Abstract
Melanotan-II had been reported to cause penile erections in men with erectile dysfunction. In the present study, we investigated the mechanisms by which systemic administration of MT-II increases intracavernosal pressure in anaesthetized rabbits.
MT-II (10 μM) had no effect on electrical field stimulation-evoked relaxations of rabbit corpus cavernosal strips in vitro.
Intravenous injection of MT-II (66 and 133 μg kg−1 elicited dose-related increases in cavernosal pressure. SHU 9119 (3 μg kg−1, i.v.), a non-selective antagonist of MC3 and MC4 receptors did not significantly affect either cavernosal pressure or systemic blood pressure but abolished the MT-II-induced increases in cavernosal pressure. SHU 9119 also inhibited the depressor response produced by MT-II.
Intracavernosal injection 100 μl of the cocktail containing phentolamine mesylate (1 mg ml−1), papaverine (20 mg ml−1) and PGE1 (20 μg ml−1) increased the cavernosal pressure by about 4 fold.
The role of NO-cyclic GMP dependent pathway to MT-II-induced increases in cavernosal pressure was investigated by bilateral transection of the pudendal nerves and by inhibition of NO synthase with L-NAME (20 mg kg−1, i.v. over 30 min). Ablation of the pudendal nerves or pretreatment with L-NAME abolished the MT-II-induced increases in intracavernosal pressure in anaesthetized rabbits.
The data suggest that activation of central melanocortin receptors by MT-II increases cavernosal pressure by the neuronal release of NO.
Keywords: MT-II, SHU 9119, cavernosal pressure, pudendal nerves, L-NAME, NO
Introduction
The melanocortins, α-MSH, β-MSH, γ-MSH and ACTH exert diverse physiological effects that are mediated by both central and peripheral mechanisms. Melanocortins have been shown to affect skin pigmentation, learning and memory, grooming behaviour, food intake, inflammation, pyretic control, pain perception and blood pressure (O'donahue & Dorsa, 1982; Boston, 2000). Central administration of melanocortins in rodents has also been reported to cause stretching, yawning and penile erection (Argiolas et al., 2000). Earlier, it was shown that subcutaneous administration of MT-II, (Ac-Nle-c[Asp-His-D-phe-Arg-Trp-Lys]-NH2), a potent synthetic cyclic heptapeptide, containing amino acids 4 – 10 of α-MSH and ACTH that represent the core melanocortin receptor binding region (Al-Obeidi et al., 1989; 1989a), was reported to cause clinically apparent erections in men with psychogenic erectile dysfunction (Wessells et al., 1998; 2000). However the mechanism(s) by which MT-II elicits erections in experimental models of erectile function are not fully elucidated.
Five subtypes of melanocortin receptors MC1, MC2, MC3, MC4 and MC5 have been cloned and characterized (Mountjoy et al., 1992; Gantz et al., 1993; Roselli-Rehfuss et al., 1993; Chhajlani & Wikberg, 1992; Adan & Gispen, 1997; Adan et al., 1999). The MC1 receptor is the α-MSH receptor which is present in melanocytes and stimulates skin pigmentation. The MC2 receptor is the ACTH receptor that is present in the adrenal glands and controls adrenal steroid production. The MC3 and MC4 receptors are distributed mostly in the hypothalamus and are known to regulate energy homeostasis (Gantz et al., 1993; Lindblom et al., 1998), while the MC5 receptor has a wide peripheral distribution and controls exocrine gland secretion (Chen et al., 1997).
The purpose of the present investigation was to investigate the mechanism(s) by which MT-II increases intracavernosal pressure in anaesthetized rabbits. We first sought to study the effects of MT-II on electrical field stimulation elicited relaxation of rabbit corpus cavernosum in vitro. This in vitro preparation is widely used to assess the role of nonadrenergic noncholinergic neurons in the relaxation of corpus cavernosum (Ignarro et al., 1990; Rajfer et al., 1992). The effects of SHU 9119 (Ac-Nle4-c[Asp5, D-Nal(2)7,Lys10] α-MSH-(4-10)NH2) (Hruby et al., 1995), a non-specific antagonist of brain MC3 and MC4 receptors and the contribution of the NO-cyclic GMP dependent pathway to MT-II-induced increases in cavernosal pressure was also investigated.
Methods
Guidelines
Animal experimentation in this study was conducted in accordance with the NIH guidelines on the care and use of laboratory animals and the Animal Welfare Act in an AAALAC accredited programme.
