Abstract
KMUP-1 (1, 3, 5 mg kg−1, i.v.), a xanthine derivative, produced dose-dependent sustained hypotensive and short-acting bradycardiac effects in anaesthetized rats. This hypotensive effect was inhibited by pretreatment with glibenclamide (5 mg kg−1, i.v.).
In endothelium-intact or denuded aortic rings preconstricted with phenylephrine, KMUP-1 caused a concentration-dependent relaxation. This relaxation was reduced by endothelium removal, the presence of NOS inhibitor L-NAME (100 μM) and sGC inhibitors methylene blue (10 μM) and ODQ (1 μM).
The vasorelaxant effects of KMUP-1 was attenuated by pretreatment with various K+ channel blockers TEA (10 mM), glibenclamide (1 μM), 4-AP (100 μM), apamin (1 μM) and charybdotoxin (ChTX, 0.1 μM).
Increased extracellular potassium levels (30 – 80 mM) caused a concentration-related reduction of KMUP-1-induced vasorelaxations. Preincubation with KMUP-1 (1, 10, 100 nM) increased the ACh-induced maximal vasorelaxations mediated by endogenous NO release, and enhanced the potency of exogenous NO-donor SNP.
The vasorelaxant responses of KMUP-1 (0.01, 0.05, 0.1 μM) together with a PDE inhibitor IBMX (0.5 μM) had an additive action. Additionally, KMUP-1 (100 μM) affected cyclic GMP metabolism since it inhibited the activity of PDE in human platelets.
KMUP-1 induced a dose-related increase in intracellular cyclic GMP levels in rat A10 vascular smooth muscle (VSM) cells, but not cyclic AMP. The increase in cyclic GMP content of KMUP-1 (0.1 – 100 μM) was almost completely abolished in the presence of methylene blue (10 μM), ODQ (10 μM), and L-NAME (100 μM).
In conclusion, these results indicate that KMUP-1 possesses the following merits: (1) stimulation of NO/sGC/cyclic GMP pathway and subsequent elevation of cyclic GMP, (2) K+ channels opening, and (3) inhibition of PDE or cyclic GMP breakdown. Increased cyclic GMP display a prominent role in KMUP-1-induced VSM relaxations.
Keywords: Soluble guanylyl cyclase, K+ channels, cyclic GMP, nitric oxide, aortic smooth muscle, phosphodiesterase
Introduction
The endothelium plays a major role in regulating vascular smooth muscle (VSM) tone through the release of a variety of vasoactive factors. Among the endothelium-derived vasodilators, nitric oxide (NO) is probably the primary mediator of endothelium-dependent relaxation in most blood vessels. NO in numerous bioregulatory pathways has not only expanded new therapeutic avenues for NO-related compounds but also led to an increased use of such compounds in pharmacological studies. In recent years, NO has been demonstrated as an important regulator of vascular functions by controlling blood vessel tone as well as blood cell interactions with the vascular wall (Ignarro, 1990; Moncada et al., 1991). The action of NO as a vasodilator is mediated by the activation of vascular smooth muscle soluble guanylyl cyclase (sGC), a signal transduction enzyme that forms the second messenger molecular cyclic GMP (Arnold et al., 1977; Lowenstein et al., 1994). The latter modulates the activity of several cyclic GMP effector proteins that lead to vasorelaxation (Schmidt et al., 1993). The membrane-bound guanylyl cyclases are receptor-like enzymes which are activated upon extracellular binding of natriuretic peptides. In contrast, soluble guanylyl cyclases act via their haem group as important intracellular receptors for NO (Wohlfart et al., 1999). Moreover, the increases in cyclic GMP with these guanylyl cyclase activators and phosphodiesterases (PDE) or cyclic GMP breakdown inhibition were associated with the relaxation of vascular and tracheal smooth muscles.
The cross talks between endogenous NO or NO donors and endothelium-derived hyperpolarizing factor (EDHF) or K+ channel activators have received a great deal of attention (Pérez-Vizcaíno et al., 1999). K+ channels play a major role in the regulation of the resting membrane potential and modulate VSM tone (Nelson & Quayle, 1995). Endothelium-derived hyperpolarizing factor activates the potassium channels and thus the subsequent potassium flux hyperpolarizes the cell membrane to relax the smooth muscle cell (Cohen & Vanhoutte, 1995). Recent findings suggest that activation of endothelium KATP channels may also release endothelium-derived NO (Feleder & Adler-Graschinsky, 1997) or endothelium-derived hyperpolarizing factor (White & Hiley, 1997). NO donors have been shown to activate KATP channels via a cyclic GMP-dependent mechanism, presumably involving activation of cyclic GMP-dependent protein kinase, in rat aortic smooth muscle cells (Kubo et al., 1993) and rabbit mesenteric artery (Murphy & Brayden, 1995), and by a cyclic GMP-independent mechanism in the rat mesenteric artery (Weidelt et al., 1997). Although most of the endothelium-dependent relaxation is due to NO, but hyperpolarization associated K+ channels opening can supplement 60 – 80% of this response if NO synthesis is blocked (Kilpatrick & Cocks, 1994). The combination activity of sGC stimulation and K+ channels opening in a molecule, such as found in nicorandil, although shown without PDE inhibition activity, is able to relax agonist-induced vasoconstriction more fully (Pérez-Vizcaíno et al., 1998). YC-1 is a representative of a class of sGC activator with PDE inhibition and leads to a long-lasting cyclic GMP-mediated inhibition of vasoconstriction (Galle et al., 1999).
KMUP-1 (7-[2-[4-(2-chlorophenyl)piperazinyl]ethyl]-1,3-dimethylxanthine), as a unique xanthine and piperazine derivative (Figure 1), structurally with six nitrogen atoms, combining the sGC stimulation, K+ channels opening and PDE inhibition activities in one molecule, is able to achieve the full function as a VSM relaxant. In this study, we demonstrated that KMUP-1, without seriously affecting the compensatory reflex system, is considered to be a favourable tool to approach the smooth muscle system. It is notable that a vasorelaxant agent such as KMUP-1, together with sGC stimulation, K+ channels opening and PDE inhibition activities, is suggested to be hopeful in the management of VSM dysfunction. This study was designed to investigate the possible mechanism of hypotensive effects and VSM relaxations of KMUP-1, including its ability in activating NO/sGC/cyclic GMP pathway, opening K+ channels and inhibiting PDE activity.
Figure 1.
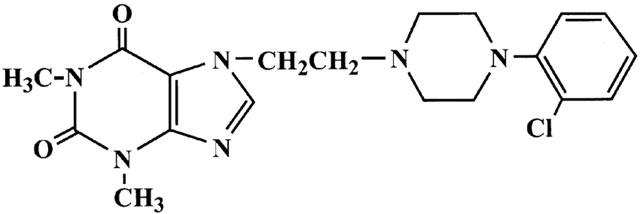
Chemical structure of KMUP-1.
