Abstract
Using the rat paw pressure test, in which increased sensitivity is induced by intraplantar injection of prostaglandin E2, we studied the action of several K+ channel blockers in order to determine what types of K+ channels could be involved in the peripheral antinociception induced by dibutyrylguanosine 3 : 5′-cyclic monophosphate (DbcGMP), a membrane permeable analogue of cyclic GMP.
DbcGMP elicited a dose-dependent (50, 75, 100 and 200 μg paw−1) peripheral antinociceptive effect. The effect of the 100 μg dose of DbcGMP was considered to be local since only a higher dose (300 μg paw−1) produced antinociception in the contralateral paw.
The antinociceptive effect of DbcGMP (100 μg paw−1) was dose-dependently antagonized by intraplantar administration of the sulphonylureas tolbutamide (20, 40 and 160 μg) and glibenclamide (40, 80 and 160 μg), selective blockers of ATP-sensitive K+ channels.
Charybdotoxin (2 μg paw−1), a selective blocker of high conductance Ca2+-activated K+ channels, and apamin (10 μg paw−1), a selective blocker of low conductance Ca2+-activated K+ channels, did not modify the peripheral antinociception induced by DbcGMP.
Tetraethylammonium (2 mg paw−1), 4-aminopyridine (200 μg paw−1) and cesium (800 paw−1), non-selective voltage-gated potassium channel blockers, also had no effect.
Based on this experimental evidence, we conclude that the activation of ATP-sensitive K+ channels could be the mechanism by which DbcGMP induces peripheral antinociception, and that Ca2+-activated K+ channels and voltage-dependent K+ channels appear not to be involved in the process.
Keywords: DbcGMP, morphine, K+ channel blockers, glibenclamide, antinociception
Introduction
In addition to the spinal and supraspinal antinociceptive sites of action of cholinergic drugs (Metys et al., 1969; Pedigo et al., 1975; Yaksh et al., 1985; Iwamoto & Marion, 1993; Brodie & Proudfit, 1984; Katayama et al., 1984), Ferreira & Nakamura (1979) described a peripheral analgesic effect of cholinergic agents. Because dibutyryl cyclic GMP (DbcGMP) mimicked ACh-induced analgesia, they suggested that cholinergic agents might cause analgesia by increasing cyclic GMP at the nociceptor level.
Duarte et al. (1990) demonstrated that intraplantar injection of ACh and sodium nitroprusside (SNP) induced antinociception in the rat paw rendered hyperalgesic with prostaglandin E2 (PGE2). The antinociceptive effect of ACh was blocked by L-NG-monomethyl-L-arginine (L-NMMA), an inhibitor of nitric oxide synthase (NOs). The antinociceptive action of both ACh and SNP was blocked by methylene blue (MB), an inhibitor of guanylate cyclase (GC), and was potentiated by MY5445, an inhibitor of cyclic GMP phosphodiesterase. In the studies by Duarte & Ferreira (1992), they described the involvement of the L-arginine/NO/cyclic GMP pathway in central morphine-induced antinociception.
Until now nothing is known about what happens after production of cyclic GMP at the nociceptor level that induces the state of antinociception. However, with respect to the participation of cyclic GMP in other biological effects such as vasodilatation, there is better evidence about the possible mechanism triggered by cyclic GMP. It is known that NO, by increasing cyclic GMP, can activate different types of potassium channels in different types of tissues (Thornbury et al., 1991; Kubo et al., 1994; Murphy & Brayden, 1995; Armstead, 1996; Zhuo et al., 1997; Carrier et al., 1997).
More recently, our group demonstrated the participation of ATP-sensitive K+ channels in the peripheral antinociception induced by morphine (Rodrigues & Duarte, 2000) and the NO donor sodium nitroprusside (Soares et al., 2000), which led us to assume that nociceptor desensitization may occur through the activation of K+ channels leading to the alteration of threshold neuronal sensitivity to pain.
The aim of the present study was to verify the possible relation between increased intracellular levels of cyclic GMP induced by DbcGMP and activation of K+ channels causing neuronal desensitization, and to determine what types of K+ channels may be involved in this effect.
