Abstract
We showed previously that interaction between NO and iron (II), both released following the decomposition of sodium nitroprusside (SNP), accounted for the late SNP-induced dopamine (DA) increase in dialysates from the striatum of freely moving rats; in addition, we showed that co-infusion of iron (II) with the NO-donor S-nitroso-N-acetylpenicillamine mimicked SNP effects on striatal DA release.
In the present study, intrastriatal co-infusion of iron (II) (given as FeSO4, 1 mM for 40 min) with the NO-donor and potential peroxynitrite generator 3-morpholinosydnonimine (SIN-1) (0.2, 0.5, 1.0 or 5.0 mM for 180 min), potentiated the SIN-1-induced increase in DA concentration in dialysates from the striatum of freely moving rats. Neither alone nor associated with iron (II) did SIN-1 induce changes in dialysate ascorbic acid or uric acid concentrations.
Neither co-infusion of a superoxide dismutase mimetic nor uric acid affected SIN-1-induced increases in dialysate DA concentration.
Infusion of the iron chelator deferoxamine (0.2 mM for 180 min) decreased dialysate DA and attenuated SIN-1-induced increases in dialysate DA concentrations.
These results suggest that iron plays a key role in SIN-1-induced release of striatal DA and do not support any role for either peroxynitrite or superoxide anion in SIN-1-induced release of striatal DA.
Keywords: NO-donor, dopamine, in vivo release, iron, peroxynitrite, microdialysis, rat striatum
Introduction
Nitric oxide (NO) is a multifunctional and ubiquitous biological messenger molecule. NO signalling plays an important role in the functioning of the central nervous system (Gartwaite & Boulton, 1995). Several in vivo studies have demonstrated that NO modulates extracellular levels of dopamine in the striatum and in the hippocampus. In these studies, either NO-generating drugs [sodium nitroprusside (SNP), 3-morpholinosydnonimine (SIN-1), S-nitroso-N-acetylpenicillamine (SNAP)] (Guevara-Guzman et al., 1994; West & Galloway, 1996; 1997; 1998; Segieth et al., 2000), NO-synthase inhibitors (Silva et al., 1995; Segovia & Mora, 1998; Desvignes et al., 1999; Segieth et al., 2000; Wegener et al., 2000), or NO-synthase substrates (Strasser et al., 1994; West & Galloway, 1997; Wegener et al., 2000), were employed. The results of these studies have often been conflicting. West & Galloway (1996; 1997) showed that SNAP increased DA efflux from the striatum of chloral hydrate-anaesthetized rats. In contrast, Guevara-Guzman et al. (1994) showed that SNAP decreased extracellular DA concentration in urethane-anaesthetized rats, as did NO gas, given directly by dissolution in degassed perfusion fluid. Segieth et al. (2000) showed that SNAP promoted DA release from the rat hippocampus in vivo at low concentrations, whilst high concentrations induced long-lasting DA decreases. More recently, Trabace & Kendrick (2000) have shown that short-lasting intrastriatal infusion of SNAP induced increases in dialysate DA at low concentrations, and decreases in dialysate DA at high concentrations; these DA changes were attributed to SNAP-induced peroxynitrite formation; at high levels, peroxynitrite may reduce extracellular DA concentration through oxidation, while at low levels it may increase DA levels in a cyclic GMP-dependent manner. We confirmed (Serra et al., 2001) that, at high concentrations, SNAP intrastriatal infusion induces non-enzymatic DA oxidation, which is inhibited by co-infusion of N-acetylcysteine (NAC) or uric acid, while, at low concentrations, it increases dialysate DA.
In a previous study (Serra et al., 2000), we showed that intrastriatal infusion of either SIN-1 or SNP produced a NO-mediated increase in DA concentration in dialysates from the striatum of freely moving rats. This effect was attenuated by exogenous ascorbic acid. In addition, SNP greatly increased dialysate DA concentration at the end of the infusion period. This effect appeared to be the consequence of an interaction between NO and iron (II), both released from the ferricyanide moiety of SNP, since it was inhibited by the iron chelator deferoxamine. In addition, we showed (Serra et al., 2001) that iron (II) greatly increased DA dialysate concentration when co-infused with either high or low concentrations of SNAP.
Iron is a transitional metal involved in many catalytic and regulatory neuronal processes (Connor, 1997), which readily reacts with NO (Le Brun et al., 1997). Iron (II) promotes oxidative stress through reactive oxygen species (ROS) formation (Jellinger, 1999). Several in vivo experimental studies have linked iron (II)-induced oxidative stress to iron (II)-induced degeneration and death of nigro-striatal neurons (Sengstock et al., 1994; 1997; Mohanakumar et al., 1996). When infused intrastriatally, iron (II) induces a delayed and infusion time-related DA increase in dialysates from the striatum of freely moving rats (Han et al., 1999). This effect has been related (Han et al., 1999) to iron (II)-induced hydroxyl radical formation (Xie et al., 1995). Interestingly, both nitric oxide (NO)-donors, with the exception of SNP, and NO gas in Ringer's solution, protected nigral neurones from iron (II)-induced oxidative stress (Rauhala et al., 1996; Mohanakumar et al., 1996) and degeneration (Lin, 1999).
SIN-1 is not a simple NO donor, since it also generates the superoxide anion, being thus a potential peroxynitrite generator (Menconi et al., 1998). Further studies on the role of NO, iron and peroxynitrite in SIN-1-induced DA release from dopaminergic terminals in the striatum of freely moving rats were therefore deemed of interest.
Methods
Animals
Male Wistar rats (Morini, R. Emilia, Italy), weighing between 280 – 330 g were used in all experiments. The rats were maintained under standard animal care conditions (12 : 12 h light/dark cycle, lights coming on at 7 a.m.; room temperature 21°C), with food and water ad libitum. Prior to the start of any experiment, the health of the rat was assessed according to published guidelines (Morton & Griffiths, 1985). All procedures were specifically licensed under the European Community directive 86/609 included in Decreto No. 116/1992 of the Italian Ministry of Public Health.
Drugs
SIN-1, deferoxamine, N-acetylcysteine (NAC), uric acid, and ferrous sulphate [FeSO4, iron (II)] were purchased from Sigma-Aldrich (Milano, Italy); manganese (III) tetrakis (4-benzoic acid) porphyrin (MnTBP) from Calbiochem (Darmstadt, Germany).
