Abstract
Adenosine, an ubiquitous neuromodulator, and its analogues have been shown to produce ‘depressant' effects in animal models believed to be relevant to depressive disorders, while adenosine receptor antagonists have been found to reverse adenosine-mediated ‘depressant' effect.
We have designed studies to assess whether adenosine A2A receptor antagonists, or genetic inactivation of the receptor would be effective in established screening procedures, such as tail suspension and forced swim tests, which are predictive of clinical antidepressant activity.
Adenosine A2A receptor knockout mice were found to be less sensitive to ‘depressant' challenges than their wildtype littermates. Consistently, the adenosine A2A receptor blockers SCH 58261 (1 – 10 mg kg−1, i.p.) and KW 6002 (0.1 – 10 mg kg−1, p.o.) reduced the total immobility time in the tail suspension test.
The efficacy of adenosine A2A receptor antagonists in reducing immobility time in the tail suspension test was confirmed and extended in two groups of mice. Specifically, SCH 58261 (1 – 10 mg kg−1) and ZM 241385 (15 – 60 mg kg−1) were effective in mice previously screened for having high immobility time, while SCH 58261 at 10 mg kg−1 reduced immobility of mice that were selectively bred for their spontaneous ‘helplessness' in this assay.
Additional experiments were carried out using the forced swim test. SCH 58261 at 10 mg kg−1 reduced the immobility time by 61%, while KW 6002 decreased the total immobility time at the doses of 1 and 10 mg kg−1 by 75 and 79%, respectively.
Administration of the dopamine D2 receptor antagonist haloperidol (50 – 200 μg kg−1 i.p.) prevented the antidepressant-like effects elicited by SCH 58261 (10 mg kg−1 i.p.) in forced swim test whereas it left unaltered its stimulant motor effects.
In conclusion, these data support the hypothesis that A2A receptor antagonists prolong escape-directed behaviour in two screening tests for antidepressants. Altogether the results support the hypothesis that blockade of the adenosine A2A receptor might be an interesting target for the development of effective antidepressant agents.
Keywords: Adenosine, A2A receptor, A2A receptor knockout mice, antidepressant, forced swim test, tail suspension test, motor activity, SCH 58261, KW 6002, ZM 241385
Introduction
There is evidence that adenosine is a neuromodulator which takes part in a variety of processes in both physiological and pathological conditions. In the central nervous system, adenosine is involved in controlling behavioural states along the continuum wakefulness-sedation (Porkka-Heiskanen, 1999), has been associated with mood changes such as anxiety (Jain et al., 1995; El Yacoubi et al., 2000a), is involved in cognitive processes (Kopf et al., 1999) and has an important role in the regulation of motor activity (Brockwell & Beninger, 1996). Research efforts made over the last 20 years have resulted in the discovery of four G-protein coupled receptors which specifically bind adenosine to produce biological effects (Olah & Stiles, 2000). These receptors, namely adenosine A1, A2A, A2B and A3 receptors, have distinct distributions and control different functions in the mammalian organism. In the brain, adenosine A1 receptors are abundant, especially in the cortex, whereas A2A receptors are mainly located in the striatum. Conversely, both adenosine A2B and A3 receptors are present in low amounts in the brain. The A2B receptor has recently been shown to constitute also a receptor for the neurotrophic factor netrin-1 (Corset et al., 2000), while the function of the A3 receptor remains to be elucidated (Impagnatiello et al., 2000).
A variety of studies have shown that blocking the A2A receptors leads to significant improvement of motor dysfunction. Among potent adenosine A2A receptor antagonists used as tools in these pharmacological studies, SCH 58261 showed an excellent selectivity profile at human adenosine receptors in a recent study (Ongini et al., 1999): A2A (Ki 0.6 nM)<A1 (Ki 287 nM)<A2B (Ki 5,011 nM)<A3 (Ki>10,000 nM). In the same study, ZM 241385, another potent non-xanthine A2A receptor antagonist (Ki 0.8 nM), also showed little affinity for A1 receptor (Ki 255 nM) and did not interact with A3 (Ki>10,000 nM); however, it displayed moderate affinity for A2B receptors (Ki 50 nM). This less favourable profile has been confirmed later (Klotz, 2000). The xanthine-like derivative KW 6002 was shown to display high affinity for A2A receptor (Ki 2.2 nM), moderate A2A versus A1 selectivity and to be active in experimental models of Parkinson's disease (Shimada et al., 1997; Shiozaki et al., 1999). Hence, adenosine A2A receptor antagonists are considered as potential drugs in the treatment of movement disorders such as Parkinson's disease (Ongini & Fredholm, 1996; Richardson et al., 1997). This activity is believed to depend upon the close anatomical and functional association between adenosine A2A and dopamine D2 receptors on the so-called indirect striato-pallidal GABAergic pathway (Ferré et al., 1997). Thus, blockade of the adenosine A2A receptors would reinstate normal movements through interactions with dopamine-mediated activity in basal ganglia.
Consistent with pharmacological data, genetic inactivation of the adenosine A2A receptor gene has shown that knockout mice are more resistant to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a neurotoxin which produces damage similar to that observed in Parkinson's disease (Chen et al., 2000).
Other data suggest that A2A receptors are involved in mediating the effects of adenosine on behavioural states. Adenosine A2A receptor knockout mice display behavioural changes, such as aggressiveness and hypoalgesia (Ledent et al., 1997). In general, adenosine and its analogues tend to produce ‘depressant' effects in animal models, believed to be relevant to human conditions. For example, stimulation of adenosine receptors or increase of adenosine levels induce a state of ‘learned helplessness' similar to that observed in an animal model of depression generally considered as reliable (Minor et al., 1994; Woodson et al., 1998). Adenosine and 2-chloroadenosine increase the immobility time in the forced swim test in mice, a widely used model of depression (Porsolt et al., 1977), while classical antidepressants have been found to reverse adenosine-mediated immobility (Kulkarni & Mehta, 1985).
The adenosine A2A receptors might be involved in these processes through their interaction with dopamine D2 receptors in the striatum, which, together with the dopamine neuronal transporters, are increased in depressed patients (D'haenen & Bossuyt, 1994; Shah et al., 1997; Laasonen-Balk et al., 1999). Consistent with these data, studies have shown that bromocriptine (Colonna et al., 1979; Sitland-Marken et al., 1990) and piribedil (Post et al., 1978; Mouret et al., 1987), two dopamine D2 receptor agonists, which are mainly used for treatment of Parkinson's disease, show some antidepressant activity. Therefore, adenosine A2A receptor antagonists, by acting on various circuitries in the brain, or more specifically by modulating mesostriatal or mesocorticolimbic dopaminergic pathways, may also possess antidepressant properties.
Within this background we have designed studies to assess whether adenosine A2A receptor antagonists or genetic inactivation of the receptors, using adenosine A2A receptor knockout mice, would be effective in established models of depression. The data show that reference adenosine A2A receptor blockers produce dose-related effects in mouse models of depression such as the forced swim or the tail suspension tests. Consistently, adenosine A2A receptor knockout mice were found to be less sensitive to ‘depressogenic' challenges than their wildtype littermates. Altogether, the data support the hypothesis that blockade of the adenosine A2A receptors might be an interesting and novel approach in the search of effective antidepressant agents.