Methods
Animals
Wistar rats were provided from National Laboratory Animal Breeding and Research Center (Taipei, Taiwan). They were housed under conditions of constant temperature and controlled illumination (light on between 7:30 and 19:30). Food and water were available ad libitum. The study was approved by the Animal Care and Use Committee of the Kaohsiung Medical University.
Drugs and chemicals
Levcromakalim was generously supplied by the SmithKline Beecham Pharmaceuticals and dissolved in ethanol at 10 mM. Sildenafil citrate was kindly supplied by the Cadila Healthcare Ltd. (Maninagar, India). Tetraethylammonium (TEA), 4-aminopyridine (4-AP), apamin, charybdotoxin (ChTX), methylene blue, glibenclamide, phenylephrine and 3-isobutyl-1-methylxanthine (IBMX) were all obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.). 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), 9-(terahydro-2-furanyl)-9H-purin-6-amine (SQ 22536) and NW-nitro-L-arginine methyl ester (L-NAME) were obtained from Research Biochemical International (Natick, MA, U.S.A.). All other reagents used were from E. Merck (Darmstadt, Germany). KMUP-1, synthesized in this laboratory, was dissolved in 10% absolute alcohol, 10% propylene glycol and 2% 1N HCl at 10 mM. Serial dilutions were made in distilled water.
Measurement of blood pressure and heart rate
The experiments were accomplished as previously described (Wu et al., 1994). In brief, male Wistar rats weighing 250 – 300 g were anaesthetized with pentobarbital sodium (50 mg kg−1, i.p.). Following tracheal cannulation, systemic arterial blood pressure and heart rates were recorded from the femoral artery with a pressure transducer (Spectramed, Model P10EZ, U.S.A.). The body temperature was maintained at 37°C by a heating pad. A femoral vein was cannulated for intravenous injection of drugs. Once the blood pressure reached steady state, KMUP-1 was administered in a series of doses. For the test of activating the KATP channels, KMUP-1 was administered via a femoral vein and the reduction in blood pressure recorded as control. Thereafter, a single dose of glibenclamide (5 mg kg−1) was administered intravenously. After 15 min, a further injection of KMUP-1 was given.
Isolated thoracic aorta preparation and measurement of tension
The aortic rings were prepared as previously described in this laboratory (Wu et al., 1994). Rats (200 – 300 g) were sacrificed after mild anaesthesia with ether and their aorta were quickly excised. Thoracic aorta were cleaned of fat and connective tissue, and cut into 3 – 4 mm wide transverse rings which were then mounted in a 10 ml organ bath and bathed at 37°C in Krebs solution (mM): NaCl 118, KCl 4.8, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 24, glucose 11; bubbled with a 95% O2+5% CO2 mixture. Isometric tension was recorded with a force displacement transducer (Grass, Model FT03, U.S.A.). In some experiments, the endothelium was removed mechanically by inserting the tip of a pair of forceps into the lumen and rolling the tissue back and forth several times on a paper towel moistened with physiological salt solution. At the beginning of each experiment, aortic rings were stretched to a resting tension of 1 g and then contracted with phenylephrine (PE, 10 μM) and, once the contractions had reached a plateau, the endothelial integrity of the preparations (E+) or absence of endothelium (E−) was verified by adding ACh (1 μM) to the superfusate. Only the aortic rings with a vasorelaxant response of >70% inhibition of preconstriction was considered E+. The preparations were then washed and allowed to equilibrate with Krebs solution for 45 min before being contracted a second time with PE.
When the stable vasoconstriction to PE (10 μM) or KCl (30 mM) was reached, concentration-response curves to KMUP-1 (1 nM – 100 μM), ACh (1 nM – 10 μM) or SNP (0.1 nM – 1 μM) were constructed. The effects of KMUP-1 on the vasorelaxant responses to ACh and SNP were investigated by preincubating the aortic rings with different concentrations of KMUP-1 (1, 10, 100 nM) for 30 min before the cumulative concentration of ACh or SNP was added. In another experiment, aortic rings were preconstricted with 30 – 80 mM KCl. When the contraction reached a steady state, cumulative concentration-response curves to KMUP-1 (1 nM – 100 μM) and levcromakalim (1 nM – 100 μM) were determined. The KCl solution was prepared by substituting NaCl with KCl (30 – 80 mM) in an equimolar amount.
To examine the possible mechanisms of vasorelaxant effects of KMUP-1, the aortic rings were pretreated with sGC inhibitors methylene blue (10 μM) and ODQ (1 μM), a NOS inhibitor L-NAME (100 μM), a K+ channel blocker TEA (10 mM), a KATP channel blocker glibenclamide (1 μM), a voltage-dependent K+ channel blocker 4-AP (100 μM) and Ca2+-dependent K+ channel blockers apamin (1 μM) and ChTX (0.1 μM) for 30 min prior to the addition of KMUP-1. Next, to observe whether the vasorelaxant effects of KMUP-1 are affected by IBMX, a nonselective inhibitor of PDE, we investigated the action of KMUP-1 (0.01, 0.05, 0.1 μM) in the presence of IBMX (0.5 μM).
Culture of A10 cells
Rat A10 VSM cells were originally obtained from American Type Culture Collection (ATCC) and grown in Dulbecco's modified Eagle's medium (DMEM) which was supplemented with 10% fetal bovine serum, 100 units ml−1 penicillin G and 100 μg ml−1 streptomycin at 37°C in a humidified 5% CO2 in air atmosphere. Cells were subcultured weekly after detachment by using culture medium containing 0.1% trypsin.
Measurement of cyclic GMP/AMP
Intracellular cyclic GMP and cyclic AMP concentrations in A10 cells were assayed as previously described by Wohlfart et al. (1999) with minor modifications given below. Cells were finally grown in 24-well plates (105 cells per well). At confluence, monolayer cells were washed with phosphate buffer solution (PBS) and then incubated with KMUP-1 (0.1 – 100 μM) in the presence of 100 μM IBMX for 20 min. Incubation was terminated by the addition of 10% trichloroacetic acid (TCA). Cell suspensions were sonicated and then centrifuged at 2500 g for 15 min at 4°C. To remove TCA, the supernatants were extracted three times with five volumes of water-saturated diethyl ether. Then, the supernatants were lyophilized and cyclic GMP or AMP of each sample was determined by using a commercially available radioimmunoassay kit (Amersham Pharmacia Biotech, Buckinghamshire, U.K.).