In vitro studies
The method was described in detail previously (Ignarro et al., 1990; Rajfer et al., 1992; Vemulapalli & Kurowski, 2001). Briefly, male New Zealand white rabbits (3 – 3.5 kg) were sedated with an intramuscular injection of a mixture of ketamine (60 mg kg−1) and xylazine (8 mg kg−1) and were anaesthetized with sodium pentobarbital (15.0 mg kg−1, i.v.) through the marginal ear vein. The rabbit penises were removed en bloc and the corpus cavernosum was dissected free from the surrounding tunica albuginea. One end of the corpus cavernosum was tied to the electrode and the strips were immersed in 25 ml organ bath chambers containing physiological salt solution with the following composition in (mM): NaCl 118, NaHCO3 25, KCl 4.7, KH2PO4 1.2, MgSO4 1.2, glucose 11, CaCl2 21.5. The physiological salt solution was maintained at 37°C and continuously aerated with 95% O2 and 5% CO2. The other end of the corpus cavernosum was tied to the force displacement transducer (FT03, Grass Instruments, Quincy, MA, U.S.A.) connected to a Grass polygraph. Optimal length – tension curves were obtained by gradual stretching and contracting the tissues with phenylephrine (10 μM). The tissues were considered to have reached optimal isometric tension if two consecutive contractions remained within 10% of each other. The tissues were then equilibrated in physiological salt solution containing indomethacin (5 μM), atropine (1 μM) and guanethidine (5 μM). An hour after the addition of inhibitors, the tissues were subjected to electrical field stimulation (EFS). EFS was achieved with the help of two parallel platinum electrodes on either side of the strips that were connected to a current amplifier and Grass S 48 stimulator. Each tissue was stimulated at 10 V, 0.5 msec pulse duration, for 10 sec at frequencies ranging from 0.5 – 16 Hz. Two control stimulations were performed in tissues contracted submaximally with phenylephrine (3 μM).
After obtaining the baseline electrical field stimulation frequency responses as outlined above, the tissues were treated with vehicle or MT-II (10 μM) and incubated for 30 min. At the end of 30 min, the tissues were contracted with phenylephrine (3 μM). After the contractile responses to phenylephrine were stabilized, the tissues were subjected to electrical field stimulations.
In vivo studies
The rabbits were sedated with a mixture of ketamine and xylazine as mentioned above. The rabbits were placed in supine position and the body temperature was maintained at 37°C with a heating pad. The left femoral artery and vein were cannulated with PE 50 tubing to record blood pressure and to administer drugs, respectively. Blood pressure was recorded by connecting the arterial catheter to a Statham pressure transducer (P23xl) and recorded on a Grass polygraph. The skin over the penis was excised and the corpus cavernosum was exposed at the root of the penis. A 25 gauge winged infusion set (needle) was inserted in the corpus cavernosum to record cavernosal pressure. Another 26 gauge needle connected to silastic tubing (Cat.NO 602-105; Dow Corning) was also inserted in the cavernosam to inject 100 μl of the cocktail containing phentolamine mesylate (1 mg ml−1), papaverine (20 mg ml−1) and PGE1 (20 μg ml−1) directly into the cavernosum to verify the position of the needle in the cavernosum. This treatment consistently increased the cavernosal pressure in all rabbits. After the cavernosal pressure returned to the basal values, the rabbits were challenged with MT-II (66 and 133 μg kg−1, i.v.) and the changes in cavernosal pressure and blood pressure were recorded.
In a second group of anaesthetized rabbits, the effects of pretreatment with SHU 9119 (a non specific melanocortin receptor antagonist) on MT-II-elicited increases in cavernosal pressure and blood pressure were also investigated. The rabbits were surgically instrumented with arterial, venous and cavernosal catheters as described above. After an equilibration period of 30 min, the rabbits were administered MT-II (133 μg kg−1). After the cavernosal pressure returned to control values, the rabbits were treated with SHU 9119 (3 μg kg−1, i.v.). Ten minutes later, the rabbits were challenged with MT-II (133 μg kg−1, i.v.) and the changes in cavernosal pressure and blood pressure were noted.
In a third group of anaesthetized rabbits, the role of pudendal nerves in modulating the effects of MT-II on intracavernous pressure was investigated. The rabbits were instrumented with arterial, venous and cavernosal catheters as described above. The pudendal nerves were bilaterally severed and MT-II (133 μg kg−1, i.v.) was administered after the stabilization of blood pressure and cavernosal pressure.