Drug administration
Iron (II), MnTBP, NAC and uric acid concentrations were chosen according to Serra et al. (2000; 2001); deferoxamine according to Desole et al. (1998).
Microdialysis probe construction
The striatal probe combined two independent microdialysis probes of concentric design with two separate inlets and a shared outlet, as previously described (Miele et al., 2000). The probes were constructed using two sections of plastic-coated silica tubing (diameter 0.15 mm; Scientific Glass Engineering, Milton Keynes, U.K.) each placed in the centre of a semi-permeable polyacrylonitrile dialysis fibres (molecular cut-off weight of 12 KD, Filtral 16 Hospal Industrie, France). Each probe had a final diameter of 0.22 mm. The tips of the dialysis fibre were sealed and joined using quick-drying epoxy glue. The two sections of silica tubing served as inlets; the outlet was made also with a section of plastic-coated silica tubing, positioned in the centre of polythene tubing. The semi-permeable membrane was coated with epoxy leaving an active length of 4 mm. The diameter of the final probe was approximately 0.50 mm. The striatal probe combining two microdialysis probes of concentric design with two separate inlets and a shared outlet, allowed separate co-infusion of drugs.
Surgery
Stereotaxic surgery was performed under chloral hydrate (400 mg kg−1 i.p.) anaesthesia. The microdialysis probes were implanted in the right striatum using the following co-ordinates from the atlas of Paxinos & Watson (1986): A/P +0.5 mm from bregma, +2.5 mm M/L, and −6.0 mm D/V from dura. Body temperature during anaesthesia was maintained at 37°C by means of an isothermal-heating pad (Harvard Apparatus, Kent, U.K.). Following surgery the animals were placed in large plastic bowls (50×55 cm), and maintained in a temperature- and light-controlled environment, with free access to food and water. Experiments were carried out 24 h after probe implantation with the animal in its home bowl. This arrangement allowed the rats free movement.
Microdialysis procedure
The composition of the Ringer solution used was as follows, in (mM) NaCl 147, KCl 4, CaCl2 1.2, MgCl2 1 (pH 6.0). A microinfusion pump (CMA/100, Microdialysis, Sweden) pumped Ringer solution at a flow rate of 1.0 μl min−1 using two separate syringes connected to the inlets by a length of polythene tubing; every 20 min, 40 μl dialysate samples were collected manually in 250 μl micro-centrifuge tubes (Alpha Laboratories, U.K.) attached to the outlet. Subsequently, a 20 μl aliquot of collected dialysate was injected into the analytical system. Drugs were added to the Ringer solution and infused via the striatal probe implanted in the striatum.
Chromatographic analysis
DA, dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), ascorbic acid and uric acid were quantified by high performance liquid chromatography with electrochemical detection (HPLC-EC) as previously described (Miele et al., 2000; Serra et al., 2000), using an Alltech 426 HPLC pump equipped with a Rheodyne injector, column 15 cm×4.6 mm i.d. Alltech Adsorbsphere C18 5U, electrochemical detector Antec CU-04-AZ and Varian Star Chromatographic Workstation. The mobile phase was citric acid 0.5 M, Na acetate 1.0 M, EDTA 12.5 mM, MeOH 10% and sodium octylsulphate 650 mg l−1 (pH 3.0); the flow rate was 1.3 ml min−1. The first sample was collected after 60 min of stabilization (time 0), then dialysates were collected, at 20 min intervals, for 40 min prior to the start of experiments.
Histology
Following the experiments, rats were killed with an overdose of chloral hydrate (800 mg kg−1 i.p.). The location of each microdialysis probe was confirmed by post-mortem histology. Brains were fixed in formal saline and 50 μm coronal sections were made with a cryostat. The slices were stained with cresyl violet and examined under a microscope.
Statistical analysis
The concentrations in the dialysate were expressed in nM (DA) or μM (DOPAC, HVA, ascorbic acid, uric acid) and given as mean±s.e.mean. Drug effects on neurochemicals were statistically evaluated in terms of changes in absolute dialysate concentrations. Statistical significance was assessed using analysis of variance (ANOVA) for difference between groups and over time. Difference within or between groups were determined by paired or unpaired t-tests with Bonferroni multiple comparison adjustment.
Results
Effects of intrastriatal infusion of increasing concentrations of SIN-1 on dialysate concentration of DA, DOPAC+HVA, ascorbic acid and uric acid
Intrastriatal infusion of SIN-1 0.2 mM (n=3) or 0.5 mM (n=3) for 180 min did not induce changes in dialysate concentrations of DA (Figure 1A), DOPAC+HVA (baseline levels 2.11±0.42 and 1.45±0.19 μM, respectively), ascorbic acid (baseline levels 7.63±1.12 and 8.32±1.15 μM, respectively) and uric acid (baseline levels 1.70±0.28 and 2.33±0.44 μM, respectively) (data not shown).
Figure 1.
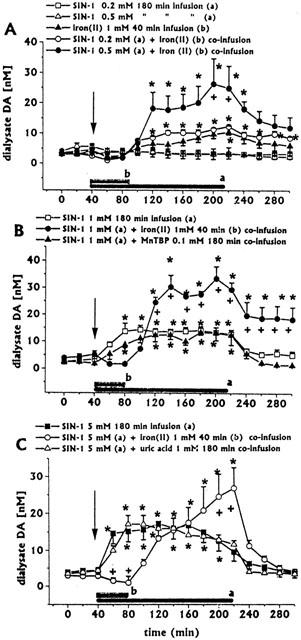
Effects of intrastriatal infusion of increasing concentrations of SIN-1 (0.2 mM (n=3, A), 0.5 mM (n=3, A), 1.0 mM (n=4, B) and 5.0 mM (n=4, C) on DA dialysate concentrations, and effects of co-infusion of either iron (II) with SIN-1 (0.2 mM (n=3, A), 0.5 mM (n=3, A), 1.0 mM (n=4, B) and 5.0 mM (n=4, C)), MnTBP with SIN-1 (1 mM (n=3, B)), or uric acid with SIN-1 (5.0 mM (n=4, C)) on SIN-1-induced changes in DA dialysate concentrations. Dialysates were collected, at 20 min intervals, for 180 min during drug infusion (horizontal black bars) and for 80 min after discontinuation of drug infusion. Values are given as mean±s.e.mean. *P<0.05 compared with baseline values. +P<0.05 iron (II)+SIN-1 0.5 mM group (A), 1.0 mM group (B) and 5.0 mM group (C) compared with respective SIN-1 groups.
