed] [Google Scholar]
- SONSALLA P.K., NICKLAS W.J., HEIKKILA R.E. Role for excitatory amino acids in methamphetamine-induced nigrostriatal dopaminergic toxicity. Science. 1989;243:398–400. doi: 10.1126/science.2563176. [DOI] [PubMed] [Google Scholar]
- SPRAGUE J.E., NICHOLS D.E. The monoamine oxidase-B inhibitor L-deprenyl protects against 3,4-methylenedioxymethamphetamine-induced lipid peroxidation and long-term serotonergic deficits. J. Pharmacol. Exp. Ther. 1995;273:667–673. [PubMed] [Google Scholar]
- STEELE T.D., MCCANN U.D., RICAURTE G.A. 3,4-Methylenedioxymethamphetamine (MDMA, ‘ecstasy'): pharmacology and toxicology in animals and humans. Addiction. 1994;89:539–551. doi: 10.1111/j.1360-0443.1994.tb03330.x. [DOI] [PubMed] [Google Scholar]
- STONE D.M., STAHL D.C., HANSON G.R., GIBB J.W. The effects of 3, 4-methylenedioxymethamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA) on monoaminergic systems in the rat brain. Eur. J. Pharmacol. 1986;128:41–48. doi: 10.1016/0014-2999(86)90555-8. [DOI] [PubMed] [Google Scholar]
- STONE D.M., HANSON G.R., GIBB J.W. Differences in the central serotonergic effects of methylenedioxymethamphetamine (MDMA) in mice and rats. Neuropharmacology. 1987;26:1657–1661. doi: 10.1016/0028-3908(87)90017-7. [DOI] [PubMed] [Google Scholar]
- STUEHR D.J., GRIFFITH O.W. Mammalian nitric oxide synthases. Adv. Enzymol. Relat. Areas Mol. Biol. 1992;65:287–346. doi: 10.1002/9780470123119.ch8. [DOI] [PubMed] [Google Scholar]
- TARASKA T., FINNEGAN K.T. Nitric oxide and the neurotoxic effects of methamphetamine and 3,4-methylenedioxymethamphetamine. J. Pharmacol. Exp. Ther. 1997;280:941–947. [PubMed] [Google Scholar]
- WHITE S.R., OBRADOVIC T., IMEL K.M., WHEATON M.J. The effects of methylenedioxymethamphetamine (MDMA, ‘ecstasy') on monoaminergic neurotransmission in the central nervous system. Progr. Neurobiol. 1996;49:455–479. doi: 10.1016/0301-0082(96)00027-5. [DOI] [PubMed] [Google Scholar]
- YEH S.Y. N-tert-butyl-alpha-phenylnitrone protects against 3,4-methylenedioxymethamphetamine-induced depletion of serotonin in rats. Synapse. 1999;31:169–177. doi: 10.1002/(SICI)1098-2396(19990301)31:3<169::AID-SYN1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- ZHANG Y., MARCILLAT O., GIULIVI C., ERNSTER L., DAVIES K. The oxidative inactivation of mitochondrial electron transport chain components and ATPase. J. Biol. Chem. 1990;265:16330–16335. [PubMed] [Google Scholar]
- ZHENG Y., LAVERTY R. Role of brain nitric oxide in (±)-3,4-methylenedioxymethamphetamine (MDMA)-induced neurotoxicity in rats. Brain Res. 1998;795:257–263. doi: 10.1016/s0006-8993(98)00313-8. [DOI] [PubMed] [Google Scholar]