Methods
Animals
Male Swiss albino CD1 mice bred by Charles River (Saint Aubin lès Elbeuf, France, and Calco, Italy), male Swiss albino CD1 mice selectively bred in our facilities (UMR CNRS 6036, Rouen, France) for high spontaneous ‘helplessness' in the tail suspension test (Vaugeois et al., 1996), or adenosine A2A receptor knockout mice and their wildtype controls bred on a CD1 background for five to ten generations (Ledent et al., 1997), weighing 20 – 30 g were used after at least one week of habituation in our own facilities. Mice were housed in groups of 15 – 20 in Makrolon cages (38×24×18 cm) with free access to water and food (U.A.R., France, and Charles River, Calco, Italy) and kept in a ventilated room at a temperature of 21°C±1°C, under a 12 h light/12 h dark cycle (light on between 0700 and 1900). Experiments were carried out between 0900 and 1900. The animals were isolated in small individual cages (27×13×13 cm) for 30 min prior testing.
The procedures described comply with ethical principles and guidelines for care and use of laboratory animals adopted by the European Community, law 86/609/CCE.
Tail suspension test
The tail suspension test is based on the observation that a mouse suspended by the tail shows alternate periods of agitation and immobility (Stéru et al., 1985). The mouse, acoustically and visually isolated, was hung on the hook by an adhesive tape placed 20 mm from the extremity of its tail and it was kept 150 mm away from the nearest object. The sum of immobility periods (duration of immobility) was measured by an observer who was unaware of the drug treatments or by a computerized device (ITEMATIC-TST) developed by ITEM-LABO (Le Kremlin-Bicêtre, France). In the latter experimental condition, a strain gauge picked up all movements of the mouse and transmitted them to a central unit which calculated the total duration of immobility during a 6-min test (Stéru et al., 1987). Using the computerized system, six animals could be tested at one time. Each mouse was used only once for each experimental session.
Forced swim test in mice
Mice were dropped individually into glass cylinders (height: 25 cm, internal diameter: 10 cm) containing 10 cm water, maintained at 23 – 25°C. The apparatus consisted of two Plexiglass cylinders placed side by side in a Makrolon cage (38×24×18 cm). Two mice were tested simultaneously for a 6-min period but a non-transparent screen placed between the two cylinders prevented mice from seeing each other. The immobility time was measured during the last 3 or 4 min of the test by an observer who was unaware of the drug treatment. A mouse was judged to be immobile when it remained floating in the water, making only the necessary movements to keep its head above water. Each mouse was used only once for each experimental session.
Reserpine test
Mice were given reserpine (2 mg kg−1 s.c.). A score of ptosis was measured for each eye 3.5 h later, as 0 (eye completely open) to 4 (eye fully closed), i.e., a maximum score of 8 per mouse. The rectal temperature was also measured 3.5 h later with a thermistor probe (Physitemp TH5, probe RM6; Clifton, U.S.A.) inserted to a depth of 2.5 cm into the rectum. The mice were then divided into vehicle and test drug groups and were introduced immediately after into the actimeters for a 30-min test session. Ptosis and rectal temperature were measured again at the end of the motor activity test.
Locomotor activity
Locomotor activity was measured with a Digiscan Animal Activity Monitor system (Omnitech Electronics Inc., Columbus, OH, U.S.A.) which monitored the horizontal (locomotion) and vertical (rearing) movements of the animals. The Digiscan analyser was interfaced with an IBM-PC compatible computer using Digipro software. The individual compartments (L=20; W=20; H=30 cm) were put in a dimly lit and quiet room. Horizontal movements, i.e., locomotion, were expressed as number of beams crossed over two (experiment with reserpine) or three 15 min periods of testing.
Drugs
SCH 58261, 5-amino-7-(2-phenylethyl)-2-(2-furyl)-pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine, and KW 6002, (E)-1,3-diethyl-8-(3,4-dimethoxystyryl)-7-methylxanthine, were synthesized at the Schering-Plough Research Institute, Kenilworth, NJ, U.S.A. ZM 241385, 4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-yl-amino]ethyl)phenol was a generous gift from Dr S. Poucher (Zeneca Pharmaceuticals, Macclesfield, U.K.). SCH 58261 (1, 3, 10 mg kg−1) and ZM 241385 (15, 30, 60 mg kg−1) were dissolved in dimethyl sulphoxide (Sigma Chemical Co., St Louis, MO, U.S.A.) and then diluted in Cremophor EL (Sigma Chemical Co., St Louis, MO, U.S.A.) and NaCl 0.9% (final concentration: 15% DMSO and 15% Cremophor EL). In another set of experiments, SCH 58261 (1, 3, 10 mg kg−1) and KW 6002 (1, 3, 10 mg kg−1) were dissolved in a suspension vehicle (methyl cellulose 0.4%, tween 80, 0.5%, benzyl alcohol, 0.8% in saline). Reserpine (Sigma Chemical Co., St Louis, MO, U.S.A.) was dissolved in distilled water containing 5% dimethyl sulphoxide and 5% Cremophor EL (Sigma Chemical Co., St Louis, MO, U.S.A.) and injected s.c. haloperidol (Haldol®, Janssen, France) was diluted in saline in order to get the appropriate doses and administered by the i.p. route. Drug solutions were prepared fresh daily in a volume of 10 ml kg−1. Doses always refer to the free bases.
Statistics
Results are expressed as means±s.e.mean. Differences between means were analysed by Student's t-test or ANOVA (with one or two factors and with or without repeated measures where appropriate). Where F ratios were significant, multiple comparisons were evaluated by the Newman-Keuls multiple comparison test. Significance levels were set at P<0.05.
Results
Response of adenosine A2A receptor knockout mice in tail suspension and forced swim test
In the tail suspension test, the duration of immobility was reduced by 30% (P<0.05) in adenosine A2A receptor knockout mice as compared to wildtype animals (Figure 1A). Similarly, in the forced swim test, A2A receptor knockout animals behaved differently from the wildtype mice as their time of immobility was reduced by 24% (P<0.001) as compared to controls (Figure 1B).
Figure 1.
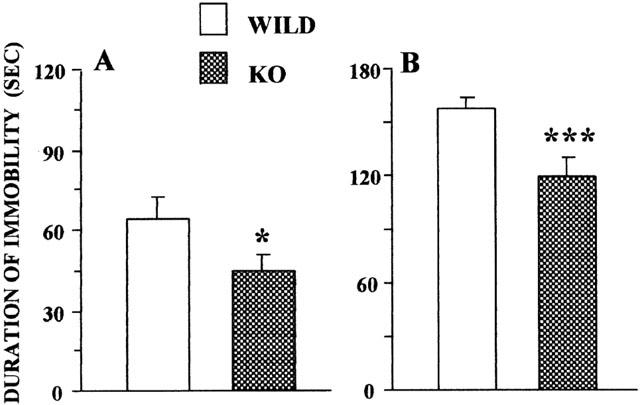
Immobility times of A2A receptor knockout (A2AR KO) and wildtype (A2AR WT) mice recorded in the tail suspension or forced swim tests. (A) duration of immobility in the tail suspension test. Means±s.e.mean of data from 29 mice per group. B: Duration of immobility in the forced swim test. Means±s.e.mean of data from 16 mice per group. *P<0.05, ***P<0.001 as compared to wildtype mice by Student's t-test.