Phosphodiesterase assay
PDE activity was determined as described by Hidaka & Asano (1976). Washed human platelets prepared as previously described (Chen et al., 1996) were resuspended in 50 mM Tris-HCl containing 5 mM MgCl2 (pH 7.5). Platelets were then disrupted by sonication, and soluble phosphodiesterase preparation was obtained by ultracentrifugation 105,000 g for 60 min (4°C). The enzyme (11.5 mg ml−1, 10 μl) was incubated with Tris-HCl (80 μl) and 10 μM cyclic GMP substrate (final concentration 1 μM containing 0.1 μCi [3H]-cyclic GMP) was added. After 20 min at 37°C, the samples were heated to 100°C for 2 min. Ophiophagus hannah snake venom (10 mg ml−1, 10 μl) was then added and incubated at 37°C for 10 min to convert the 5′-GMP to the uncharged nucleosides, guanosine. An ion-exchange resin (200 μl) was added to bind all unconverted cyclic GMP. After centrifuging, the supernatant was removed for determination in a liquid scintillation counter.
Statistical evaluation of data
The results are expressed as mean±s.e.mean. Statistical differences were determined by independent and paired Student's t-test in unpaired and paired samples, respectively. Whenever a control group was compared with more than one treated group, the one way ANOVA or two way repeated measures ANOVA was used. When the ANOVA manifested a statistical difference, the Dunnett's or Student-Newman-Keuls test was applied. The P value less than 0.05 was considered to be significant in all experiments. Analysis of the data and plotting of the figures were done with the aid of software (SigmaStat and SigmaPlot, Version 5.0, San Rafael, CA, U.S.A.; GraphPad PRISM™, Version 2.0, San Diego, CA, U.S.A.) run on an IBM compatible computer and a Power Macintosh.
Results
Effects on blood pressure and heart rate
Acute intravenous injection of KMUP-1 (1, 3, 5 mg kg−1) caused a dose-related hypotensive effect, which reached a steady state at 5 min and persisted for over 2 h. Meanwhile, KMUP-1 produced a short-acting bradycardic effect or occasionally a reflex tachycardia in pentobarbital-anaesthetized Wistar rats (Figure 2). Intravenous administration of levcromakalim (0.1 mg kg−1) produced a similar reduction of mean arterial blood pressure (45±4 mmHg) and induced a significant tachycardia, which persisted for 3 h. Moreover, after pretreatment with a selective KATP channels blocker glibenclamide (5 mg kg−1, i.v.), the hypotensive effects of KMUP-1 (1, 3, 5 mg kg−1, i.v.) were partially and significantly inhibited, but levcromakalim (0.025, 0.05, 0.1 mg kg−1, i.v.)-induced hypotensive effects were almost completely blocked (Figure 3).
Figure 2.
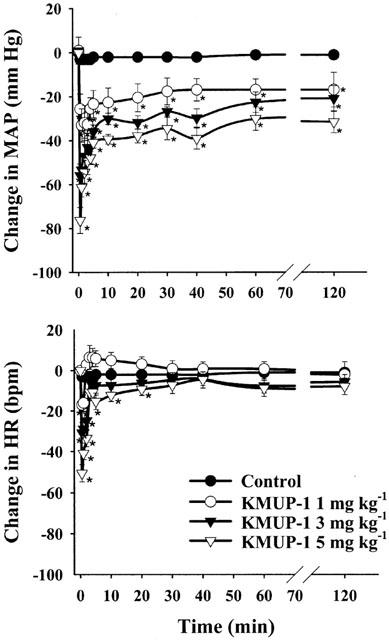
Effects of KMUP-1 (1, 3, 5 mg kg−1) on mean arterial blood pressure (MAP) and heart rate (HR) in Wistar rats, anaesthetized with pentobarbital. 10% Propylene glycol plus 10% ethanol mixed with 2% HCl was used for KMUP-1 as control. Vertical bars represent s.e.mean change from the baseline value, which was 115±10 mmHg and 370±21 b.p.m. for MAP and HR, respectively. Each point represents the mean±s.e.mean of eight experiments. *Indicates P<0.05, Two way repeated measures ANOVA followed by Student-Newman-Keuls test.
Figure 3.
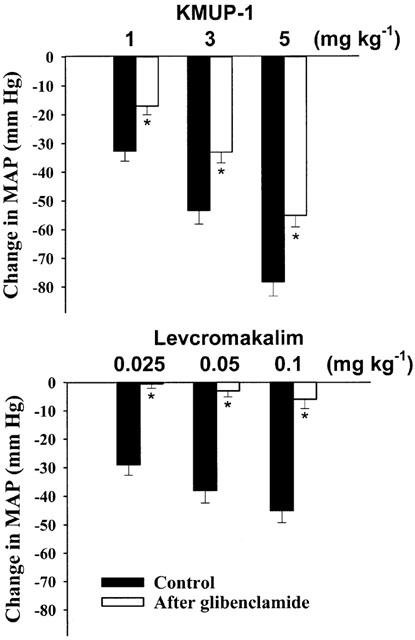
Effects of intravenous injection of KMUP-1 and levcromakalim induced hypotensive responses before and after i.v. injection of glibenclamide (5 mg kg−1) in anaesthetized rats. Each value represents the mean±s.e.mean of 6 – 8 experiments. *Indicates P<0.05, Paired Student's t-test.
Endothelium-independent effect
We investigated the vasorelaxant effects of KMUP-1 in isolated aortic rings with and without endothelium. Cumulative concentrations of KMUP-1 (1 nM – 100 μM) produced concentration-dependent vasorelaxations both in endothelium-denuded (E−) and endothelium-intact (E+) aortic rings, indicating that KMUP-1-induced vasorelaxation is more likely endothelium-independent. However, KMUP-1 did have a significant shift in the response curve after endothelium denudation suggesting that at least part of the observed effect is endothelium-dependent (Figure 4).
Figure 4.
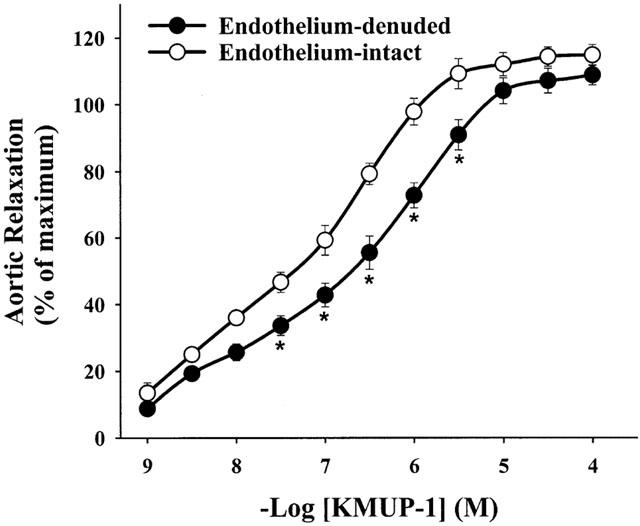
Vasorelaxations induced by cumulative concentrations of KMUP-1 in endothelium-intact and endothelium-denuded rat aortic rings preconstricted with phenylephrine (1 μM). KMUP-1 induced relaxant responses were significantly different in endothelium-intact and endothelium-denuded aortic rings at the indicated KMUP-1 concentrations. Each point represents the mean±s.e.mean of 8 – 10 experiments. *Indicates P<0.05, Two way repeated measures ANOVA followed by Student-Newman-Keuls test.
