Abstract
To further explore the effect of antioxidants in preventing diabetes-induced vascular and neural dysfunction we treated streptozotocin-induced diabetic rats daily with subcutaneous injections of 10 mg kg−1 of M40403 (n=11) and compared the results obtained from 17 control rats and 14 untreated diabetic rats. M40403 is a manganese(II) complex with a bis(cyclo-hexylpyridine)-substituted macrocyclic ligand that was designed to be a selective functional mimetic of superoxide dismutase. Thus, M40403 provides a useful tool to evaluate the roles of superoxide in disease states.
Treatment with M40403 significantly improved diabetes-induced decrease in endoneurial blood flow, acetylcholine-mediated vascular relaxation in arterioles that provide circulation to the region of the sciatic nerve, and motor nerve conduction velocity (P<0.05). M40403 treatment also reduced the appearance of superoxide in the aorta and epineurial vessels and peroxynitrite in epineurial vessels. Treating diabetic rats with M40403 reduced the diabetes-induced increase in thiobarbituric acid reactive substances in serum but did not prevent the decrease in lens glutathione level. Treating diabetic rats with M40403 did not improve sciatic nerve Na+/K+ ATPase activity or the sorbitol, fructose or myo-inositol content of the sciatic nerve.
These studies provide additional evidence that diabetes-induced oxidative stress and the generation of superoxide and perhaps peroxynitrite may be partially responsible for the development of diabetic vascular and neural complications.
Keywords: Diabetes, vasodilation, diabetic neuropathy, acetylcholine, oxygen radicals, superoxide, peroxynitrite, endothelium
Introduction
Oxidative stress is an important component of diabetes and its complications (Pieper et al., 1993; Cameron & Cotter, 1995; 1999; Pieper & Siebeneich, 1997; 1998; Cameron et al., 1998; Keegan et al., 1999; Pieper, 2000; Obrosova et al., 2000; Cakatay et al., 2000; Haak et al., 2000; Andrew et al., 2000; Gocmen et al., 2000; Ishii et al., 1998; Ammar et al., 2000). Moreover, treatment of streptozotocin-induced diabetic rats with antioxidants has demonstrated that oxidative stress and vascular dysfunction may be a major factor in the development of diabetic neuropathy (Cameron et al., 1993; 1994; 1998; Karasu et al., 1995; Keegan et al., 1999; Cameron & Cotter, 1999). Previously, we have shown that acetylcholine-induced vasodilation by arterioles that provide circulation to the region of the sciatic nerve is impaired early in diabetes and is accompanied by a reduction of endoneurial blood flow (EBF) and an increase in superoxide in these vessels (Terata et al., 1999; Coppey et al., 2000). These changes preceded the slowing of motor nerve conduction velocity (MNCV) and the reduction in Na+/K+ ATPase activity in the sciatic nerve, suggesting that vascular dysfunction rather than hyperglycemia-induced metabolic abnormalities of the nerve is responsible for the nerve disorders associated with the early onset of diabetes. We have also demonstrated that treating streptozotocin-induced diabetic rats with α-lipoic acid or hydroxyethyl starch deferoxamine (HES-DFO) prevents the impairment of vascular function and accumulation of superoxide and peroxynitrite induced by diabetes in the arterioles that provide circulation to the region of the sciatic nerve as well as the reduction in endoneurial blood flow and slowing of motor nerve conduction velocity (Coppey et al., 2001).
In spite of these studies identification of the particular free radical(s) involved in diabetic complications has not been accomplished. This stems from the fact that selective antioxidants have not been available. In numerous disease states the use of native superoxide dismutase (SOD) enzymes both pre-clinically and clinically shed light on the importance of O.2− in disease and, thus, the therapeutic potential of exogenous SOD enzymes (Huber et al., 1980; Flohe, 1988; Uematsu et al., 1994). However, the native SOD enzyme has not been evaluated in animal models of diabetes. Thus, the role of superoxide in this condition is to date not defined. There are drawbacks or problematic issues associated with the use of the native enzymes as therapeutic agents (e.g., solution instability, immunogenicity of non-human enzymes, bell-shaped dose-response curves, high susceptibility to proteolytic digestion) and as pharmacological tools (e.g., they do not penetrate cells or cross the blood-brain barrier, limiting the dismutation of superoxide only to the extracellular space or compartments). To overcome the limitations associated with native enzyme therapy, we have developed a series of SOD mimetics that catalytically remove O.2−. M40403 is a prototypic example of a stable, low molecular weight, manganese-containing, non-peptidic molecule possessing the function and catalytic rate of native SOD enzymes, but with the advantage of being a much smaller molecule (MW 483 vs MW 30,000 for the mimetic and native enzyme, respectively) (Salvemini et al., 1999). An important property of these SOD mimetics is that they catalytically remove superoxide at a high rate without interacting with other biologically important reactive species including nitric oxide, peroxynitrite, hydrogen peroxide, oxygen or hydroxyl radicals (Riley et al., 1996, 1997). This property is not shared by other classes of SOD mimetics or scavengers, including several metalloporphyrins such as tetrakis-(N-ethyl-2-pyridyl) porphyrin and tetrakis-(benzoic acid)porphyrin, that interact with other reactive species such as nitric oxide and peroxynitrite that clearly play important roles in inflammation (Patel & Day, 1999). The purpose of our study was to evaluate the role of superoxide by using M40403 in the development of diabetic vascular and neural complications.
Methods
Materials
Unless stated otherwise all chemicals used in these studies were obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.). M40403 was synthesized at MetaPhore Pharmaceuticals, Inc (St. Louis, MO, U.S.A.) as described previously (Salvemini et al., 1999).