Intrastriatal infusion of SIN-1 1 mM (n=4) for 180 min induced increases in dialysate DA, with a peak (about six times baseline levels) 60 min after the start of infusion. (Figure 1B). Dialysate DA returned to baseline within 60 min after SIN-1 discontinuation. DOPAC+HVA (baseline levels 2.00±0.34 μM), ascorbic acid (baseline levels 7.58±2.37 μM) and uric acid (baseline levels 1.82±0.17 μM) dialysate concentrations baseline levels were unaffected (data not shown).
Intrastriatal infusion of SIN-1 5 mM (n=4) for 180 min induced increases in dialysate DA, with a peak (about five times baseline levels) 80 min after the start of infusion. (Figure 1C). Dialysate DA returned to baseline within 20 min after SIN-1 discontinuation. Dialysate DOPAC+HVA concentrations were significantly decreased (Figure 2), while ascorbic acid (baseline levels 8.97±2.42 μM) and uric acid (baseline levels 1.89±0.22 μM) were unaffected (data not shown).
Figure 2.
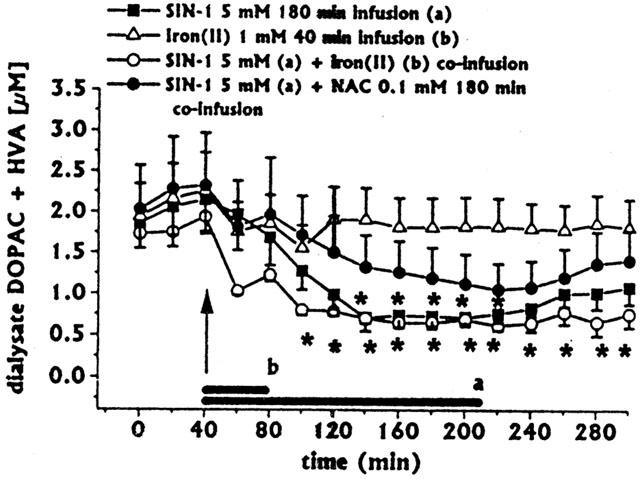
Effect of intrastriatal infusion of SIN-1 (n=4) or iron (II) (n=3) on DOPAC+HVA dialysate concentrations, and effects of iron (II) (n=4) or NAC+iron (II) (n=3) co-infusion with SIN-1, on SIN-1-induced changes in DOPAC+HVA concentrations. Dialysates were collected, at 20 min intervals, for 180 min during drug infusion (horizontal black bars) and for 80 min after discontinuation of drug infusion. Values are given as mean±s.e.mean. *P<0.05 compared with baseline values.
Functional integrity of dopaminergic terminals exposed to SIN-1 5.0 mM
The SIN-1 5.0 mM-induced increase in dialysate DA in the range of that obtained with SIN-1 1.0 mM infusion, and the SIN-1 5.0 mM-induced decrease in dialysate DOPAC+HVA, raised the question as to whether exposure to SIN-1 5.0 mM might compromise the functional integrity of dopaminergic terminals. To address this important point, additional experiments were done. All the questions concerning repeated dialysis procedures within the same animal have been thoroughly addressed in a previous paper (Enrico et al., 1997).
SIN-1 5.0 mM was infused intrastriatally for 180 min (n=3). Baseline levels of dialysate neurochemicals were the following: DA, 3.83±0.51 nM; DOPAC+HVA, 1.47±0.27 μM; ascorbic acid, 11.28±0.86 μM; uric acid 1.74±0.23 μM. Four days after the intrastriatal infusion of SIN-1 5.0 mM, dopaminergic terminals were challenged with a d-amphetamine 2.0 mM 15 min infusion, according to Miele et al. (2000). Baseline dialysate levels of DA (2.68±0.58 mM) and DOPAC+HVA (0.927±0.17 μM) did not statistically differ from baseline levels detected before SIN-1 infusion. On the contrary, dialysate ascorbic acid (7.65±0.90 μM) and uric acid (5.95±1.56 μM) resulted statistically (P<0.05) lower and, respectively, higher than baseline levels detected before SIN-1 infusion. The increase in dialysate uric acid may reflect a periprobe tissue reaction (Enrico et al., 1997).
Following d-amphetamine 2.0 mM 15 min infusion, dialysate DA concentrations increased up to 800 – 1600% of baseline value. These increases are in the range of those obtained in the previous study (Miele et al., 2000) with d-amphetamine systemic (2.0 mg kg−1) administration or intrastriatal (2.0 mM) 15 min infusion.
Effect of iron (II) co-infusion of SIN-1-induced changes in dialysate neurochemicals concentrations
Intrastriatal infusion of iron (II) (1 mM for 40 min, n=3) induced a late moderate increase in dialysate DA concentration, with a peak (about three times baseline levels) 140 min after iron (II) discontinuation (Figure 1A), whilst all other neurochemicals (DOPAC+HVA, ascorbic acid, uric acid) dialysate concentrations were unaffected (data not shown).
Iron (II) (1 mM for 40 min) co-infusion with SIN-1 (0.2 mM for 180 min, n=3) induced an early (40 min after iron (II) discontinuation) and long-lasting increase (even after SIN-1 discontinuation) in dialysate DA, with a peak (about four times baseline levels) at the end of SIN-1 infusion (Figure 1A). During the 40 min of iron (II) and SIN-1 co-infusion, dialysate DA concentrations fell below control values (Figure 1A). All other neurochemicals (DOPAC+HVA, ascorbic acid, uric acid) dialysate concentrations were unaffected (data not shown).
Iron (II) (1 mM for 40 min) co-infusion with SIN-1 (0.5 mM for 180 min, n=4) induced an early (40 min after iron (II) discontinuation) and long-lasting increase (even after SIN-1 discontinuation) in dialysate DA, with a peak (about seven times baseline levels) 20 min before SIN-1 discontinuation (Figure 1A). During the 40 min of iron (II) and SIN-1 co-infusion, dialysate DA concentrations fell below control values (Figure 1A). All other neurochemicals (DOPAC+HVA, ascorbic acid, uric acid) dialysate concentrations were unaffected (data not shown).
