Abstract
In the absence of indomethacin, anandamide did not contract the guinea-pig bronchus at concentrations up to 100 μM. In the presence of indomethacin (10 μM), anandamide induced concentration-related contractions with a pEC50 value of 5.18±0.11. It was significantly less potent than capsaicin (pEC50 7.01±0.1). The anandamide uptake inhibitor AM404, produced only a 14.1±3.22% contraction at 100 μM. All experiments were conducted in the presence of PMSF (20 μM).
The vanilloid receptor antagonist, capsazepine (10 μM), significantly attenuated the contractile effect of anandamide, the response to 100 μM anandamide being 40.53±7.04% in the presence of vehicle and 1.57±8.93% in the presence of 10 μM capsazepine. The contractile actions of anandamide and AM404 were markedly enhanced by the peptidase inhibitor thiorphan.
The log concentration-response curve of anandamide was unaltered by the CB1 receptor antagonist, SR141716A. The pEC50 values for anandamide were 4.88±0.08 and 5.17±0.19 in the presence of vehicle and SR141716A (1 μM) respectively.
The lipoxygenase inhibitors 5,8,11,14-eicosatetraynoic acid (ETYA) and 5,8,11 eicosatriynoic acid (ETI) reduced the effect of 100 μM anandamide from 34.7±1.9% (vehicle) to 7.7±5% (ETYA, 10 μM) and from 41.85±4.25% (n=6) (vehicle) to 10.31±3.54 (n=6) (ETI, 20 μM). Neither inhibitor significantly affected contraction of the tissue by substance P.
This study provides evidence that anandamide acts on vanilloid receptors in the guinea-pig isolated bronchus. These data raise the possibility that the contractile action of anandamide may be due, at least in part, to lipoxygenase metabolites of this fatty acid amide that are vanilloid receptor agonists.
Keywords: Anandamide, bronchus, vanilloid, cannabinoid, lipoxygenase, cyclooxygenase, ETYA, ETI
Introduction
There is mounting evidence that anandamide, and its analogue AM404, can activate vanilloid receptors, both in cells transfected with the VR1 receptor (Smart et al., 2000; Jerman et al., 2000, Ross et al., 2001) and in tissues which natively express this receptor (Zygmunt et al., 1999; 2000). These studies have shown that AM404, the anandamide transport inhibitor is significantly more potent than anandamide at vanilloid receptors. Capsaicin is known to produce a vanilloid receptor-mediated contractile response in isolated guinea-pig bronchi by releasing sensory neuropeptides (Holzer, 1991). A recent report demonstrated that anandamide induces capsazepine-sensitive contractions of the guinea-pig bronchus (Spina et al., 2000). However, there is also evidence of a CB1 receptor mediated contractile action of anandamide in guinea-pig isolated lung parenchyma (Calignano et al., 2000). Studies in the isolated pawskin have suggested that anandamide may act on CB1 receptors to attenuate capsaicin-mediated CGRP release from sensory nerves (Richardson et al., 1988b).
There is evidence that vanilloid receptors may be activated by endogenous products of lipoxygenases (Hwang et al., 2000). These compounds include 12- and 15-hydroperoxyeicosatetraenoic acids (HPETE) and 5- and 15-hydroxyeicosatetraenoic acids (HETE) and leukotriene B4. It has also been shown that lipoxygenase products, including lipoxin A4, may be mediators of the excitatory effects of arachidonic acid on capsaicin-sensitive sensory nerves in the guinea-pig bronchus (Manzini & Meini, 1991). These effects were only observed in the presence of the cyclo-oxygenase inhibitor, indomethacin. Some studies have demonstrated lipoxygenase mediated hydroxylations of anandamide analogous to those observed for arachidonic acid (Burstein et al., 2000). Thus, it is possible that such compounds may be implicated in the contractile action of anandamide in the guinea-pig bronchus. In this study we investigate the nature of the receptors mediating contraction of the guinea-pig bronchus by anandamide and AM404, and the effects of inhibition of cyclo-oxygenase and lipoxygenase on this action.
Methods
Drugs and chemicals
Anandamide (arachidonyl ethanolamide), was obtained from Biomol research laboratories. AM404 (4-hydroxyphenyl arachidonyl amide), capsaicin, capsazepine, and (+)-WIN55212 [(R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenyl-methanonemesylate] were obtained from Tocris and SR141716A [N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride] from Sanofi Recherche. Bovine serum albumin (BSA), cell culture media, non-enzymatic cell dissociation solution, 5,8,11-eicosatriynoic acid (ETI), 5,8,11,14-eicosatetraynoic acid (ETYA), G418, L-glutamine, Krebs salts, penicillin with streptomycin, thiorphan, phenylmethylsulphonyl fluoride (PMSF), and Triton X-100 were all obtained from Sigma-Aldrich. 45Ca2+ (5 – 50 mCi mg−1 calcium) were obtained from Amersham Pharmacia Biotech (U.K.). Rat VR1 transfected CHO cells were a gift from Novartis, London.