Enhanced ACh-induced vasorelaxant responses
To investigate whether KMUP-1 has a direct effect on the action of endogenous NO released from the endothelium upon exposure to ACh, we analysed the ACh-induced vasorelaxant responses in an experimental set-up in which E+ rat aortic rings were preconstricted with PE (10 μM) or KCl (30 mM) and dilated by cumulative concentration of ACh (1 nM – 10 μM) in the presence of different concentrations of KMUP-1 (1, 10, 100 nM). Preincubation with KMUP-1 significantly increased the maximal vasorelaxant responses mediated by endogenous NO in aortic rings that was released upon exposure to acetylcholine (Figure 5A and C).
Figure 5.
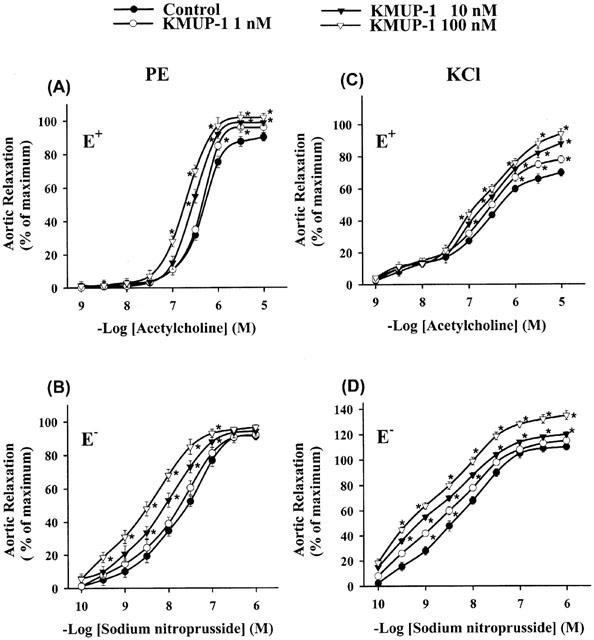
Effects of KMUP-1 on vasorelaxations induced by cumulative concentrations of ACh in endothelium-intact (E+) or SNP in endothelium-denuded (E−) rat aortic rings preconstricted with phenylephrine (10 μM, left panel) and KCl (30 mM, right panel). Each point represents the mean±s.e.mean of 6 – 8 experiments. *Indicates P<0.05, ANOVA followed by Student-Newman-Keuls test.
Potentiated SNP-induced vasorelaxant responses
Next, we evaluated the effects of KMUP-1 on the endothelium-independent vasorelaxation by the exogenous NO donor. E− aortic rings were pretreated with KMUP-1, and the responses to SNP were studied according to a protocol identical to that for ACh-induced vasorelaxations. Pretreatment with KMUP-1 (1, 10, 100 nM) markedly increased the potency of SNP, as it shifted the concentration-response curve for SNP to the left (Figure 5B and D).
Effects on K+ channels
Figure 6 shows the cumulative concentration-response curves to KMUP-1 and levcromakalim against high K+ (30 – 80 mM)-induced vasoconstriction in E+ rat thoracic aorta. KMUP-1 (1 nM – 100 μM) and the KATP channel opener levcromakalim (1 nM – 100 μM) caused concentration-dependent relaxations of isolated aortic rings precontracted with 30 mM K+ solution. Progressively increasing the concentration of K+ (40, 60, 80 mM) resulted in significant concentration-dependent reductions of the vasorelaxation induced by KMUP-1 (Figure 6A) or levcromakalim (Figure 6B). Additionally, KMUP-1 and levcromakalim (1 nM – 100 μM) produced concentration-dependent vasorelaxations in E+ rat aortic rings preconstricted with phenylephrine and completely relaxed the rings at 3 μM (-log EC50=7.58±0.13) and 30 μM (-log EC50=7.30±0.10), respectively. Aortic relaxations of KMUP-1 were reduced by a K+ channel blocker TEA (-log EC50=5.93±0.11), a KATP channel blocker glibenclamide (-log EC50=5.57±0.13), a voltage-dependent K+ channel blocker 4-AP (-log EC50=6.29±0.14) and Ca2+-dependent K+ channel blockers apamin (-log EC50=5.64±0.12) and ChTX (-log EC50=5.99±0.11). Levcromakalim-induced vasorelaxations was also attenuated by the TEA (-log EC50=6.01±0.13), glibenclamide (-log EC50<4.0) and 4-AP (-log EC50=5.78±0.12) but not by the Ca2+-dependent K+ channel blockers apamin and ChTX (Figure 7).
Figure 6.
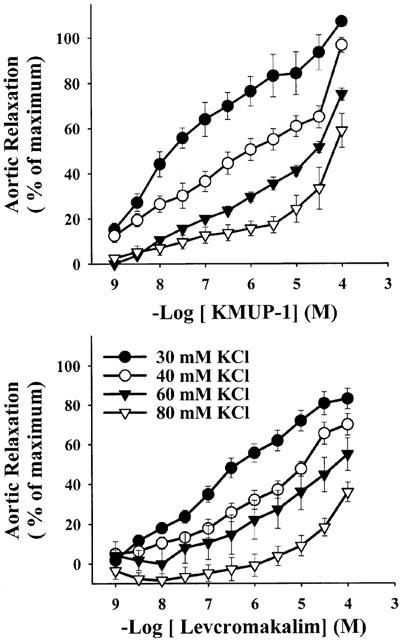
The vasorelaxant effects of KMUP-1 and levcromakalim on rat thoracic aortic rings precontracted with different concentrations of KCl (30 – 80 mM). Each point represents the mean±s.e.mean of eight experiments.
Figure 7.
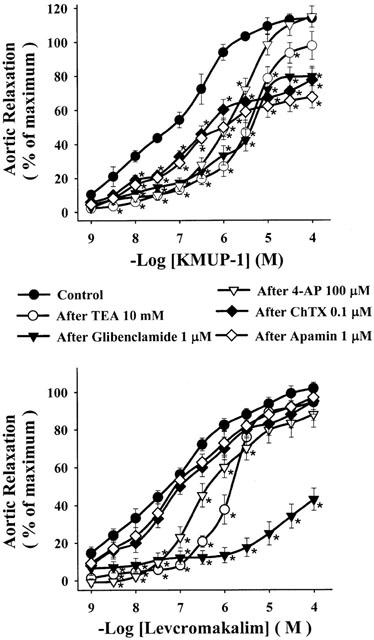
Effects of KMUP-1 and levcromakalim on rat aortic rings precontracted by phenylephrine in the absence and presence of various K+ channel blockers. Each point represents the mean±s.e.mean of 6 – 8 experiments. *Indicates P<0.05, two way repeated measures ANOVA followed by Student-Newman-Keuls test.