Iron (II) (1 mM for 40 min) co-infusion with SIN-1 (1.0 mM for 180 min, n=4) induced an early (40 min after iron (II) discontinuation) and long-lasting increase (even after SIN-1 discontinuation) in dialysate DA, with a peak (about nine times baseline levels) 20 min before SIN-1 discontinuation (Figure 1B). During the 40 min of iron (II) and SIN-1 co-infusion, dialysate DA concentrations fell below control values (Figure 1B). All other neurochemicals (DOPAC+HVA, ascorbic acid, uric acid) dialysate concentrations were unaffected (data not shown).
Iron (II) (1 mM for 40 min) co-infusion with SIN-1 (5.0 mM for 180 min, n=3) induced a late (80 min after iron (II) discontinuation) increase in dialysate DA, with a peak (about nine times baseline levels) at the end of SIN-1 infusion (Figure 1, panel 5). During the 40 min of iron (II) and SIN-1 co-infusion, dialysate DA concentrations fell below control values (Figure 1B). Dialysate DA returned to baseline within 40 min after SIN-1 discontinuation. Dialysate DOPAC+HVA concentrations decreased significantly (Figure 2), while ascorbic acid and uric acid were unaffected (data not shown).
Effect of NAc-cysteine co-infusion on SIN-1+iron (II)-induced changes in DA and DOPAC+HVA dialysate concentrations
We showed previously (Serra et al., 2001) that intrastriatal infusion of NAC protected DA from SNAP-induced non-enzymatic oxidation. In the present study, the increase in dialysate DA given by co-infusion of iron (II) with SIN-1 1.0 mM was greater than that given by co-infusion of iron (II) with SIN-1 5.0 mM (Figure 1B,C). These data suggest that, at high concentrations, SIN-1 might also induce DA non-enzymatic oxidation. Therefore, the study of NAC co-infusion on SIN-1-induced changes in DA and DOPAC+HVA dialysate concentrations was deemed of interest.
Intrastriatal co-infusion of NAC 0.1 mM+SIN-1 5.0 mM (for 180 min) with iron (II) (1 mM for 40 min, n=3) induced a significant early and long-lasting increase in dialysate DA, compared with iron (II)+SIN-1 5.0 mM group; in addition, return of dialysate DA to baseline was significantly delayed (Figure 3). Also, NAC co-infusion inhibited iron (II)+ SIN-1-induced decreases in dialysate DOPAC+HVA (Figure 2). Ascorbic acid and uric acid dialysate concentrations were unaffected (data not shown).
Figure 3.
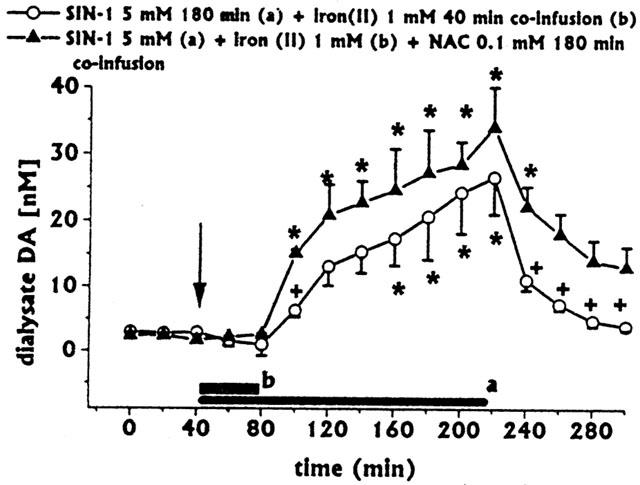
Effect of intrastriatal co-infusion of SIN-1 with iron (II) on dialysate DA (same group as in Figure 1C) and effects of NAC co-infusion (n=3) on iron (II)+SIN-1 induced changes in dialysate DA. Dialysates were collected, at 20 min intervals, for 180 min during drug infusion (horizontal black bars) and for 80 min after discontinuation of drug infusion. Values are given as mean±s.e.mean. *P<0.05 compared with baseline values; +P<0.05 compared with iron (II)+SIN-1.
Effect of superoxide dismutase (SOD) mimetic MnTBP co-infusion on SIN-induced increases in dialysate DA concentration
Peroxynitrite is formed by reaction of NO with superoxide anions, thus SIN-1 is a potential peroxynitrite generator, since it releases both NO and superoxide anions (Menconi et al., 1998). A role for peroxynitrite in SNAP-induced increases in striatal DA has been suggested by Trabace & Kendrick (2000). Ascorbic acid is a natural inhibitor of peroxynitrite generation (Jackson et al., 1998; Kirsch & de Groot, 2000), while uric acid is a natural scavenger of peroxynitrite (Hooper et al., 1998); however, in this study, SIN-1 infusion affected neither ascorbic acid nor uric acid dialysate levels. SOD is too large a molecule to cross the dialysis membrane used; therefore, we used the cell-permeant SOD mimetic MnTBP (Patel & Day, 1999), in order to assess the role of peroxynitre in SIN-1-induced increases in dialysate DA.
Co-infusion of MnTBP (0.1 mM for 180 min, n=3) did not affect SIN-1 (1 mM)-induced increases in dialysate DA (Figure 1B).
Effect of uric acid co-infusion on SIN-induced changes in DA and ascorbic acid dialysate concentrations
Uric acid is a strong natural scavenger of peroxynitrite (Hooper et al., 1998). Although in the present study SIN-1 infusion failed to modify dialysate uric acid concentrations, the study of uric acid co-infusion on SIN-1-induced changes in DA and dialysate concentrations was deemed of interest.
Co-infusion of uric acid 1 mM with SIN-5 mM for 180 min (n=4) did not affect SIN-1-induced increases in dialysate DA (Figure 1C). In addition, uric acid co-infusion did not affect SIN-1-induced decreases in dialysate DOPAC+HVA concentrations (data not shown).
Effect of intrastriatal infusion of deferoxamine on SIN-1 induced increases in dialysate DA
The finding that exogenous iron (II) potentiated SIN-1-induced increases in dialysate DA prompted us to study the effect of the endogenous iron chelator deferoxamine, to evaluate any potential role of endogenous iron in the SIN-1 induced increase in striatal DA release.