45Ca2+ uptake experiments
rVR1 transfected CHO cells were maintained in MEM alpha minus media containing 2 mM L-glutamine supplemented with 10% hyclone foetal bovine serum, 350 μg ml−1 G418, 100 u ml−1 penicillin and 100 μg ml−1 streptomycin. Cells were maintained in 5% CO2 at 37°C. For 45Ca2+ uptake assay, cells were plated in 24-well plates at 5×105 cells ml−1. Cells were incubated for 10 min at 37°C with 100 μl of anandamide and 1 μCi 45Ca2+ in a total volume of 1 ml of assay buffer (Minimum Essential Medium containing 0.25 mg ml−1 BSA). A basal stimulation was measured in the presence of the appropriate vehicle equivalent. The cells were incubated with PMSF (100 μM) and either vehicle or ETYA (10 μM) for 20 min at 37°C, prior to the addition of anandamide. Following incubation, plates were placed on ice and washed three times with ice-cold assay buffer. Cells were incubated with assay buffer containing 0.5% Triton X-100 at 45°C for 20 min, before a 200 μl aliquot was removed for scintillation counting. The counts per min above basal for each datum point were expressed as a percentage of the response to 1 nM resiniferatoxin.
Guinea-pig bronchus
Bronchus and lower trachea were obtained from Dunkin-Hartley guinea-pigs weighing 300 – 800 g. Animals were stunned, exsanguinated and the lungs with attached bronchi and trachea were quickly removed. The main bronchi and lower 1 cm of trachea were dissected and 3 mm rings prepared. Each ring was mounted in a 4 ml organ bath at an initial tension of 0.5 g. The baths contained Krebs solution which was kept at 37°C and bubbled with 95% O2 and 5% CO2. The composition of the Krebs solution was (mM): MgSO4.7H2O 1.29, NaCl 118.2, KCl 4.75, KH2PO4 1.19, NaHCO3 25.0, glucose 11.0, CaCl2 6H2O 2.54. It also contained 10 μM indomethacin (unless otherwise stated). Contractions were monitored by computer (Apple Macintosh LCIII and Performa 475) using a data recording and analysis system (MacLab) that was linked to either UF1 transducers (Pioden Controls) or Model 1030 transducers (UFI, CA, U.S.A.). All agonist additions were made cumulatively without washout in a volume of 10 μl. Top stocks of drugs were 10 mM in dimethylsulphoxide (DMSO), except indomethacin which was a 20 mg ml−1 stock in ethanol. In control experiments, DMSO was added instead of agonist or antagonist and it had no contractile action when added alone (n=6, data not shown). Antagonists were added 30 min prior to the addition of agonists. Similarly, enzyme inhibitors were added 30 min (PMSF and ETYA) or 1 h (ETI) prior to the addition of agonists. In all experiments with anandamide and AM404 (unless otherwise stated), the tissues were preincubated with 20 μM PMSF for 30 min prior to the addition of the cannabinoid.
Analysis of data
Peak contractions were calculated as a percentage of the contraction induced by 100 μM histamine added at the end of each experiment. Values have been expressed as means and variability as s.e.mean or as 95% confidence limits. The values for pEC50 (−log EC50) are calculated from the effective concentration producing 50% of the maximum response inducible by that compound. EC50 and maximal effects (Emax) and the s.e.mean or 95% confidence limits of these values have been calculated by non-linear regression analysis using the equation for a sigmoid concentration-response curve (GraphPad Prism). KB values for capsazepine and its 95% confidence limits were determined by symmetrical (2+2) dose parallel line assays (see Ross et al., 2001). This method was also used to determine whether log concentration-response plots deviated significantly from parallelism.
Results
Log concentration-responses curves
In some tissues anandamide is rapidly metabolised to arachidonic acid and ethanolamide by the action of fatty acid amide hydrolase (FAAH), this enzyme can be inhibited by phenylmethyl sulphonyl fluoride (PMSF). Unless otherwise stated all the experiments with anandamide were carried out in the presence of 20 μM PMSF.
