Abstract
In myometrial strips from near-term non-labouring human uterus, addition of oxytocin (OT) evoked dose-dependent (10 – 3000 nM) phasic contractions that were antagonized by atosiban (1 μM) and relaxed by addition of the nitric oxide donor S-nitroso L-cysteine (Cys-NO). In near-term labouring myometrium, however, addition of OT was ineffective at raising additional tone.
In both labouring and non-labouring tissue, Cys-NO mediated relaxation of spontaneous or OT-induced contractions (IC50=1 μM) was unaffected by prior addition of the guanylyl cyclase (GC) inhibitors ODQ (1H-[1,2,4]oxadiazolo[4,3,-α]quinoxalin-1-one; 1 μM), or methylene blue (MB; 10 μM).
Elevation of intracellular cyclic GMP accompanying 30 μM Cys-NO addition in non-labouring tissue (7.5 fold) or in labouring tissues (2.5 fold) was completely blocked in tissues that had been pre-treated with ODQ or MB.
Charybdotoxin (ChTx), iberiotoxin (IbTx) and kaliotoxin (KalTx) all shifted the Cys-NO inhibition curve to the right and reduced the degree of relaxation produced by maximal Cys-NO treatment (100 μM in non-labouring tissue; in labouring tissue, KalTx prevented Cys-NO mediated relaxation in both stimulated and unstimulated tissue.
Addition of the NO-donor S-nitroso N-acetyl penicillamine (SNAP) produced a dose-dependent relaxation of pregnant myometrium while 3-morpholinosyndonimine (SIN-1) did not. The failure of SIN-1 to relax OT-induced contractions was not due to a failure of the donor to stimulate myometrial GC.
We demonstrate that despite the ability of NO to stimulate myometrial GC in pregnant uterine muscle, relaxations are independent of cyclic GMP action. Effects of K+-channel inhibitors suggests that NO-induced relaxation in human uterine smooth muscle may be subserved by direct or indirect activation of one or more calcium-activated K+-channels.
Keywords: Oxytocin, nitric oxide, S-nitroso L-cysteine, S-nitroso N-acetyl penicillamine, 3-morpholinosyndonimine, sodium nitroprusside, cyclic GMP, Ca2+-activated potassium channels, potassium channel toxins, myometrium (human)
Introduction
Preterm labour, initiation of birth prior to the thirty-seventh week of gestation, is a significant problem worldwide and many cases cannot be explained on the basis of associated risk factors, let alone physiological mechanisms (Buxton et al., 2000). Regulation of the signal(s) that initiate myometrial smooth muscle contraction at the time of birth is(are) poorly understood and therapeutic approaches to preventing myometrial contractions in patients in preterm labour are found wanting (Koks et al., 1998). Indeed, if a test were available that predicted all women who would experience preterm labour as well as when they could expect its onset, we would fail to prevent their delivery of a preterm foetus, as there is, to date, no effective means of halting the labour and maintaining the pregnancy until term. While tocolytics based on β-adrenergic receptor and calcium channel antagonists are in use, their efficacy and safety are questionable (Koks et al., 1998). Knowledge of the actions of nitric oxide (NO) as a vascular and gastrointestinal smooth muscle relaxant together with initial clinical evidence (Lees et al., 1994) have suggested the use of NO in the treatment of preterm labour.
NO is clearly an effective agent in relaxing spontaneous contractions in both animal (Kuenzli et al., 1996; 1998) and human myometrium (Bradley et al., 1998). It has been claimed that NO (Izumi & Garfield, 1995) promotes human uterine relaxation (Buhimschi et al., 1995), as it does in other smooth muscle, by the elevation of cyclic guanosine 5′-monophosphate (cyclic GMP) (Yallampalli et al., 1993). However, several other studies have demonstrated that a rise in cyclic GMP fails to cause a relaxation of some smooth muscles, including human myometrium (Diamond, 1983; Word et al., 1991). Our own data in guinea-pig uterine smooth muscle demonstrated that a nitric oxide donor produced relaxation despite the inhibition of guanylyl cyclase (GC) by methylene blue (Kuenzli et al., 1996). Furthermore, enormous concentrations of cyclic GMP analogues were required to produce any demonstrable relaxation of the uterine smooth muscle. Indeed, we have now shown in both monkey and non-pregnant human myometrium that NO-induced relaxation may be independent of a cyclic GMP mechanism (Kuenzli et al., 1998; Bradley et al., 1998). These and other studies where GC-independent actions of NO have been noted (Bolotina et al., 1994), suggest that intracellular cyclic GMP elevation is neither necessary nor sufficient for NO induced relaxation of the uterine smooth muscle and that other pathways such as ion channel/pump regulation by NO may be involved (Buxton et al., 2000). Such a finding is provocative since cyclic GMP elevation is clearly the mechanistic foundation of the action of NO in the regulation of vascular smooth muscle (Murad, 1986) as has been recognized, in part, with the awarding of the 1998 Nobel Prize in Physiology or Medicine.
There are exceptions to the assumption that cyclic GMP subserves all actions of NO however. These exceptions are now seen in a variety of systems such as renal arterioles (Trottier et al., 1998), cerebral microvessels (Wu et al., 2000), pituitary hormone secretion (Pinilla et al., 1998; 1999), regulation of neuronal cell ion channels (Ahern et al., 1999; Summers et al., 1999), and apoptosis (Brune et al., 1996). Exceptions are also seen in non-vascular smooth muscles. In canine airway, Jenssen et al. (2000) have recently suggested that the cyclic GMP-independent actions of NO-donors can be ascribed to the chemistry of the NO-species liberated. Their data, taken together with the work of Jones et al. (1994) suggest that the actions of NO are cyclic GMP-dependent or cyclic GMP-independent in airway smooth muscle based on the NO species delivered by a particular NO donor. While such a result is unexpected based on the chemistry of NO in warm, oxygenated physiological buffers (Kishnani & Fung, 1996; Stamler et al., 1992), the possible role of calcium in the cyclic GMP-independent actions of NO is intriguing.
Here we have pursued the hypothesis that the relaxations of spontaneous and agonist-mediated myometrial contraction in both non-labouring and labouring near-term pregnant human myometrium are independent of cyclic GMP elevation and blocked by calcium-activated potassium channel inhibition.
Methods
Preparation and treatment of uterine samples
Contractile studies were performed on samples of pregnant human uterine tissue obtained from near-term, informed, and consenting women undergoing caesarean section. Tissue samples were transported to the laboratory in buffer (4°C) containing (in mM) 120 NaCl, 5 KCI, 0.587 KH2PO4, 0.589 Na2HP04, 2.5 MgCl2, 20 α-,D-glucose, 2.5 CaCl2, 25 Tris, and 5 NaHCO3; this buffer was also used during the course of organ bath experiments. Strips of myometrium (0.5×1×4 mm) were carefully cut from the centre of the tissue section, mounted in organ baths (Radnoti, Monrovia, CA, U.S.A.), and attached to isometric force transducers (Kent Scientific, Litchfield, CT, U.S.A.) by silk thread. Transducer voltages were amplified and converted to digital signals by an analogue-to-digital board mounted within a computer system running the Workbench data acquisition system (Strawberry Tree, Sunnyvale, CA, U.S.A.). Strips were maintained at 37°C, aerated with 100% O2, and loaded with initial tensions of 1.2 g mm−2. Initial tensions were based on empirical results obtained during pilot experiments that established force-length relationships in pregnant human myometrium and correspond to tissue lengths that are ∼1.5× the resting tissue length. During the course of a 1 h equilibration period, tissues were repeatedly challenged with oxytocin (1 μM) to ensure contractile viability, followed by bath washout and relaxation periods.