Infusion of deferoxamine (0.2 mM) for 180 min (n=3) decreased dialysate DA (Figure 4A), whilst it did not affect dialysate DOPAC+HVA (Figure 4B), ascorbic acid or uric acid concentrations (data not shown).
Figure 4.
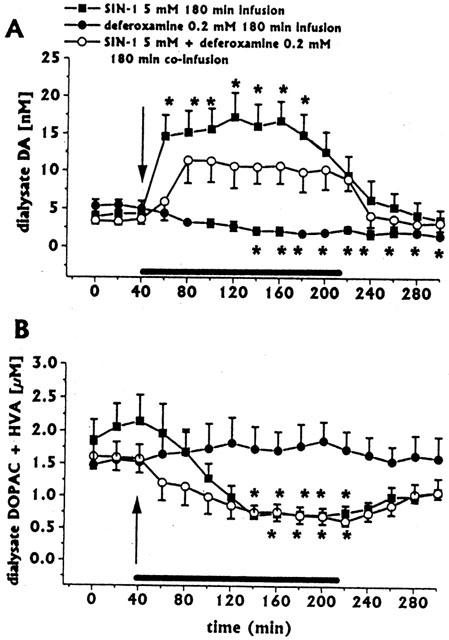
Effect of intrastriatal infusion of deferoxamine (n=4) on dialysate DA (A) and DOPAC+HVA concentrations (B), and effects of deferoxamine co-infusion on SIN-1-induced changes in dialysate DA (same group as in Figure 1C) and DOPAC+HVA (same group as in Figure 2). Dialysates were collected, at 20 min intervals, for 180 min during drug infusion (horizontal black bar) and for 80 min after discontinuation of drug infusion. Values are given as mean±s.e.mean. *P<0.05 compared with baseline values.
Co-infusion of deferoxamine 0.2 mM for 180 min (n=4) attenuated the SIN-1-induced increase in dialysate DA concentration (Figure 4A). Over time ANOVA revealed that the increase was still statistically significant (P<0.001), but no significant point resulted with the Bonferroni test.
Deferoxamine co-infusion did not affect SIN-1-induced decreases in dialysate DOPAC+HVA (Figure 4B).
Discussion
The results of the present study have shown that intrastriatal infusion of the NO-donor and potential peroxynitrite generator SIN-1 induces concentration-unrelated increases in dialysate DA. In fact, at lower concentrations (0.2 – 0.5 mM), SIN-1 does not induce changes in dialysate DA, while the increase given by the higher concentration (5.0 mM) tested does not differ from the increase given by the concentration of 1.0 mM. In addition, the higher SIN-1 concentration (5.0 mM) decreased dialysate DOPAC+HVA levels. At all concentrations tested, SIN-1 infusion did not affect dialysate ascorbic acid and uric acid concentrations.
The SIN-1 5.0 mM-induced increase in dialysate DA in the range of that obtained with SIN-1 1.0 mM infusion, and the SIN-1 5.0 mM-induced decrease in dialysate DOPAC+HVA, raise the question as to whether exposure to SIN-1 might compromise the functional integrity of dopaminergic terminals. West & Galloway (1998) claim that it is unlikely that exposure of striatal dopaminergic terminals to SIN-1 1.0 mM might compromise the functional integrity of nerve terminals via a neurotoxic disruption of plasma membrane. In the present study, instrastriatal d-amphetamine infusion 4 days after exposure to SIN-1 5.0 mM increased dialysate DA levels up to 800 – 1600% of baseline value. These increases are in the range of those obtained in a previous study with systemic or intrastriatal d-amphetamine administrations (Miele et al., 2000), and should be considered as a reliable test of functional integrity of dopaminergic terminals previously exposed to SIN-1.
SIN-1, besides NO, generates superoxide anions, being thus a potential peroxynitrite generator (Menconi et al., 1998). According to Trabace & Kendrick (2000), peroxynitrite plays a key role in NO-donors (SNAP or (Z)-1-[2-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2 diolate (NOC-18))-induced changes in striatal DA release. At high extracellular levels, peroxynitrite reduces extracellular DA and DOPAC through oxidation, while at low concentrations it increases striatal DA release in a cyclic GMP-dependent manner. In the present study, however, we were not able to detect SIN-1-induced changes in dialysate DA or DOPAC attributable to peroxynitrite formation. In fact: (i) co-infusion of the SOD mimetic MnTBP failed to inhibit SIN-1-induced increases in dialysate DA; (ii) uric acid is a natural strong scavenger of peroxynitrite (Hooper et al., 1998). However, co-infusion of uric acid with SIN-1 did not affect SIN-1-induced changes in dialysate DA. In addition, at all concentrations tested, SIN-1 failed to induce changes in endogenous uric acid dialysate concentrations. This finding might represent indirect evidence for lack of peroxynitrite formation; (iii) at all concentrations tested, SIN-1 failed to induce changes in dialysate ascorbic acid concentrations. Again, this finding might be indirect evidence for lack of peroxynitrite formation, since ascorbic acid is highly effective to counteract the oxidizing properties of peroxynitrite (Jackson et al., 1998; Kirsch & de Groot, 2000). On the whole, all these findings cast doubt on SIN-1-induced in vivo intrastriatal generation of peroxynitrite.
The present study was designed mainly to ascertain the role of iron in the NO donors-induced increase in striatal DA release. In a previous study (Serra et al., 2000), we showed that interaction between NO and iron (II), both released following decomposition of SNP, accounted for the late SNP-induced dopamine (DA) increase in dialysates from the striatum of freely moving rats. This conclusion was based on the observation that the iron chelator deferoxamine greatly inhibited the late SNP-induced increase in dialysate DA; in addition, we showed (Serra et al., 2001) that the late SNP-induced increase in dialysate DA could be mimicked by co-infusing iron (II) with either high or low concentrations of SNAP. In the present study, the amount of NO released by SIN-1 0.2 or 0.5 mM failed to trigger dopamine release; however, a concentration-related increase in dialysate DA occurred when iron (II) was co-infused with SIN-1 0.2 or 0.5 mM. Also, co-infusion with iron (II) potentiated SIN-1 1.0 and 5.0 mM effects on dialysate DA. However, the increase obtained with SIN-1 5.0 mM was lower than that with SIN-1 1.0 mM. In addition, iron (II) co-infusion with SIN-1 5 mM resulted in an early and long-lasting decrease in dialysate DOPAC+HVA. These effects seem to be the consequence of extracellular non-enzymatic oxidation of both DA and DOPAC, which are catechol-containing molecules (Miller et al., 1996); in fact, NAC co-infusion resulted in a greater and long-lasting increase in dialysate DA and restored DOPAC+HVA dialysate levels.