Methods
Animals
The experiments were performed on 180 – 250 g male Wistar rats from CEBIO-UFMG (Animal House of Universidade Federal de Minas Gerais). The animals were housed in a temperature-controlled room (23±1°C) on an automatic 12-h light/dark cycle (0600 to 1800 h of light phase). All testing was concluded during the light phase (1200 to 1700 h). Food and water were freely available until the beginning of the experiments. Naïve animals were used throughout.
Measurement of hyperalgesia
Hyperalgesia was induced by a subcutaneous injection of prostaglandin E2 (PGE2, 2 μg) into the plantar surface of the rat's hindpaw and measured by the paw pressure test described by Randall & Selitto (1957). An analgesimeter (Ugo-Basile, Italy) with a cone-shaped paw-presser with a rounded tip was used to apply a linearly increasing force to the rat's right hindpaw. The weight in grams required to elicit nociceptive responses such as paw flexion or struggle was determined as the nociceptive threshold. A cut-off value of 300 g was used to prevent damage to the paws. The nociceptive threshold was measured in the right paw and is reported as the average of three consecutive trials recorded before (zero time) and 3 h after PGE2 injection (peak of effect). The results were calculated by the difference between these two averages (Δ of nociceptive threshold) and expressed as grams.
Experimental protocol
DbcGMP was injected subcutaneously into the right hindpaw 2 h after the local injection of PGE2. In the protocol used to determine whether DbcGMP acts at sites outside the injected paw, PGE2 was injected into both hindpaws while DbcGMP was injected 2 h later into the left paw (Ferreira & Nakamura, 1979). The nociceptive threshold was always measured in the right hindpaw. All the K+ channel blockers were injected subcutaneously into the right hindpaw. The sulphonylureas (glibenclamide and tolbutamide) were administered 5 min before DbcGMP while all the other K+ channel blockers were injected 45 min after DbcGMP (Wild et al., 1991; Ocaña & Baeyens, 1993; Yonehara & Takiuchi, 1997).
Chemicals
Prostaglandin E2 (Sigma, U.S.A.) was used as the hyperalgesic agent and DbcGMP (Sigma, U.S.A.) as the antinociceptive drug. The K+ channel blockers were glibenclamide (Sigma, U.S.A.), tolbutamide (ICN Biomedicals, U.S.A.), charybdotoxin (ChTx, Sigma, U.S.A.), apamin (Apa, Sigma, U.S.A.), tetraethylammonium chloride (TEA, Sigma, U.S.A.), 4-aminopyridine (4-AP, Sigma, U.S.A.), and cesium (Cs, Mitsuwa's Pure Chemicals, Japan). All drugs were dissolved in isotonic saline, except the sulphonylureas, which were dissolved in Tween 80 (2% in saline), and injected in a volume of 100 μl per paw.
Statistical analysis
Data were analysed statistically by one-way analysis of variance (ANOVA) with post-hoc Bonferroni's test for multiple comparisons. Probabilities less than 5% (P<0.05) were considered statistically significant.
Results
Antinociceptive action of DbcGMP
Figure 1 shows that intraplantar (right paw) administration of DbcGMP (50, 75, 100 and 200 μg) antagonized the hyperalgesic effect of PGE2 (2 μg paw−1), in a dose-dependent manner. Maximal antinociceptive effect of DbcGMP is at 1 h after administration and last for plus 2 h (data not shown). DbcGMP at the dose of 100 μg paw−1 when injected into the left hindpaw (contralateral) did not produce antinociception in the right hindpaw, whereas DbcGMP at doses of 300 μg, when injected in to the left hindpaw induced a potent antinociceptive effect in the contralateral paw (Figure 2).
Figure 1.

Effect of DbcGMP on the nociceptive threshold in rats with PGE2-induced hyperalgesia. DbcGMP (μg paw−1) was administered 2 h after local administration of 100 μl of PGE2 (2 μg). The antinociceptive response was measured in the paw pressure test as described in Methods. Each column represents the mean±s.e.mean (n=10). *Indicates a significant difference from the PGE2+vehicle injected control (P<0.05, ANOVA+Bonferroni's test).