Effects on nitric oxide synthase and soluble guanylyl cyclase
Pretreatment with a NOS inhibitor L-NAME (-log EC50=6.20±0.12) and two sGC inhibitors, methylene blue (-log EC50=5.89±0.10) and ODQ (-log EC50=5.96±0.15), the relaxations elicited by KMUP-1 (-log EC50=7.05±0.09) were significantly reduced (Figure 8). On the other hand, pretreatment with an adenylyl cyclase inhibitor SQ 22536 did not exhibit any significant inhibition on the effects induced by KMUP-1, suggesting that the cyclic AMP pathway can be excluded (data not shown).
Figure 8.
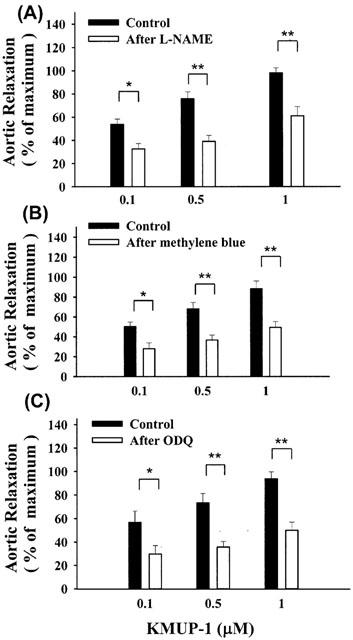
The vasorelaxant effects of KMUP-1 on rat aortic rings precontracted by phenylephrine in the absence and presence of L-NAME (100 μM), methylene blue (10 μM) and ODQ (1 μM). Each value represents the mean±s.e.mean of eight experiments. *Indicates P<0.05, **indicates P<0.01, paired Student's t-test.
Inhibition effects on phosphodiesterase
The effects of KMUP-1 on aortic rings were investigated after inhibition of PDE activity by IBMX, a nonselective PDE inhibitor. KMUP-1 (0.01, 0.05, 0.1 μM)-induced vasorelaxations (-log EC50=7.03±0.10) had an additive effect in the presence of IBMX (0.5 μM) (-log EC50=10.60±0.13) (Figure 9). In further experiments, KMUP-1 inhibited the PDE activity (29±3.1%, n=3, each performed in triplicate) at 100 μM while in comparison with sildenafil (94±4.8%, n=3).
Figure 9.
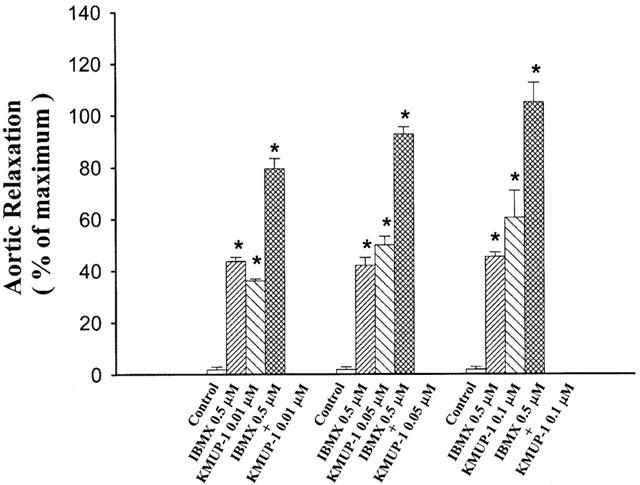
Additive effects of KMUP-1 and IBMX on rat aortic rings precontracted by phenylephrine. Each value represents the mean±s.e.mean of eight experiments. *Indicates P<0.05 as compared with the control value, ANOVA followed by Dunnett's test.
Measurement of vascular cyclic GMP and cyclic AMP
We examined the effect of KMUP-1 on cyclic GMP levels in the presence of nonselective PDE inhibitor IBMX (100 μM) in rat A10 VSM cells. The amount of basal release of cyclic GMP was 1750±154 fmol 105 cells−1 (n=3). KMUP-1 (1, 10, 100 μM) significantly increased the cyclic GMP levels in a concentration-dependent manner. SNP and sildenafil (100 μM) also elicited significant elevation of cyclic GMP accumulation (Figure 10). The rises of cyclic GMP were fully eliminated by pretreatment with methylene blue (10 μM), ODQ (10 μM) and L-NAME (100 μM) (Figure 11). However, KMUP-1 did not show any significant increase on the cyclic AMP levels in cultured A10 cells as compared with the basal value (3700±250 fmol 105 cells−1, n=3). In contrast, isoprenaline (1 – 100 μM) displayed significant increases of cyclic AMP accumulation in a dose-related manner (Figure 12).
Figure 10.
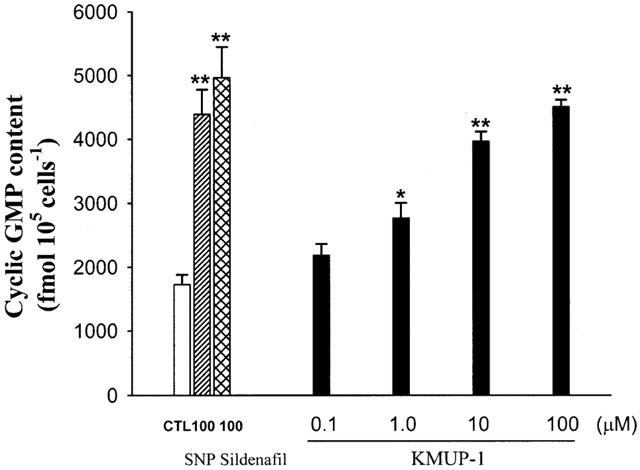
Effects of sodium nitroprusside, sildenafil and KMUP-1 on cyclic GMP levels in rat A10 vascular smooth muscle cells. Each value represents the mean±s.e.mean of three independent triplicate experiments. *Indicates P<0.05, **indicates P<0.01 as compared with the control value, ANOVA followed by Dunnett's test. CTL: solvent control (10% alcohol+10% propylene alcohol+2% HCl).
Figure 11.