Methods
Animals
Male Sprague-Dawley (Harlan Sprague Dawley, Indianapolis, IN, U.S.A.) rats 8 – 9 weeks of age were used for these studies. The animals were housed in a certified animal care facility and food (Harlan Teklad, #7001, Madison, WI, U.S.A.) and water were provided ad libitum. All institutional and NIH guidelines for use of animals were followed. Diabetes was induced by intravenously injecting streptozotocin (60 mg kg−1 in 0.9% NaCl, adjusted to a pH 4.0 with 0.2 M sodium citrate). Control rats were injected with vehicle alone. The rats were anaesthetized with methoxyflurane before injection. Diabetes was verified 48 h later by evaluating blood glucose levels with the use of glucose-oxidase reagent strips (Lifescan Inc., Milpitas, CA, U.S.A.). Rats having blood glucose level of 300 mg dl−1 (16.7 mM) or greater were considered to be diabetic. At this time the diabetic rats were randomly divided into two groups one to receive M40403 and the other to receive vehicle. All studies were conducted approximately 3 – 4 weeks after the verification of diabetes. Rats treated with M40403 received a subcutaneous injection of 10 mg kg−1 daily. The M40403 was dissolved in sterile saline and control and non-treated diabetic rats received vehicle alone. Treatments with M40403 were started on the day hyperglycemia was verified.
Motor nerve conduction velocity
MNCV was determined as previously described using a noninvasive procedure in the sciatic-posterior tibial conducting system in a temperature controlled environment (Terata et al., 1999; Yorek et al., 1993).
Endoneurial blood flow
Immediately after determination of MNCV, sciatic nerve endoneurial nutritive blood flow was determined using the hydrogen clearance method as described by Cameron et al. (1991; 1997) and adapted by our laboratory (Terata et al., 1999; Coppey et al., 2000). The hydrogen clearance data was fitted by computer to a mono- or bi-exponential curve using commercial software (Prism, GraphPad, San Diego, CA, U.S.A.) and nutritive blood flow, (ml min−1 100 g−1), calculated using the equation described by Young (1980) and vascular conductance, (ml min−1 100 g−1 mm Hg−1) determined by dividing nutritive blood flow by the average mean arterial blood pressure.
Vascular reactivity
Videomicroscopy was used to investigate in vitro vasodilatory responsiveness of epineurial arterioles supplying the region of the sciatic nerve (branches of the superior gluteal and internal pudendal arteries) to acetylcholine (10−4 and 10−8 mol l−1) or sodium nitroprusside (10−4 mol l−1) as previously described (Terata et al., 1999; Coppey et al., 2000).
Detection of superoxide and peroxynitrite
Hydroethidine (Molecular Probes Inc., Eugene, OR, U.S.A.), an oxidative fluorescent dye, was used to evaluate in situ levels of superoxide (O2−) in epineurial vessels as described previously (Coppey et al., 2000; 2001). Hydroethidine is permeable to cells and in the presence of O2− is oxidized to fluorescent ethidium bromide, where it is trapped by intercalating with DNA. This method provides sensitive detection of O2− in situ. Superoxide levels were also measured in the aorta by lucigenin-enhanced chemiluminescence as described previously (Miller et al., 1998; Coppey et al., 2000; 2001).
One of two mechanisms by which acetylcholine mediates vascular relaxation in arterioles that provide circulation to the sciatic nerve is through the production of nitric oxide (Terata et al., 1999). The chemistry of nitric oxide is very complex, and several biochemical pathways other than nitric oxide production can influence nitric oxide action. For example, superoxide anion can interact with nitric oxide to form peroxynitrite (Wattanapitayakul et al., 2000). This reaction reduces the efficacy of nitric oxide to act as a signal transduction agent. Peroxynitrite is a highly reactive intermediate known to nitrate protein tyrosine residues and cause cellular oxidative damage (Pryor & Squadrito, 1995; Beckman, 1996). To determine whether the diabetes-induced formation of superoxide by arterioles that provide circulation to the region of the sciatic nerve promotes the formation of peroxynitrite we measured 3-nitrotyrosine, a stable biomarker of tissue peroxynitrite formation, immunoreactivity using a commercial kit from Vector Laboratories (Burlingame, CA, U.S.A.) (Coppey et al., 2001)
Sciatic nerve Na+/K+ ATPase activity and sorbitol, fructose and myo-inositol content
The left sciatic nerve was removed, desheathed, and divided into three samples for determination of Na+/K+ ATPase activity, conjugated diene levels (see below) and sorbitol, fructose and myo-inositol content as previously described (Coppey et al., 2001).
Additional biological parameters
Lens glutathione (GSH), serum TBARS and sciatic nerve conjugated diene levels were determined as additional markers of oxidative stress. Lens glutathione levels were determined according to Lou et al. (1988). Lens were weighed and homogenized in 1 ml of cold 10% trichloroacetic acid and centrifuged for 15 min at 1000×g. The supernatant (100 μl) was mixed with 0.89 ml of 1.0 M Tris, pH 8.2, and 0.02 M EDTA. Afterwards, 10 μl of dithionitrobenzene (DTNB) was added and change in absorbance measured at 412 nm. A glutathione standard curve (100 – 500 ng) was performed for each assay. The data were recorded as μg mg wet weight−1. TBARS level in serum was determined by the method of Mihara et al. (1980) as modified by Siman & Eriksson (1997). Briefly, 200 μl of serum was boiled in 0.75 ml of phosphoric acid (0.19 M), 0.25 ml thiobarbituric acid (0.42 mM) and 0.3 ml water for 60 min. Afterwards, the samples were precipitated with methanol/NaOH and centrifuged for 5 min. The supernatant was measured fluorometrically at excitation wavelength 532 nm and emission wavelength 553 nm. Standards were prepared by the acid hydrolysis of 1,1,3,3-tetraethoxypropane. The data was reported as μg ml−1 serum. Sciatic nerve conjugated diene level were determined according to the method of Recknagel & Ghoshal (1996) and Low & Nickander (1991). Briefly, a segment of the sciatic nerve was extracted with chloroform and methanol. The lipid extract was evaporated and redissolved in 1 ml cyclohexane. Conjugated diene levels were determined by measuring the absorbance at 233 nm with extraction blanks used as references. An extinction coefficient of 2.52×104 M was used to determine the amount of conjugated diene present. The data was reported as μmol mg wet weight−1. Serum free fatty acid and triglyceride levels were determined using commercial kits from Roche Diagnostics, Mannheim, Germany and Sigma Chemical Co. (St. Louis, MO, U.S.A.) respectively.