Figure 2.

Exclusion of an outside paw antinociceptive effect of DbcGMP. DbcGMP (μg) was administered into the left (L) paw 2 h after PGE2 (2 μg) administration into both hindpaws, right (R) and left. The analgesic response of the right hindpaw was measured by the paw pressure test as described in Methods. Each column represents the mean±s.e.mean (n=10). *Indicates a significant difference from the PGE2+vehicle injected control (P<0.05, ANOVA+Bonferroni's test).
Antagonism of DbcGMP-induced antinociception by tolbutamide and glibenclamide
The intraplantar injection of tolbutamide (20, 40 and 160 μg) reduced the peripheral antinociception induced by DbcGMP (100 μg) in a dose-dependent manner (Figure 3). The other K+ ATP channel blocker tested, glibenclamide (40, 80 and 160 μg paw−1), also significantly inhibited the DbcGMP-induced peripheral antinociceptive effect (Figure 4). Maximal dose of sulphonylureas, when injected in contralateral paw, did not antagonize the antinociception (not shown). Neither sulphonylurea tested significantly modified the nociceptive threshold in control animals (data not shown), or induced any overt behavioural effect at the doses used. Furthermore, glibenclamide had no significant effect on plasma glucose level (results not shown).
Figure 3.
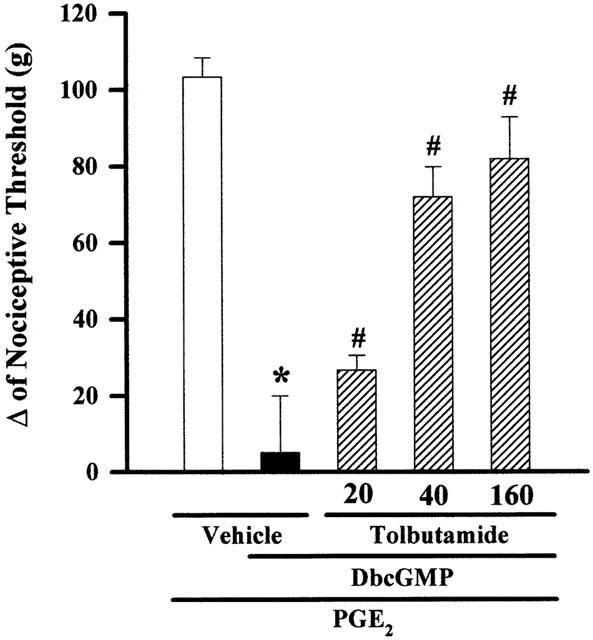
Tolbutamide induces a dose-dependent antagonism of the DbcGMP-induced antinociception of hyperalgesic paws (PGE2, 2 μg). Antagonism induced by intraplantar administration of tolbutamide of the peripheral antinociception produced by DbcGMP in hyperalgesic paws (PGE2, 2 μg). Tolbutamide (μg paw−1) was administered 5 min before DbcGMP (100 μg paw−1). Each column represents the mean±s.e.mean (n=5). *, # Indicate significant differences compared to PGE2+vehicle- and PGE2+DbcGMP+vehicle-injected controls, respectively (P<0.05, ANOVA+Bonferroni's test).
Figure 4.

Glibenclamide induces a dose-dependent antagonism of the DbcGMP-induced antinociception of hyperalgesic paws (PGE2, 2 μg). Glibenclamide (μg paw−1) was administered 5 min before DbcGMP (100 μg paw−1). Each column represents the mean±s.e.mean (n=5). *, # Indicate significant differences compared to PGE2+vehicle- and PGE2+DbcGMP+vehicle-injected controls, respectively (P<0.05, ANOVA+Bonferroni's test).