Indomethacin present
In the presence of indomethacin, capsaicin and anandamide (20 μM PMSF) induced concentration-related contractions of the isolated guinea-pig bronchus with pEC50 values of 7.01±0.01 (n=10) and 5.18±0.11 (n=9) respectively. The maximal contraction of 37.02±3.91% induced by anandamide was significantly less (P<0.01, unpaired t-test) than that of 74.81±3.69% which was obtained with capsaicin. In the absence of PMSF the pEC50 for anandamide was significantly lower (4.15±0.64, n=4; P<0.05, unpaired t-test) (Figure 1). The rate of onset of the contractions by anandamide was slow (26±2 min to reach a plateau, n=12) in comparison to capsaicin (5.42±0.34, n=19). The anandamide uptake inhibitor, AM404, has previously been shown to be a full agonist at VR1 receptors (Jerman et al., 2000; Zygmunt et al., 2000). However, AM404 had little effect in the bronchus, producing a maximum contraction of 14.15±3.22% (n=7) at 100 μM (20 μM PMSF), which was not significantly different from the baseline tension (P>0.05, unpaired t-test).
Figure 1.
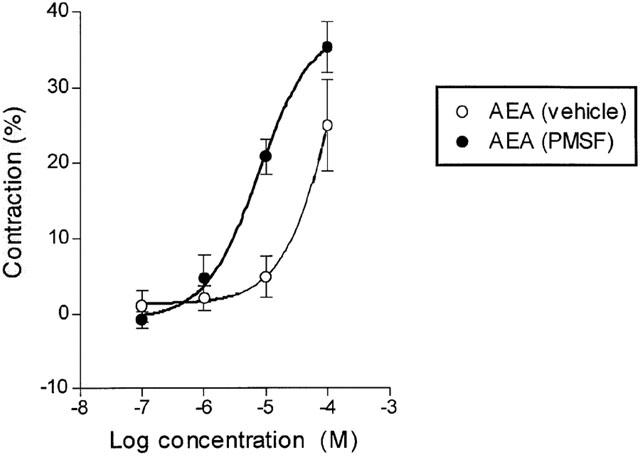
Log concentration-response curve for contraction of the guinea-pig isolated bronchus by anandamide in the presence of either PMSF vehicle or 20 μM PMSF. The experiments were carried out in the presence of indomethacin (10 μM). Each symbol represents the contraction calculated as a percentage of the maximum contraction induced by 100 μM histamine±s.e.mean.
Indomethacin absent
In this series of experiments the effect of capsaicin and anandamide were compared in the presence of 10 μM indomethacin or the vehicle equivalent (Figure 2). The pEC50 and Emax values were not significantly different (P>0.05, unpaired t-test) for capsaicin in the presence of vehicle or indomethacin (10 μM). The pEC50 values were 7.33±0.08 (vehicle) and 7.19±0.04 (indomethacin), and the Emax values were 74.18±5.01 (vehicle) and 82.30±6.31 (indomethacin). In the absence of indomethacin (vehicle equivalent present), anandamide (20 μM PMSF) did not contract the tissue, a concentration of 100 μM produced a relaxation of the tissue of 23.8±8.8% (n=6).
Figure 2.
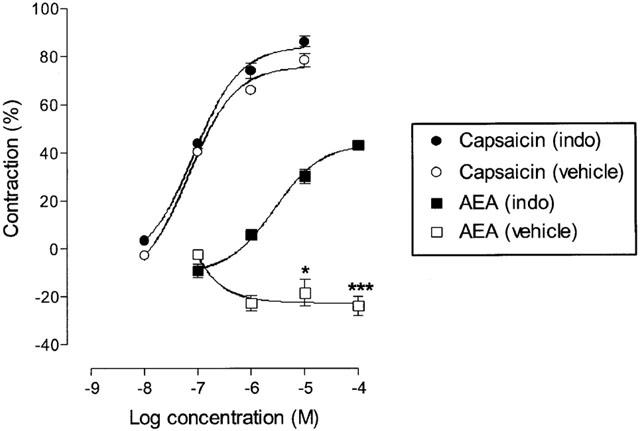
Log concentration-response curves for contraction of the isolated guinea-pig bronchus by capsaicin and anandamide (20 μM PMSF) in the presence of either indomethacin vehicle or indomethacin (10 μM). Each symbol represents the contraction calculated as a percentage of the maximum contraction induced by 100 μM histamine±s.e.mean. The action of anandamide was significantly attenuated by indomethacin, ***P<0.001, *P<0.05, Student's unpaired t-test.
Effect of SR141716A
The CB1 receptor antagonist, SR141716A (1 μM) did not significantly affect the contractions induced by capsaicin (Figure 3a), the pEC50 values being 7.39±0.12 and 7.42±0.12 (P>0.05, unpaired t-test, n=5) in the presence of vehicle and SR141716A respectively. Similarly, the contractions induced by anandamide were unaffected by the CB1 receptor antagonist (Figure 3b), the pEC50 values being 4.88±0.08 and 5.17±0.19 (P>0.05, unpaired t-test, n=6) in the presence of vehicle and SR141716A respectively.
Figure 3.