Effects of adenosine A2A receptor antagonists in the tail suspension test in CD1 mice
SCH 58261 (1, 3, 10 mg kg−1, i.p.) dose-dependently reduced the immobility time by 51, 86 and 92%, respectively (Figure 2A). Similarly, another adenosine A2A receptor antagonist, KW 6002 (0.1, 1, 10 mg kg−1, p.o.) dose-dependently decreased the total immobility time, after oral administration, by 40, 74 and 91%, respectively (Figure 2B).
Figure 2.
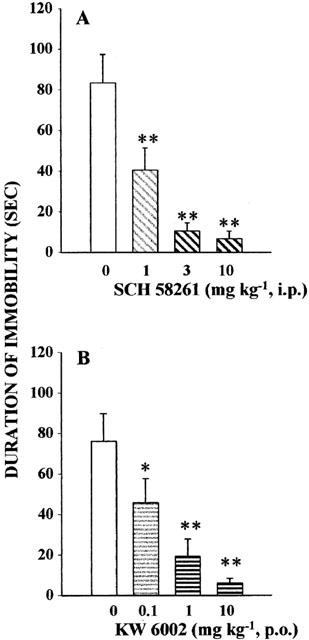
Effects of SCH 58261 (A) and KW 6002 (B) in the mouse tail suspension test. Mice were injected with vehicle or SCH 58261 (1, 3, 10 mg kg−1, i.p.) 30 min before the test; or received vehicle or KW 6002 (1, 3, 10 mg kg−1, p.o.), 60 min before the test. Data are mean±s.e.mean of 10 animals per group. **P<0.01 versus vehicle-treated group by one-way ANOVA followed by Student-Newman-Keuls test.
Under a repeated treatment schedule (3 mg kg−1 i.p., twice daily for 8 days), SCH 58261 decreased by 44% the duration of immobility in the tail suspension test. The immobility times (mean±s.e.mean) were 128±16 s for nine controls and 72±12 s for nine SCH 58261-treated mice [F(2,33)=4.61, P<0.05].
In another set of experiments, the tail suspension test was carried out in mice which were pre-screened before the assay. Specifically, 140 mice showing the highest immobility time (score ⩾115 s) considered as ‘High-Immobility' animals (HI), were selected on day 1 from a sample of 256 tested mice (mean total immobility time: 166±3 s). On the following day, from the 140 HI tested mice, 108 mice scored over 115 s (mean total immobility time: 181±4 s). The time of immobility of vehicle-injected HI mice on day 3 did not differ significantly from mean scores obtained with the same animals during the screening procedure (i.e., trials 1 and 2). On day 3, mice were injected i.p., 30 min before the test, with either vehicle, SCH 58261 (1, 3, 10 mg kg−1, i.p.) or ZM 241385 (15, 30, 60 mg kg−1, i.p.). The two adenosine A2A receptor antagonists SCH 58261 and ZM 241385 decreased significantly [F(6,102)=8.78, P<0.001] the immobility time of screened HI animals (Figure 3).
Figure 3.
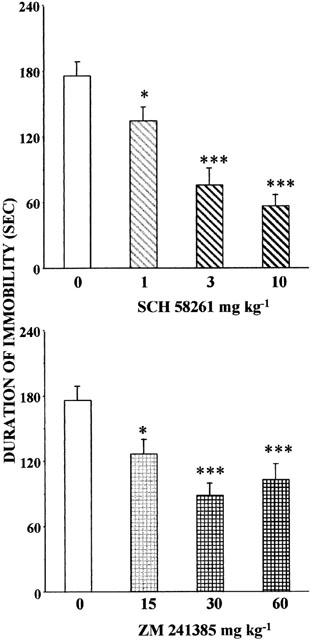
Effects of SCH 58261 and ZM 241385 in tail suspension test in screened male CD1 mice. Pre-test selection consisted of one trial on two consecutive days. For each selected ‘high-immobility' mouse (HI), the mean score was calculated and used as the pretest score. On day 3, mice were injected with vehicle, SCH 58261 (1, 3, 10 mg kg−1 i.p.) or ZM 241385 (15, 30, 60 mg kg−1 i.p.) 30 min before the test. Means±s.e.mean. of data from 17 controls and 13 – 15 mice in treated groups. *P<0.05, ***P<0.001 (one-way ANOVA followed by Student-Newman-Keuls test).
The tail suspension test in selectively bred ‘Helpless' mice
SCH 58261 was studied in the tail suspension test using selective bred ‘Helpless' CD1 mice. Specifically, experiments were carried out in male and female Swiss albino CD1 ‘Helpless' mice from the seventh generation of selective breeding for this behavioural trait in our laboratory at the University of Rouen. The reference antidepressant drug imipramine (30 mg kg−1, i.p.) reduced by 69% the immobility time [F(1,42)=96.40, P<0.001]. SCH 58261 also significantly [F(1,34)=26.80, P<0.001] shortened by 49% the immobility time, i.e. increased struggling time as compared to vehicle-treated animals (Figure 4).
Figure 4.
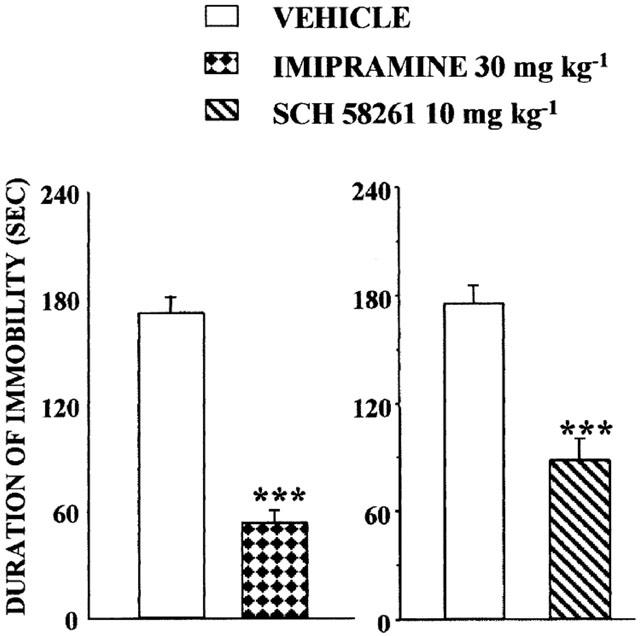
Effects of SCH 58261 or imipramine in the tail suspension test performed in a genetic mouse model of depression. Outbred CD1 mice were used as the foundation population of a line of mice that was selectively bred for its high spontaneous helplessness (immobility scores ⩾115 s=helpless) in the tail suspension test. Mice of both sexes (7th generation) were injected with SCH 58261 10 mg kg−1 i.p. (right panel) or imipramine 30 mg kg−1 i.p. (left panel) 30 min before the test. Testing was for 6 min. Means±s.e.mean. of data from 18 – 22 in each group. ***P<0.001 (one-way ANOVA followed by Student-Newman-Keuls test) as compared to vehicle-injected groups.
Effects of adenosine A2A receptor antagonists in the forced swim test in CD1 mice
SCH 58261 was administered 30 min before the test at doses ranging from 1 to 10 mg kg−1, i.p. The higher dose of 10 mg kg−1 reduced the immobility time by 61% (Figure 5A). KW 6002, p.o., decreased the total immobility time at the doses of 1 and 10 mg kg−1, after oral administration, by 75 and 79%, respectively (Figure 5B).
Figure 5.