In a fourth group of anaesthetized rabbits, the role of nitric oxide in modulating the effects of MT-II on cavernosal pressure was investigated. In these studies, the rabbits were infused with L-NAME (20 mg kg−1, i.v.) over 30 min. At the end of 30 min, the rabbits were challenged with MT-II (133 μg kg−1, i.v.) and the changes in cavernosal pressure and blood pressure were noted.
Materials
Melanotan-II and SHU 9119 were purchased from Bachem Bioscience (King of Prussia, PA, U.S.A.). L-NAME, phenylephrine, PGE1, atropine and guanethidine were purchased from Sigma Chemical Co (St. Louis, MO, U.S.A.).
Data analysis
The data are presented as means±s.e.mean of n independent observations. Relaxation (in vitro experiments) was calculated as the percentage of phenylephrine induced contraction. The data was analysed by paired t-test in the same tissues and by unpaired t-test when the responses in different tissues were compared. The data from in vivo experiments were analysed by anova. A P value of <0.05 was considered significant.
Results
Effects of MT-II on EFS-induced relaxation of rabbit corpus cavernosum
The contraction of rabbit corpus cavernosal strips with phenylephrine (3 μM) in the presence of noradrenergic, cholinergic and cyclo-oxygenase inhibitors produced a tension of 2.9 to 3.3 g. Stimulation of the nonadrenergic noncholinergic neurons of the corpus cavernosum caused a frequency-dependent relaxation of the phenylephrine contracted tissues (Figure 1). MT-II at 10 μM had no effect on electrical field stimulation-induced relaxations (Figure 1). MT-II at doses as high as 30 μM also had no affect on electrical field stimulation elicited relaxations (data not shown).
Figure 1.

MT-II does not affect electrical field stimulation-induced relaxation of rabbit corpus cavernosum in vitro. The rabbit corpus cavernosum strips were incubated with MT-II (10 μM) and 10 min later contracted with phenylephrine and electrically stimulated. See methods for details. n=5 tissues per group.
Effects of MT-II on cavernosal pressure in anaesthetized rabbits
The baseline cavernosal pressure in different groups ranged from 10.1±1.5 to 11.2±1.8 mmHg and was not significantly different between groups (P>.05). The resting blood pressure in various groups of anaesthetized rabbits averaged between 54.5±3 to 58.5.0±2 mmHg and was not statistically significant between groups. Intracavernosal administration of 100 μl of the cocktail containing papaverine (20 mg ml−1), phentolamine mesylate (1 mg ml−1) and PGE1 (20 μg ml−1) increased the cavernosal pressure by about 4 fold whereas the high dose of MT-II administered as an intravenous bolus increased the cavernosal pressure by 3.2 fold (Figure 2). The duration of the increases in cavernosal pressure after the administration of the cocktail lasted up to 43±2 min. MT-II-induced increases in cavernosal pressure lasted up to 20±2 and 41.5±2 min at the low and high doses, respectively. The increases in cavernosal pressure produced by the high dose of MT-II was accompanied by slight but significant reduction in blood pressure (Figure 3).
Figure 2.
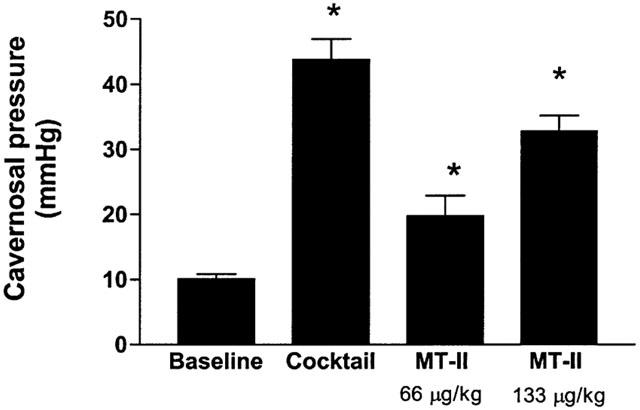
Effects of cocktail and MT-II on cavernosal pressure in anaesthetized rabbits. One hundred μls of the cocktail (see methods for composition) was injected into the cavernosum and flushed with 100 μl of saline. MT-II was administered intravenously into the femoral vein and the changes in cavernosal pressure were recorded. n=6 rabbits per group. *P<0.05 vs baseline.