NO was added to organ baths or in vitro reactions in the form of S-nitroso-L-cysteine (Cys-NO); 3-morpholinosyndonimine (SIN-1); or sodium nitroprusside (SNP), from a fresh daily concentrated stock solution for which water served as the solvent. S-nitroso N-acetyl penicillamine (SNAP) was diluted in 50% dimethyl sulphoxide (DMSO) and diluted 1 : 1000 or more before addition to experiments. Dilutions of DMSO diluent served as a control for SNAP addition. Cys-NO was prepared by the method described by Gibson et al. (1992). Treatment of NO donors by bubbling the donor stock solution with oxygen for 30 min prior to use was employed as the donor control. Such solutions were considered ‘spent' (Kuenzli et al., 1996) and did not cause relaxation of the tissue. Concentration-response curves for oxytocin (OT) and NO-donors were constructed in a non-cumulative manner and the effect of NO-donors was evaluated by addition 2 min following OT and quantified as described below. Effects of SNAP and SIN-1 were evaluated in a cumulative fashion in some experiments, while effects of potassium channel (K+Ca) and GC blockers were evaluated based on single concentrations determined by experience (Bradley et al., 1998) to be the lowest concentrations that produced the maximal inhibition observed.
Simultaneous measurement of tissue tension and intracellular cyclic GMP concentration
Agonist stimulated tissue strips mounted within organ baths were treated with Cys-NO and flash-frozen in liquid nitrogen precisely 30 s after Cys-NO addition; control unstimulated samples were frozen at baseline between spontaneous contractions. For unstimulated, labouring tissues, samples were frozen at the average tension between the peak and nadir of phasic contractions. Without allowing samples to thaw, tissues were homogenized with a Duall™ glass-glass homogeniser in 1 ml of 7% trichloroacetic acid (TCA) dissolved in acetone while constantly immersed in a methanol-dry ice slurry. Precipitated protein was removed by centrifugation, TCA was removed by triplicate extraction with H2O-saturated diethyl ether, and heating samples to 70°C for 10 min evaporated residual ether. After lyophilization, samples were resuspended in phosphate buffer and assayed for cyclic GMP by enzyme-linked immunoassay (ELISA) using antibodies obtained from Cayman Chemical (Ann Arbor, MI, U.S.A.). Protein present in the acid precipitate was neutralized with NaOH and assayed by the method of Lowry (Butcher & Lowry, 1976).
To inhibit GC activities, tissue strips mounted in organ baths were pretreated with methylene blue (MB; 10 μM) or 1 μM ODQ (1H-[1,2,4]oxadiazolo[4,3,-α]quinoxalin-1-one) for 15 min at 37°C in the dark before the addition of either Cys-NO or ion channel blockers. Guanylyl cyclase treatments were considered irreversible. Preliminary experiments were performed to determine time courses of cyclic GMP generation in response to treatment with Cys-NO, or after pretreatment with GC inhibitors as shown.
Direct measurement of soluble guanylyl cyclase activity
Myometrial smooth muscle tissues obtained from pregnant and non-pregnant human donors and canine tracheal smooth muscle tissue to serve as a control preparation were homogenized in cyclase buffer at pH 6.8 containing (mM) KH2PO4 5, K2HPO4 5, EGTA 5, AEBSF 1 (4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride, a serine protease inhibitor). The homogenate was centrifuged at 350×g for 15 min and the resulting supernatant filtered through a 100 μM nylon filter. The filtrate was then centrifuged at 46,000×g for 30 min and the supernatant employed in GC assays at a 1 : 10 dilution. The pellet was resuspended and protein measured as above. GC activity assays were carried out in cyclase buffer with the addition of 1 mM GTP, 1 mM MgCl2, and 10 μM zaprinast to inhibit cyclic GMP degradation. Reactions were initiated by the addition of cytosolic protein; carried out for 10 min (30°C); stopped by heating (95°C) for 5 min; centrifuged briefly to clarify and the reaction, and the supernatant assayed for cyclic GMP content by ELISA.
Data analysis
Contractile activity was quantified by integration of the area under each contractile record using software written specifically for this purpose. To evaluate the effects of additions, spontaneous or agonist-stimulated activity was quantified for periods of 5 min before and after experimental additions; effects were then expressed as a percentage of the amount of activity present immediately before addition to control for any differences in contractile activity during the course of an experiment and to control for any cumulative effects of treatments. Values for cyclic GMP content were evaluated by one-way analysis of variance, and a t-test was employed as in Figure 4 to compare effects of treatments on contractile tension. Results presented are statistical means±s.e.mean and ‘n' always refers to the number of patients, each contributing a minimum of four tissue strip samples to each experimental set (an ‘n' of 3=12 tissue strips). ELISA assays for cyclic GMP concentrations were performed in duplicate (by dilution) for each tissue strip or in vitro sample. Curve fitting, data analysis and presentation graphics were performed with the aid of GraphPad Prism graphics software (Carlsbad, CA, U.S.A.).
Figure 4.
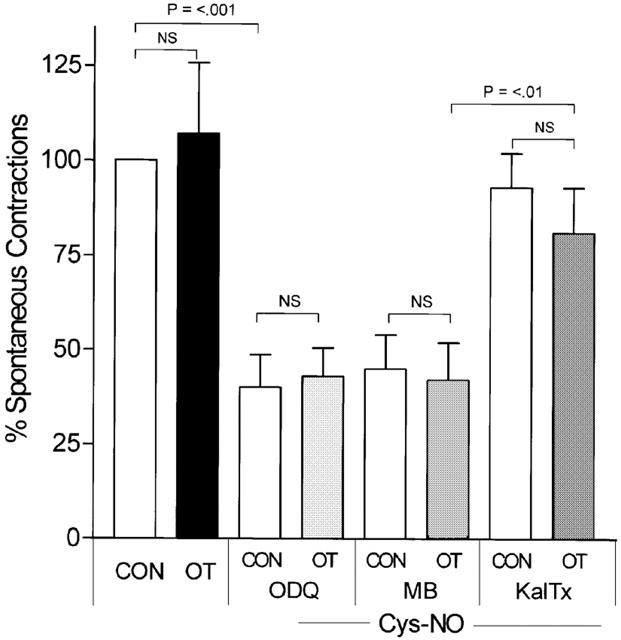
Cys-No-induced relaxation of spontaneous contractions in labouring myometrium are unaffected by guanylyl cyclase inhibitors but blocked by kaliotoxin. Addition of OT (10 μM) to labouring myometrium had no effect on spontaneous contractions (CON), while addition of Cys-NO (30 μM) caused significant relaxation regardless of pretreatment with the guanylyl cyclase inhibitors ODQ (1 μM) or MB (10 μM), or simultaneous stimulation by OT (10 μM). Addition of kaliotoxin to tissues for 1 min prior to addition of Cys-NO, however, significantly blunted the NO-induced relaxation in a fashion unaffected by OT.