Data analysis
The results are presented as mean±s.e.mean. Comparisons between the groups for MNCV, EBF, sciatic nerve Na+/K+ ATPase activity, sciatic nerve sorbitol, fructose and myo-inositol content, serum TBARS, sciatic nerve conjugated diene, serum free fatty acid and triglyceride, aorta lactate/pyruvate ratio and lens glutathione levels were conducted using independent unpaired Student's t-tests. Dose response curves for acetylcholine-induced relaxation were compared using a two way repeated measures analysis of variance with autoregressive covariance structure using proc mixed program of SAS (Terata et al., 1999; Coppey et al., 2000). Whenever significant interactions were noted specific treatment-dose-effects were analysed using a Bonferroni adjustment. A P value of less 0.05 was considered significant. All computations were performed using SAS for Windows version 6.12.
Results
Body weight and plasma glucose levels
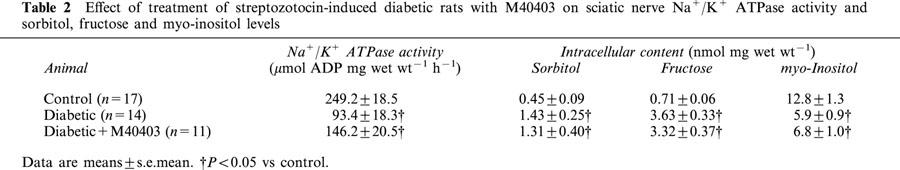
For these studies we used 17 control rats, 14 untreated diabetic rats and 11 diabetic rats treated with M40403. Data in Table 1 show that streptozotocin-induced diabetic rats treated with or without M40403 on average gained less weight than age-matched control rats over the 3 – 4 week experimental period of this study. At the time of experimentation plasma glucose levels were increased 3 – 4 fold in diabetic rats and diabetic rats treated with M40403 compared to control rats. Treating diabetic rats with M40403 had no significant effect on weight gain or blood glucose levels compared to non-treated diabetic rats.
Table 1.
Change in body weight and blood glucose levels
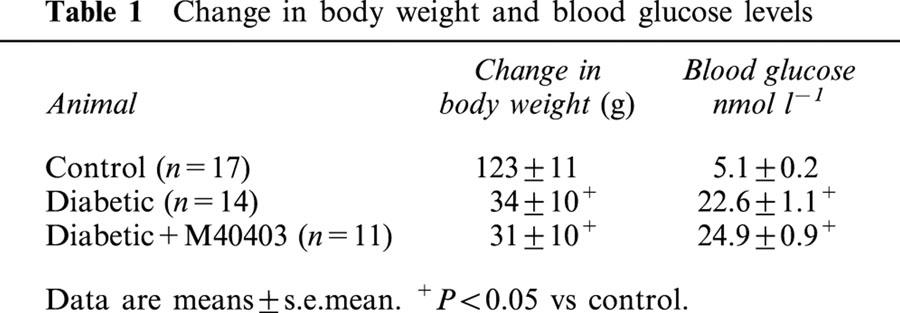
Sciatic nerve Na+/K+ ATPase activity and sorbitol, fructose and myo-inositol content
Data in Table 2 demonstrate that diabetes causes a significant decrease in sciatic nerve Na+/K+ ATPase activity. Treating diabetic rats with M40403 did not prevent the diabetes-induced decrease in Na+/K+ ATPase activity compared to control rats. Data in Table 2 also demonstrate that diabetes causes a significant increase in the sorbitol and fructose content and a decrease in myo-inositol levels in the sciatic nerve and this was not improved by treating diabetic rats with M40403.
Table 2.
Effect of treatment of streptozotocin-induced diabetic rats with M40403 on sciatic nerve Na+/K+ ATPase activity and sorbitol, fructose and myo-inositol levels
Serum triglyceride and free fatty acid levels
Data in Figure 1 demonstrate that diabetes causes a significant increase in serum triglyceride and free fatty acid levels (P<0.05). Treating diabetic rats with M40403 did not affect the diabetes-induced increase in serum free fatty acid levels. In contrast, serum triglyceride levels were significantly reduced in diabetic rats treated with M40403 compared to untreated diabetic rats (P<0.05). However, serum triglyceride levels in diabetic rats treated with M40403 remained significantly elevated compared to control rats (P<0.05).
Figure 1.
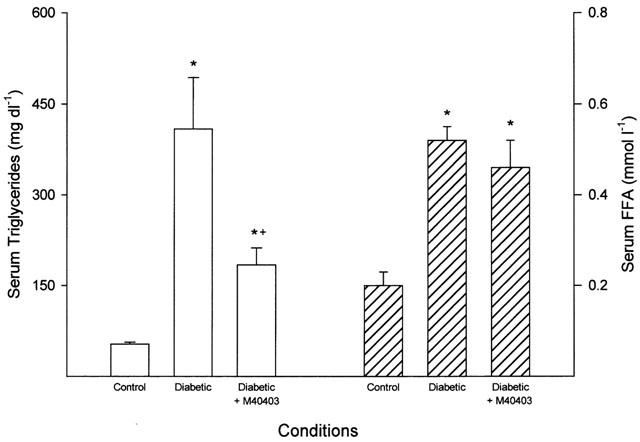
Serum triglyceride and free fatty acid levels. Data are presented as the mean±s.e.mean for 17 control rats, 14 untreated diabetic rats and 11 diabetic rats treated with M40403. The * denotes a significant difference compared to control, P<0.05. The+denotes a significant difference compared to untreated diabetic rats, P<0.05.
Evaluation of oxidative stress
Data in Figure 2 demonstrate that diabetes causes an significant increase in thiobarbituric acid reactive substances (TBARS) in serum (P<0.05). Treating diabetic rats with M40403 significantly reduced the increase in serum TBARS level caused by diabetes (P<0.05). Data in Figure 2 also demonstrate that conjugated diene level in the sciatic nerve was significantly increased (P<0.05) by diabetes and was reduced by about 40% when diabetic rats were treated with M40403. The level of conjugated dienes in the sciatic nerve of diabetic rats treated with M40403 remained significantly increased compared to control rats (P<0.05). Lens GSH level was significantly decreased in streptozotocin-induced diabetic rats compared to control rats (0.5±0.1 vs 1.5±0.1 μg mg wet wt−1, respectively, P<0.05, n=14 and 17, respectively). Treating diabetic rats with M40403 did not improve the decrease in lens GSH level induced by diabetes (0.4±0.1 μg mg wet wt−1, P<0.05 compared to control, n=11).
Figure 2.