Figure 3.
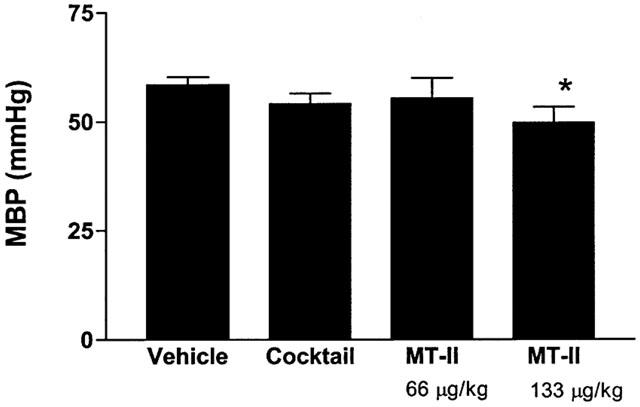
Effects of cocktail and MT-II on systemic blood pressure in anaesthetized rabbits. Refer to Figure 2 legend for details. n=6 per group. *P<0.05 vs baseline.
Effect of MT-II on cavernosal pressure in rabbits treated with SHU 9119
The effects of MT-II on cavernosal pressure in anaesthetized rabbits treated with SHU 9119 are summarized in Figure 4. The baseline cavernosal pressure in these groups of anaesthetized rabbits ranged between 10.1±1.2 and 10.3±1 mmHg. MT-II (133 μg kg−1, i.v.) increased cavernosal pressure 10.2±1.3 to 29.5±1.5 mmHg (P<0.05 vehicle). Injection of SHU 9119 (3 μg kg−1) itself did not significantly modify cavernosal pressure. However, SHU 9119 abolished the MT-II-induced (133 μg kg−1, i.v.) increases in cavernosal pressure in anaesthetized rabbits. Similarly, SHU 9119 did not affect systemic blood pressure but prevented the MT-II (133 μg kg−1, i.v.) induced depressor response (Figure 5).
Figure 4.
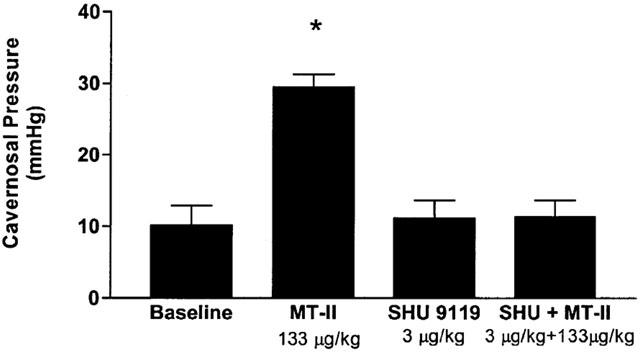
SHU 9119 abolished the MT-II induced increases in cavernosal pressure in anaesthetized rabbits. The rabbits were first injected with MT-II (133 μg kg−1, i.v.). After the cavernosal pressure returned to basal values, the rabbits were administered SHU 9119 3 μg kg−1 i.v. and 10 min later challenged with MT-II (133 μg kg−1, i.v.) and changes in cavernous pressure were recorded. n=5 per group. *P<0.05 vs baseline.
Figure 5.
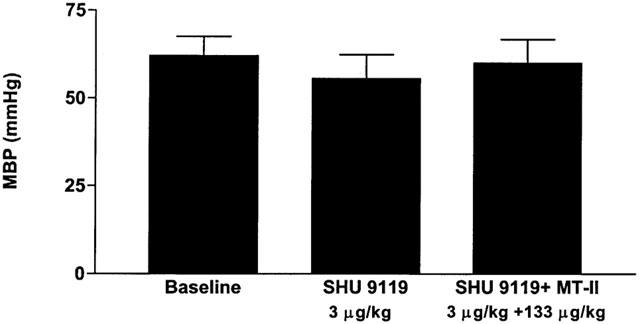
Effects of MT-II on systemic blood pressure in rabbits treated with SHU 9119 (3 μg kg−1, i.v.). Refer to Figure 4 legend for details. n=5 per group.