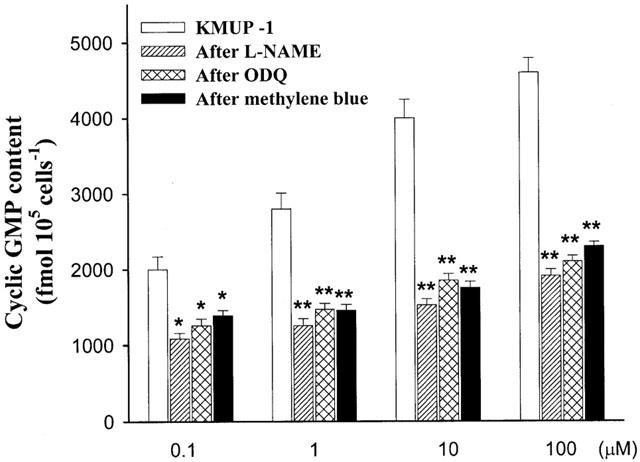
Effects of KMUP-1 on cyclic GMP levels in rat A10 vascular smooth muscle cells in the absence and presence of L-NAME (100 μM), ODQ (10 μM) and methylene blue (10 μM). The amount of basal release of cyclic GMP was 1750±154 fmol 105 cells−1. Each value represents the mean±s.e.mean of three independent triplicate experiments. *Indicates P<0.05, **indicates P<0.01, ANOVA followed by Bonferroni t-test.
Figure 12.
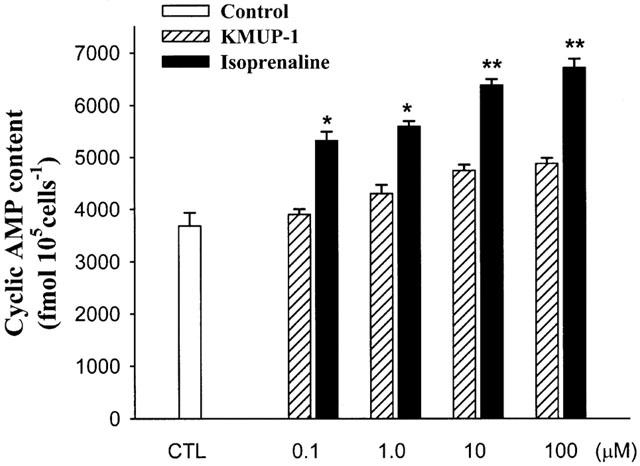
Effects of KMUP-1 and isoprenaline on cyclic AMP levels in rat A10 vascular smooth muscle cells. Each value represents the mean±s.e.mean of three independent triplicate experiments. *Indicates P<0.05, **indicates P<0.01 as compared with the control value, ANOVA followed by Dunnett's test. CTL: solvent control (10% alcohol+10% propylene alcohol+2% HCl).
Discussion
NO is an important regulator of arterial blood pressure and the vascular function. Its direct effect on vascular tone is brought about by cyclic GMP formation in VSM cells. Several NO-related compounds, such as SNP, and endogenous NO activate sGC, elevate cyclic GMP and relax smooth muscles (Katsuki & Murad, 1977). The role of sGC has been underestimated in the past due to the lack of compounds, which modulate enzyme activity specifically. This situation changed once compounds with a higher specificity towards sGC could be identified. Besides NO itself, YC-1 represents the first activating pharmacophore of intracellular sGCs in a biological milieu (Ko et al., 1994). In this study, we have demonstrated that KMUP-1 is another novel sGC activator with K+ channels opening and PDE or cyclic GMP breakdown inhibition activities.
In our in vivo study, we found that intravenous administration of KMUP-1 produced a significant dose-dependent reduction in mean arterial blood pressure, bradycardiac effect, and sometimes slightly delayed reflex tachycardia in pentobarbital-anaesthetized rats. The bradycardia effect is consistent with the in vitro finding of Mohan & Paterson (2000). These two authors reported that the NO donor and cyclic GMP analogue attenuates the heart rate and inotropic responses caused by sympathetic nerve stimulation in the isolated guinea-pig atria. In comparison with levcromakalim, the tachycardia reflex response produced by KMUP-1 is not so significant. The reason might be due to the stimulation of cyclic GMP-mediated reduction in the heart rate and thus overcomes the reflex tachycardia response. To date, the signalling pathway responsible for inhibition of sympathetic neurotransmission regulated by NO and cyclic GMP is still few, however, it can involve cyclic GMP activation of protein kinase G (Mery et al., 1991) and phosphodiesterases that decrease cyclic AMP-dependent phosphorylation of neuronal Ca2+ channels. Moreover, pretreatment with the KATP channel inhibitor glibenclamide significantly blocked the hypotensive responses of KMUP-1, indicating that KMUP-1 may have the KATP channels opening activity. However, levcromakalim-induced hypotensive responses were nearly abolished by glibenclamide, in contrast to KMUP-1. Therefore, we suggest that the hypotensive and bradycardia effects of KMUP-1 are not only KATP channels opening, but also have the possibility to activate the NO/cGMP pathway in the regulation of cardiac contractility and heart rate changes.
KMUP-1 produced aortic relaxation against the contractions induced by the low concentration (30 mM) of KCl. Under the condition where the aortic smooth muscle is contracted with a low concentration of KCl, the equilibrium potential for K+ (EK) would be more negative than the actual membrane potential (Hamilton et al., 1986). The action mechanism of KMUP-1 might stimulate K+ efflux, which in turn causes hyperpolarization and subsequent relaxation of aortic smooth muscle. On the other hand, KMUP-1 did diminish the relaxation contracted by 80 mM KCl, because the high K+ reduced the Ek to a value of membrane potential that was less negative than that required to close voltage-dependent Ca2+ channels. Under these conditions, the KMUP-1-induced increases in K+ efflux would not hyperpolarize aortic smooth muscle sufficiently to inhibit transmembrane Ca2+ influx. Such an experiment easily allows Ca2+ channel blockers to be distinguished from K+ channel opener, as demonstrated by Hamilton et al. (1986). In the present study, we found that the pharmacological characteristics of KMUP-1 against KCl (30 – 80 mM)-induced contractions are similar to those of levcromakalim that has been described as a K+ channel opener. From the above results, we suggested that the KMUP-1 is more likely a K+ channel opener but not a Ca2+ channel blocker. Besides, KMUP-1 and levcromakalim produced concentration-dependent relaxations of rat aortic rings precontracted with phenylephrine. The potency of KMUP-1 was greater than the KATP channel opener levcromakalim. The vasorelaxant effect of KMUP-1 significantly inhibited by a K+ channel blocker TEA, a KATP channel blocker glibenclamide, a voltage-dependent K+ channel blocker 4-AP and Ca2+-dependent K+ channel blockers apamin and ChTX. This result further supports the hypothesis that the vasorelaxant effect of KMUP-1 is mediated by opening of the K+ channels.