Materials
Generally, compounds were made up in water except for ODQ (RBI; Natick, MA, U.S.A.), which was dissolved in 50% dimethyl sulphoxide (DMSO) and zaprinast (1,4-Dihydro-5-(2-propoxyphenyl)-7H-1,2,3-triazolo(4,5-δ)pyrimidin-7-one; Sigma-RBI) which was dissolved in 100% DMSO. The presence of DMSO was controlled for and in no circumstance was present in experiments at a final concentration exceeding 0.01%. Atosiban was obtained from Ferring AB (Limhamn, Sweden). Potassium channel specific toxins were obtained from Alomone Labs (Jerusalem, Israel). All other compounds were reagent grade and were obtained from Sigma Chemical (St. Louis, MO, U.S.A.).
Results
Human near-term non-labouring uterine smooth muscles were, while variable from both woman to woman and tissue piece to tissue piece, generally quiescent (Figure 1) compared to tissues obtained from non-pregnant women (Bradley et al., 1998). In non-labouring tissues, addition of OT led to the immediate generation of phasic contractions that could be completely relaxed by addition of Cys-NO (Figure 1). The ability of Cys-NO or SNAP to relax the tissue was independent of time of addition following OT stimulation at all times tested between 15 s and 10 min. Pre-treatment of tissues with the GC inhibitor ODQ did not prevent the ability of the tissue to respond to subsequent stimulation by OT or inhibition by Cys-NO or SNAP. Pre-treatment of tissues with potassium channel antagonists such as IBTx however, prevented the inhibition of contraction seen following Cys-NO addition.
Figure 1.
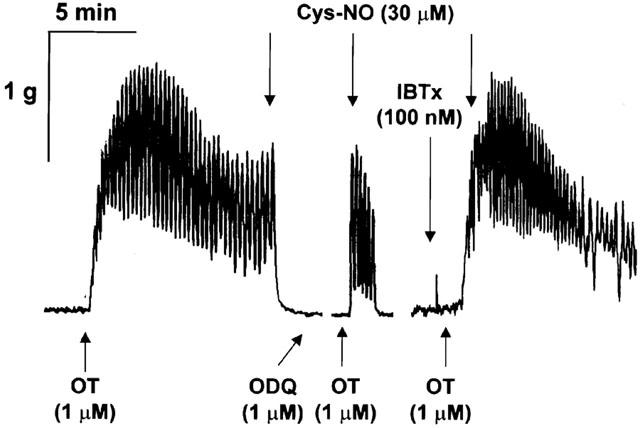
Agonist-mediated contraction/relaxation of pregnant myometrium. Strips of near-term pregnant non-labouring myometrium approximately 0.5×1×4 mm were dissected from samples taken from consenting women at the time of caesarean section within 90 min of donation. Tissues were hung in organ baths and tension recorded as described in Methods. Addition of agonists was accomplished by addition of 20 μl of concentrated stock to a 20 ml organ bath. Addition of the GC inhibitor ODQ (1 μM) was made 15 min prior to re-challenge with OT while IBTx (100 nM) was given a 1-min pretreatment. Breaks in the tracing occur where rest and washout periods are subtracted from the record. In the presence of ODQ, relaxations to Cys-NO could be seen during the initial contraction as illustrated or following up to 10 min in the presence of OT (not shown). Recording is representative of five experiments of four-tissue strips each and is made up from three periods of recording from a single continuous record of a single tissue strip.
OT was able to stimulate contractions in non-labouring tissues in a dose-dependent fashion with an EC50 of 0.8 μM (Figure 2). Prior addition of the OT receptor antagonist atosiban at a concentration of 1 μM blocked the ability of OT to stimulate contractions in non-labouring tissues by 80%. In labouring tissues, spontaneous activity was high and approximated that seen following maximal OT stimulation of non-labouring tissues (Figure 2). OT stimulation of labouring tissues did not elicit significant increases in tension, while treatment of unstimulated labouring tissues with 1 μM atosiban produced a significant inhibition of spontaneous contractions.
Figure 2.
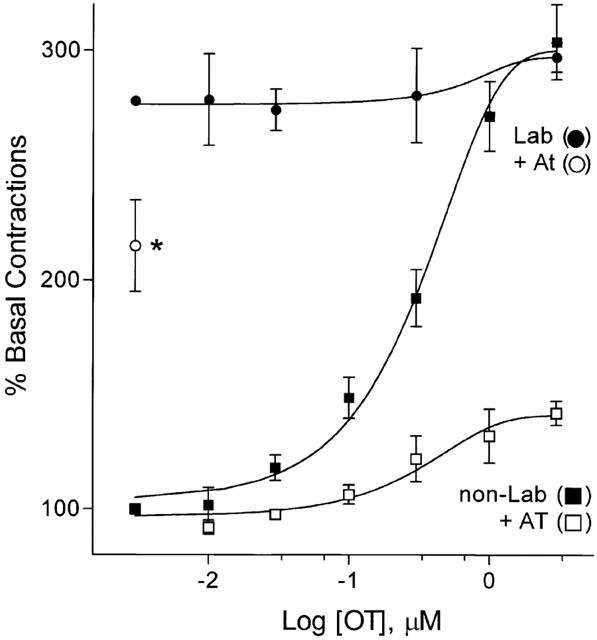
Oxytocin stimulation of labouring versus non-labouring myometrium. Experiments conducted as in Figure 1 were carried out in both labouring (Lab) and non-labouring (non-Lab) tissues (determined by the attending obstetric physician). In non-labouring tissues, concentration-dependent OT-stimulated contractions could be antagonized by addition of the OT receptor antagonist atosiban (1 μM), while in labouring tissues, addition of OT had no significant effect. Furthermore, addition of 1 μM atosiban to labouring tissues in the absence of OT only reduced tension modestly consistent with the presence of other endogenous mediators of contractile activity in labouring tissue. Data plotted as percentage of basal tension (0.72±0.31 g mm−3) are mean±s.e.mean of 6 – 11 samples (four replicates/sample).