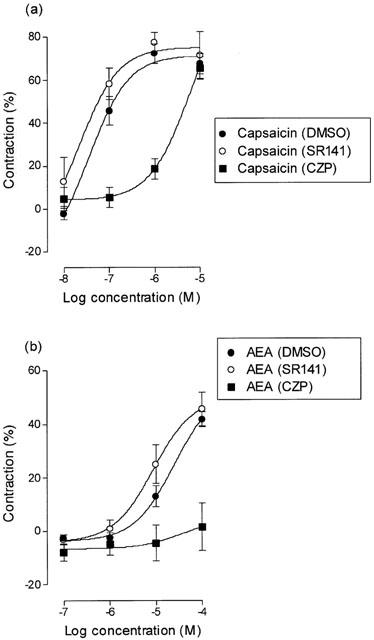
Log concentration-response curves for contraction of the guinea-pig isolated bronchus by (a) capsaicin in the presence of vehicle, SR141716A (SR141, 1 μM) and capsazepine (CZP, 10 μM) or (b) by anandamide (with 20 μM PMSF) in the presence of vehicle, SR141716A (1 μM) and capsazepine (10 μM). The experiments were carried out in the presence of indomethacin (10 μM). Each symbol represents the contraction calculated as a percentage of the maximum contraction induced by 100 μM histamine±s.e.mean.
Effect of cannabinoid receptor agonist, CP55940
The cannabinoid receptor ligand, CP55940 failed to induce contractions of the tissue at concentrations of 10 nM – 10 μM. Ten micro molar produced a relaxation of the tissue of 4.68±5.64% (n=8), which was not significantly different from the baseline values. Pre-treatment of the tissue with CP55940 caused a slight rightward shift in the log concentration response curve to capsaicin, but the pEC50 values were not significantly different, being 7.15±0.17 in the presence of vehicle and 6.88±0.12 in the presence of 100 nM CP55940 (P>0.05, unpaired t-test, n=5).
Effect of capsazepine
The VR1 receptor antagonist, capsazepine (10 μM) induced a parallel rightward shift in the log concentration-response curve of capsaicin (Figure 3a), the pEC50 values being 7.39±0.12 and 5.35±0.16 (n=5) in the presence of vehicle and capsazepine respectively. The pKB value for capsazepine was 6.62 (95% confidence limits 6.84 – 6.37). At the same concentration, capsazepine significantly inhibited the contractions induced by anandamide (Figure 3b). The response to 100 μM was 40.53±7.04% in the presence of vehicle and 1.57±8.93% in the presence of 10 μM capsazepine (P<0.01, unpaired t-test, n=6). Measurement of KB values for capsazepine against anandamide was not possible because the low potency of anandamide would require using inappropriately high concentrations at which this compound may have non-specific effects.
Desensitization experiments
In these experiments, tissues were exposed to a 10 μM concentration of capsaicin and left for 2 h, at which point the contraction had returned to baseline. After desensitization of the tissues with 10 μM capsaicin, 100 μM anandamide produced a relaxation of 8.64±2.84% (n=6), which was not significantly different from the baseline values (P>0.05, unpaired t-test). Desensitized tissues were unresponsive to capsaicin (data not shown).
Effect of thiorphan
Thiorphan is a peptidase inhibitor, which has been shown to markedly enhance the response to capsaicin. The response to 100 μM anandamide in the isolated bronchus was significantly enhanced (P<0.01, unpaired t-test) when thiorphan (10 μM) was present, contractions being 29.92±9.2 (n=4) and 62.36±12.2 (n=4) in the presence of vehicle and thiorphan respectively (Figure 4c). The response to AM404 was significantly (P<0.05, unpaired t-test) enhanced by thiorphan from 2.82±5.86 (vehicle) to 33.13±10.7 (n=4) (thiorphan) (Figure 4b).
Figure 4.
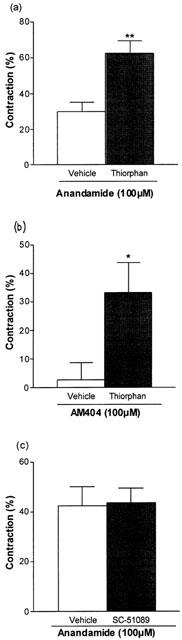
Contraction of the guinea-pig bronchus by (a) 100 μM anandamide (20 μM PMSF) in the presence of vehicle or thiorphan (10 μM), (b) 100 μM AM404 (20 μM PMSF) in the presence of vehicle or thiorphan (10 μM) and (c) 100 μM anandamide in the presence of vehicle or the EP1 receptor antagonist, SC-51089 (10 μM). The experiments were carried out in the presence of indomethacin (10 μM). The bars represent the contraction calculated as a percentage of the maximum contraction induced by 100 μM histamine±s.e.mean. Anandamide and AM404 were significantly enhanced by thiorphan, **P<0.01, *P<0.05, Student's unpaired t-test.