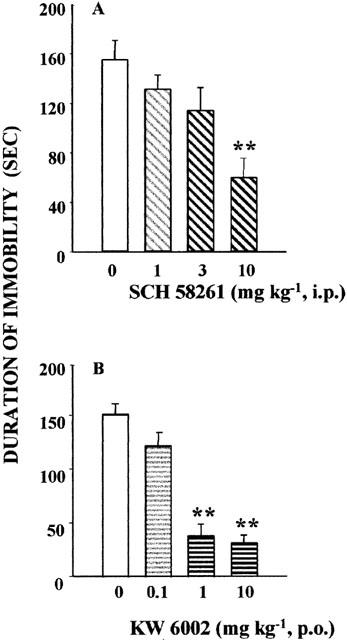
Effects of SCH 58261 (A; 1, 3, 10 mg kg−1, i.p.) and KW 6002 (B; 1, 3, 10 mg kg−1, p.o.) in the mouse forced swim test. Mice were injected with vehicle or SCH 58261 30 min before the test; or received vehicle or KW 6002, 60 min before the test. The duration of immobility was recorded during the last 4-min of the 6-min testing period. Data are mean±s.e.mean. of 10 animals per group. *P<0.05, **P<0.01 versus vehicle treated group (one-way ANOVA followed by Student-Newman-Keuls test).
Role of dopamine D2 receptors in mediating anti-immobility and stimulant motor effects of acute SCH 58261 in CD1 mice
To assess whether the dopamine D2 receptors are involved in mediating anti-immobility and stimulant motor effects of SCH 58261, we studied its interaction with haloperidol. Mice received increasing doses of haloperidol (0, 100, 200 μg kg−1 s.c.) as a pretreatment 15 min before the administration of an effective dose (10 mg kg−1 i.p.) of SCH 58261 in either locomotor activity or forced swim tests. In the locomotor activity test, there was a significant haloperidol-SCH 58261 interaction [F(2,47)=4.11, P<0.05]. As expected, haloperidol by itself reduced motor activity. However, the stimulant effects of SCH 58261 were not changed by the concomitant presence of haloperidol (Figure 6). In the forced swim test, the two-way ANOVA also showed a significant interaction between the two factors [F(3,72)=5.04, P<0.01]. Here haloperidol produced no effects over the dose range used (Figure 6, lower panel). However, the effects of SCH 58261 were reversed in the presence of haloperidol (50, 100, 200 μg kg−1 i.p.).
Figure 6.
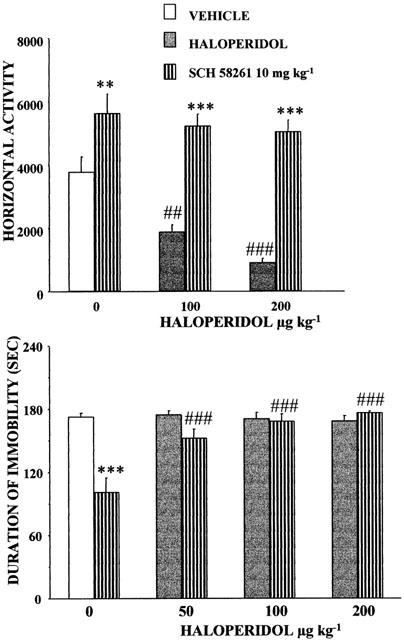
Effects of haloperidol on stimulation of locomotor activity and anti-immobility response induced by SCH 58261. Mice were injected with saline (open bars) or increasing doses of haloperidol (50, 100, 200 μg kg−1 i.p.) (hatched bars). Fifteen minutes later, they were injected with vehicle or SCH 58261 (10 mg kg−1 i.p.). Upper panel – locomotor activity test – Immediately after the second treatment, mice were introduced into the actimeters. The horizontal activity was measured for 45 min. Means±s.e.mean. of data from 8 mice per group. Two-way ANOVAs: (interaction of haloperidol×SCH 58261): F(2,47)=4.11, P=0.02. Lower panel – forced swim test – mice pretreated with haloperidol or saline received vehicle or SCH 58261 30 min before testing. The duration of immobility was recorded during the last 3-min of the 6-min testing period. Means±s.e.mean of data from 14 controls and 8 – 11 mice in treated groups. Two-way ANOVAs: (interaction of haloperidol×SCH 58261): F(3,72)=5.04, P<0.01. Post hoc comparisons: **P<0.01, ***P<0.001 as compared with respective SCH 58261 untreated control groups; ##P<0;01; ###P<0.001 as compared with respective haloperidol-untreated control groups.
The reserpine model in CD1 mice
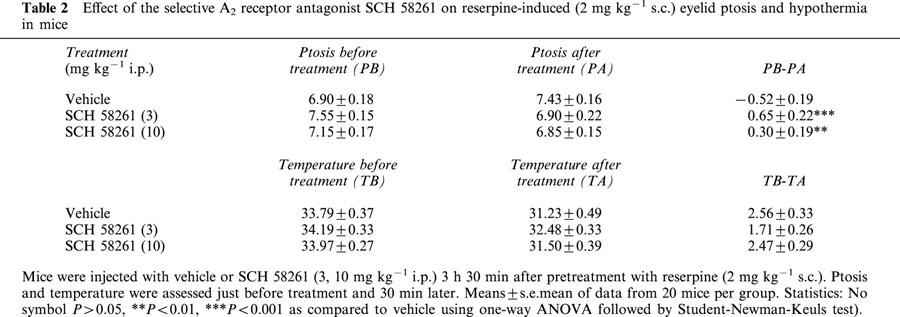
The vesicular monoamine uptake blocker reserpine (2 mg kg−1 s.c.) produced akinesia, hypothermia and ptosis (eye closure). SCH 58261 (3, 10 mg kg−1 i.p.), given 210 min after reserpine reversed ptosis but not akinesia nor hypothermia. Specifically, it did not reverse significantly [F(2,60)=1.64, P>0.05] reserpine-induced akinesia (Table 1). The same animals were also checked for hypothermia and eyelid ptosis before and after the locomotor activity test. Concerning reserpine-induced hypothermia, the effect caused by SCH 58261 did not reach a statistically significant level [F(2,60)=2.53, P=0.08]. Only eyelid ptosis induced by reserpine was very weakly attenuated, although in a significant [F(2,60)=9.04, P<0.001] manner, in SCH 58261-treated animals (Table 2).
Table 1.
Effect of the selective A2A receptor antagonist SCH 58261 on motor activity in reserpine-pretreated (2 mg kg−1 s.c.) mice
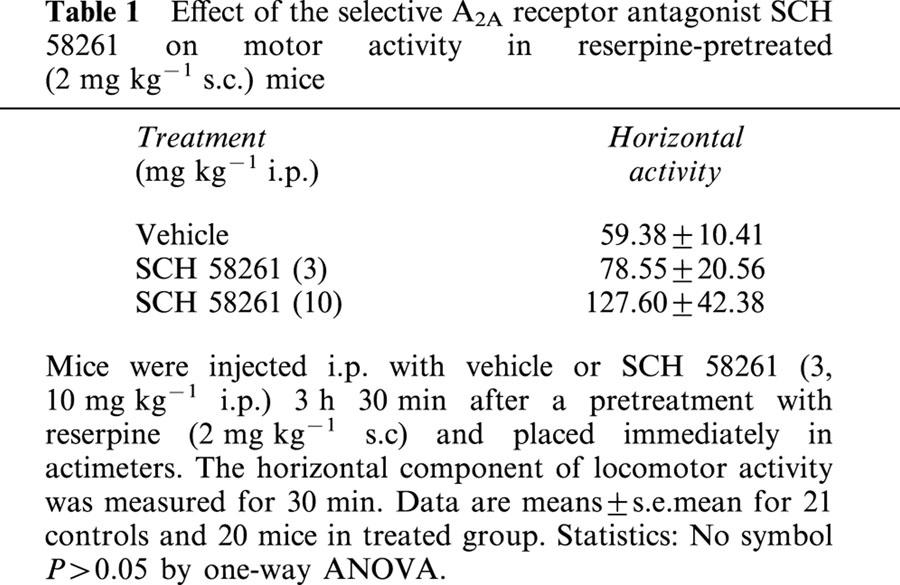
Table 2.