In non-labouring tissues stimulated with 1 μM OT (a concentration that produced consistent contractions of the muscle and did not alter cyclic GMP), the addition of Cys-NO led to a dose-dependent relaxation (IC50=1 μM) that was unaltered by pre-treatment of tissues with 1 μM ODQ (Figure 3), a concentration that blocks myometrial GC. Cys-NO mediated relaxation was blocked by pre-treatment of tissues with each of three scorpion toxins at a concentration known (Garcia et al., 1999) to specifically block one or more calcium-activated K+-channels (K+Ca). In labouring tissues, where addition of OT (1 μM) had no significant effect on tension, treatment with Cys-NO still produced significant relaxation of the tissue (Figure 4) regardless of the presence of OT or the presence of GC inhibitors. Cys-NO mediated relaxation was prevented by pre-treatment of tissues with KalTx in a fashion independent of the presence of OT.
Figure 3.
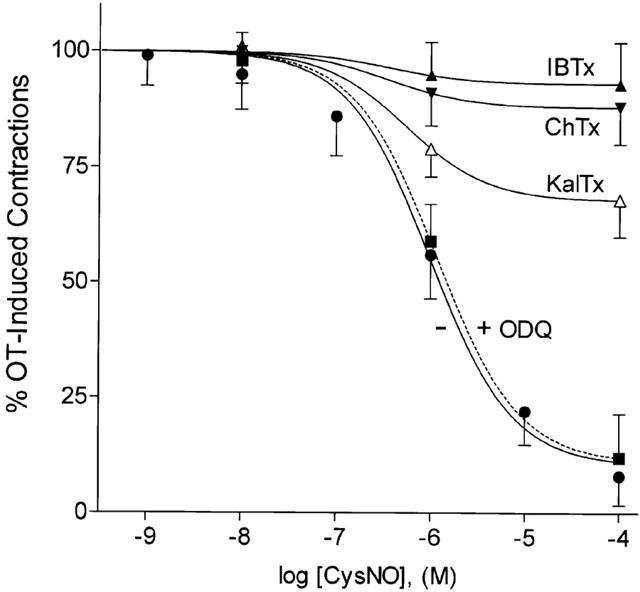
No-donors relax agonist-evoked contractions in human myometrium in a cyclic GMP-independent manner. Non-labouring tissues were stimulated with 1 μM OT (100%) and the tension elicited (2.38±0.76 g mm−3, n=8), relaxed by addition (2 min following OT) of increasing concentrations (IC50=1 μM) of the NO-donor Cys-NO in a non-cumulative manner. Prior treatment of tissues with ODQ (1 μM) for 15 min prior to addition of Cys-NO following addition of diluent or OT (1 μM) had not effect on either basal tension (0.57±0.16 g mm−3, n=6) or agonist evoked contractions (2.24±0.35 g mm−3) and did not prevent NO-mediated relaxation. Prior addition (1 min prior to OT) of any one of three scorpion toxins (KalTx, ChTx, or IBTx) at a concentration of 100 nM however, markedly reduced the ability of NO to relax the tissue.
Measurement of cyclic GMP in non-labouring tissues revealed that treatment with Cys-NO caused a 7.5 fold increase in cyclic GMP content (Figure 5) which was prevented by prior incubation of the tissue with the GC inhibitors ODQ (1 μM) or MB (10 μM), but not by the K+Ca-channel inhibitor IBTx (100 nM). Furthermore, the combination of IBTx and a GC inhibitor did not prevent the blockade of cyclic GMP accumulation produced by the GC inhibitor alone (Figure 5). In labouring tissues, basal cyclic GMP content (9.0±1.92 pmol mg protein−1, n=5) was significantly lower (P<0.05) than that seen in non-labouring tissues (15.8±1.09 pmol mg protein−1, n=12; mean±s.e.mean) and this was not altered by the presence of 1 μM OT (9.8±1.34 pmol mg protein−1, n=5 vs 16.9±1.29 pmol mg protein−1, n=12). Addition of Cys-NO to labouring tissues produced a 3.6 fold increase in cyclic GMP that was unaltered by the presence of OT (1 μM) and was completely blocked by pre-treatment of tissues with a GC inhibitor (Figure 6). Treatment of labouring tissues with KalTx, however, in the absence of GC inhibitors, did not alter the accumulation of cyclic GMP seen following addition of Cys-NO.
Figure 5.
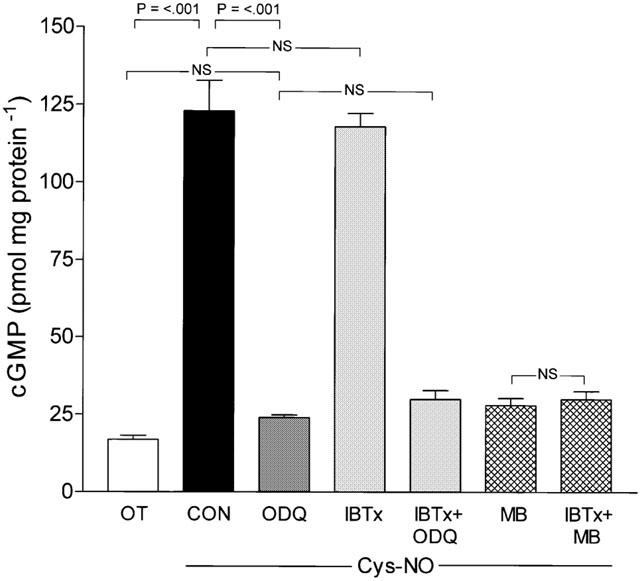
Nitric oxide-stimulated cyclic GMP accumulation in non-labouring myometrium is blocked by GC inhibitors but not by scorpion toxin. Cys-No caused a 7.5 fold increase in basal cyclic GMP content (15.8±1.09 pmol mg protein−1) in non-labouring tissues snap frozen during contractile experiments. OT (10 μM) alone (16.9±4.5 pmol mg protein−1) had no effect on basal cyclic GMP content. Cys-NO (30 μM) stimulation of ODQ pretreated tissues (15 min, 1 μM) led to near complete inhibition of Cys-NO stimulation of cyclic GMP accumulation (P<0.001) and thus no significant elevation of cyclic GMP content over baseline (Basal vs Cys-NO with ODQ, P>0.05). Cys-NO stimulated cyclic GMP accumulation was not altered by IBTx (100 nM) treatment, which also did not alter the ability of ODQ and MB to block guanylyl cyclase. Data expressed as pmol of cyclic GMP mg protein−1 are mean±s.e.mean, n=12).
Figure 6.