Effect of SC-51089
SC51089 is an EP1 receptor antagonist with a pA2 value of 6.5 (Hallinan et al., 1993). In the guinea-pig bronchus this compound markedly attenuated the contractile action of PGE2. 100 nM PGE2 elicited a contraction of 51.9±5.66% (n=5) in the presence of vehicle and 4.55±6.31% (n=5) in the presence of the SC51089 (10 μM). The contractile effect of anandamide in this tissue was unaffected by SC51089. One hundred micro molar anandamide induced a 43.53±5.91% (n=4) contraction in the presence of vehicle and 42.40±7.81% (n=4) in the presence of the antagonist (10 μM) (Figure 4c).
Effect of ETYA
ETYA is an inhibitor of all lipoxygenase and cyclo-oxygenase enzymes (Tobias & Hamilton, 1979) with ID50 values of 12 and 4 μM respectively. A 30-min pre-treatment with the ETYA (10 μM) did not affect the contractile response to substance P (Figure 5a). However, it did produce a modest (Figure 5b), but significant, attenuation of the pEC50 values for capsaicin (P<0.05, unpaired t-test), but the Emax values were not significantly different (P>0.05, unpaired t-test). The pEC50 and Emax values were 7.16±0.05 and 71.48±3.14% (n=5) in the presence of vehicle and 6.66±0.07 and 64.36±5.81% (n=7) in the presence of ETYA. After treatment with ETYA the response to 100 μM anandamide (Figure 5c) was significantly reduced from 34.74±1.9% in the presence of vehicle to 7.68±5% (P<0.01, unpaired t-test, n=7) in the presence of the enzyme inhibitor. ETYA did not significantly affect the maximum contraction due to histamine (data not shown). 45Ca2+ uptake experiments in rVR1 transfected cells were carried out to check whether this compound has a direct action on VR1 receptors. In rVR1 transfected CHO cells, pretreatment with ETYA did not affect the basal levels of 45Ca2+ uptake and had no significant effect on the log concentration-response curve for stimulation of 45Ca2+ uptake by anandamide (Figure 5d).
Figure 5.
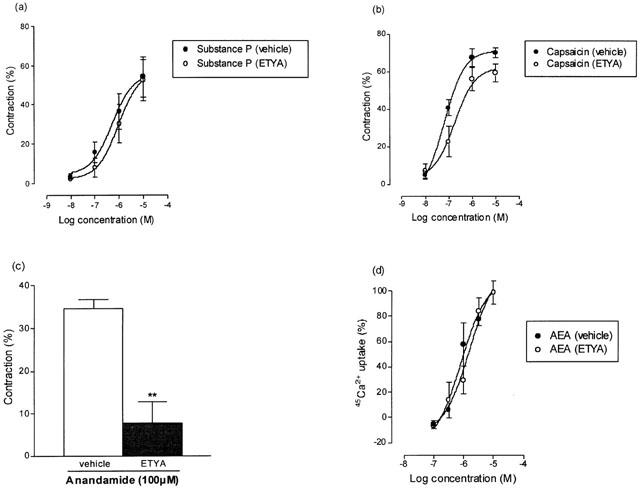
The effects of the lipoxygenase and cyclooxygenase inhibitor, ETYA (10 μM) on: (a) the contractile action of substance P in the bronchus, (b) the contractile action of capsaicin in the bronchus, (c) the contractile action of 100 μM anandamide (20 μM PMSF) in the bronchus and (d) the stimulation of 45Ca2+ uptake in VR1 transfected CHO cells by anandamide. The symbols/bars represent the contraction calculated as a percentage of either the maximum contraction induced by 100 μM histamine (bronchus) or the maximum 45Ca2+ uptake by 10 nM RTX (VR1 transfected cells)±s.e.mean. The contractile action of anandamide was significantly attenuated by ETYA **P<0.01, Student's unpaired t-test.
Effect of ETI
ETI is a non-selective inhibitor of lipoxygenasese (Hammarstrom, 1977) with an ID50 of 24 μM. A 1 h incubation with 20 μM ETI caused a rightward shift in the log concentration-response curve for anandamide (Figure 6b). The responses to 10, 30 and 100 μM anandamide were significantly reduced (P<0.001, unpaired t-test) from 21.32±2.74%, 40.33±4.55% and 41.85±4.25% to −4.80±3.86%, 5.82±5.57% and 10.31±3.54% after pretreatment with ETI. ETI had no effect on the contractile action of substance P in this tissue (Figure 6a).
Figure 6.