Effect of the selective A2 receptor antagonist SCH 58261 on reserpine-induced (2 mg kg−1 s.c.) eyelid ptosis and hypothermia in mice
Discussion
This paper shows that the adenosine A2A receptor may represents a novel target for the discovery of new antidepressants. Specifically, adenosine A2A receptor knockout mice displayed reduction of immobility in functional assays in vivo, such as tail suspension and forced swim tests which are predictive of clinical antidepressant activity. Adenosine A2A receptor antagonists were active in the same tests in normal mice.
Adenosine A2A receptor knockout mice were previously found to display reduced locomotor activities in an open field when compared to control mice (Ledent et al., 1997; Chen et al., 1999; El Yacoubi et al., 2000b). Conversely, in the two experimental paradigms used here, the forced swim and the tail suspension tests, their activities were enhanced as compared to those of wildtype mice, suggesting that the neuronal pathways underlying the two behaviours are at least partly different. Reduction of immobility by antidepressants cannot be explained by a non-specific behavioural stimulation as many antidepressants tend to decrease motor activity (Tucker & File, 1986; Perrault et al., 1992). In addition, direct dopamine D2 receptor agonists, which are known to reduce motor activity when administered in mice (Boulay et al., 1999), have been shown to increase mobility time in the forced swim test (Borsini et al., 1988; Duterte-Boucher et al., 1988).
In the tail suspension test, antipsychotics and anxiolytics increase immobility time (Porsolt et al., 1987), whereas adenosine A2A receptor antagonists decrease it. Moreover, adenosine A2A receptor antagonists produce antidepressant-like effects at low doses in comparison to classical antidepressant drugs, such as imipramine and fluoxetine. The efficacy of adenosine A2A receptor antagonists in reducing immobility time in the tail suspension test was confirmed and extended by studies on two different groups of mice. Specifically, the drugs SCH 58261 and ZM 241385 (Ongini et al., 1999; Fredholm & Lindström, 1999), the latter having a lower selectivity profile, were effective in mice previously screened for depressive behaviour (high immobility time). Interestingly, SCH 58261 at 10 mg kg−1 reduced immobility in the tail suspension test performed with mice that were selectively bred for their spontaneous ‘helplessness' in this test, i.e., a genetic mouse model useful for screening potential antidepressants (Vaugeois et al., 1996). In the same experimental procedure, the tricyclic antidepressant imipramine (30 mg kg−1) induced similar effects. In this study, the effects of repeated administration of SCH 58261 compared to single dose appeared to be attenuated. However, the significance of this result remains doubtful given that data were obtained in different experimental conditions. Nevertheless, if some degree of tolerance to the antidepressant-like effect following chronic treatment with A2A receptor antagonists could be confirmed in future studies, it might be related to an up-regulation of A2A receptor specifically in brain areas implicated in goal-directed behaviours. Since a lack of tolerance to motor stimulant effects of SCH 58261 has been observed in rats (Halldner et al., 2000), this specific aspect clearly warrants further study.
As part of this pharmacological characterization, SCH 58261 and KW 6002 were also examined in the forced swim test where both drugs reduced the duration of immobility in mice. These results support earlier findings by Sarges et al. (1990) showing that a weakly selective A2A receptor antagonist, CP 66, 713 (25 fold selectivity A2A vs A1), was effective in the forced swim test.
Additional experiments were carried out with the more selective compound SCH 58261. An interaction study with the dopamine D2 receptor antagonist (Seeman, 1980) haloperidol was performed with the aim to discriminate an escape-directed behaviour (i.e. a loss of motivation to avoid the stressful situation) from its motor stimulant effects (Svenningsson et al., 1997b; Popoli et al., 1998; El Yacoubi et al., 2000c). Here the anti-immobility effect elicited by SCH 58261 was prevented by a low dose (0.05 mg kg−1) of the dopamine D2 receptor antagonist, demonstrating a high sensitivity of the goal-directed behaviour to haloperidol. It is worth comparing this finding with those of other studies showing that dopamine D2 receptor antagonists block anti-immobility effects of antidepressants (Borsini et al., 1985; Borsini & Meli, 1990). SCH 58261-induced stimulant motor effects were not counteracted by haloperidol administered at moderate doses (0.1 – 0.2 mg kg−1) used in the present work. Moreover, adenosine A2A receptor antagonists effectively reduce catalepsy induced by high doses (in the mg kg−1 range) of dopamine D2 antagonists, a screening test for potential antiparkinsonian drugs (Kanda et al., 1994; Kafka & Corbett, 1996). Altogether, these data suggest that targeting with drugs the dopamine D2 and adenosine A2A receptors may result in swings in opposite directions of the physiological balance that exists between the neurotransmitter and neuromodulator, depending on the neuronal systems implicated in a particular function.
Therefore, dopamine transmission through dopamine D2 receptors appears to be critically involved in the anti-immobility effect elicited by SCH 58261. It has been suggested that disturbances in dopamine transmission are involved in the pathophysiology of mood disorders. For example, the antidepressant bupropion is a dopamine and noradrenaline reuptake inhibitor that has a direct enhancing action upon dopamine transmission (Cooper et al., 1980; Davidson & Connor, 1998), whereas a depressive syndrome is frequently encountered in subjects affected by Parkinson's disease, where dopamine depletion is observed (Allain et al., 2000). Dopamine transmission in both frontal cortex and nucleus accumbens has been implicated in the mechanism of action of antidepressants (Tanda et al., 1994; Fibiger, 1995). One tentative explanation for the dissociation between the two behaviours studied here may reside in the peculiar physiology and pharmacology of dopamine neurones originating in the ventral tegmental area in the midbrain and projecting to the prefrontal cortex. They are known to have higher turnover rates and enhanced burst firing compared to mesolimbic and nigrostriatal dopamine neurones, and to have high responsiveness to mild stressors (Deutch & Roth, 1990). In contrast to nigrostriatal dopamine neurones, mesoprefrontal dopamine neurones may also not be well suited for maintaining homeostasis due to the absence or low sensitivity of synthesis- and impulse-regulating autoreceptors (Deutch & Roth, 1990). Due to the blockade of synthesis- and impulse-regulating autoreceptors projecting to dorsal and ventral striatum, haloperidol by itself induces release of dopamine to reach a synaptic concentration allowing a competition with haloperidol, which could explain, in part, the lack of antagonism of SCH 58261-induced effects in the motor activity test. On the contrary, dopamine release elicited by haloperidol in the frontal cortex would be much weaker in intensity (Moghaddam & Bunney, 1990), allowing the reversal of the anti-immobility effect caused by the selective adenosine A2A receptor antagonist. This hypothesis is supported by the evidence that antidepressants mostly increase extracellular dopamine release in the frontal cortex than in the nucleus accumbens (Tanda et al., 1994).