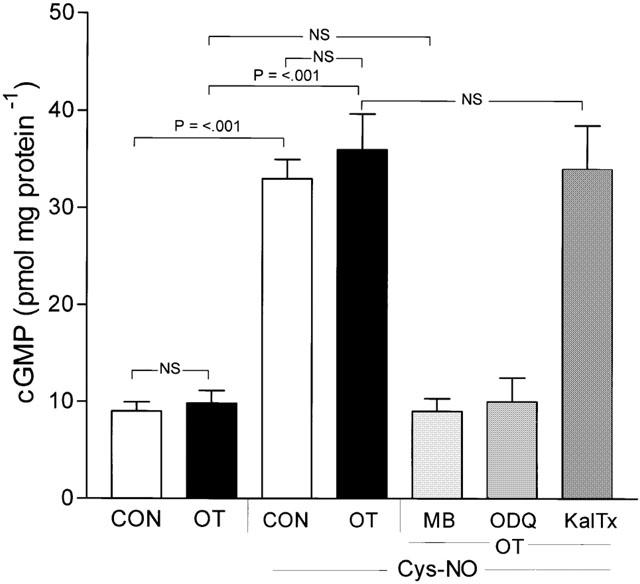
Cyclic GMP accumulation in labouring myometrium is prevented by GC inhibitors. Spontaneously active labouring tissues (CON) were snap frozen and cyclic GMP content (9±2.1 pmol mg protein−1) determined by ELISA as described in the text. OT stimulation (10 μM) had no effect on cyclic GMP content (P>0.05), while Cys-NO stimulation (30 μM) led to a significant elevation in cyclic GMP (33±4 pmol mg protein−1) that was blocked by prior treatment of tissues with GC inhibitors ODQ (1 μM) or MB (10 μM), but not KalTx. Data are mean±s.e.mean n=5.
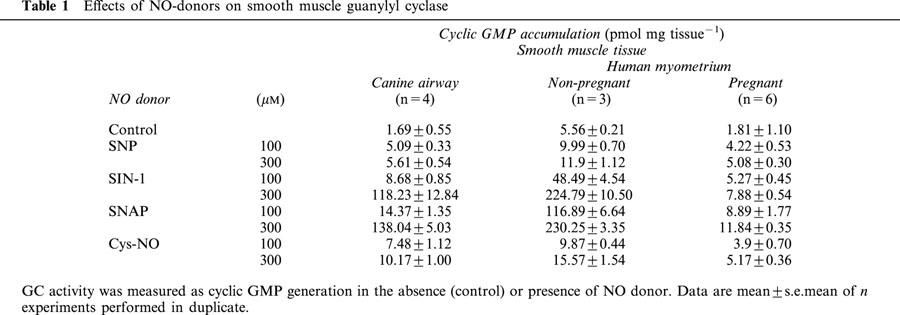
The likely absence of a role for cyclic GMP in the action of NO to relax human myometrium at term is further demonstrated by studies examining the role of the NO donors SNAP and SIN-1 which are well characterized in their ability to relax smooth muscles (Darkow et al., 1997; Janssen et al., 2000; Kiris et al., 1999). Indeed, in pregnant human myometrial smooth muscle from non-labouring patients at term, addition of SNAP but not SIN-1 resulted in a dose-dependent relaxation of OT-stimulated contractions (Figure 7). When 100 μM SNAP was added to pregnant, non-labouring tissues at peak tension following stimulation with 1 μM OT and recordings quantified over 5 min after NO-donor addition, results demonstrated a significant effect of SNAP but not SIN-1 to relax the tissues (Control=100% vs SNAP=76.5±6.5%, P<0.05; Control 100% vs SIN-1=98±3.2%, NS). The failure of SIN-1 to relax the tissue was not the result of a failure of this NO-donor to liberate NO and activate the soluble myometrial GC since direct analysis of GC activity in the presence of 100 μM or 300 μM SIN-1 revealed stimulation that was greater than that seen for the same concentration of Cys-NO which does relax myometrium (Figure 8). Indeed, careful measurement of GC activity in canine smooth muscle where both SIN-1 and SNAP relax agonist-induced contractions (Janssen et al., 2000) revealed that both donors cause significant dose-dependent stimulation of the enzyme (Table 1). This effect of SIN-1 and SNAP to stimulate GC is seen in both pregnant and non-pregnant human myometrium even though SIN-1 fails to relax agonist-induced contraction in myometrium (Figure 7).
Figure 7.
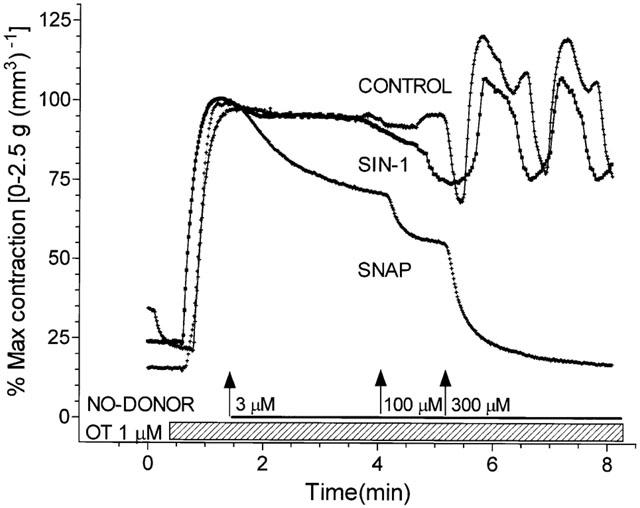
Dose-dependent relaxation of pregnant myometrium by SNAP. Tissues were stimulated with 1 μM OT at the time indicated by the hatched bar and allowed to contract to peak tension. In some tissues (as in the tracing shown) the NO-donors SNAP or SIN-1 were added in a cumulative fashion as indicated by the labelled open bar, to clearly illustrate the ability of SNAP to relax the tissue. SIN-1 did not produce relaxation. Data illustrated are representative of four tissues each receiving SNAP and SIN-1 additions. Similar results were obtained in six experiments where NO-donor was added at 100 μM to pregnant non-labouring tissues at peak tension following OT stimulation and recordings quantified over 5 min after NO-donor demonstrating a significant effect of SNAP but not SIN-1 to relax the tissues when (Control=100% vs SNAP=77.5±6.5%, P<0.05; Control 100% vs SIN-1=98±3.2%, NS). Tissue failing to respond to SIN-1, responded to SNAP while SNAP sensitive tissues failed to respond to SIN-1.
Figure 8.
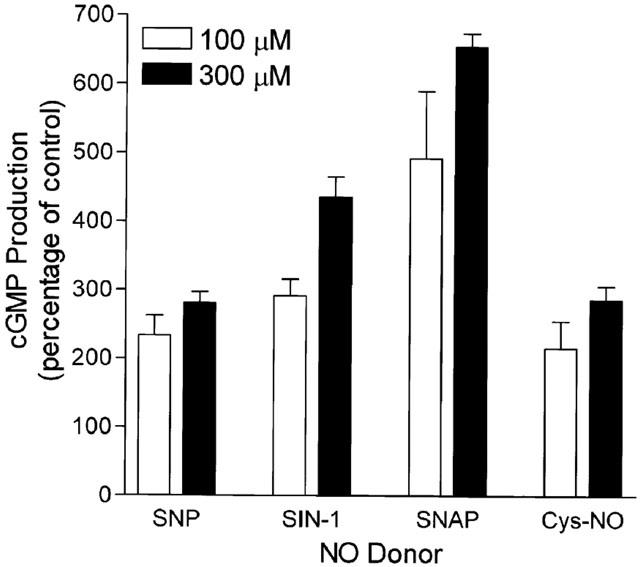
NO-donors stimulate GC in pregnant non-labouring myometrium. Guanylyl cyclase was assayed in smooth muscle homogenates and determined by ELISA. NO-donors were added to the reaction buffer followed immediately by addition of the homogenate sample. Control experiments established that cyclic GMP generation under our conditions was linear over time from 2 – 20 min and over a range protein from one-third to three times that routinely added in the assay. Data, included in Table 1, are mean±s.e.mean of duplicate observations in replicate experiments (n=6).