Effect of ChTX, apamin, TEA, 4-AP and Cesium on DbcGMP-induced antinociception
Figure 5 shows that Charybdotoxin (2 μg), apamin (10 μg), TEA (2 mg), 4-AP (200 μg) and cesium (800 μg), injected into the paw, did not modify significantly the antinociception induced by DbcGMP (100 μg paw−1).
Figure 5.
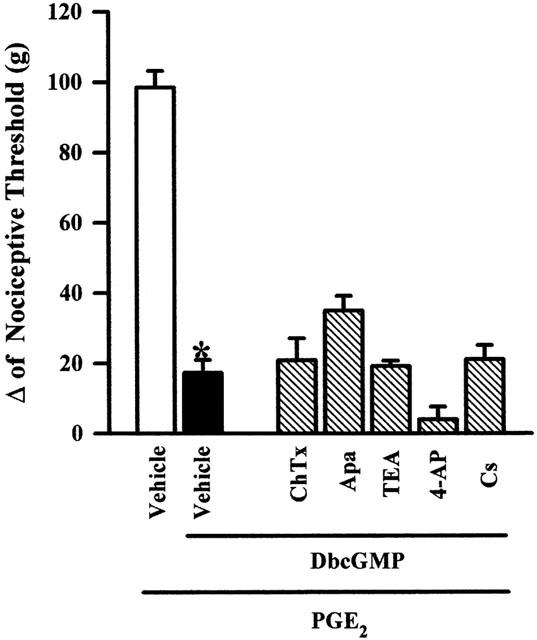
Effect of intraplantar administration of apamin (10 μg), charybdotoxin (2 μg), 4-AP (200 μg), TEA (2 mg) and cesium (800 μg) on the peripheral antinociception induced by DbcGMP in hyperalgesic paws (PGE2, 2 μg). Drugs were administered 45 min after DbcGMP (100 μg paw−1). Each column represents the mean±s.e.mean (n=5). No statistically significant difference was found between the groups treated with PGE2+DbcGMP+vehicle and PGE2+DbcGMP+apamin, charybdotoxin, 4-AP, TEA or Cs. *Indicates a significant difference from the PGE2+ vehicle injected control (P<0.05, ANOVA+Bonferroni's test).
Discussion
In the present study, DbcGMP, a permeable cyclic GMP analogue, had a dose-dependent peripheral antinociceptive effect on the hyperalgesia induced by PGE2. Several studies have also demonstrated an antinociceptive effect of this compound through the activation of the L-arginine/NO/cyclic GMP pathway. Ferreira et al. (1991) reported that the antinociceptive effect of low doses of morphine administered into paws treated with PGE2 was inhibited by two blockers of the enzymatic synthesis of NO from L-arginine, L-NIO and L-NMMA and also by methylene blue, a guanilate cyclase inhibitor. Also, the antinociceptive effect was potentiated by MY5445, an inhibitor of the cyclic GMP-degrading enzyme. The activation of the NO/cyclic GMP pathway in primary sensory neurons seems to contribute to the antinociceptive action of dipyrone at the spinal and peripheral level since it was observed that the analgesia induced by intraplantar (Duarte et al., 1992), intraperitoneal and intrathecal administration was abolished by pretreatment with L-NMMA or methylene blue.
Furthermore, Dray et al. (1992) reported that DbcGMP inhibited the depolarization response evoked by bradykinin in the ventral roots of the medulla. Vocci et al. (1978) observed that DbcGMP injected i.c.v. reduced the tail flick reflex response to thermal stimuli in mice. In rats, injection of DbcGMP into the ventricles (Cohn et al., 1978) or i.t. (Jurna, 1984) also caused antinociception, suggesting that DbcGMP may be involved in analgesia also at the central level.
Recently Germany et al. (1996) demonstrated the participation of the NO/cyclic GMP pathway in the antinociception induced by intracerebroventricular administration of bradykinin, since this antinociception was antagonized by prior administration of NG-nitro-L-arginine methylester (L-NAME) and methylene blue.