The adenosine A2A receptor has been visualized by autoradiography in the prefrontal cortex of the mouse (Johansson et al., 1996) and rat (Ishiwata et al., 2000) with densities equal respectively to about one tenth and one fifth that found in the striatum. The selective adenosine A2A receptor antagonist [3H] SCH 58261 was also found to label the postmortem human prefrontal cortex, with a binding density about one third that detected in rostral putamen (Svenningsson et al., 1997a). A role of adenosine A2A receptor located in the striatum cannot be completely excluded, since dopamine transmission in this structure plays an important role in determining the individual flexibility to cope with available sensory information (Cools, 1980), and dopamine D2 receptor densities are modified in striatum of depressed patients relative to controls (D'haenen & Bossuyt, 1994; Shah et al., 1997).
The stimulant motor effects elicited by SCH 58261 in reserpine-pretreated mice were mild in our experimental conditions. Shiozaki et al. (1999) have reported a reversal of reserpine-induced akinesia by another adenosine A2A receptor antagonist. Further work will be necessary to explain the lack of reserpine-induced akinesia in the present study. The absence of effects upon eyelid ptosis and hypothermia induced by reserpine suggests that adenosine A2A receptors are not involved in the modulation of noradrenergic neuronal pathways underlying these behaviours (Bourin et al., 1983).
The models of depression that we have used have however some limitations. As previously mentioned by several authors (Willner, 1990, Weiss & Kilts, 1998), one of the major drawbacks of the forced swim and tail suspension tests is the positive response elicited after an acute administration of antidepressants. Although it is widely accepted that these tests are useful to screen potential antidepressants, selective A2A receptor antagonists should be further examined both in other preclinical models such as learned helplessness or chronic mild stress and after repeated treatments. It is also worth noting that useful and robust information can only emerge when selective adenosine A2A receptor antagonists will be studied in patients such as those affected by Parkinson's disease.
In conclusion, these data support the hypothesis that adenosine A2A receptor antagonists enhance the activity of mice in the forced swim and tail suspension tests by a prolongation of escape-directed behaviour, rather than by a generalized motor stimulant effect. The positive effect is likely mediated by an increase in dopaminergic transmission, possibly in frontal cortex. Modulation of monoamine activity as a therapeutic strategy dominates antidepressant research. However, all antidepressants developed so far exert their therapeutic effects with an undesirable delay, and there is still a need to fill this therapeutic gap. In this sense, adenosine A2A receptor antagonists might offer a novel approach to the treatment of depression.
Acknowledgments
The authors are most grateful to the individuals and companies mentioned under Methods for their generous supply of drugs. Malika El Yacoubi is supported by a Lilly Institute. Catherine Ledent and Marc Parmentier are supported by the Fonds Médical Reine Elisabeth and the Pôles d'Attraction Interuniversitaires.
Abbreviations
- DMSO
dimethyl sulphoxide
- KW 6002
(E)-1,3-diethyl-8-(3,4-dimethoxystyryl)-7-methylxanthine
- SCH 58261
5-amino-7-(2-phenylethyl)-2-(2-furyl)-pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine
- ZM 241385
4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-yl-amino]ethyl)phenol
References
- ALLAIN H., SCHUCK S., MAUDUIT N. Depression in Parkinson's disease. Br. Med. J. 2000;320:1287–1288. doi: 10.1136/bmj.320.7245.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORSINI F., PULVIRENTI L., SAMANIN R. Evidence of dopamine involvement in the effect of repeated treatment with various antidepressants in the behavioural ‘despair' test in rats. Eur. J. Pharmacol. 1985;110:253–256. doi: 10.1016/0014-2999(85)90219-5. [DOI] [PubMed] [Google Scholar]
- BORSINI F., LECCI A., MANCINELLI A., D'ARANNO V., MELI A. Stimulation of dopamine D-2 but not D-1 receptors reduces immobility time of rats in the forced swimming test: implication for antidepressant activity. Eur. J. Pharmacol. 1988;148:301–307. doi: 10.1016/0014-2999(88)90107-0. [DOI] [PubMed] [Google Scholar]
- BORSINI F., MELI A.The forced swimming test: its contribution to the understanding of the mechanisms of action of antidepressants Dopamine and mental depression 1990Oxford: Pergamon Press; 63–76.Gessa, G.L. & Serra G. (eds) [Google Scholar]
- BOULAY D., DEPOORTERE R., PERRAULT G., BORELLI E., SANGER D.J. Dopamine D2 receptor knock-out mice are insensitive to the hypolocomotor and hypothermic effects of dopamine D2/D3 receptor agonists. Neuropharmacology. 1999;38:1389–1396. doi: 10.1016/s0028-3908(99)00064-7. [DOI] [PubMed] [Google Scholar]
- BOURIN M., PONCELET M., CHERMAT R., SIMON P. The value of the reserpine test in psychopharmacology. Arzneimittelforschung. 1983;33:1173–1176. [PubMed] [Google Scholar]
- BROCKWELL N.T., BENINGER R.J. The differential role of A1 and A2 adenosine receptor subtypes in locomotor activity and place conditioning in rats. Behav. Pharmacol. 1996;7:373–383. doi: 10.1097/00008877-199608000-00009. [DOI] [PubMed] [Google Scholar]
- CHEN J.-F., HUANG Z., MA J., ZHU J., MORATALLA R., STANDAERT D., MOSKOWITZ M.A., FINK J.S., SCHWARZSCHILD M.A. A2A adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J. Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN J.-F., STAAL R., XU K., BEILSTEIN M., SONSALLA P.K., SCHWARZSCHILD M.A. Novel neuroprotection by adenosine A2A receptor inactivation in an animal model of Parkinson's disease. Drug Dev. Res. 2000;50:71. [Google Scholar]
- COLONNA L., PETIT M., LÉPINE J.P. Bromocriptine in affective disorders. J. Affect. Dis. 1979;1:173–177. doi: 10.1016/0165-0327(79)90002-8. [DOI] [PubMed] [Google Scholar]
- COOLS A.R. Role of the neostriatal dopaminergic activity in sequencing and selecting behavioural strategies: facilitation of processes involved in selecting the best strategy in a stressful situation. Behav. Brain Res. 1980;1:361–378. doi: 10.1016/0166-4328(80)90035-2. [DOI] [PubMed] [Google Scholar]