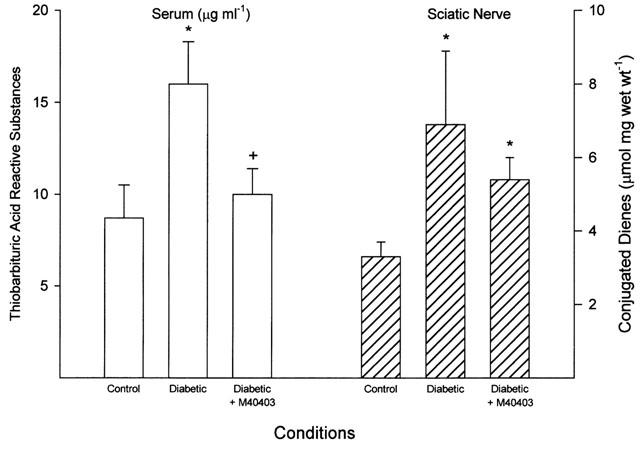
Serum thiobarbituric acid reactive substances and sciatic nerve conjugated diene level. Serum samples were collected and used to determine thiobarbituric acid reactive substances level (left side of figure). The sciatic nerve was also collected and a portion used to determine the conjugated diene level (right side of figure). Data are presented as the mean±s.e.mean for 17 control rats, 14 untreated diabetic rats and 11 diabetic rats treated with M40403. The * denotes a significant difference compared to control, P<0.05. The+denotes a significant difference compared to untreated diabetic rats, P<0.05.
Data in Figure 3 demonstrate that treating streptozotocin-induced diabetic rats with M40403 markedly decreased the diabetes-induced increase in the level of superoxide in epineurial vessels as measured by hydroethidine fluorescence compared to paired analysis of untreated diabetic rats. Similar results were obtained in two additional studies using control, untreated diabetic rats and diabetic rats treated with M40403. We also measured the superoxide level in the aorta by lucigenin-enhanced chemiluminescence. These studies demonstrated that the superoxide level is significantly increased in the aorta of diabetic rats compared to control rats (3.1±0.3 vs 2.0±0.1 mean RLU min−1 mm−1, respectively, P<0.05, n=14 and 17, respectively) and treating diabetic rats with M40403 prevented the diabetes-induced increase of superoxide (2.3±0.2 mean RLU min−1 mm−1 P<0.05 compared to untreated diabetic rat, n=11).
Figure 3.
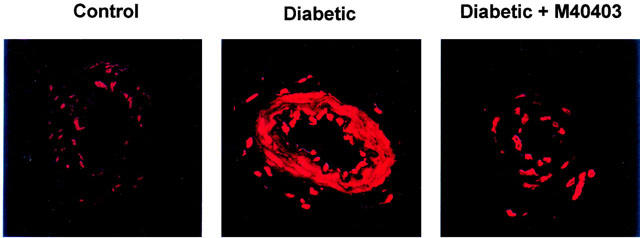
Detection of superoxide level in arterioles from control, diabetic rats and diabetic rats treated with M40403. The duration of diabetes and treatments for these studies was 3 weeks. Fluorescent photomicrographs of confocal microscopic sections of arterioles that provide circulation to the region of the sciatic nerve from the three individual groups of animals were examined on the same day. Arterioles were labeled with the oxidative dye hydroethidine. Recording of fluorescent were taken at identical laser and photomultiplier settings for both control and untreated and treated diabetic rats. Shown is a representative sample of one set of animals. This experiment was repeated three separate times on separate sets of animals on three different days with similar results.
Data in Figure 4 visually demonstrate that diabetes induces the formation of 3-nitrotyrosine (as indicated by the blue staining), a marker for peroxynitrite, in presumably endothelial cells and the adventitia of these arterioles. Treating diabetic rats with M40403 prevented the formation of 3-nitrotyrosine as indicated by the lack of staining in these arterioles.
Figure 4.
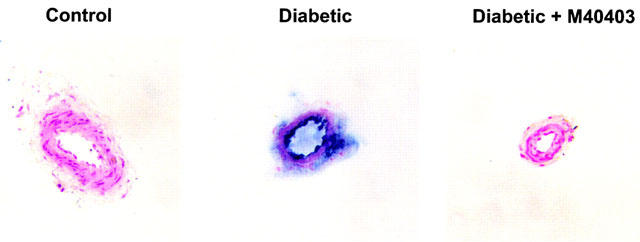
Detection of peroxynitrite in arterioles from control, diabetic rats, and diabetic rats treated with M40403. Arterioles from control, diabetic rats and diabetic rats treated with M40403 were collected and treated for determination of 3-nitrotyrosine immunostaining. Shown is a representative sample of one set of animals. This experiment was repeated three separate times with similar results.
Endoneurial blood flow and motor nerve conduction velocity
Data in Figure 5 demonstrate that treating diabetic rats with M40403 prevents the decrease in EBF compared to untreated diabetic rats. Likewise, data in Figure 6 demonstrate that treating diabetic rats with M40403 prevents the slowing in MNCV.
Figure 5.
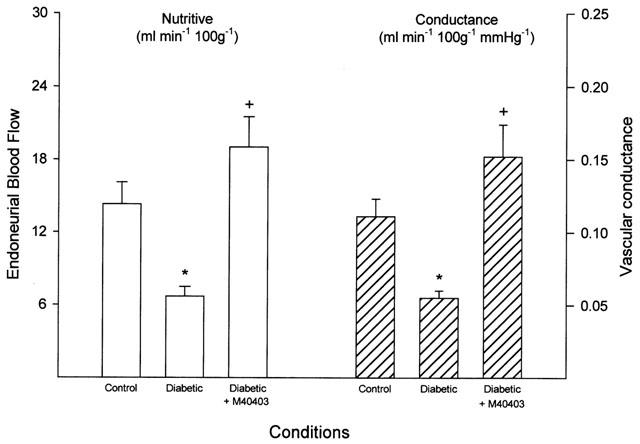
Determination of endoneurial blood flow. Endoneurial blood flow reported as nutritive flow (left) or conductance (right) was determined. Data are presented as the mean±s.e.mean for 17 control rats, 14 untreated diabetic rats and 11 diabetic rats treated with M40403. The * denotes a significant difference compared to control, P<0.05. The+denotes a significant difference compared to untreated diabetic rats, P<0.05.
Figure 6.