To exclude central effects of analgesics many strategies can be used (Stein, 1993). In the present study, we used the strategy of evaluating the efficacy of i.p.s.i.-versus contralateral paw administration because the route and site of administration would the same. DbcGMP, at dose of 100 μg, was ineffective when administered into the contralateral paw, suggesting that at this dose, DbcGMP has a peripheral site of action.
The sulphonylureas tolbutamide and glibenclamide reversed the peripheral antinociceptive effect of DbcGMP in a dose-dependent manner. These drugs specifically block ATP-sensitive K+ channels, with no effect on Ca2+-activated or voltage-dependent K+ channels (Amoroso et al., 1990; Davies et al., 1991; Nichols & Lederer, 1991; Edwards & Weston, 1993).
The central antinociceptive action of various substances such as R-PIA, an A1 adenosine receptor agonist (Ocaña & Baeyens, 1994), prolactin (Shewade & Ramaswany, 1995) and various 5-HT1A serotonin receptor agonists (Robles et al., 1996) also appears to be related to the activation of ATP-sensitive K+ channels. Intracerebroventricular administration of glibenclamide (Ocaña et al., 1995) antagonized the central antinociceptive effect of morphine in mice, as measured by the hot-plate algesimetric test (Ocaña et al., 1990). Some studies using the tail-flick test have reported similar results, i.e., reversal of the central antinociceptive effect of morphine by glibenclamide.
In a study carried out in our laboratory using the rat paw compression test, Rodrigues & Duarte (2000) and Soares et al. (2000) demonstrated that glibenclamide and tolbutamide respectively reversed the peripheral antinociceptive action of μ opioids receptor agonists and of the nitric oxide donor, sodium nitroprusside, in a dose-dependent manner.
The peripheral antinociceptive action of DbcGMP seems to occur only through specific activation of ATP-sensitive K+ channels since blockers of other types of K+ channels, such as Ca2+-dependent (apamin and charybdotoxin) and voltage-dependent (4-AP, TEA and Cs) channels, did not reverse such action. The results were still negative when these blockers were applied according to the same protocol as for the sulphonylureas, 5 min before DbcGMP (results not shown).
Ocaña et al. (1995), in a study of the central antinociceptive effect of morphine and fentanyl, Rodrigues & Duarte (2000), in a study of the peripheral antinociceptive effect of the same drugs, and Soares et al. (2000), in a study of peripheral antinociception induced by sodium nitroprusside, also observed that 4-AP and TEA did not reverse the effect of these agonists. Similarly, the antinociceptive action of R-PIA (Ocaña & Baeyens, 1994) and of 5-HT1A serotonin receptor agonists (Robles et al., 1996) was not reversed by either 4-AP or TEA.
It is important to point out that, as mentioned earlier, on the one hand, several studied demonstrated the participation of the NO-cyclic GMP pathway in the analgesia induced by certain drugs such as opioids, and on the other, various studies have associated analgesia with the activation of ATP-sensitive K+ channels. Thus, our results establish a link between these data by indicating that ATP-sensitive K+ channels are involved in the peripheral antinociceptive effect of DbcGMP. This suggestion is based on the fact that the peripheral antinociceptive effect of this drug was reversed by tolbutamide and glibenclamide.
Finally, we still do not know whether the activation of ATP-sensitive potassium channels in the nociceptive terminal occurs directly via cyclic GMP or through the activation of PKG, this being an area for future investigation.
Acknowledgments
Research supported by Conselho Nacional de Pesquisa (CNPq, grants 000521719/95) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, CBS-1800/95).