Table 1.
Effects of NO-donors on smooth muscle guanylyl cyclase
Discussion
Pregnant human myometrium relaxes in response to NO delivered in the form of Cys-NO whether or not the tissue is labouring. Because NO-donors are ineffective at relaxing myometrium or stimulating cyclic GMP accumulation in our hands if they have first been bubbled with oxygen, we attribute the effects of Cys-NO and other NO-donors we employ to the liberation of NO consistent with current understanding of the breakdown of nitroso-containing drugs in oxygenated solution at neutral pH (Stamler et al., 1992). Because we add NO-donors to 20 ml tissue baths containing small pieces of myometrium (∼0.5×1×4 mm) suspended in 37°C oxygenated buffer, we cannot accurately assess the exact delivery of NO, in either time or concentration, to myometrial smooth muscle cells in our preparation.
The effect of NO to relax pregnant myometrium is not likely to be due to the choice of OT as the agonist since Cys-NO mediated relaxations can be elicited both in OT stimulated non-labouring tissues as well as labouring tissues where it is clear from use of atosiban to block the effects of endogenous OT that relaxation can be produced that exceeds the effect of OT receptor blockade (Figures 2 and 4). Furthermore, in data not presented here, non-labouring tissues from pregnant women that were stimulated to contract with prostaglandin F2α (100 nM) were relaxed by addition of Cys-NO (10 μM). The effect of Cys-NO is reasonably potent in human pregnant tissue with an apparent EC50 of 1 μM.
The ability of Cys-NO to relax exogenous agonist-induced contractions in labouring tissues was not readily observable, per se, since these tissues were significantly more spontaneously active than non-labouring tissues and addition of up to 3 μM OT did not produce significant additional contractile activity. Since addition of the OT receptor antagonist atosiban to labouring tissue significantly reduced spontaneous activity, it is probable that at least this component of activity is directly due to endogenous OT stimulation. Addition of Cys-NO to these preparations relaxes the tissue to a degree (60%) that exceeds that produced by atosiban alone (35%). Although it is clear from our data that 30 μM Cys-NO produces a more complete relaxation in non-labouring pregnant myometrium (87% inhibition) than that seen in labouring tissues (60% inhibition) the effects of Cys-NO in both states of the tissue are very clearly independent of the accumulation of cyclic GMP that follows addition of Cys-NO (Figures 5 and 6). This cyclic GMP-independence is striking since it is possible to completely block the change in intracellular cyclic GMP accumulation without any effect on the ability of Cys-NO to relax the tissue. It is not inconceivable that only a very minor change in intracellular cyclic GMP accumulation in one or more restricted regions of the smooth muscle cell that can not be measured, might be necessary and sufficient for the full effect of cyclic GMP in the tissue and that we miss such changes in determining total cyclic GMP content. While subcellular compartmentalization of cyclic nucleotide action has been seen in muscle tissues as first described by us (Buxton & Brunton,1983), such compartments were not established on the basis of a failure to adequately determine cellular changes in cyclic nucleotide, but rather, were due to distinctions in the ability of all cyclic nucleotide to interact with its protein kinase. Furthermore, we do not complicate our interpretation that the effect of NO in myometrial tissues during contractions is cyclic GMP-independent by the addition of phosphodiesterase inhibitors to the preparation. We follow changes in cyclic GMP by freezing tissues in the contractile bath within seconds of knowing their physiologic status thus ensuring a meaningful relationship between our contractile and biochemical data. Furthermore, while pregnant myometrium is multicellular, we hang pieces of tissue that are predominantly muscle by cell volume. The ability of Cys-NO to elicit large increases in cyclic GMP is consistent with its action on a soluble GC enzyme found in the smooth muscle cells. If this change in cyclic GMP subserved relaxation, then the ability to block its accumulation should prevent the relaxation. This is not the case.
The notion that an NO-donor can act in a cyclic GMP-independent manner is not unique to myometrial smooth muscle (Kosonen et al., 1998) and furthermore, there are now several reports of NO-induced relaxation in smooth muscles possessing significant cyclic GMP-independent components (Stuart-Smith et al., 1994; Zhou & Torphy, 1991). Perkins et al. (1998) working in airway smooth muscle, have shown that the S-nitroso glutathione (GSNO) induced relaxation of the muscle is due to reversible oxidation of thiols (Perkins et al., 1998). While GSNO-induced relaxation of acetylcholine contracted canine trachealis was correlated with an immediate, several fold-increase in cyclic GMP in their study, subsequent addition of the thiol reducing agent dithiothreitol (DTT), reversed the relaxation. While the Perkins study does not investigate potential distinctions between S-nitroso thiols and other NO-donors, it does offer a plausible mechanism for cyclic GMP-independent effects of NO due to nitrosonium cation transfer from the NO-donor to one or more protein acceptors.
The possibility that a smooth muscle might relax to an NO-donor in a fashion independent of cyclic GMP accumulation, as we propose, is underscored by the effect of SNAP and SIN-1 in pregnant human myometrium. While addition of SNAP elicited dose-dependent relaxation, addition of SIN-1 did not. The failure of SIN-1 to relax the tissue could not be attributed to a failure of the donor to stimulate the GC enzyme from pregnant tissues since SIN-1 was more efficacious than sodium nitroprusside or Cys-NO in stimulating cyclic GMP production (Figure 8, Table 1). Furthermore, the use of SIN-1 under our exact conditions does cause relaxation of human trachealis smooth muscle pre-contracted with acetylcholine (data not shown). While the failure of SIN-1 to relax myometrium was not anticipated, and is not yet explained, we see the same phenomenon in guinea-pig myometrium (data not shown). Furthermore, it follows from the work of Janssen et al. (2000) that such a distinction could have a mechanistic basis.
These authors demonstrated that SNAP and SIN-1 both relax agonist-mediated contractions in canine trachealis. Although they did not measure cyclic GMP in their study, the ability of DTT to reverse the SNAP-mediated calcium rise they measured, together with producing a rightward shift in the concentration-relaxation relationship for SNAP, was taken to mean that thiol oxidation (presumably involving NO+) was the mechanism of action of SNAP. In addition, the absence of an effect of SIN-1 on intracellular calcium together with a less dramatic effect of DTT on the SIN-1 concentration-relaxation relationship were taken to mean that this compound does not generate nitrosonium cation (NO+) as is the proposed case for SNAP, but rather liberates neutral nitric oxide radical (NO·) which, serves to activate GC leading to cyclic GMP accumulation and subsequent relaxation via one or more actions of cyclic GMP-dependent protein kinase.