In this report, we have shown that KMUP-1 increased both the vasorelaxant response induced by endogenous NO (ACh-elicited endothelium-derived) and exogenous NO (released from the NO-donor SNP) in PE- or KCl-preconstricted aortic rings. However, there was one noteworthy difference between these two interactions. The potency of exogenous NO-donor SNP was considerably potentiated in the presence of KMUP-1 as shown in Figure 5. In contrast, KMUP-1 at low concentrations slightly increased the effectiveness, i.e. the maximal response to ACh at a given PE or KCl concentration, but not the potency of ACh-induced NO-mediated vasorelaxations. These distinct effects of KMUP-1 on exogenous and endogenous NO confirm that NO-donor SNP and ACh do not necessarily induce identical signal transduction processes (Galle et al., 1999), and provide a tool to further study the nature and action of endogenous NO. Dora et al. (2000) demonstrated that the phenylephrine-initiated increase of vascular tone could influence per se tonic NO release. Thus in this PE-preconstricted aortic rings, it may be not the best model to evaluate the effects of tonic NO release on KMUP-1 vascular action. Many VSM constrictors usually induced per se tonic NO release in vessels, among them, phenylephrine and high potassium (250 mM KCl) were chosen to evaluate NO releasing in the endothelial cell (Dora et al., 1997). In the present study, lower potassium (30 mM KCl), which induced less calcium influx associated NO releases on both endothelial and VSM cells, was used as a preconstrictor to evaluate the enhancing activity of KMUP-1 on ACh and SNP in aortic smooth muscles, respectively. However, we can not exclude the possibility that vasorelaxant responses of KMUP-1 are modulated by the activation of endothelial KATP channels (Feleder & Adler-Graschinsky, 1997). We suggested that KMUP-1-induced vasorelaxation might be partly attributed to both ACh-mediated NO release and K+ channels opening-associated NO release from endothelial cells.
Under the physiology conditions, NO is released from the endothelial cells and diffuses to the adjacent vascular smooth muscle cells where it activates soluble guanylyl cyclase, increases cyclic GMP and induces vasorelaxation (Pérez-Vizcaíno et al., 1998). There is growing evidence that the KATP channels activity may be modulated by NO. Kubo et al. (1993) demonstrated that nitric oxide donors cause activation of KATP channels in rat aorta by means of a cyclic GMP-dependent mechanism, possibly cyclic GMP-dependent protein kinase. Since KMUP-1 in endothelium-denuded aortic rings or during the inhibition of NOS is still able to evoke a vasorelaxant effect, we cannot, therefore, exclude the possibility that the direct action of KMUP-1 on VSM component is dependent on K+ channels opening activity. Furthermore, we show that the vasorelaxant effects of KMUP-1 are significantly blunted but not inhibited by pretreatment with sGC inhibitors methylene blue and ODQ. The results further support that the opening of K+ channels are involved in the underlying mechanism of relaxations in addition to the stimulation of sGC/cyclic GMP pathway.
In this study, we have demonstrated that the combination of KMUP-1 and IBMX, a nonselective PDE inhibitor, had an additive effect on VSM relaxations in rat aorta. Additionally, we observed that KMUP-1 also affected cyclic GMP breakdown at 100 μM, because it inhibited the enzyme activity of PDE in human platelets. In further experiments, KMUP-1 significantly raised the intracellular cyclic GMP levels in a concentration-dependent manner in rat A10 VSM cells, but did not find any significant increase on the cyclic AMP. The augment in cyclic GMP level of KMUP-1 was almost completely inhibited by pretreatment with methylene blue, ODQ and L-NAME. These results further confirm that KMUP-1 activate the NO/sGC/cyclic GMP pathway and inhibit the PDE or cyclic GMP breakdown, and therefore elevate the intracellular cyclic GMP levels leading to the VSM relaxation.
In summary, the results presented here provide the evidence that the VSM relaxant activities of KMUP-1, a novel xanthine and piperazine derivative, are most likely mediated via activation of NO/sGC/cyclic GMP pathway, stimulating the K+ channels and inhibition of PDE or cyclic GMP metabolism (Figure 13). The negative logarithm EC50 results of KMUP-1 indicate that K+ channels opening and cyclic GMP increasing are the major determinants for its vasorelaxant and hypotensive effects in rats. Obtained accumulation of cyclic GMP derived from NO/sGC/cyclic GMP pathway further enhances the K+ efflux, leading blunt of Ca2+ influx-associated contractility in aortic smooth muscles.
Figure 13.
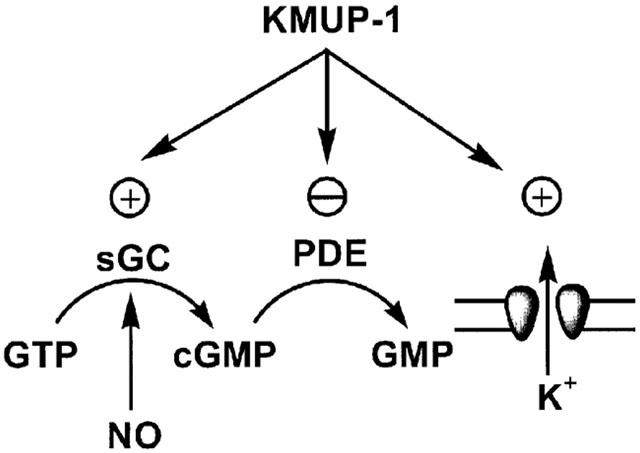
Proposed mechanism of action of KMUP-1 on the intracellular cyclic GMP synthesis and metabolism, K+ channels opening and phosphodiesterase inhibition.
Acknowledgments
The work was supported by grants No. NSC-89-2320-B-037-056-M59 to Dr. Ing-Jun Chen and NSC-89-2320-B-037-081 to Dr. Bin-Nan Wu from the National Science Council, Taiwan and Vital Pharm Co., Ltd. (Kaohsiung, Taiwan).