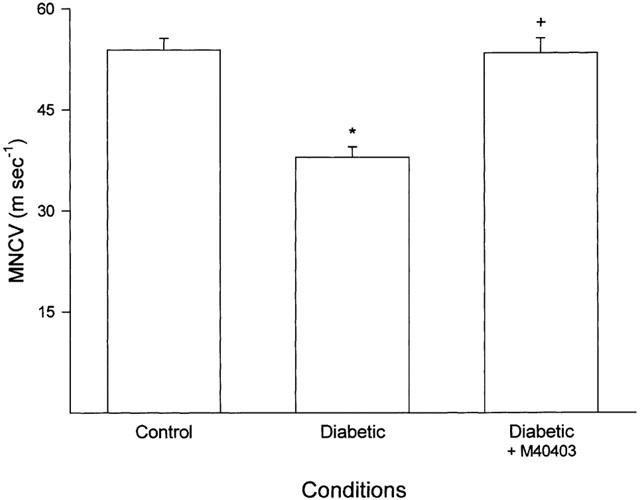
Determination of motor nerve conduction velocity. Data are presented as the mean±s.e.mean for 17 control rats, 14 untreated diabetic rats and 11 diabetic rats treated with M40403. The * denotes a significant difference compared to control, P<0.05. The+denotes a significant difference compared to untreated diabetic rats, P<0.05.
Arteriolar vascular reactivity
As demonstrated in Figure 7, diabetes causes a significant decrease (P<0.05) in acetylcholine (10−4 and 10−8 mol l−1) mediated vascular relaxation in arterioles that provide circulation to the region of the sciatic nerve. Treating diabetic rats with M40403 significantly improves the diabetes-induced impairment in acetylcholine mediated vascular relaxation (P<0.05). In contrast, maximal vasodilation induced by sodium nitroprusside (10−4 M), endothelium-independent, in these vessels was not affected by diabetes or treatment of diabetic rats with M40403 (112.8±8.5, 105.1±8.8 and 113.9±7.1 in control (n=17), untreated diabetic (n=14) and diabetic rats treated with M40403 (n=11), respectively. Baseline diameters of the vessels used in these studies was not different for control, untreated diabetic and diabetic rats treated with M40403 (129±9, 111±9 and 117±7 μm, respectively).
Figure 7.
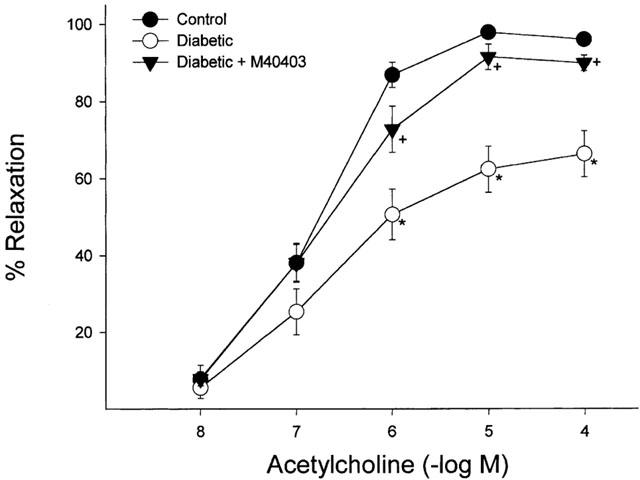
Determination of the effect of treatment with M40403 on acetylcholine-mediated vascular relaxation in arterioles that provide circulation to the region of the sciatic nerve. Pressurized arterioles (40 mm Hg) were constricted with U46619 (30 – 50%) and incremental doses of acetylcholine were added to the bathing solution while recording steady state vessel diameter. For control, diabetic and diabetic rats treated with M40403 the number of experimental observations was 17, 14 and 11, respectively. The * denotes that the response to acetylcholine was significantly attenuated in the diabetic rat, P<0.05. The+denotes that the response to acetylcholine was significantly different compared to the untreated diabetic rats, P<0.05.
Discussion
M40403 has been described to be a nonpeptidyl mimetic of SOD (Salvemini et al., 1999; Salvemini & Riley, 2000). M40403 was derived from the macrocyclic ligand, 1,4,7,10,13-pentaazacyclopentadecane, containing the added bis(cyclo-hexylpyridine) substitution pattern (Salvemini et al., 1999). M40403 selectively catalyzes the dismutation of superoxide (O2.−) with rates approaching that of the native Mn SOD (Salvemini & Riley, 2000). M40403 has a molecular weight of 484.4 and a catalytic rate >2×108 M−1 s−1, at the pH of ∼6.5 (Salvemini et al., 1999). It is thermodynamically stable and stable for up to 10 h in whole rat blood at 37°C (Salvemini et al., 1999; Salvemini & Riley, 2000). After intravenous injection into rats, M40403 distributes widely into the heart, lungs, brain, liver, and kidneys, while retaining its intact chemical identity (Salvemini et al., 1999; Salvemini & Riley, 2000). The compound is excreted intact in urine and feces with no detectable dissociation of the manganese (Salvemini & Riley, 2000). M40403 does not react with nitric oxide, hydrogen peroxide, or peroxynitrite (Salvemini & Riley, 2000). This unique selectivity of M40403 activity for superoxide in the presence of other reactive oxygen species makes it possible to dissect the role of superoxide in disease models such as diabetes in which other reactive oxygen species may be implicated.
Treating diabetic rats with M40403 we found that it inhibited the generation of superoxide by aorta and epineurial vessels, the formation of peroxynitrite by epineurial vessels, the reduction in endoneurial blood flow, the slowing of MNCV and impairment of endothelium-dependent vasodilation of arterioles that provide circulation to the sciatic nerve. It also improved the diabetes induced changes in serum TBARS and sciatic nerve conjugated diene level, two additional markers of oxidative stress. In contrast, lens glutathione level remained reduced in diabetic rats treated with M40403. Treating diabetic rats with M40403 did not improve the metabolic related defects in the sciatic nerve such as the decrease in Na+/K+ ATPase activity and the reciprocal change in sorbitol and myo-inositol levels. This result raises the question whether these metabolic changes are a primary contributor to diabetes-induced vascular and neural dysfunction.