We have measured cyclic GMP in canine airway stimulated by SNAP or SIN-1 and other NO-donors (Table 1) as well as the effects of these donors on both pregnant and non-pregnant human myometrium. It is clear from our data and that of Jones et al. (1994) in canine airway, that both SNAP and SIN-1 stimulate GC in a dose-dependent fashion. We suggest that the strict distinctions regarding the oxidation states of NO liberated by SNAP and SIN-1 donors as proposed by Janssen et al. (2000) is unreasonable as the sole distinction in the actions of these compounds. A role for NO in mediating calcium-dependent K+ efflux independent of changes in cyclic GMP or the action of its kinase, is consistent with relaxation whatever the upstream biochemical pathway, and is consistent with our data.
Relaxations to Cys-NO can be prevented by the use of selective toxins. The short chain scorpion toxins, kaliotoxin from the species Androctonus mauretanicus, charybdotoxin from the scorpion Leiurus quinquestriatus hebraeus, and iberiotoxin from Buthus tamulus, are specific inhibitors of high conductance K+Ca (Garcia et al., 1999). The ability of these agents to block the relaxation to NO suggests that one or more channels of this type is expressed in near-term human myometrium and may be the cyclic GMP-independent target for the action of NO. The mechanism underlying NO-induced cyclic GMP-independent relaxation would then be expected to follow from an elevation in intracellular calcium leading to activation of the K+Ca. Opening of K+Ca would lead to activation of the voltage-dependent channels permeable to potassium and cause hyperpolarization of the cell resulting in relaxation.
While such a mechanism is plausible, it is not yet well established in myometrium although there is recent electrophysiological evidence supporting it (Shimano et al., 2000). Direct activation of Ca2+-activated K+-channels by nitric oxide not withstanding, ascribing K+-channel activation to the role of NO is awkward even with the knowledge that scorpion toxins block the effects of NO donors. That is, any mechanism that leads to relaxation in smooth muscle tissues with elevated calcium levels, as is the case in either spontaneously active or agonist-stimulated myometrium, can be expected to involve K+-channel activity.
Thus, we predict that the target for NO that mediates relaxation in uterine smooth muscle is distinct from that found in other tissues and may be unique to the myometrial cell. If we are correct, the search for a tocolytic based on this unique K+-channel, or K+-channel associated target may provide selective relaxation of preterm uterine contractions from any cause, while drug-discovery based on cyclic GMP-dependent mechanisms will likely fail.
Acknowledgments
The authors are grateful to Shannon Martin for technical assistance with tissue acquisition and performing contractile studies, and to Zosia Targosz for clerical assistance with the manuscript. This work was supported in part by NIH HD56422 and HL35416 to I.L.O. Buxton and a grant from the Robert Z. Hawkins Foundation.
Abbreviations
- AEBSF
4-(2-Aminoethyl) benzenesulphonyl fluoride hydrochloride
- ChTx
charybdotoxin
- Cys-NO
S-nitroso L-cysteine
- GC
guanylyl cyclase
- IBTx
iberiotoxin
- KalTx
kaliotoxin
- K+Ca
Ca2+-activated potassium channels
- SIN-1
3-morpholinosyndonimine
- SNAP
S-nitroso N-acetyl penicillamine
References
- AHERN G.P., HSU S.F., JACKSON M.B. Direct actions of nitric oxide on rat neurohypophysial K+ channels. J. Physiol. (Lond) 1999;520:165–176. doi: 10.1111/j.1469-7793.1999.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLOTINA V.M., NAJIBI S., PALACINO J.J., PAGANO P.J., COHEN R.A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- BRADLEY K.K., BUXTON I.L.O., BARBER J.E., MCGAW T., BRADLEY M.E. Nitric oxide relaxes human myometrium by a cGMP-independent mechanism. Am. J. Physiol. 1998;44:C1668–C1673. doi: 10.1152/ajpcell.1998.275.6.C1668. [DOI] [PubMed] [Google Scholar]
- BRUNE B., MOHR S., MESSMER U.K. Protein thiol modification and apoptotic cell death as cGMP-independent nitric oxide (NO) signaling pathways. Rev. Physiol. Biochem. Pharmacol. 1996;127:1–30. doi: 10.1007/BFb0048263. [DOI] [PubMed] [Google Scholar]
- BUHIMSCHI I., YALLAMPALLI C., DONG Y., GARFIELD R.E. Involvement of a nitric oxide-cyclic guanosine monophosphate pathway in control of human uterine contractility during pregnancy. Am. J. Obstet. Gynecol. 1995;172:1577–1584. doi: 10.1016/0002-9378(95)90500-6. [DOI] [PubMed] [Google Scholar]
- BUTCHER E.C., LOWRY O.H. Measurement of nanogram quantities of protein by hydrolysis followed by reaction with orthophthalaldehyde or determination of glutamate. Anal. Biochem. 1976;76:502–523. doi: 10.1016/0003-2697(76)90343-2. [DOI] [PubMed] [Google Scholar]
- BUXTON I.L.O., BRUNTON L.L. Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. J. Biol. Chem. 1983;258:10233–10239. [PubMed] [Google Scholar]
- BUXTON I.L.O., CROW W., MATHEW S. Regulation of uterine contraction: mechanisms in preterm labor. AACN Clin. Iss. 2000;11:271–282. doi: 10.1097/00044067-200005000-00010. [DOI] [PubMed] [Google Scholar]
- DARKOW D.J., LU L., WHITE R.E. Estrogen relaxation of coronary artery smooth muscle is mediated by nitric oxide and cGMP. Am. J Physiol. 1997;272:H2765–H2773. doi: 10.1152/ajpheart.1997.272.6.H2765. [DOI] [PubMed] [Google Scholar]
- DIAMOND J. Lack of correlation between cyclic GMP elevation and relaxation of nonvascular smooth muscle by nitroglycerin, nitroprusside, hydroxylamine and sodium azide. J. Pharmacol. Exp. Ther. 1983;225:422–426. [PubMed] [Google Scholar]
- GARCIA M.L., HANNER M., KNAUS H.G., SLAUGHTER R., KACZOROWSKI G.J. Scorpion toxins as tools for studying potassium channels. Methods Enzymol. 1999;294:624–639. doi: 10.1016/s0076-6879(99)94035-1. [DOI] [PubMed] [Google Scholar]