- COOPER B.R., HESTER T.J., MAXWELL R.A. Behavioral and biochemical effects of the antidepressant bupropion (Wellbutrin): evidence for selective blockade of dopamine uptake in vivo. J. Pharmacol. Exp. Ther. 1980;215:127–134. [PubMed] [Google Scholar]
- CORSET V., NGUYEN-BA-CHARVET K.T., FORCET C., MOYSE E., CHEDOTAL A., MEHLEN P. Netrin-1-mediated axon outgrowth and cAMP production requires interaction with adenosine A2b receptor. Nature. 2000;407:747–750. doi: 10.1038/35037600. [DOI] [PubMed] [Google Scholar]
- DAVIDSON J.R., CONNOR K.M. Bupropion sustained release: a therapeutic overview. J. Clin. Psychiatry. 1998;59:25–31. [PubMed] [Google Scholar]
- DEUTCH A.Y., ROTH R.H. The determinants of stress-induced activation of the prefrontal cortical dopamine system. Prog. Brain Res. 1990;85:367–403. doi: 10.1016/s0079-6123(08)62691-6. [DOI] [PubMed] [Google Scholar]
- D'HAENEN H.A., BOSSUYT A. Dopamine D2 receptors in depression measured with single photon emission computed tomography. Biol. Psychiatry. 1994;35:128–132. doi: 10.1016/0006-3223(94)91202-5. [DOI] [PubMed] [Google Scholar]
- DUTERTE-BOUCHER D., LECLÈRE J.-F., PANISSAUD C., COSTENTIN J. Acute effects of direct dopamine agonists in the mouse behavioral despair test. Eur. J. Pharmacol. 1988;154:185–190. doi: 10.1016/0014-2999(88)90096-9. [DOI] [PubMed] [Google Scholar]
- EL YACOUBI M., LEDENT C., PARMENTIER M., COSTENTIN J., VAUGEOIS J.-M. The anxiogenic-like effects of caffeine in two experimental procedures measuring anxiety in the mouse is not shared by selective A2A adenosine receptor antagonists. Psychopharmacology. 2000a;148:153–163. doi: 10.1007/s002130050037. [DOI] [PubMed] [Google Scholar]
- EL YACOUBI M., LEDENT C., MÉNARD J.-F., PARMENTIER M., COSTENTIN J., VAUGEOIS J.-M. The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A2A receptors. Br. J. Pharmacol. 2000b;129:1465–1473. doi: 10.1038/sj.bjp.0703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL YACOUBI M., LEDENT C., PARMENTIER M., COSTENTIN J., VAUGEOIS J.-M. SCH 58261 and ZM 241385 differentially prevent the motor effects of CGS 21680 in mice: evidence for a functional ‘atypical' adenosine A2A receptor. Eur. J. Pharmacol. 2000c;401:63–77. doi: 10.1016/s0014-2999(00)00399-x. [DOI] [PubMed] [Google Scholar]
- FERRÉ S., FREDHOLM B.B., MORELLI M., POPOLI P., FUXE K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- FIBIGER H.C.Neurobiology of depression: focus on dopamine Depression and mania: from neurobiology to treatment 1995New York: Raven Press; 1–17.Gessa, G., Fratta, W., Pani, L. & Serra, G. (eds) [Google Scholar]
- FREDHOLM B.B., LINDSTRÖM K. Autoradiographic comparison of the potency of several structurally unrelated adenosine receptor antagonists at adenosine A1 and A2A receptors. Eur. J. Pharmacol. 1999;380:197–202. doi: 10.1016/s0014-2999(99)00533-6. [DOI] [PubMed] [Google Scholar]
- HALLDNER L., LOZZA G., LINDSTRÖM K., FREDHOLM B.B. Lack of tolerance to motor stimulant effects of a selective adenosine A2A receptor antagonist. Eur. J. Pharmacol. 2000;406:345–354. doi: 10.1016/s0014-2999(00)00682-8. [DOI] [PubMed] [Google Scholar]
- IMPAGNATIELLO F., BASTIA E., ONGINI E., MONOPOLI A. Adenosine receptors in neurological disorders. Emerging Therapeutic Targets. 2000;4:635–663. [Google Scholar]
- ISHIWATA K., OGI N., SHIMADA J., NONAKA H., TANAKA A., SUZUKI F., SENDA M. Further characterization of a CNS adenosine A2A receptor ligand [11C] KF18446 with in vitro autoradiography and in vivo tissue uptake. Ann. Nucl. Med. 2000;14:81–89. doi: 10.1007/BF02988585. [DOI] [PubMed] [Google Scholar]
- JAIN N., KEMP N., ADEYEMO O., BUCHANAN P., STONE T.W. Anxiolytic activity of adenosine receptor activation in mice. Br. J. Pharmacol. 1995;116:2127–2133. doi: 10.1111/j.1476-5381.1995.tb16421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHANSSON B., GEORGIEV V., KUOSMANEN T., FREDHOLM B.B. Long-term treatment with some methylxanthines decreases the susceptibility to bicuculline- and pentylenetetrazol-induced seizures in mice. Eur. J. Neurosci. 1996;762:153–164. doi: 10.1111/j.1460-9568.1996.tb01539.x. [DOI] [PubMed] [Google Scholar]
- KAFKA S.H., CORBETT R. Selective adenosine A2A receptor/dopamine D2 receptor interactions in animal models of schizophrenia. Eur. J. Pharmacol. 1996;295:147–154. doi: 10.1016/0014-2999(95)00668-0. [DOI] [PubMed] [Google Scholar]
- KANDA T., SHIOZAKI S., SHIMADA J., SUZUKI F., NAKAMURA J. KF17837: a novel selective adenosine A2A receptor antagonist with anticataleptic activity. Eur. J. Pharmacol. 1994;256:263–268. doi: 10.1016/0014-2999(94)90551-7. [DOI] [PubMed] [Google Scholar]
- KLOTZ K.N. Adenosine receptors and their ligands. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:382–391. doi: 10.1007/s002100000315. [DOI] [PubMed] [Google Scholar]
- KOPF S.R., MELANI A., PEDATA F., PEPEU G. Adenosine and memory storage: effect of A1 and A2 receptor antagonists. Psychopharmacology. 1999;146:214–219. doi: 10.1007/s002130051109. [DOI] [PubMed] [Google Scholar]
- KULKARNI S.K., MEHTA A.K. Purine nucleoside-mediated immobility in mice: Reversal by antidepressants. Psychopharmacology. 1985;85:460–463. doi: 10.1007/BF00429665. [DOI] [PubMed] [Google Scholar]
- LAASONEN-BALK T., KUIKKA J., VIINAMÄKI H., HUSSO-SAASTAMOINEN M., LEHTONEN J., TIIHONEN J. Striatal dopamine transporter density in major depression. Psychopharmacology. 1999;144:282–285. doi: 10.1007/s002130051005. [DOI] [PubMed] [Google Scholar]
- LEDENT C., VAUGEOIS J.-M., SCHIFFMANN S.N., PEDRAZZINI T., EL YACOUBI M., VANDERHAEGHEN J.-J., COSTENTIN J., HEATH J.K., VASSART G., PARMENTIER M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2A receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- MINOR T.R., WINSLOW J.L., CHANG W.C. Stress and adenosine: II. Adenosine analogs mimic the effect of inescapable shock on shuttle-escape performance in rats. Behav. Neurosci. 1994;108:265–276. doi: 10.1037//0735-7044.108.2.265. [DOI] [PubMed] [Google Scholar]
- MOGHADDAM B., BUNNEY B.S. Acute effects of typical and atypical antipsychotic drugs on the release of dopamine from prefrontal cortex, nucleus accumbens, and striatum of the rat: an in vivo microdialysis study. J. Neurochem. 1990;54:1755–1760. doi: 10.1111/j.1471-4159.1990.tb01230.x. [DOI] [PubMed] [Google Scholar]