Abbreviations
- Cyclic AMP
adenosine 3′,5′-cyclic monophosphate
- cyclic GMP
guanosine 3′,5′-cyclic monophosphate
- KATP channels
ATP-sensitive potassium channels
- NO
nitric oxide
- NOS
nitric oxide synthase
- PDE
phosphodiesterase
- PE
phenylephrine
- sGC
soluble guanylyl cyclase
- SNP
sodium nitroprusside
- VSM
vascular smooth muscle
References
- ARNOLD W.P., MITTAL C.K., KATSUKI S., MURAD F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc. Natl. Acad. Sci. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN S.J., WANG M.H., CHEN I.J. Antiplatelet and calcium inhibitory properties of eugenol and sodium eugenol acetate. Gen. Pharmacol. 1996;27:629–633. doi: 10.1016/0306-3623(95)02089-6. [DOI] [PubMed] [Google Scholar]
- COHEN R.A., VANHOUTTE P.M. Endothelium-dependent hyperpolarization. Beyond nitric oxide and cyclic GMP. Circulation. 1995;87 Suppl. V:V18–V25. doi: 10.1161/01.cir.92.11.3337. [DOI] [PubMed] [Google Scholar]
- DORA K.A., DOYLE M.P., DULING B.R. Elevation of intracellular calcium in smooth muscle causes endothelial cell generation of NO in arterioles. Proc. Natl. Acad. Sci. U.S.A. 1997;94:6529–6534. doi: 10.1073/pnas.94.12.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DORA K.A., HINTON J.M., WALKER S.D., GARLAND C.J. An indirect influence of phenylephrine on the release of endothelium-derived vasodilators in rat small mesenteric artery. Br. J. Pharmacol. 2000;129:381–387. doi: 10.1038/sj.bjp.0703052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELEDER E.C., ADLER-GRASCHINSKY E. Endothelium-mediated and Nω-nitro-L-arginine methyl ester-sensitive responses to cromakalim and diazoxide in the rat mesenteric bed. Eur. J. Pharmacol. 2000;319:229–238. doi: 10.1016/s0014-2999(96)00843-6. [DOI] [PubMed] [Google Scholar]
- GALLE J., ZABEL U., HÜBNER U., HATZELMANN A., WAGNER B., WANNER C., SCHMIDT H.H.H.W. Effects of the soluble guanylyl cyclase activator, YC-1, on vascular tone, cyclic GMP levels and phosphodiesterase activity. Br. J. Pharmacol. 1999;127:195–203. doi: 10.1038/sj.bjp.0702495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILTON T.C., WEIR S.W., WESTON A.H. Comparison of the effects of BRL 34915 and verapamil on electrical and mechanical activity in rat portal vein. Br. J. Pharmacol. 1986;88:103–111. doi: 10.1111/j.1476-5381.1986.tb09476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIDAKA H., ASANO T. Human blood platelet 3′:5′-cyclic nucleotide phosphodiesterase: isolation of low-Km and high-Km phosphodiesterase. Biochim. Biophys. Acta. 1976;429:485–497. doi: 10.1016/0005-2744(76)90296-5. [DOI] [PubMed] [Google Scholar]
- IGNARRO L.J. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu. Rev. Pharmacol. Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- KATSUKI S., MURAD F. Regulation of adenosine cyclic 3′,5′-monophosphate and guanosine cyclic 3′,5′-monophosphate levels and contractility in bovine tracheal smooth muscle. Mol. Pharmacol. 1977;13:330–341. [PubMed] [Google Scholar]
- KILPATRICK E.V., COCKS T.M. Evidence for differential roles of nitric oxide (NO) and hyperpolarization in endothelium-dependent relaxation of pig isolated coronary artery. Br. J. Pharmacol. 1994;112:557–565. doi: 10.1111/j.1476-5381.1994.tb13110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KO F.N., WU C.C., KUO S.C., LEE F.Y., TENG C.M. YC-1, a novel activator of platelet guanylate cyclase. Blood. 1994;84:4226–4233. [PubMed] [Google Scholar]
- KUBO M., NAKAYA Y., MATSUOKA S., SAITO K., KURODA Y. Atrial natriuretic factor and isosorbide dinitrate modulate the gating of ATP-sensitive K+ channels in cultured vascular smooth muscle. Circ. Res. 1993;74:471–476. doi: 10.1161/01.res.74.3.471. [DOI] [PubMed] [Google Scholar]
- LOWENSTEIN C.J., DINERMAN J.L., SNYDER S.H. Nitric oxide: A physiology messenger. Ann. Intern. Med. 1994;120:227–237. doi: 10.7326/0003-4819-120-3-199402010-00009. [DOI] [PubMed] [Google Scholar]
- MERY P.F., LOHMANN S.M., WALTER U., FISCHMEISTER R. Ca2+ current is regulated by cyclic GMP-dependent protein kinase in mammalian cardiac myocytes. Proc. Natl. Acad. Sci. 1991;88:1197–1201. doi: 10.1073/pnas.88.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOHAN R.M., PATERSON D.J. Activation of sulphonylurea-sensitive channels and the NO-cGMP pathway decreases the heart rate response to sympathetic nerve stimulation. Cardiovasc. Res. 2000;47:81–89. doi: 10.1016/s0008-6363(00)00057-2. [DOI] [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M., HIGGS E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- MURPHY M.E., BRAYDEN J.E. Nitric oxide hyperpolarizes rabbit mesenteric arteries via ATP-sensitivity potassium channels. J. Physiol. 1995;486:47–58. doi: 10.1113/jphysiol.1995.sp020789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON M.T., QUAYLE J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- PÉREZ-VIZCAÍNO F., COGOLLUDO A.L., VILLAMOR E., TAMARGO J. Role of K+ channel opening and stimulation of cyclic GMP in the vasorelaxant effects of nicorandil in isolated piglet pulmonary and mesenteric arteries: relative efficacy and interactions between both pathways. Br. J. Pharmacol. 1998;123:847–854. doi: 10.1038/sj.bjp.0701693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PÉREZ-VIZCAÍNO F., COGOLLUDO A.L., ZARAGOZÁ-ARNÁEZ F., FAJARDO S., IBARRA M., LÓPEZ-LÓPEZ J.G., TAMARGO J. Vasodilator effects of sodium nitroprusside, levcromakalim and their combination in isolated rat aorta. Br. J. Pharmacol. 1999;128:1419–1426. doi: 10.1038/sj.bjp.0702924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMIDT H.H.H.W., LOHMANN S.L., WALTER U. The nitric oxide and cGMP signal-transduction pathway. Biochim. Biophys. Acta. 1993;1178:153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- WEIDELT T., BOLDT W., MARKWARDT F. Acetylcholin-induced K+ currents in smooth muscle cells of intact rat small arteries. J. Physiol. 1997;500:617–630. doi: 10.1113/jphysiol.1997.sp022047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE R., HILEY C.R. Endothelium and cannabinoid receptor involvement in levcromakalim vasorelaxation. Eur. J. Pharmacol. 1997;339:157–160. doi: 10.1016/s0014-2999(97)01397-6. [DOI] [PubMed] [Google Scholar]
- WOHLFART P., MALINSKI T., RUETTEN H., SCHINDLER U., LINZ W., SCHOENAFINGER K., STROBEL H., WIEMER G. Release of nitric oxide from endothelial cells stimulated by YC-1, an activator of soluble guanylyl cyclase. Br. J. Pharmacol. 1999;128:1316–1322. doi: 10.1038/sj.bjp.0702921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU B.N., HWANG T.L., LIAO C.F., CHEN I.J. Vaninolol: a new selective β1-adrenoceptor antagonist derived from vanillin. Biochem. Pharmacol. 1994;48:101–109. [PubMed] [Google Scholar]