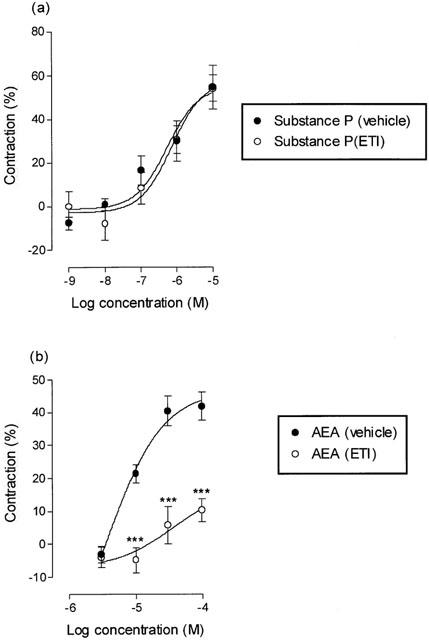
The effect of the lipoxygenase inhibitor, ETI (20 μM) on the log concentration-response curves for (a) substance P and (b) anandamide (20 μM PMSF) in the guinea-pig bronchus. The experiments were carried out in the presence of indomethacin (10 μM). The symbols/bars represent the contraction calculated as a percentage of the maximum contraction induced by 100 μM histamine±s.e.mean. The contractile action of anandamide was significantly attenuated by ETI, ***P<0.001, Student's unpaired t-test.
Discussion
The data presented confirm the findings of Spina et al. (2000) that the contractile action of anandamide in the isolated guinea-pig bronchus is mediated by vanilloid receptors. We have extended these findings to investigate the role of cyclo-oxygenase and lipoxygenase in the contractile action of anandamide. Evidence for vanilloid receptor activation by anandamide is threefold. Firstly, the log concentration-response curve of anandamide is shifted markedly to the right in the presence of the vanilloid receptor antagonist, capsazepine. Secondly, anandamide is inactive in tissues in which the vanilloid receptors have been desensitized by pre-treatment with capsaicin. Thirdly, the contractile action of anandamide is markedly enhanced by the peptidase inhibitor thiorphan, thus implicating the release of neuropeptides in the contractile action of the endogenous cannabinoid. We found no evidence of a CB1 receptor mediated action in the isolated guinea-pig bronchus. The CB1 receptor antagonist, SR141716A, did not shift the log-concentration response curve of anandamide to the right. In addition, the CB1/CB2 receptor agonist CP55940 did not contract this preparation. During the preparation of this paper Tucker et al. (2001) have also reported results implicating the vanilloid receptor in the contractile action of anandamide in the guinea-pig bronchus.
It is notable that Richardson et al. (1998a) found that anandamide attenuated capsaicin-evoked neuropeptide release from spinal cord of hyperalgesic animals in an SR141716A-sensitive manner. In the bronchus however, there is no evidence of CB1 receptors on primary afferent fibres as CP55940 did not significantly attenuate the contractile action of capsaicin. Finally, we have also excluded the possibility that anandamide may be acting on EP1 prostanoid receptors to contract the bronchus, the EP1 receptor antagonist, SC-51089 having no effect on the contractile action of anandamide.
The lipoxygenase and cyclo-oxygenase inhibitor ETYA, has been shown to inhibit the contraction of the guinea-pig trachea induced by arachidonic acid (Mitchell, 1982). The inhibition of the contractile action of anandamide by ETYA suggests that the action of anandamide may be due, at least in part, to lipoxygenase metabolites of this fatty acid amide that are vanilloid receptor agonists. A direct action of ETYA on vanilloid receptors is unlikely as it had no effect either alone or on the 45Ca2+ uptake stimulated by anandamide in rVR1 transfected cells. A second lipoxygenase inhibitor, ETI also markedly attenuated the contractile action of anandamide in this tissue. It is important to note that both the lipoxygenase inhibitors used may have non-selective actions and the possibility remains that the inhibition of anandamide-induced contraction of this tissue by ETI and ETYA is not due to prevention of the formation of lipoxygenase products. A non-specific effect of ETYA or ETI seems unlikely in that neither of these compounds altered the contractile action of substance P or histamine in this preparation. However, both substance P and neurokinin A are released from capsaicin-sensitive nerves in the bronchus (Maggi, 1995) and it is possible that the lipoxygenase inhibitors may have a non-specific effect on the contractile action of neurokinin A.