The finding that infusion of deferoxamine decreased dialysate DA concentrations suggests that endogenous iron might have a role in striatal DA release. Deferoxamine is also a Ca2+ chelator, therefore it might inhibit DA release by chelating Ca2+; however, the very low in vivo affinity for calcium allows the ruling out of this hypothesis (Anghileri et al., 1992). The ability of deferoxamine to decrease DA release might be related to inhibition of tyrosine hydroxylase activity, since iron is essential for enzyme activity. Very recently, however, Liu et al. (2001) demonstrated that iron-binding affinity of iron-chelator drugs is not correlated with tyrosine hydroxylase inhibitory activity; this finding allows the ruling out of the above hypothesis. In contrast, the hypothesis that endogenous iron might have a role in striatal DA release is supported by the finding that deferoxamine co-infusion attenuated SIN-1-induced increases in dialysate DA, probably by chelating endogenous free iron.
Understanding the chemistry of NO is important in order to clarify the activity of NO in in vivo striatal DA release. NO is a simple hydrophobic gaseous molecule that is highly diffusible and reactive. The following forms are important for its biological action: *NO radical, which can be oxidized to nitrosonium cation (NO+), or reduced to nitronyl anion (NO−). NO readily reacts with either iron (II), to form Fe (III)-NO− complexes, or with iron (III), to form Fe (II)-NO+ complexes (Le Brun et al., 1997). NO may also interact with ascorbic acid (Millar, 1995); in addition, ascorbic acid may either protect NO from destruction by superoxide anions (Dudgeon et al., 1998; Jackson et al., 1998), or scavenge it (Whiteman & Halliwell, 1996). Ascorbic acid may trigger decomposition of the NO-donor SNP both in biological tissue in vitro (Bates et al., 1991; Reiser et al., 1999) and in the striatal extracellular space in vivo (Serra et al., 2000). In addition, ascorbic acid potentiates decomposition SNAP in vitro in striatal slices (Reiser et al., 1999) and in the striatal extracellular space in vivo (Serra et al., 2001), but not that of SIN-1 in vitro (Reiser et al., 1999). In the present study, SIN-1-induced increases in dialysate DA occurred without changes in dialysate ascorbic acid concentrations. This finding suggests that endogenous ascorbic acid does not interfere with decomposition in vivo; in addition, it prompts us to speculate that either scavenging properties of ascorbic acid were not involved in SIN-1-induced changes of dialysate DA, or, if they were, oxidative stress did not exceed the homeostatic regulation of extracellular ascorbic acid (Miele & Fillenz, 1996; Rice, 2000).
The results of the present study allow merely an insight into the mechanism by which the NO-donors increase striatal DA release from dopaminergic terminals. West & Grace (2000) have shown that intrastriatal infusion of either a NO-generator, or a NO-synthase substrate, increased the mean firing rate of nigral DA neurons in anaesthetized rats, suggesting that striatal NO-synthase-containing interneurones play an intermediary role in modulating the striatal efferent regulation of nigral DA neurones. The results of this study do not allow the recognition of a nigral component in the NO-donor-induced increase in striatal DA release; however, they strongly suggest that iron plays a key role in exogenous NO-induced release of striatal DA.
Acknowledgments
The research was supported by University of Sassari (ex 60% fund).
Abbreviations
- ANOVA
analysis of variance
- DA
dopamine
- DOPAC
dihydroxyphenylacetic acid
- HVA
homovanillic acid
- MnTBP
manganese(III) tetrakis (4-benzoic acid) porphyrin
- NAC
N-acetylcysteine NO, nitric oxide
- SIN-1
3-morpholinosydnonimine
- SNAP
S-nitroso-N-acetylpenicillamine
- SNP
sodium nitroprusside
- SOD
superoxide dismutase
References
- ANGHILERI L.J., MALEKI P., ROBERT J. Changes in calcium uptake by liver induced by ferric lactate. Arch. Biochem. Biophys. 1992;292:529–533. doi: 10.1016/0003-9861(92)90026-s. [DOI] [PubMed] [Google Scholar]
- BATES J.N., BAKER M.T., GUERRA R. , JR, HARRISON D.G. Nitric oxide generation from nitroprusside by vascular tissue. Evidence that reduction of the nitroprusside anion and cyanide loss are required. Biochem. Pharmacol. 1991;42:S157–S165. doi: 10.1016/0006-2952(91)90406-u. [DOI] [PubMed] [Google Scholar]
- CONNOR J.R. Evidence for iron mismanagement in the brain in neurological disorders Metal and Oxidative Damage in Neurological Disorders 1997New York: Plenum Press; 23–40.ed. Connor, J.R. pp [Google Scholar]
- DESOLE M.S., SCIOLA L., SIRCANA S., GODANI C., MIGHELI R., DELOGU M.R., PIRAS G., DE NATALE G., MIELE E. Protective effect of deferoxamine on sodium nitroprusside-induced apoptosis in PC12 cells. Neurosci. Lett. 1998;247:1–4. doi: 10.1016/s0304-3940(98)00260-2. [DOI] [PubMed] [Google Scholar]
- DESVIGNES C., BERT L., VINET L., DENOROY L., RENAUD B., LAMBAS-SENAS L. Evidence that neuronal nitric oxide synthase inhibitor 7-nitroindazole inhibits monoamine oxidase in the rat: in vivo effects on extracellular striatal dopamine and 3,4-dihydroxyphenylacetic acid. Neurosci. Lett. 1999;261:175–178. doi: 10.1016/s0304-3940(99)00026-9. [DOI] [PubMed] [Google Scholar]