In a previous study we found that treating diabetic rats with α-lipoic acid prevented the generation of superoxide and peroxynitrite in epineurial vessels, reduced serum TBARS and improved lens GSH levels (Coppey et al., 2001). The level of conjugated dienes in the sciatic nerve remained increased in diabetic rats treated with α-lipoic acid. Both M40403 and α-lipoic acid treatment was found to prevent the decrease in endoneurial blood flow, slowing of motor nerve conduction velocity and impairment in acetylcholine-mediated vasodilation in arterioles that provide circulation to the region of the sciatic nerve. Therefore, treatment of diabetic rats with either M40403 or α-lipoic acid seem to have a similar efficacy in preventing oxidative stress and vascular and neural dysfunction in diabetic rats. This occurs even though the antioxidant properties of these two compounds are different. M40403 is a mimetic of superoxide dismutase whereas; α-lipoic acid is a naturally occurring free radical scavenger and transition metal chelator (Salvemini et al., 1999; Keegan et al., 1999).
Our studies have shown that treating diabetic rats with M40403 or α-lipoic acid improved vascular and neural function (Coppey et al., 2001). One common feature of these treatments in diabetic rats was the prevention of the generation of superoxide/peroxynitrite in vascular tissue. This suggests that the formation of superoxide may be a main factor in diabetes-induced vascular disease. This is supported by the present studies. Unlike the non-selective antioxidant properties of α-lipoic acid, M40403 is selective in preventing superoxide formation and does not directly influence the generation of other reactive oxygen species (Salvemini et al., 1999; Salvemini & Riley, 2000). Since treatment of diabetic rats with M40403 prevented vascular and neural dysfunction we conclude that the generation of superoxide and subsequent formation of peroxynitrite are likely responsible for the diabetes-induced vascular and neural defects associated with the early stages of diabetic neuropathy. Combined, these results suggest that diabetes-induced vascular dysfunction is caused by oxidative stress via the formation of superoxide and that vascular dysfunction rather than metabolic related defects of the sciatic nerve is responsible for the early neural defects associated with diabetic neuropathy.
In diabetes the production of superoxide anion radicals plays a role in impaired endothelium-dependent relaxation (Pieper et al., 1997). We have shown that in diabetes the accumulation of superoxide and peroxynitrite in epineurial vessels accompanies the reduction in EBF and impairment of endothelium-dependent vasodilation in these vessels and precedes the slowing of MNCV (Coppey et al., 2000). In addition, Mayhan (1997) has demonstrated that treating the basilar artery from diabetic rats with SOD improves nitric oxide synthase-dependent vasodilation. As further evidence that oxidative stress is a contributing factor in the development of diabetic neuropathy, antioxidant therapy has been shown to improve neural function in diabetic animal models (Cameron et al., 1993; 1994; 1998; Karasu et al., 1995; Keegan et al., 1999; Cameron & Cotter, 1999). In diabetes, one possible explanation for reactive oxygen species mediated endothelial dysfunction comes from studies by Soriano et al. (2001). These studies have implicated the generation of reactive oxygen species with the activation of poly (ADP-ribose) polymerase in vascular tissue from diabetic mice and endothelial cells exposed to increased concentration of glucose. In the endothelium, the activation of poly (ADP-ribose) polymerase has been linked to vascular dysfunction, the activation of nuclear factor κB (NF-κB) and reduction in cellular ATP and pyridine nucleotide levels. In summary, these studies provide evidence that oxidative stress induced by diabetes contributes to vascular and neural dysfunction and that superoxide plays a significant role in these events.
Acknowledgments
This work was supported by a National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-25295, by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases DK-58005, by a Diabetes Center Grant from the Veterans Affairs and International Juvenile Diabetes Foundation, and by a research grant from the American Diabetes Association.
Abbreviations
- DTNB
dithionitrobenzene
- EBF
endoneurial blood flow
- GSH
glutathione
- H2O2
hydrogen peroxide
- HES-DFO
hydroxyethyl starch deferoxamine
- M40403
dichloro[(4aR, 13aR, 17aR,21aR)-1,2,3,4,4a,5,6,12,13,13a,14, 15,16,17,17a,18,19,20,21,21a - eicosahydro - 11,7 - nitrilo - 7H-dibenzo[1,4,7,10]tetraazacycloheptadecine-κN5,κN13,κN18,κN21,κN22]manganese
- MNCV
motor nerve conduction velocity
- Na+/K+ ATPase
sodium/potassium ATPase
- NF-κB
nuclear factor κB
- O.2−
superoxide anion
- OH.
hydroxyl radical
- PSS
Krebs Henseleit physiological saline solution
- RLU
relative light units
- SOD
superoxide dismutase
- TBARS
thiobarbituric acid reactive substances
References
- AMMAR R.F., JR, GUTTERMAN D.D., BROOKS L.A., DELLSPERGER K.C. Free radicals mediate endothelial dysfunction of coronary arterioles in diabetes. Cardiovasc. Res. 2000;47:595–601. doi: 10.1016/s0008-6363(00)00094-8. [DOI] [PubMed] [Google Scholar]