Abbreviations
- 4-AP
4-aminopyridine
- Ach
acetylcholine
- ATP
adenosine 5′-triphosphate
- ChTX
charybdotoxin
- DbcGMP
N2, 2′-O- dibutyrylguanosine 3:5′-cyclic monophosphate
- KATP
ATP-sensitive K+ channels
- L paw
left paw
- L-NMMA
L-NG- monomethylarginine
- NO
nitric oxide
- NOS
nitric oxide synthase
- PGE2
prostaglandin E2
- R paw
right paw
- TEA
tetraethylammonium
References
- AMOROSO S., SCHMID-ANTOMARCH H., FOSSET M., LADZUNKY M. Glucose, sulfonylureas, and neurotransmitter release: Role of ATP-sensitive K+ channels. Science. 1990;247:852–854. doi: 10.1126/science.2305257. [DOI] [PubMed] [Google Scholar]
- ARMSTEAD W.M. Role of ATP-sensitive K+ channels in cGMP-mediated pial artery vasodilatation. Am. J. Physiol. 1996;270:H423–H426. doi: 10.1152/ajpheart.1996.270.2.H423. [DOI] [PubMed] [Google Scholar]
- BRODIE M.S., PROUDFIT H.K. Hypoalgesia induced by local injection of carbachol into nucleus raphe magnum. Brain Res. 1984;291:337–342. doi: 10.1016/0006-8993(84)91266-6. [DOI] [PubMed] [Google Scholar]
- CARRIER G.O., FUCHS L.C., WINECOFF A.P., GIULUMIAN A.D., WHITE R.E. Nitrovasodilatadors relax mesenteric microvessels by cGMP-induced stimulation of Ca2+-activated K+ channels. Am. J. Physiol. 1997;273:H76–H83. doi: 10.1152/ajpheart.1997.273.1.H76. [DOI] [PubMed] [Google Scholar]
- COHN M.L., COHN L., TAYLOR F.H. Guanosine 3′ : 5′-monophosphate, a central system regulator of analgesia. Science. 1978;199:319–322. doi: 10.1126/science.202029. [DOI] [PubMed] [Google Scholar]
- DAVIES N.W., STANDEN N.B., STANFIELD P.R. ATP-dependent K+ channels of muscle cells – their properties, regulation, and functions. J. Bioenerg. Biomembr. 1991;23:509–535. doi: 10.1007/BF00785809. [DOI] [PubMed] [Google Scholar]
- DUARTE I.D.G., FERREIRA S.H. The molecular mechanism of central analgesia induced by morphine or carbachol and the L-arginine-nitric oxide-cGMP pathway. Eur. J. Pharmacol. 1992;221:171–174. doi: 10.1016/0014-2999(92)90789-7. [DOI] [PubMed] [Google Scholar]
- DUARTE I.D.G., LORENZETTI B.B., FERREIRA S.H.Acetylcholine induces peripheral analgesia by the release of nitric oxide Nitric oxide from L-arginine: a bioregulatory system 1990London: Science Publishers; 165–170.ed. Moncada, S. & Higgs, E.A. pp [Google Scholar]
- DUARTE I.D.G., SANTOS I.R., LORENZETTI B.B., FERREIRA S.H. Analgesia by direct antagonism of nociceptor sensitization involves the arginine-nitric oxide-cGMP pathway. Eur. J. Pharmacol. 1992;217:225–227. doi: 10.1016/0014-2999(92)90881-4. [DOI] [PubMed] [Google Scholar]
- DRAY A., PATEL I.A., PERKINS M.N., RUEFR A. Bradykinin-induced activation of nociceptors: receptor and mechanist studies on the neonatal rat spinal cord-tail preparation in vivo. Br. J. Pharmacol. 1992;107:1129–1134. doi: 10.1111/j.1476-5381.1992.tb13418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS G., WESTON A.H. The pharmacology of ATP-sensitive K+ channels. Annu. Rev. Pharmacol. Toxicol. 1993;33:597–637. doi: 10.1146/annurev.pa.33.040193.003121. [DOI] [PubMed] [Google Scholar]
- FERREIRA S.H., NAKAMURA M. Prostaglandin hyperalgesia, the peripheral analgesic activity of morphine, enkephalin and opioid antagonists. Prostaglandin. 1979;18:191–200. doi: 10.1016/0090-6980(79)90104-7. [DOI] [PubMed] [Google Scholar]