- MOURET J., LEMOINE P., MINUIT M.P. Marqueurs polygraphiques, cliniques et thérapeutiques des dépressions dopamine dépendantes. C.R. Acad. Sci. Paris (Série 3) 1987;305:301–306. [PubMed] [Google Scholar]
- OLAH M.E., STILES G.L. The role of receptor structure in determining adenosine receptor activity. Pharmacol. Ther. 2000;85:55–75. doi: 10.1016/s0163-7258(99)00051-0. [DOI] [PubMed] [Google Scholar]
- ONGINI E., DIONISOTTI S., GESSI S., IRENIUS E., FREDHOLM B.B. Comparison of CGS 15943, ZM 241385 and SCH 58261 as antagonists at human adenosine receptors. Naunyn Schmiedebergs Arch. Pharmacol. 1999;359:7–10. doi: 10.1007/pl00005326. [DOI] [PubMed] [Google Scholar]
- ONGINI E., FREDHOLM B.B. Pharmacology of adenosine A2A receptors. Trends Pharmacol. Sci. 1996;17:364–372. [PubMed] [Google Scholar]
- PERRAULT G., MOREL E., ZIVKOVIC B., SANGER D.J. Activity of litoxetine and other serotonin uptake inhibitors in the tail suspension test in mice. Pharmacol. Biochem. Behav. 1992;42:45–47. doi: 10.1016/0091-3057(92)90444-k. [DOI] [PubMed] [Google Scholar]
- POPOLI P., REGGIO R., PEZZOLA A., FUXE K., FERRE S. Adenosine A1 and A2A receptor antagonists stimulate motor activity: evidence for an increased effectiveness in aged rats. Neurosci. Lett. 1998;251:201–204. doi: 10.1016/s0304-3940(98)00533-3. [DOI] [PubMed] [Google Scholar]
- PORKKA-HEISKANEN T. Adenosine in sleep and wakefulness. Ann. Med. 1999;31:125–129. doi: 10.3109/07853899908998788. [DOI] [PubMed] [Google Scholar]
- PORSOLT R.D., BERTIN A., JALFRE M. Behavioural despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- PORSOLT R.D., CHERMAT R., LENEGRE A., AVRIL I., JANVIER S., STÉRU L. Use of the automated tail suspension test for the primary screening of psychotropic agents. Arch. Int. Pharmacodyn. Ther. 1987;288:11–30. [PubMed] [Google Scholar]
- POST R.M., GERNER R.H., CARMAN J.S., GILLIN J.C., JIMERSON D.C., GOODWIN F.K., BUNNEY W.E. Effects of a dopamine agonist piribedil in depressed patients. Arch. Gen. Psychiatry. 1978;35:609–615. doi: 10.1001/archpsyc.1978.01770290091008. [DOI] [PubMed] [Google Scholar]
- RICHARDSON P.J., KASE H., JENNER P.G. Adenosine A2A receptor antagonists as new agents for the treatment of Parkinson's disease. Trends Pharmacol. Sci. 1997;18:338–344. doi: 10.1016/s0165-6147(97)01096-1. [DOI] [PubMed] [Google Scholar]
- SARGES R., HOWARD H.R., BROWNE R.G., LEBEL L.A., SEYMOUR P.A., KOE B.K. 4-Amino[1,2,4]triazolo[4,3-a]quinoxalines. A novel class of potent adenosine receptor antagonists and potential rapid-onset antidepressants. J. Med. Chem. 1990;33:2240–2254. doi: 10.1021/jm00170a031. [DOI] [PubMed] [Google Scholar]
- SEEMAN P. Brain dopamine receptors. Pharmacol. Rev. 1980;32:229–313. [PubMed] [Google Scholar]
- SHAH P.J., OGILVIE A.D., GOODWIN G.M., EBMEIER K.P. Clinical and psychometric correlates of dopamine D2 binding in depression. Psychol. Med. 1997;27:1247–1256. doi: 10.1017/s0033291797005382. [DOI] [PubMed] [Google Scholar]
- SHIMADA J., KOIKE N., NONAKA H., SHIOZAKI S., YANAGAWA K., KANDA T., KOBAYASHI H., ICHIMURA M., NAKAMURA J., KASE H., SUZUKI F. Adenosine A2A antagonists with potent anti-cataleptic activity. Bioorg. Med. Chem. Lett. 1997;18:2349–2352. [Google Scholar]
- SHIOZAKI S., ISHIKAWA S., NAKAMURA J., KITAMURA S., YAMADA K., KUWANA Y. Actions of adenosine A2A receptor antagonist KW-6002 on drug-induced catalepsy and hypokinesia caused by reserpine or MPTP. Psychopharmacology. 1999;147:90–95. doi: 10.1007/s002130051146. [DOI] [PubMed] [Google Scholar]
- SITLAND-MARKEN P.A., WELLS B.G., FROEMMING J.H., CHU C.-C., BROWN C.S. Psychiatric applications of bromocriptine therapy. J. Clin. Psychiatry. 1990;51:68–82. [PubMed] [Google Scholar]
- STÉRU L., CHERMAT R., THIERRY B., MICO J.A., LENEGRE A., STÉRU M., SIMON P., PORSOLT R.D. The automated tail suspension test: a computerized device which differentiates psychotropic drugs. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1987;11:659–671. doi: 10.1016/0278-5846(87)90002-9. [DOI] [PubMed] [Google Scholar]
- STÉRU L., CHERMAT R., THIERRY B., SIMON P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- SVENNINGSSON P., HALL H., SEDVALL G., FREDHOLM B.B. Distribution of adenosine receptors in the postmortem human brain: an extended autoradiographic study. Synapse. 1997a;27:322–335. doi: 10.1002/(SICI)1098-2396(199712)27:4<322::AID-SYN6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- SVENNINGSSON P., NOMIKOS G.G., ONGINI E., FREDHOLM B.B. Antagonism of adenosine A2A receptors underlies the behavioural activating effect of caffeine and is associated with reduced expression of messenger RNA for NGFI-A and NGFI-B in caudate-putamen and nucleus accumbens. Neuroscience. 1997b;79:753–764. doi: 10.1016/s0306-4522(97)00046-8. [DOI] [PubMed] [Google Scholar]
- TANDA G., CARBONI E., FRAU R., DI CHIARA G. Increase of extracellular dopamine in the prefrontal cortex: a trait of drugs with antidepressant potential. Psychopharmacology. 1994;115:285–288. doi: 10.1007/BF02244785. [DOI] [PubMed] [Google Scholar]
- TUCKER J.C., FILE S.E. The effects of tricyclic and ‘atypical' antidepressants on spontaneous locomotor activity in rodents. Neurosci. Biobehav. Rev. 1986;10:115–121. doi: 10.1016/0149-7634(86)90022-9. [DOI] [PubMed] [Google Scholar]
- VAUGEOIS J.-M., ODIEVRE C., LOISEL L., COSTENTIN J. A genetic mouse model of helplessness sensitive to imipramine. Eur. J. Pharmacol. 1996;316:R1–R2. doi: 10.1016/s0014-2999(96)00800-x. [DOI] [PubMed] [Google Scholar]
- WEISS J.M., KILTS C.D.Animal models of depression and schizophrenia The American Psychiatric Press textbook of psychopharmacology 1998Washington DC: American Psychiatric Press, Inc.89–131.Schatzberg, A.F. & Nemeroff, C.B. (eds) [Google Scholar]
- WILLNER P. Animal models of depression: an overview. Pharmacol. Ther. 1990;45:425–455. doi: 10.1016/0163-7258(90)90076-e. [DOI] [PubMed] [Google Scholar]
- WOODSON J.C., MINOR T.R., JOB R.F. Inhibition of adenosine deaminase by erythro-9-(2-hydroxy-3-nonyl)adenine(EHNA) mimics the effect of inescapable shock on escape learning in rats. Behav. Neurosci. 1998;112:399–409. doi: 10.1037//0735-7044.112.2.399. [DOI] [PubMed] [Google Scholar]