- ANDREW R., SKYRME-JONES P., O'BRIEN R.C., BERRY K.L., MEREDITH I.T. Vitamin E supplementation improves endothelial function in type I diabetes mellitus: a randomized placebo-controlled study. J. Am. Coll. Cardiol. 2000;36:94–102. doi: 10.1016/s0735-1097(00)00720-8. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S. Oxidative damage and tyrosine nitration from peroxynitrite. Chem. Res. Toxicol. 1996;9:836–844. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- CAKATAY U., TELCI A., KAYALI R., SIVAS A., AKCAY T. Effect of α-lipoic acid supplementation on oxidative protein damage in the streptozotocin-diabetic rat. Res. Exp. Med. 2000;199:243–251. doi: 10.1007/s004330050007. [DOI] [PubMed] [Google Scholar]
- CAMERON N.E., COTTER M.A. Neurovascular dysfunction in diabetic rats: potential contribution of autoxidation and free radicals examined using transition metal chelating agents. J. Clin. Invest. 1995;96:1159–1163. doi: 10.1172/JCI118104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMERON N.E., COTTER M.A. Effects of antioxidants on nerve and vascular dysfunction in experimental diabetes. Diabetes Res. Clin. Practice. 1999;45:137–146. doi: 10.1016/s0168-8227(99)00043-1. [DOI] [PubMed] [Google Scholar]
- CAMERON N.E., COTTER M.A., ARCHIBALD V., DINES K.C., MAXFIELD E.K. Anti-oxidant and pro-oxidant effects on nerve conduction velocity, endoneurial blood flow and oxygen tension in non-diabetic and streptozotocin-diabetic rats. Diabetologia. 1994;37:449–459. doi: 10.1007/s001250050131. [DOI] [PubMed] [Google Scholar]
- CAMERON N.E., COTTER M.A., BASSO M., HOHMAN T.C. Comparison of the effects of inhibitors of aldose reductase and sorbitol dehydrogenase on neurovascular function, nerve conduction and tissue polyol pathway metabolites in streptozotocin-diabetic rats. Diabetologia. 1997;40:271–281. doi: 10.1007/s001250050674. [DOI] [PubMed] [Google Scholar]
- CAMERON N.E., COTTER M.A., HORROBIN D.H., TRITSCHLER H.J. Effects of α-lipoic acid on neurovascular function in diabetic rats: interaction with essential fatty acids. Diabetologia. 1998;41:390–399. doi: 10.1007/s001250050921. [DOI] [PubMed] [Google Scholar]
- CAMERON N.E., COTTER M.A., LOW P.A. Nerve blood flow in early experimental diabetes in rats: relation to conduction deficits. Am. J. Physiol. 1991;261:E1–E8. doi: 10.1152/ajpendo.1991.261.1.E1. [DOI] [PubMed] [Google Scholar]
- CAMERON N.E., COTTER M.A., MAXFIELD E.K. Anti-oxidant treatment prevents the development of peripheral nerve dysfunction in streptozotocin-diabetic rats. Diabetologia. 1993;36:299–304. doi: 10.1007/BF00400231. [DOI] [PubMed] [Google Scholar]
- COPPEY L.J., DAVIDSON E.P., DUNLAP J.A., LUND D.D., YOREK M.A. Slowing of motor nerve conduction velocity in streptozotocin-induced diabetic rats is preceded by impaired vasodilation in arterioles that overlie the sciatic nerve. Int. J. Exp. Diabetes Res. 2000;1:131–143. doi: 10.1155/EDR.2000.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COPPEY L.J., GELLETT J.S., DAVIDSON E.P., DUNLAP J.A., LUND D.D., YOREK M.A.Effect of antioxidant treatment of streptozotocin-induced diabetic rats on endoneurial blood flow, motor nerve conduction velocity and vascular reactivity of epineurial arterioles of the sciatic nerve Diabetes 2001(in press) [DOI] [PubMed]
- FLOHE L. Superoxide dismutase for therapeutic use: clinical experience, dead ends and hopes. Mol. Cell. Biochem. 1988;84:123–131. doi: 10.1007/BF00421046. [DOI] [PubMed] [Google Scholar]
- GOCMEN C., SECILMIS A., KUMCU E.K., ERTUG P.U., ONDER S., DIKMEN A., BAYSAL F. Effects of vitamin E and sodium selenate on neurogenic and endothelial relaxation of corpus cavernosum in the diabetic mouse. Eur. J. Pharmacol. 2000;398:93–98. doi: 10.1016/s0014-2999(00)00242-9. [DOI] [PubMed] [Google Scholar]
- HAAK E., USADEL K.H., KUSTERER K., AMINI P., FROMMEYER R., TRITSCHLER H.J., HAAK T. Effects of alpha-lipoic acid on microcirculation in patients with peripheral diabetic neuropathy. Exp. Clin. Endocrinol. Diabetes. 2000;108:168–174. doi: 10.1055/s-2000-7739. [DOI] [PubMed] [Google Scholar]
- HUBER W., MENANDER-HUBER K.B., SAIFER M.G.P., WILLIAMS L.D. Bioavailability of superoxide dismutase: implications for the anti-inflammatory action mechanism of orgotein. A.A.S. 1980;7:185–195. [PubMed] [Google Scholar]
- ISHII H., KOYA D., KING G.L. Protein kinase C activation and its role in the development of vascular complications in diabetes mellitus. J. Mol. Med. 1998;76:21–31. doi: 10.1007/s001090050187. [DOI] [PubMed] [Google Scholar]
- KARASU C., DEWHURST M., STEVENS E.J., TOMLINSON D.R. Effects of anti-oxidant treatment on sciatic nerve dysfunction in streptozotocin-diabetic rats; comparison with essential fatty acids. Diabetologia. 1995;38:129–134. doi: 10.1007/BF00400086. [DOI] [PubMed] [Google Scholar]
- KEEGAN A., COTTER M.A., CAMERON N.E. Effects of diabetes and treatment with the antioxidant α-lipoic acid on endothelial and neurogenic responses of corpus cavernosum in rats. Diabetologia. 1999;42:343–350. doi: 10.1007/s001250051161. [DOI] [PubMed] [Google Scholar]
- LOU M.F., DICKERSON J.E., JR, GARADI R., YORK B.M., JR Glutathione depletion in the lens of galactosemic and diabetic rats. Exp. Eye Res. 1988;46:517–530. doi: 10.1016/s0014-4835(88)80009-5. [DOI] [PubMed] [Google Scholar]
- LOW P.A., NICKANDER K.K. Oxygen free radical effects in sciatic nerve in experimental diabetes. Diabetes. 1991;40:873–877. doi: 10.2337/diab.40.7.873. [DOI] [PubMed] [Google Scholar]
- MAYHAN W.G. Superoxide dismutase partially restores impaired dilatation of the basilar artery during diabetes mellitus. Brain Res. 1997;760:204–209. doi: 10.1016/s0006-8993(97)00282-5. [DOI] [PubMed] [Google Scholar]