- FERREIRA S.H., DUARTE I.D.G., LORENZETTI B.B. The molecular mechanism of action of peripheral morphine analgesia: stimulation of the cGMP system via nitric oxide release. Eur. J. Pharmacol. 1991;201:121–122. doi: 10.1016/0014-2999(91)90333-l. [DOI] [PubMed] [Google Scholar]
- GERMANY A., GONZÁLEZ P., CONTRERAS E. Possible role of nitric oxide in the antinociception action of intraventricular bradykinin in mice. Eur. J. Pharmacol. 1996;310:123–127. doi: 10.1016/0014-2999(96)00384-6. [DOI] [PubMed] [Google Scholar]
- IWAMOTO E.T., MARION L. Characterisation of the antinociceptive action produced by intrathecally administered muscarinic agonists in rat. J. Pharmacol. Exp. Ther. 1993;266:329–338. [PubMed] [Google Scholar]
- JURNA I. Cyclic nucleotides and aminophylline produce different effects on nociceptive motor and sensory response in the rat spinal cord. Naunyn-Schimiedeberg Arch. Pharmacol. 1984;327:23–30. doi: 10.1007/BF00504987. [DOI] [PubMed] [Google Scholar]
- KATAYAMA Y., WATKINS L.R., BECKER D.P., HAYES R.L. Non- opiate analgesia induced by carbachol microinjection into the pontine parabrachial region of the cat. Brain Res. 1984;296:263–283. doi: 10.1016/0006-8993(84)90063-5. [DOI] [PubMed] [Google Scholar]
- KUBO M., NAKAYA Y., MATSUOKA S., SAITO K., KURODA Y. Atrial natriuretic factor and isosorbide dinitrate modulate the gating of ATP-sensitive K+ channel in cultured vascular smooth muscle cells. Circ. Res. 1994;74:470–476. doi: 10.1161/01.res.74.3.471. [DOI] [PubMed] [Google Scholar]
- METYS J., WAGNER N., METYSODA J., HERZ A. Studies on the central antinociceptive actions of cholinomimetics agents. Int. J. Neuropharmacol. 1969;8:413–425. doi: 10.1016/0028-3908(69)90058-6. [DOI] [PubMed] [Google Scholar]
- MURPHY M.E., BRAYDEN J.E. Nitric oxide hyperpolarizes rabbit mesenteric arteries via ATP-sensitive K+ channels. J. Physiol. 1995;486:47–58. doi: 10.1113/jphysiol.1995.sp020789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHOLS C.G., LEDERER J.W. Adenosine triphosphate-sensitive K+ channels in the cardiovascular system. Am. J. Physiol. 1991;261:H1675–H690. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- OCAÑA M., BAEYENS M. Role of ATP-sensitive K+ channels in antinociception induced by R-PIA, an adenosine A1 receptor agonist. Naunyn-Schmiedeberg's Arch. Pharmacol. 1984;350:57–62. doi: 10.1007/BF00180011. [DOI] [PubMed] [Google Scholar]
- OCAÑA M., BAEYENS M. Differential effects of K+ channel blockers on antinociception induced by α2-adrenoceptor, GABAB and κ-opioid receptor agonists. Br. J. Pharmacol. 1993;110:1049–1054. doi: 10.1111/j.1476-5381.1993.tb13919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OCAÑA M., BAEYENS M. Role of ATP-sensitive K+ channels in antinociception induced by R-PIA, an adenosine A1 receptor agonist. Naunyn-Schmiedeberg's Arch. Pharmacol. 1994;350:57–62. doi: 10.1007/BF00180011. [DOI] [PubMed] [Google Scholar]
- OCAÑA M., DEL POZO E., BARRIOS M., BAEYENS J.M. An ATP-dependent K+ channel blocker antagonises morphine analgesia. Eur. J. Pharmacol. 1990;186:377–378. doi: 10.1016/0014-2999(90)90466-j. [DOI] [PubMed] [Google Scholar]