- DUDGEON S., BENSON D.P., MACKENZIE A., PAISLEY-ZYSZKIEWICZ K., MARTIN W. Recovery by ascorbate of impaired nitric-oxide dependent relaxation resulting from oxidant stress in rat aorta. Br. J. Pharmacol. 1998;125:782–786. doi: 10.1038/sj.bjp.0702120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENRICO P., ESPOSITO G., MURA M.A., FRESU L., DE NATALE G., MIELE E., DESOLE M.S., MIELE M. Effect of morphine on striatal dopamine metabolism and ascorbic acid and uric acid release in freely moving rats. Brain Res. 1997;745:173–182. doi: 10.1016/s0006-8993(96)01146-8. [DOI] [PubMed] [Google Scholar]
- GARTHWAITE J., BOULTON C.L. Nitric oxide signaling in the central nervous system. Ann. Rev. Physiol. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- GUEVARA-GUZMAN R., EMSON P.C., KENDRICK K.M. Modulation of in vivo striatal transmitter release by nitric oxide and cyclic GMP. J. Neurochem. 1994;62:807–810. doi: 10.1046/j.1471-4159.1994.62020807.x. [DOI] [PubMed] [Google Scholar]
- HAN J., CHENG F.C., YANG Z., DRYHURST G. Inhibitors of mitochondrial respiration, iron (II), and hydroxyl radical evoke release and extracellular hydrolysis of glutathione in rat striatum and substantia nigra: potential implications to Parkinson's disease. J. Neurochem. 1999;73:1683–1695. doi: 10.1046/j.1471-4159.1999.731683.x. [DOI] [PubMed] [Google Scholar]
- HOOPER D.C., SPITSIN S., KEAN B.B., CHAMPION J.M., DICKSON G.M., CHAUDRY L., KOPROWSKY H. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc. Natl. Acad. Sci. U.S.A. 1998;95:675–680. doi: 10.1073/pnas.95.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRSCH M., DE GROOT H. Ascorbate is a potent antioxidant against peroxynitrite-induced oxidation reactions. Evidence that ascorbate acts by re-reducing substrate radicals produced by peroxynitrite. J. Biol. Chem. 2000;275:16702–16708. doi: 10.1074/jbc.M909228199. [DOI] [PubMed] [Google Scholar]
- JACKSON T.S., XU A., VITA A.J., KEANEY J.F. , JR Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ. Res. 1998;83:916–922. doi: 10.1161/01.res.83.9.916. [DOI] [PubMed] [Google Scholar]
- JELLINGER K.A. The role of iron in neurodegeneration. Prospect for pharmacotherapy of Parkinson's disease. Drugs Aging. 1999;14:115–140. doi: 10.2165/00002512-199914020-00004. [DOI] [PubMed] [Google Scholar]
- LE BRUN N.E., ANDREWS S.C., MOORE G.R., THOMSON A.J. Interaction of nitric oxide with non-haem iron sites of escherichia coli bacterioferritin: reduction of nitric oxide to nitrous oxide and oxidation of iron(II) to iron(III) Biochem. J. 1997;326:173–179. doi: 10.1042/bj3260173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN A.M. Recovery by NO of the iron-attenuated dopamine dynamics in nigrostriatal system of the rat brain. Neurosci. Res. 1999;34:133–139. doi: 10.1016/s0168-0102(99)00054-1. [DOI] [PubMed] [Google Scholar]
- LIU Z.D., LOCKWOOD M., ROSE S., THEOBALD A.E., HIDER R.C. Structure-activity investigation of the inhibition of 3-hydroxypyridin-4-ones on mammalian tyrosine hydroxylase. Biochem. Pharmacol. 2001;61:285–290. doi: 10.1016/s0006-2952(00)00551-7. [DOI] [PubMed] [Google Scholar]
- MENCONI M.J., UNNO N., SMITH M., AGUIRRE D.E., FINK M.P. Nitric oxide donor-induced hyperpermeability of cultured intestinal epithelial monolayers: role of superoxide radical, hydroxyl radical, and peroxynitrite. Biochem. Biophys. Acta. 1998;1425:189–203. doi: 10.1016/s0304-4165(98)00072-5. [DOI] [PubMed] [Google Scholar]
- MIELE M., FILLENZ M. In vivo determination of extracellular brain ascorbate. J. Neurosci. Meth. 1996;70:15–19. doi: 10.1016/S0165-0270(96)00094-5. [DOI] [PubMed] [Google Scholar]
- MIELE M., MURA M.A., ENRICO P., ESPOSITO G., SERRA P.A., MIGHELI R., ZANGANI D., MIELE E., DESOLE M.S. On the mechanism of d-amphetamine-induced changes in glutamate, ascorbic acid and uric acid release in the striatum of freely moving rats. Br. J. Pharmacol. 2000;129:582–588. doi: 10.1038/sj.bjp.0703066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLAR J. The nitric oxide/ascorbate cycle: how neurones may control their own oxygen supply. Med. Hypoth. 1995;45:21–26. doi: 10.1016/0306-9877(95)90194-9. [DOI] [PubMed] [Google Scholar]
- MILLER J.W., SELHUB J., JOSEPH J.A. Oxidative damage caused by free radicals produced during catecholamine autoxidation: protective effects of O-methylation and melatonin. Free Rad. Biol. Med. 1996;21:241–249. doi: 10.1016/0891-5849(96)00033-0. [DOI] [PubMed] [Google Scholar]
- MOHANAKUMAR K.P., HANBAUER I., CHIUEH C.C. Neuroprotection by nitric oxide against hydroxyl radical-induced nigral toxicity. J. Chem. Neuroanatom. 1996;14:195–205. doi: 10.1016/s0891-0618(98)00032-5. [DOI] [PubMed] [Google Scholar]
- MORTON D.B., GRIFFITHS P.H.M. Guidelines on the recognition of pain, distress and discomfort in experimental animals and a hypothesis for assessment. Vet. Rec. 1985;116:431–436. doi: 10.1136/vr.116.16.431. [DOI] [PubMed] [Google Scholar]
- PATEL M., DAY B.J. Metalloporphyin class of therapeutic catalytic antioxidants. Trends Pharmacol. Sci. 1999;20:359–364. doi: 10.1016/s0165-6147(99)01336-x. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1986. [Google Scholar]