Thus our data suggest that, in this tissue, anandamide may be metabolized to hydroperoxyeicosatetraenoyl ethanolamides and lipoxin ethanolamides that, like the hydroperoxy-derivatives of arachidonic acid (HPETEs) and lipoxin A4, may be vanilloid receptor agonists. Earlier studies have suggested that the presence of the cyclo-oxygenase inhibitor, indomethacin, may encourage arachidonic acid to pass through the lipoxygenase pathway, and aspirin has been shown to trigger the biosynthesis of lipoxins (Serhan, 1997). Such biosynthetic processes may also be applicable to anandamide. It is notable that the rate of onset of the vanilloid receptor mediated action of anandamide (26 min) in this tissue was markedly slower than that previously observed (5 min) in the mouse vas deferens (Ross, et al., 2001). This may be indicative of the conversion of anandamide to active metabolites in the guinea-pig bronchus. In this study we found that, similar to earlier findings with arachidonic acid, anandamide did not contract the tissue in the absence of indomethacin. The contractile action of capsaicin was unaffected by the exclusion of the cyclo-oxygenase inhibitor. In the absence of indomethacin, anandamide appears to cause a relaxation of the tissue, the nature of this effect and the receptors involved is the subject of ongoing investigations. It is, of course, possible that anandamide is being metabolized to arachidonic acid which, in turn, is metabolised to activators of vanilloid receptors. However, this appears to be unlikely because inhibition of FAAH metabolism of anandamide by PMSF caused a modest enhancement of the contractile action of anandamide.
It would appear that ETYA also attenuates the contractile action of capsaicin, although the effect was modest in comparison to that of the lipoxygenase inhibitor on anandamide. This raises the possibility that the increase in intracellular calcium caused by VR1 receptor activation leads to the release of arachidonic acid and/or anandamide, whose hydroxylation by lipoxygenase may lead to the formation of compounds which are themselves vanilloid receptor agonists.
We have extended investigations in the bronchus to study the anandamide uptake inhibitor AM404. It is surprising that, in this tissue, AM404 is significantly less potent than anandamide. In a number of previous investigations AM404 has been shown to be significantly more potent as a vanilloid receptor agonist than anandamide (Jerman et al., 2000; Zygmunt et al., 2000; Ross et al., 2001). In the bronchus, the fact that thiorphan enhances the contractile action of AM404 implicates vanilloid receptor activation in the contractile action of this compound. However, the fact that anandamide is significantly more potent than AM404 in the guinea-pig bronchus preparation is further evidence that metabolism to active products may amplify its contractile action. Recent findings suggest that both anandamide and AM404 may have low efficacy at the VR1 receptor (Ross et al., 2001). The low potency of anandamide and AM404 at vanilloid receptors in this study is in line with this hypothesis and may reflect a low vanilloid receptor reserve in the guinea-pig bronchus. There are numerous examples of the potency/Emax of a low efficacy agonist being markedly affected by receptor reserve differences between native tissues and high expression transfected cell lines. In addition, there is evidence of considerable species variability in vanilloid receptor pharmacology (Szallasi & Blumberg, 1999). Such differences may account for the low potency of AM404 and anandamide at VR1 receptors in the guinea-pig bronchus as compared with that observed in cells transfected with the rat and human VR1 receptor.
The agonist action of anandamide in this tissue is only evident at high concentrations (10 and 100 μM). The physiological relevance of the activation of VR1 receptors by anandamide is the subject of debate. There is, however, evidence that the ligand binding site on the vanilloid receptor may be intracellular (Jung et al., 1999). The release of anandamide at or near this binding site, and indeed its rapid hydroxylation to HPETE ethanolamides and other lipoxygenase products, may be a physiologically relevant means of vanilloid receptor activation.
Acknowledgments
The Wellcome Trust for Grant 047980 to R.A. Ross and R.G. Pertwee, MRC/Novartis and NIDA for Grants to R.G. Pertwee and Novartis for the gift of the rVR1 transfected cells.
Abbreviations
- AM404
(4-hydroxyphenyl arachidonylamide)
- Anandamide
(arachidonyl ethanolamide)
- Capsaicin
(3-methoxy-4-hydroxybenzyl-8-methyl-6-nonenamide)
- CB1
cannabinoid receptor
- CZP
capsazepine
- DMSO
dimethylsulphoxide
- ETI
5,8,11-eicosatriynoic acid
- ETYA
5,8,11,14-eicosatetraynoic acid
- PMSF
phenylmethylsulphonyl fluoride
- VR1
vanilloid receptor