- MIHARA M., UCHIYAMA M., FUKUZAMA K. Thiobarbituric acid value of fresh homogenate of rat as a parameter of lipid peroxidation in aging, CC14 intoxication, and vitamin E deficiency. Biochem. Med. 1980;23:302–311. doi: 10.1016/0006-2944(80)90040-x. [DOI] [PubMed] [Google Scholar]
- MILLER F.J., GUTTERMAN D.D., RIOS C.D., HEISTAD D.D., DAVIDSON B.L. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ. Res. 1998;82:1298–1305. doi: 10.1161/01.res.82.12.1298. [DOI] [PubMed] [Google Scholar]
- OBROSOVA I.G., FATHALLAH L., GREENE D.A. Early changes in lipid peroxidation and antioxidative defense in diabetic rat retina: effect of DL-α-lipoic acid. Eur. J. Pharmacol. 2000;398:139–146. doi: 10.1016/s0014-2999(00)00286-7. [DOI] [PubMed] [Google Scholar]
- PATEL M., DAY B.J. Metalloporphyrin class of therapeutic catalytic antioxidants. TIPS. 1999;20:359–364. doi: 10.1016/s0165-6147(99)01336-x. [DOI] [PubMed] [Google Scholar]
- PIEPER G.M.Hyperglycemia and diabetes-induced vascular dysfunction: role of oxidative stress Oxidative Stress and Vascular Disease 2000Kluwer Academic; 305–322.Keaney JF, edp [Google Scholar]
- PIEPER G.M., LANGENSTROER P., GROSS G.J. Hydroxyl radicals mediate injury to endothelium-dependent relaxation in diabetic rat. Mol. Cell. Biochem. 1993;122:139–145. doi: 10.1007/BF01076098. [DOI] [PubMed] [Google Scholar]
- PIEPER G.M., LANGENSTROER P., SIEBENEICH W. Diabetic-induced endothelial dysfunction in rat aorta: a role of hydroxyl radicals. Cardiovasc. Res. 1997;34:145–156. doi: 10.1016/s0008-6363(96)00237-4. [DOI] [PubMed] [Google Scholar]
- PIEPER G.M., SIEBENEICH W. Diabetes-induced endothelial dysfunction is prevented by long-term treatment with the modified iron chelator, hydroxyethyl starch conjugated-deferoxamine. J. Cardiovasc. Pharmacol. 1997;30:734–738. doi: 10.1097/00005344-199712000-00006. [DOI] [PubMed] [Google Scholar]
- PIEPER G.M., SIEBENEICH W. Oral administration of the antioxidant N-acetylcysteine, abrogates diabetes-induced endothelial dysfunction. J. Cardiovasc. Pharmacol. 1998;32:101–105. doi: 10.1097/00005344-199807000-00016. [DOI] [PubMed] [Google Scholar]
- PRYOR W.A., SQUADRITO G.I. The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am. J. Physiol. 1995;268:L699–L722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- RECKNAGEL R.O., GHOSHAL A.K. Lipoperoxidation as a vector in carbon tetrachloride hepatotoxicity. Lab. Invest. 1996;15:132–145. [PubMed] [Google Scholar]
- RILEY D.P., HENKE S.L., LENNON P.J., WEISS R.H., NEUMANN W.L., RIVERS W.J., ASTON K.W., SAMPLE K.R., LING C.S., SHIEH J.J., BUSCH D.H., SZULBINSKI W. Synthesis, characterization and stability of manganese (II) C-substituted 1,4,7,10,13-pentaazacyclopentadecane complexes exhibiting superoxide dismutase activity. Inorg. Chem. 1996;35:5213–5231. [Google Scholar]
- RILEY D.P., LENNON P.J., NEUMANN W.L., WEISS R.H. Toward the rational design of superoxide dismutase mimics: Mechanistic studies for the elucidation of substituent effects on the catalysis activity of macrocyclic manganese (II) complexes. J. Am. Chem. Soc. 1997;119:6522. [Google Scholar]
- SALVEMINI D., RILEY D.P. M40403, superoxide dismutase mimetic. Drugs of the Future. 2000;25:1027–1033. [Google Scholar]
- SALVEMINI D., WANG Z.-Q., ZWEIER J.L., SAMOUILOV A., MACARTHUR H., MISKO T.P., CURRIE M.G., CUZZOCREA S., SIKORSKI J.A., RILEY D.P. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science. 1999;286:304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- SIMAN C.M., ERIKSSON U.J. Vitamin C supplementation of the maternal diet reduces the rate of malformation in the offspring of diabetic rats. Diabetologia. 1997;40:1416–1424. doi: 10.1007/s001250050844. [DOI] [PubMed] [Google Scholar]
- SORIANO F.G., VIRAG L., JAGTAP P., SZABO E., MABLEY J.G., LIAUDET L., MARTON A., HOYT D.G., MURTHY K.G.K., SALZMAN A.L., SOUTHAN G.J., SZABO C. Diabetic endothelial dysfunction: the role of poly (ADP-ribose) polymerase activation. Nature Med. 2001;7:108–113. doi: 10.1038/83241. [DOI] [PubMed] [Google Scholar]
- TERATA K., COPPEY L.J., DAVIDSON E.P., DUNLAP J.A., GUTTERMAN D.D., YOREK M.A. Acetylcholine-induced arteriolar dilation is reduced in streptozotocin-induced diabetic rats with motor nerve dysfunction. Br. J. Pharmacol. 1999;128:837–843. doi: 10.1038/sj.bjp.0702856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UEMATSU T., NAGASHIMA S., UMEMURA K., KANAMARU M., NAKASHIMA M. Pharmacokinetics and safety of intravenous recombinant human superoxide dismutase (NK341) in healthy subjects. Inter. J. of Clin. Pharm. Therap. 1994;32:638–641. [PubMed] [Google Scholar]
- WATTANAPITAYAKUL S.K., WEINSTEIN D.M., HOLYCROSS B.J., BAUER J.A. Endothelial dysfunction and peroxynitrite formation are early events in angiotensin-induced cardiovascular disorders. FASEB J. 2000;14:271–278. doi: 10.1096/fasebj.14.2.271. [DOI] [PubMed] [Google Scholar]
- YOREK M.A., WIESE T.J., DAVIDSON E.P., DUNLAP J.A., STEFANI M.R., CONNER C.E., LATTIMER S.A., KAMIJO M., GREENE D.A., SIMA A.A.F. Reduced motor nerve conduction velocity and Na+-K+ -ATPase activity in rats maintained on L-fucose diet. Diabetes. 1993;42:1401–1406. doi: 10.2337/diab.42.10.1401. [DOI] [PubMed] [Google Scholar]
- YOUNG W. H2 clearance measurement of blood flow: a review of technique and polarographic principles. Stroke. 1980;11:552–564. doi: 10.1161/01.str.11.5.552. [DOI] [PubMed] [Google Scholar]