- OCAÑA M., DEL POZO E., BARRIOS M., BAEYENS J.M. Subgroups among μ-opioid receptor agonists distinguished by ATP-sensitive K+ channel-acting drugs. Br. J. Pharmacol. 1995;114:1296–1302. doi: 10.1111/j.1476-5381.1995.tb13346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEDIGO N.W., DEWEY W.L., HARRIS L.S. Determination and characterisation of the antinociceptive activity of intraventricular administered acetylcholine in mice. J. Pharmacol. Exp. Ther. 1975;193:845–852. [PubMed] [Google Scholar]
- RANDALL L.D., SELITTO J.J. A method for measurement of analgesic activity on inflamed tissues. Arch. Int. Pharmacol. 1957;113:233–249. [PubMed] [Google Scholar]
- ROBLES L.I., BARRIOS M., DELPOZO E., DORDAL A., BAEYENS J. Effects of K+ channel blockers and openers on antinociception induced by agonists of 5-HT1A receptors. Eur. J. Pharmacol. 1996;295:181–188. doi: 10.1016/0014-2999(95)00643-5. [DOI] [PubMed] [Google Scholar]
- RODRIGUES A.R.A., DUARTE I.D.G. The peripheral antinociceptive effect induced by morphine is associated with ATP-sensitive K+ channels. Br. J. Pharmacol. 2000;129:110–114. doi: 10.1038/sj.bjp.0703038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOARES A.C., LEITE R., TATSUO M.A.K.F., DUARTE I.D.G. Activation of ATP-sensitive K+ channels: mechanism of peripheral antinociceptive action of the nitric oxide donor, sodium nitroprusside. Eur. J. Pharmacol. 2000;400:67–71. doi: 10.1016/s0014-2999(00)00355-1. [DOI] [PubMed] [Google Scholar]
- SHEWADE D.G., RAMASWAMY S. Prolactin induced analgesia is dependent on ATP sensitive K+ channels. Clin. Exp. Pharmacol. Physiol. 1995;22:635–636. doi: 10.1111/j.1440-1681.1995.tb02079.x. [DOI] [PubMed] [Google Scholar]
- STEIN C. Peripheral mechanisms of opioid analgesia. Anesth. Analg. 1993;76:182–191. doi: 10.1213/00000539-199301000-00031. [DOI] [PubMed] [Google Scholar]
- THORNBURY K.D., WARD S.M., DALZIEL H.H., CARL A., WESTFALL D.P., SANDERS K.M. Nitric oxide and nitrocysteine mimic nonadrenergic, noncholinergic hyperpolarization in canine proximal colon. Am. J. Phisiol. 1991;26:G553–G557. doi: 10.1152/ajpgi.1991.261.3.G553. [DOI] [PubMed] [Google Scholar]
- VOCCI F.J., PETTY S.K., DEWEY W.L. Antinociceptive action of butyryl derivates of guanosine 3′ : 5′-cyclic monophosphate. J. Pharmacol. Exp. Ther. 1978;207:892–898. [PubMed] [Google Scholar]
- WILD K.D., VANDERAH T., MOSBERG H.I., PORRECA F. Opioid δ receptor subtypes are associated with different K+ channels. Eur. J. Pharmacol. 1991;193:135–136. doi: 10.1016/0014-2999(91)90215-c. [DOI] [PubMed] [Google Scholar]
- YAKSH T.L., DIRKSEN R., HARTY G.L. Antinociceptive effects of intrathecally injected cholinomimetic drugs in the rat and cat. Eur. J. Pharmacol. 1985;117:81–88. doi: 10.1016/0014-2999(85)90474-1. [DOI] [PubMed] [Google Scholar]
- YONEHARA N., TAKIUCHI S. Involvement of calcium-activated potassium channels in the inhibitory prejunctional effect of morphine on peripheral sensory nerves. Regulat. Peptides. 1997;68:147–153. doi: 10.1016/s0167-0115(96)02102-7. [DOI] [PubMed] [Google Scholar]
- ZHUO P., BÉNY J.L., FLAMMER J., LÜSCHER T.F. Relaxation by bradykinin in porcine ciliary artery- Role of nitric oxide and K+-channels. Invest. Ophthal. Visual Sci. 1997;38:1761–1767. [PubMed] [Google Scholar]