- RAUHALA P., MOHANAKUMAR K.P., SZIRAKI I., LIN A.M., CHIUEH C.C. S-nitrosothiols and nitric oxide, but not sodium nitroprusside, protect nigrostriatal dopamine neurons against iron-induced oxidative stress in vivo. Synapse. 1996;23:58–60. doi: 10.1002/(SICI)1098-2396(199605)23:1<58::AID-SYN7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- REISER M., SCHILD L., KEILHOFF G., WOLF G. Interaction of nitric oxide donors and ascorbic acid on D-[3H] aspartate efflux from rat striatal slices. Neurochem. Res. 1999;24:61–67. doi: 10.1023/a:1020980013915. [DOI] [PubMed] [Google Scholar]
- RICE M.E. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23:209–216. doi: 10.1016/s0166-2236(99)01543-x. [DOI] [PubMed] [Google Scholar]
- SEGIETH J., FOWLER L., WHITON P., PEARCE B. Nitric-oxide mediated regulation of dopamine release in the hippocampus in vivo. Neuropharmacology. 2000;39:571–577. doi: 10.1016/s0028-3908(99)00178-1. [DOI] [PubMed] [Google Scholar]
- SEGOVIA G., MORA F. Role of nitric oxide in modulating the release of dopamine, glutamate and GABA in striatum of the freely moving rat. Brain Res. Bull. 1998;45:275–279. doi: 10.1016/s0361-9230(97)00402-4. [DOI] [PubMed] [Google Scholar]
- SENGSTOCK G.J., OLANOV C.W., DUNN A.J., BARONE S., JR, ARENDASH G.W. Progressive changes in striatal dopaminergic markers, nigral volume, and rotational behavior following iron infusion into the rat substantia nigra. Exp. Neurol. 1994;130:82–94. doi: 10.1006/exnr.1994.1187. [DOI] [PubMed] [Google Scholar]
- SENGSTOCK G.J., ZAWIA N.H., OLANOV C.W., DUNN A.J., ARENDASH G.W. Intranigral iron infusion in the rat. Acute elevation in nigral lipid peroxidation and striatal dopaminergic markers with ensuing nigral degeneration. Biol. Trace Elem. Res. 1997;58:177–195. doi: 10.1007/BF02917470. [DOI] [PubMed] [Google Scholar]
- SERRA P.A., ESPOSITO G., DELOGU M.R., MIGHELI R., ROCCHITTA G., GRELLA G., MIELE E., MIELE M., DESOLE M.S. Analysis of 3-morpholinosydnonimine and sodium nitroprusside effects on dopamine release in the striatum of freely moving rats: role of nitric oxide, iron and ascorbic acid. Br. J. Pharmacol. 2000;131:836–842. doi: 10.1038/sj.bjp.0703635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERRA P.A., ESPOSITO G., DELOGU M.R., MIGHELI R., ROCCHITTA G., MIELE E., DESOLE M.S., MIELE M. Analysis of S-nitroso-N-acetylpenicillamine effects on dopamine release in the striatum of freely moving rats: role endogenous ascorbic acid and oxidative stress. Br. J. Pharmacol. 2001;132:941–949. doi: 10.1038/sj.bjp.0703887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVA M.T., ROSE S., HINDMARSH J.G., AISLAITNER G., GORROD J.W., MOORE P.K., JENNER P., MARSDEN C.D. Increased striatal dopamine efflux in vivo following inhibition of cerebral nitric oxide synthase by the novel monosodium salt of 7-nitro indazole. Br. J. Pharmacol. 1995;114:257–258. doi: 10.1111/j.1476-5381.1995.tb13219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRASSER A., MCCARRON R.M., ISHII H., STAMIROVIC D., SPATZ M. L-arginine induces dopamine release from striatum in vivo. Neuroreport. 1994;5:2298–2300. doi: 10.1097/00001756-199411000-00023. [DOI] [PubMed] [Google Scholar]
- TRABACE L., KENDRICK K.M. Nitric oxide can differentially modulate neurotransmitter concentrations via soluble guanylate cyclase and peroxynitrite formation. J. Neurochem. 2000;75:1664–1674. doi: 10.1046/j.1471-4159.2000.0751664.x. [DOI] [PubMed] [Google Scholar]
- WEGENER G., VOLKE V., ROSENBERG R. Endogenous nitric oxide decreases hippocampal levels of serotonin and dopamine in vivo. Brit. J. Pharmacol. 2000;130:575–580. doi: 10.1038/sj.bjp.0703349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEST A.R., GALLOWAY M.P. Intrastriatal infusion of (+/−)-S-nitroso-N-acetylpenicillamine release vesicular dopamine via an ionotropic glutamate receptor-mediated mechanism: an in vivo microdialysis study in chloral hydrate-anesthetized rats. J. Neurochem. 1996;66:1971–1980. doi: 10.1046/j.1471-4159.1996.66051971.x. [DOI] [PubMed] [Google Scholar]
- WEST A.R., GALLOWAY M.P. Endogenous nitric oxide facilitates striatal dopamine and glutamate efflux in vivo: role of ionotropic glutamate receptor-dependent mechanisms. Neuropharmacology. 1997;36:1571–1581. doi: 10.1016/s0028-3908(97)00148-2. [DOI] [PubMed] [Google Scholar]
- WEST A.R., GALLOWAY M.P. Nitric oxide and potassium chloride-facilitated striatal dopamine efflux in vivo: role of calcium-dependent release mechanism. Neurochem. Int. 1998;33:493–501. doi: 10.1016/s0197-0186(98)00054-0. [DOI] [PubMed] [Google Scholar]
- WEST A.R., GRACE A.A. Striatal nitric oxide signaling regulates the neuronal activity of midbrain dopamine neurons in vivo. J. Neurophysiol. 2000;83:1796–1808. doi: 10.1152/jn.2000.83.4.1796. [DOI] [PubMed] [Google Scholar]
- WHITEMAN M., HALLIWELL B. Protection against peroxynitrite-dependent tyrosine nitration and α1-antiproteinase inactivation by ascorbic acid. A comparison with other biological antioxidants. Free Radic. Res. 1996;25:275–283. doi: 10.3109/10715769609149052. [DOI] [PubMed] [Google Scholar]
- XIE X.C., STPYREK J., PORTER W.H., YOKEL R.A. Hydroxyl radical generation in rat brain is initiated by iron, not aluminum, as determined by microdialysis with salicylate trapping and GC-MS analysis. Neurotoxicology. 1995;16:489–496. [PubMed] [Google Scholar]