Effect of bilateral transection of pudendal nerves on MT-II-induced increases in cavernosal pressure in anaesthetized rabbits
The effect of bilateral transection of pudendal nerves on MT-II elicited increases in cavernosal pressure was investigated in anaesthetized rabbits. Bilateral transection of pudendal nerves did not affect either the resting blood pressure (60±4 mmHg vs 60±4 mmHg after bilateral transection of pudendal nerves) or cavernosal pressure (10±1 mmHg vs 10±1 mmHg after bilateral transection of pudendal nerves). Intravenous injection of MT-II (133 μg kg−1) to these rabbits failed to increase cavernosal pressure suggesting MT-II-induced increases in cavernosal pressure is dependent upon intact pudendal nerve supply to the corpus cavernosum.
Effects of MT-II on cavernosal pressure in L-NAME treated anaesthetized rabbits
The role of the NO-cyclic GMP dependent pathway in MT-II-induced increases in cavernosal pressure was investigated in anaesthetized rabbits pretreated with L-NAME (20 mg kg−1, i.v.). Systemic administration of L-NAME increased blood pressure from 58±5 to 80±6 mmHg (P<0.05) without affecting cavernosal pressure (12.8±3 mmHg (baseline) vs 15.0±3 mmHg in L-NAME treated rabbits). MT-II (133 μg kg−1, i.v.) failed to increase cavernosal pressure in L-NAME pretreated anaesthetized rabbits. The transection of the pudental nerves or L-NAME treatment did not alter intracavernosal pressure in response to intracavernous administration of the phentolamine/papaverine/PGE cocktail (data not shown).
Discussion
Penile erection is mediated by a neurogenic and vascular process during which the vascular smooth muscles of the corpus cavernosum are relaxed and as a result the blood flow to the cavernosum is greatly increased. The increased blood flow to the cavernosum increases the cavernosal pressure which facilitates an erection (Andersson & Wagner, 1995). The results of the present study suggest that MT-II increases cavernosal pressure in anaesthetized rabbits. Inhibition of melanocortin MC4 and MC3 receptors with SHU 9119 or inhibition of NO synthase with L-NAME or bilateral ablation of pudendal nerves abolished the MT-II-induced increases in intracavernosal pressure in anaesthetized rabbits. These results suggest that activation of brain melanocortin receptors by MT-II increases cavernosal pressure by the neuronal release of NO.
Systemic administration of MT-II increased cavernosal pressure in anaesthetized rabbits, an effect that was abolished by SHU 9119 (3 μg kg−1, i.v.) a non-selective antagonist of melanocortin receptors. MT-II could increase cavernosal pressure by a direct action in the corpus cavernosum or indirectly via a centrally mediated mechanism. Electrical field stimulation of the nonadrenergic noncholinergic neurons of the rabbit penile smooth muscle relaxes rabbit corpus cavernosum by the neuronal release of NO (Ignarro et al., 1990; Burnett et al., 1992; Rajfer et al., 1992). Sildenafil, a potent cyclic GMP-specific type 5 phosphodiesterase inhibitor augments electrical field stimulation-induced relaxation of the rabbit penile smooth muscle by preventing the inactivation of cyclic GMP and promoting its accumulation in the corpus cavernosum (Jeremy et al., 1997). In the present study, MT-II failed to affect electrically evoked relaxation of rabbit corpus cavernosum in vitro. These data argue against a direct action of MT-II in the corpus cavernosum.
MT-II is a potent agonist of MC1, MC3, MC4 and MC5 receptors while SHU 9119, a cyclic lactam analogue of α-MSH is a potent antagonist of MC4 and MC3 receptors (Hruby et al., 1995; Fan et al., 1997) and an agonist for MC1 and MC5 receptors. In our study, prior injection of SHU 9119 abolished MT-II-elicited increases in cavernosal pressure suggesting activation of central MC3 and MC4 melanocortin receptors by MT-II contributes to the increases in cavernosal pressure. In line with this contention, the hypothalamus contains high concentrations of MC3 and MC4 receptors and SHU 9119 inhibits MT-II-elicited increases in cavernosal pressure by inhibiting these receptors in the CNS. In contrast, HS014 another melanocortin receptor antagonist was recently reported to block the α-MSH-induced penile erections in some (Argiolas et al., 2000) but not in other studies (Vergoni et al., 1998). The reasons for the discrepancy are not known. HS014 is a cyclic MSH analogue that is a potent and selective antagonist at MC4 receptors and a partial agonist at MC1 and MC5 receptors. HS104 selectivity for the MC4 receptors is 34, 17 and 220 fold higher than for the MC1, MC3 and MC5 receptors, respectively (Schioth et al., 1998). These data may argue that central MC4 receptor activation is responsible for the pro-erectile effects of MT-II. It can be argued that intravenous administration of MT-II could increase cavernosal pressure in rabbits via a direct effect by inhibiting MC receptors in the urogenital tract. However, to our knowledge, the localization of MC receptors in the cavernosal tissue has not been reported. Therefore, it remains to be investigated whether intravenous injection of MT-II increases cavernosal pressure by inhibiting MC receptors in the erectile tissue.