References
- BURSTEIN S.H., ROSSETTI R.G., YAGEN B., ZURIER R.B. Oxidative metabolism of anandamide. Prostaglandins and other Lipid Mediators. 2000;61:29–41. doi: 10.1016/s0090-6980(00)00053-8. [DOI] [PubMed] [Google Scholar]
- CALIGNANO A., KATONA I., DESARNAUD F., GIUFFRIDA A., LA RANA G., MACKIE K., FREUND T.F., PIOMELLI D. Bidirectional control of airway responsiveness by endogenous cannabinoids. Nature. 2000;408:96–101. doi: 10.1038/35040576. [DOI] [PubMed] [Google Scholar]
- HALLINAN E.A., HAGEN T.J., HUSA R.K., TSYMBALOV S., RAO S.N., VANHOECK J.P., RAFFERTY M.F., STAPELFELD A., SAVAGE M.A., REICHMAN M. N-substituted dibenzoxazepines as analgesic PGE2 antagonists. J. Med. Chem. 1993;36:3293–3299. doi: 10.1021/jm00074a010. [DOI] [PubMed] [Google Scholar]
- HAMMARSTROM S. Selective inhibition of platelet n-8 lipoxygenase by 5,8,11-eicosatriynoic acid. Biochim. Biophys. Acta. 1977;487:517–591. doi: 10.1016/0005-2760(77)90221-1. [DOI] [PubMed] [Google Scholar]
- HOLZER P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurones. Pharmacol. Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- HWANG S.W., HAWOON C., KWAK J., LEE S.-Y., KANG C.-J., JUNG J., CHO S., MIN K.H., SUH Y.-G., KIM G., OH U. Direct activation of capsaicin receptors by products of lipoxygenase: endogenous capsaicin-like substances. Proc. Nat. Acad. Sci. (U.S.A.) 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JERMAN J.C., BROUGH S.J., DAVIS J.B., MIDDLEMISS D.N., SMART D. The anandamide transport inhibitor AM404 is an agonist at the rat vanilloid receptor (VR1) Br. J. Pharmacol. (Proc. Suppl.) 2000;129:73P. [Google Scholar]
- JUNG J., HWANG S.W., KWAK J., LEE S.Y., KANG C.J., KIM W.B., KIM D., OH U. Capsaicin binds to the intracellular domain of the capsaicin-activated ion channel. J. Neurosci. 1999;19:529–538. doi: 10.1523/JNEUROSCI.19-02-00529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGGI C.A. The mammalian tachykinin receptors. Gen. Pharmacol. 1995;26:911–944. doi: 10.1016/0306-3623(94)00292-u. [DOI] [PubMed] [Google Scholar]
- MANZINI S., MEINI S. Involvement of capsaicin-sensitive nerves in the bronchomotor effects of arachidonic acid and melittin: a possible role for lipoxin A4. Br. J. Pharmacol. 1991;103:1027–1032. doi: 10.1111/j.1476-5381.1991.tb12295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL H.W. Effect of ETYA and BW 755c on arachidonate-induced contractions in the guinea-pig isolated trachea. Br. J. Pharmacol. 1982;76:527–529. doi: 10.1111/j.1476-5381.1982.tb09250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON J.D., AANONSEN L., HARGREAVES K.M. Antihyperalgesic effects of spinal cannabinoids. Eur. J. Pharmacol. 1998a;345:145–153. doi: 10.1016/s0014-2999(97)01621-x. [DOI] [PubMed] [Google Scholar]
- RICHARDSON J.D., KILO S., HARGREAVES K.M. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998b;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- ROSS R.A., BROCKIE H.C., GIBSON M., CRAIB S.J., LESLIE M., PASHMI G., DI MARZO V., PERTWEE R.G. Structure-activity relationship for the endogenous cannabinoid, anandamide and certain of its analogues at vanilloid receptors in transfected cells and vas deferens. Br. J. Pharmacol. 2001;132:631–640. doi: 10.1038/sj.bjp.0703850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERHAN C.N. Lipoxins and novel aspirin-triggered 15-epi-lipoxins: a jungle of cell-cell interactions or a therapeutic opportunity. Prostaglandins. 1997;53:107–137. doi: 10.1016/s0090-6980(97)00001-4. [DOI] [PubMed] [Google Scholar]
- SMART D., GUNTHORPE M.J., JERMAN J.C., NASIR S., GRAY J., MUIR A.I., CHAMBERS J.K., RANDALL A.D., DAVIS J.B. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br. J. Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPINA D., TUCKER R.C., PAGE C.P. Effect of the putative vanilloid receptor agonist, anandamide on the baseline tone in the guinea-pig bronchus. Proc. Aust. Soc. Clin. Exp. Pharmacol. Toxicol. 2000;7:60. [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–211. [PubMed] [Google Scholar]
- TOBIAS L.D., HAMILTON J.G. The effect of 5,8,11,14-eicosatetraynoic acid on lipid metabolism. Lipids. 1979;14:181–193. doi: 10.1007/BF02533870. [DOI] [PubMed] [Google Scholar]
- TUCKER R.C., KAGAYA M., PAGE C.P., SPINA D. The endogenous cannabinoid agonist, anandamide stimulates sensory nerves in guinea-pig airways. Br. J. Pharmacol. 2001;132:1127–1135. doi: 10.1038/sj.bjp.0703906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYGMUNT P.M., CHUANG H.-H., MOVAHED P., JULIUS D., HÖGESTÄTT E.D. The anandamide transport inhibitor AM404 activates vanilloid receptors. Eur. J. Pharmacol. 2000;396:39–42. doi: 10.1016/s0014-2999(00)00207-7. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H., SORGÅRD M., DI MARZO V., JULIUS D., HÖGESTÄTT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