- GIBSON A., BABBEDGE R., BRAVE S.R., HART S.L., HOBBS A.J., TUCKER J.F., WALLACE P., MOORE P.K. An Investigation of some S-nitrosothiols, and of hydroxy-arginine, on the mouse anococcygeus. Br. J. Pharmacol. 1992;107:715–721. doi: 10.1111/j.1476-5381.1992.tb14512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IZUMI H., GARFIELD R.E. Relaxant effects of nitric oxide and cyclic GMP on pregnant rat uterine longitudinal smooth muscle. Eur. J. Obstet. Gynecol. Rep. Biol. 1995;60:171–180. doi: 10.1016/0028-2243(95)02096-b. [DOI] [PubMed] [Google Scholar]
- JANSSEN L.J., PREMJI M., LU-CHAO H., COX G., KESHAVJEE S. NO(+) but not NO radical relaxes airway smooth muscle via cGMP-independent release of internal Ca(2+) Am. J. Physiol. 2000;278:L899–L905. doi: 10.1152/ajplung.2000.278.5.L899. [DOI] [PubMed] [Google Scholar]
- JONES K.A., LORENZ R.R., WARNER D.O., KATUSIC Z.S., SIECK G.C. Changes in cytosolic cGMP and calcium in airway smooth muscle relaxed by 3-morpholinosydnonimine. Am. J. Physiol. 1994;266:L9–L16. doi: 10.1152/ajplung.1994.266.1.L9. [DOI] [PubMed] [Google Scholar]
- KIRIS T., KARASU A., YAVUZ C., ERDEM T., UNAL F., HEPGUL K., BALOGLU H. Reversal of cerebral vasospasm by the nitric oxide donor SNAP in an experimental model of subarachnoid haemorrhage. Acta Neurochim. 1999;141:1323–1328. doi: 10.1007/s007010050437. [DOI] [PubMed] [Google Scholar]
- KISHNANI N.S., FUNG H.L. Nitric oxide generation from pharmacological nitric oxide donors. Methods Enzymol. 1996;268:259–265. doi: 10.1016/s0076-6879(96)68028-8. [DOI] [PubMed] [Google Scholar]
- KOKS C.A., BROLMANN H.A., DE KLEINE M.J., MANGER P.A. A Randomized Comparison of nifedipine and ritodrine for supression of preterm labor. Eur. J. Obstet. Gynecol. Rep. Biol.. 1998;77:171–176. doi: 10.1016/s0301-2115(97)00255-8. [DOI] [PubMed] [Google Scholar]
- KOSONEN O., KANKAANRANTA H., LAHDE M., VUORINEN P., YLITALO P., MOILANEN E. Nitric oxide-releasing oxatriazole derivatives inhibit human lymphocyte proliferation by a cyclic GMP-independent mechanism. J. Pharmacol. Exp. Ther. 1998;1998;286:215–220. [PubMed] [Google Scholar]
- KUENZLI K.A., BRADLEY M.E., BUXTON I.L.O. Cyclic GMP-independent effects of nitric oxide on guinea-pig uterine contractility. Br. J. Pharmacol. 1996;119:737–743. doi: 10.1111/j.1476-5381.1996.tb15734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUENZLI K.A., BUXTON I.L., BRADLEY M.E. Nitric oxide regulation of monkey myometrial contractility. Br. J. Pharmacol. 1998;124:63–68. doi: 10.1038/sj.bjp.0701799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEES C., CAMPBELL S., JAUNIAUX E., BROWN R., RAMSAY B., GIBB D., MONCADA S. Arrest of preterm labor and prolongation of gestation with glyceryl trinitrate, a nitric oxide donor. Lancet. 1994;343:1325–1326. doi: 10.1016/s0140-6736(94)92468-6. [DOI] [PubMed] [Google Scholar]
- MURAD F. Cyclic guanosine monophosphate as a mediator of vasodilation. J. Clin. Invest. 1986;78:1–5. doi: 10.1172/JCI112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERKINS W.J., PABELICK C., WARNER D.O., JONES K.A. cGMP-independent mechanism of airway smooth muscle relaxation induced by S-nitrosoglutathione. Am. J. Physiol. 1998;275:468–474. doi: 10.1152/ajpcell.1998.275.2.C468. [DOI] [PubMed] [Google Scholar]
- PINILLA L., GONZALEZ D., TENA-SEMPERE M., AGUILAR E. Nitric oxide (NO) stimulates gonadotropin secretion in vitro through a calcium-dependent, cGMP-independent mechanism. Neuroendocrinology. 1998;68:180–186. doi: 10.1159/000054364. [DOI] [PubMed] [Google Scholar]
- PINILLA L., TENA-SEMPERE M., AGUILAR E. Nitric oxide stimulates growth hormone secretion in vitro through a calcium- and cyclic guanosine monophosphate-independent mechanism. Horm. Res. 1999;51:242–247. doi: 10.1159/000023378. [DOI] [PubMed] [Google Scholar]
- SHIMANO M., NAKAYA Y., FUKUI R., KAMADA M., HAMADA Y., MAEDA K., AONO T. Activation of Ca2+-activated K+ channels in human myometrium by nitric oxide. Gynecol. Obstet. Invest. 2000;49:249–254. doi: 10.1159/000010254. [DOI] [PubMed] [Google Scholar]
- STAMLER J.S., SINGEL D.J., LOSCALZO J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- STUART-SMITH K., BYNOE T.C., LINDEMAN K.S., HIRSHMAN C.A. Differential effects of nitrovasodilators and nitric oxide on porcine tracheal and bronchial muscle in vitro. J. Applied Physiol. 1994;77:1142–1147. doi: 10.1152/jappl.1994.77.3.1142. [DOI] [PubMed] [Google Scholar]
- SUMMERS B.A., OVERHOLT J.L., PRABHAKAR N.R. Nitric oxide inhibits L-type Ca2+ current in glomus cells of the rabbit carotid body via a cGMP-independent mechanism. J. Neurophysiol. 1999;81:1449–1457. doi: 10.1152/jn.1999.81.4.1449. [DOI] [PubMed] [Google Scholar]
- TROTTIER G., TRIGGLE C.R., O'NEILL S.K., LOUTZENHISER R. Cyclic GMP-dependent and cyclic GMP-independent actions of nitric oxide on the renal afferent arteriole. Br. J. Pharmacol. 1998;125:563–569. doi: 10.1038/sj.bjp.0702090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WORD R.A., CASEY M.L., KAMM K.E., STULL J.T. Effects of CGMP on [Ca2+]i, myosin light chain phosphorylation, and contraction in human myometrium. Am. J. Physiol. 1991;260:C861–C867. doi: 10.1152/ajpcell.1991.260.4.C861. [DOI] [PubMed] [Google Scholar]
- WU S.N., HWANG T., TENG C.M., LI H.F., JAN C.R. The mechanism of actions of 3-(5′-(hydroxymethyl-2′-furyl)-1-benzyl indazole (YC-1) on Ca(2+)-activated K(+) currents in GH(3) lactotrophs. Neuropharmacology. 2000;39:1788–1799. doi: 10.1016/s0028-3908(00)00025-3. [DOI] [PubMed] [Google Scholar]
- YALLAMPALLI C., GARFIELD R.E., BYAM-SMITH M. Nitric oxide inhibits uterine contractility during pregnancy but not during delivery. Endocrinology. 1993;133:1899–1902. doi: 10.1210/endo.133.4.8404632. [DOI] [PubMed] [Google Scholar]
- ZHOU H.L., TORPHY T.J. Relationship between cyclic guanosine monophosphate accumulation and relaxation of canine trachealis induced by nitrovasodilators. J. Pharmacol. Exp. Ther. 1991;258:972–978. [PubMed] [Google Scholar]