Central administration of melanocortin peptides such as α-MSH have been shown to induce erection in rats (Argiolas & Melis, 1995). Therefore, the notion that the effects of MT-II are centrally mediated is supported by the finding that ablation of the efferent nerves (pudendal nerves) to the corpus cavernosum prevented the MT-II-induced increases in cavernosal pressure. The observations that NO synthase inhibition with L-NAME abolished the effects of MT-II suggested that the MT-II action is dependent upon the neuronal release of NO most likely at the central sites. In support of this argument, NO in the central nervous system has been implicated to cause penile erections (Argiolas et al., 2000). Recently, Andersson et al. (1999) demonstrated that subcutaneous administration of apomorphine increased cavernosal pressure in rats that was abolished by bilateral cavernous nerve transection or pretreatment with L-NAME. These data clearly suggest that central NO plays an important role in modulating penile erections. In addition, the profile of side effects such as nausea and vomiting observed after the subcutaneous administration of MT-II to humans also suggest a central mechanism of action (Wessells et al., 1998).
Melanocortins had been reported to exert profound cardiovascular effects in rodents (Versteeg et al., 1998). In our study, MT-II-induced increases in cavernosal pressure was accompanied by slight but significant reductions in systemic blood pressure especially at the high dose. Pretreatment with SHU 9119 abolished the depressor effects of MT-II suggesting that endogenous melanocortins may play a role in regulating the cardiovascular system. These results confirm and extend the published results that central and peripheral administration of α-MSH reduced blood pressure in anaesthetized rats, an effect that was abolished by SHU 9119 (Li et al., 1996; Adan & Gispen, 1997). As mentioned above, because of the limited selectivity of the SHU 9119 for MC4 and MC3 receptors, we cannot conclude which one of melanocortin receptors mediates the depressor effects of MT-II.
The advent of sildenafil had significantly changed the treatment of erectile dysfunction. However, the interaction of sildenafil with organic nitrates causes marked hypotension (Webb et al., 1999) and is therefore contraindicated in patients taking nitrates. Therefore, there is a clinical need for newer agents to treat erectile dysfunction that are devoid of side effects associated with PDE5 inhibitors. MT-II had been reported to cause erections in men with erectile dysfunction (Wessells et al., 2000). Our results that intravenous administration of MT-II increases cavernosal pressure in rabbits supports the contention that MT-II may be used to treat erectile dysfunction.
In conclusion our results suggest that intravenous injection of MT-II increases cavernosal pressure in anaesthetized rabbits, an effect that was abolished by pretreatment with SHU 9119. Inhibition of NO synthase with L-NAME or bilateral transection of the pudendal nerve supply to the cavernosum also abolished the MT-II elicited increases in cavernosal pressure. The data suggest that activation of brain MC3 and/or MC4 receptors by MT-II increases cavernosal pressure by the neuronal release of NO.
Abbreviations
- ACTH
adrenocorticotropin
- MSH
melanocyte-stimulating hormone
- MT-II
melanocortin agonist melanotan-II
- NO
nitric oxide
- L-NAME
NG-nitro-L-arginine
References
- ADAN R.A.H., GISPEN W.H. Brain melanocortin receptors: From cloning to function. Peptides. 1997;18:1279–1287. doi: 10.1016/s0196-9781(97)00078-8. [DOI] [PubMed] [Google Scholar]
- ADAN R.A.H., SZKLARCZYK A.W., OOSTEROM J., BRAKKEE J.H., NIJENHUIS W.A.J., SCHAAPER W.M.M., MELOEN J.H., GISPEN W.H. Characterization of melanocortin receptor ligands on cloned brain melanocortin receptors and on grooming behavior in the rat. Eur. J. Pharmacol. 1999;378:249–258. doi: 10.1016/s0014-2999(99)00465-3. [DOI] [PubMed] [Google Scholar]
- AL-OBEIDI F., DE LAURO CASTRUCCI A.M., HADLEY M.E., HRUBY V.J. Potent and prolonged acting cyclic lactam analogues of α-melanotropin: Design based on molecular dynamics. J Med. Chem. 1989;32:2555–2561. doi: 10.1021/jm00132a010. [DOI] [PubMed] [Google Scholar]
- AL-OBEIDI F., HADLEY A.M., PETTITT B.M., HRUBY V.J. Design of a new class of super potent cyclic α-melanotropins based on quenched dynamic simulations. J. Amer. Chem. Soc. 1989a;111:3413–3416. [Google Scholar]
- ANDERSSON K.E., GEMALMAZ H., WALDECK K., CHAPMAN T.N., TUTTLE J.B., STEERS W.D. The effect of sildenafil on apomorphine-evoked increases in intracavernous pressure in the awake rat. J. Urol. 1999;161:1707–1712. [PubMed] [Google Scholar]
- ANDERSSON K.E., WAGNER G. Physiology of penile erection. Physiological Rev. 1995;75:191–236. doi: 10.1152/physrev.1995.75.1.191. [DOI] [PubMed] [Google Scholar]
- ARGIOLAS A., MELIS M.R.Neuromodulation of penile erection 199547Pergamon Press, UK.235–255.In progress in neurobiology edited by Kerkut GA and Phillis JW [PubMed] [Google Scholar]
- ARGIOLAS A., MELIS M.R., MURGIA S., SCHIOT H.B. ACTH- and alpha-MSH-induced grooming, stretching, yawning and penile erection in male rats: Sites of action in the brain and role of melanocortin receptors. Brain Res. Bull. 2000;51:425–431. doi: 10.1016/s0361-9230(99)00270-1. [DOI] [PubMed] [Google Scholar]
- BOSTON B.A.Peripheral effects of melanocortin receptors 2000Humana Press, Totowa, NJ (USA)45–55.In melanocortin receptors edited by Cone, RDpages [Google Scholar]
- BURNETT A.L., LOWENSTEIN C.J., BREDT D.S., CHANG T.S.K., SNYDER S.H. Nitric Oxide: A physiologic mediator of penile erection. Science. 1992;257:401–403. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- CHEN W., KELLY W.A., OPITZ-ARAYA X., THOMAS R.E., LOW J., CONE R.D. Exocrine gland dysfunction in MC5-R deficient mice: Evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell. 1997;91:789–798. doi: 10.1016/s0092-8674(00)80467-5. [DOI] [PubMed] [Google Scholar]
- CHHAJLANI V., WIKBERG J.E.S. Molecular cloning and expression of the human melanocyte stimulating hormone receptor cDNA. FEBS Lett. 1992;309:417–420. doi: 10.1016/0014-5793(92)80820-7. [DOI] [PubMed] [Google Scholar]
- FAN W., BOSTON B.A., KESTERSON R.A., HRUBY V.J., CONE R.D. The role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- GANTZ I., MIWA H., KONDA Y., SHIMOTO Y., TASHIRO T., WATSON S.J., DELVALLE J., YAMADA T. Molecular cloning expression and gene localization of a fourth melanocortin receptor. J. Biol. Chem. 1993;268:15174–15179. [PubMed] [Google Scholar]
- HRUBY V.J., LU D., SHARMA S.D., CASTRUCCI A.L., KESTERSON R.A., AL-OBEIDI F.A., HADLEY M.E., CONE R.D. Cyclic lactam α-melanotropin analogues of Ac-Nle4-cyclo [Asp5, D-Phe7, Lys10] α-melanocyte-stimulating-(4-10)-NH2 with bulky aromatic amino acids at position 7 show antagonist potency and selectivity at specific melanocortin receptors. J. Med. Chem. 1995;38:3454–3461. doi: 10.1021/jm00018a005. [DOI] [PubMed] [Google Scholar]
- IGNARRO L.J., BUSH P.A., BUGA G.M., WOOD K.S., FUKUTO J.M., RAJFER J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem. Biophys. Res. Commun. 1990;170:843–850. doi: 10.1016/0006-291x(90)92168-y. [DOI] [PubMed] [Google Scholar]
- JEREMY J., BALLARD S.A., NAYLOR A.M., MILLER M.A.W., ANGELINI G.D. Effects of sildenafil, a type-5 cGMP phosphodiesterase inhibitor, and papaverine on cyclic GMP and cyclic AMP levels in the rabbit corpus cavernosum in vitro. Br. J. Urol. 1997;79:958–963. doi: 10.1046/j.1464-410x.1997.00206.x. [DOI] [PubMed] [Google Scholar]
- LINDBLOM J., SCHIOTH H.B., LARSSON A., WIKBERG J.E., BERGSTROM L. Auto-radiographic discrimination of melanocortin receptors indicate that the MC3 subtype dominates in the medial rat brain. Brain Res. 1998;810:161–171. doi: 10.1016/s0006-8993(98)00918-4. [DOI] [PubMed] [Google Scholar]
- LI SI-JAI, , VARGA K., ARCHER P., HRUBY V.J., SHARMA S.D., KESTERSON R.A., CONE R.D., KUNOS G. Melanocortin antagonists define two distinct pathways of cardiovascular control by alpha- and gamma-melanocyte-stimulating hormones. J. Neuroscience. 1996;16:5182–5186. doi: 10.1523/JNEUROSCI.16-16-05182.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOUNTJOY K.G., ROBINS L.S., MORTRUD M.T., CONE R.D. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- O'DONAHUE T.L., DORSA D.M. The opiomelanotropinergic neuronal and endocrine systems. Peptides. 1982;3:353–395. doi: 10.1016/0196-9781(82)90098-5. [DOI] [PubMed] [Google Scholar]
- RAJFER J., ARONSON W.J., BUSH P.A., DOREY F.J., IGNARRO L.J. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N. Eng. J. Med. 1992;326:90–94. doi: 10.1056/NEJM199201093260203. [DOI] [PubMed] [Google Scholar]
- ROSELLI-REHFUSS L., MOUNTJOY K.G., ROBBINS L.S., MORTRUDT M.T., LOW M.J., TATRO J.B., ENTWISTLE M.L., SIMERLY R.B., CONE R.D. Identification of a receptor for gamma-MSH and other proopiomelanocortin peptides in the hypothalamus and limbic systems. Proc. Natl. Acad. Sci. USA. 1993;90:8856–8860. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIOTH H.B., MUTULIS F., MUCENIECE R., PRUSIS P., WIKBERG J.E.S. Discovery of novel melanocortin 4 receptor selective MSH analogues. Br. J. Pharmacol. 1998;124:75–82. doi: 10.1038/sj.bjp.0701804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VEMULAPALLI S., KUROWSKI S. Phentolamine mesylate relaxes rabbit corpus cavernosum by a nonadrenergic nonchlinergic mechanism. Fundamental & Clinical Pharmacology. 2001;15:1–7. doi: 10.1046/j.1472-8206.2001.00010.x. [DOI] [PubMed] [Google Scholar]
- VERGONI A.V., BERTOLINI A., MUTULIS F., WIKBERG J.E.S., SCHIOTH H.B. Differential influence of a selective melanocortin MC4 receptor antagonist (HS014) on melanocortin-induced behavioral effects in rats. Eur. J. Pharmacol. 1998;362:95–101. doi: 10.1016/s0014-2999(98)00753-5. [DOI] [PubMed] [Google Scholar]
- VERSTEEG D.H.G., VAN BERGEN P., ADAN R.A.H., DE WILDT D.J. Melanocortins and cardiovascular regulation. Eur. J. Pharmacol. 1998;360:1–14. doi: 10.1016/s0014-2999(98)00615-3. [DOI] [PubMed] [Google Scholar]
- WEBB D.J., FREESTONE S., ALLEN M., MUIRHEAD G. Sildenafil citrate and blood-pressure-lowering drugs: Results of drug interaction studies with organic nitrate and a calcium antagonist. Am. J. Cardiol. 1999;83:21C–28C. doi: 10.1016/s0002-9149(99)00044-2. [DOI] [PubMed] [Google Scholar]
- WESSELLS H., FUCIARELLI K., HANSEN J., HADLEY M.E., HRUBY V.J., DORR R., LEVINE N. Synthetic melanotropic peptide initiates erections in men with psycogenic erectile dysfunction: Double-blind, placebo controlled crossover study. J. Urol. 1998;160:389–393. [PubMed] [Google Scholar]
- WESSELLS H., LEVINE N., HADLEY M.E., DORR R., HRUBY V. Melanocortin receptor antagonists, penile erection, and sexual motivation: human studies with melanotan II. Int. J. Impot. Res. 2000;12 suppl. 4:S74–S79. doi: 10.1038/sj.ijir.3900582. [DOI] [PubMed] [Google Scholar]


