Abstract
Recent studies indicate that nitroglycerin (NTG) can produce beneficial clinical effects in healing anal fissures through the relaxation of the internal anal sphincter. The in vivo relaxation effects of NTG on the anorectal smooth muscle have not been studied and it is not known whether this tissue may also exhibit pharmacological tolerance toward NTG.
We have developed an in vivo procedure in the anaesthetized rat that permits continual monitoring of anorectal pressure after intravenous (i.v.) and intra-rectal application of NTG. The relaxant effects of NTG were quantified via the area-under-the-contraction-waveforms vs time curve (AUEC).
AUEC decreased significantly after intra-rectal bolus doses of NTG (5 – 25 μg), in a dose- and time-dependent manner. Sustained relaxation effects on anorectal pressure were also observed after continuous intra-rectal infusions of NTG.
Two-hours of i.v. NTG infusion led to a significant reduction in the mean arterial blood pressure (MAP) response toward a i.v. NTG (30 μg) bolus challenge. In contrast, relaxation of the anorectal pressure toward the challenge dose was not altered after NTG infusion.
In isolated tissues, cyclic GMP accumulation was significantly decreased after NTG pre-incubation in the rat aorta but not in the rat anorectal smooth muscle and anal sphincter.
These results indicate that the relaxation response toward NTG was not diminished in the anorectum under conditions that produced vascular tolerance. Thus, NTG causes significant and sustained in vivo relaxation of anorectal smooth muscle in the anaesthetized rat without evidence of tolerance development.
Keywords: Nitroglycerin, nitrate tolerance, cyclic GMP, anorectal smooth muscle, relaxation, nitric oxide
Introduction
Organic nitrates, such as nitroglycerin (NTG), have long been used in treating cardiovascular disease because of their potent relaxation effects on the vascular smooth muscle. These effects were generated by in vivo metabolic conversion of these compounds to nitric oxide (NO), which stimulates the production of cyclic GMP, leading to vascular relaxation (Fung et al., 1992; Munzel et al., 1996). Recent studies suggest that NO is also released as an important mediator from enteric inhibitory neurons innervating the internal anal sphincter muscle, contributing to the relaxation of this tissue (Rattan & Chakder, 1997; Stebbing, 1998). Since internal anal sphincter hypertonia may play an important role in disease states such as anal fissure and anal pain caused by external haemorrhoids, it is not surprising that organic nitrates have been shown to exert a beneficial effect in treating these disease conditions (Gorfine, 1995a,1995b; Lysy et al., 1998).
One of the major drawbacks of NTG therapy in cardiovascular disease is the rapid development of pharmacological tolerance, both in vitro and in vivo (Fung & Bauer, 1994; Munzel et al., 1996). The mechanisms for this phenomenon are complex and multi-factorial, including processes such as decreased tissue sulphydryl availability, reduced bioactivation of the parent drug, physiological compensation and tissue oxidative stress, etc. (Fung & Bauer, 1994; Munzel et al., 1996; Needleman & Johnson, 1973). It is presently unknown whether the relaxation effects of NTG on the anorectal tissue are similarly affected by the development of pharmacological tolerance.
The objective of the present study, therefore, was to examine the in vitro and in vivo NTG-induced relaxation response in the anorectal tissue. Specifically, we examined (1) the in vivo effects of intra-rectal NTG administration on anorectal pressure, using both bolus and continuous dosing regimens, (2) the presence of pharmacological tolerance in haemodynamic vs anorectal relaxation by intravenous (i.v.) NTG in the same animal, and (3) the differences in tissue cyclic GMP accumulation response in rat aorta vs anorectal tissues after NTG incubation.
Methods
Animal preparation
All procedures were performed according to protocols approved by the Institutional Animal Care and Use Committee, University at Buffalo. Male Sprague-Dawley rats, 300 – 400 g in body weight, were used in the study. When blood pressure measurements were planned, the animal was catheterized 1 – 2 days before the study at the left femoral artery for blood pressure monitoring, the left femoral vein for bolus drug administration and the right jugular vein for drug infusion (Booth et al., 1996). On the day of the study, each rat was anaesthetized by intramuscular ketamine 90 mg kg−1, xylazine 9 mg kg−1, and this dose was repeated as necessary (generally every 2 – 4 h). The diuretic effect of anaesthesia was offset by rehydration with intra-peritoneal saline through an implanted 24 ga angiocath. The volume of saline infused was varied according to the observed urinary output, generally totaling around 2 ml every hour administered through several doses.
Measurement of anorectal pressure
Anorectal pressure was monitored via a water-filled LV balloon made of latex rubber (3 mm diameter, 7 mm length, Kent Scientific Corp., CT, U.S.A.) connected through a saline-filled PE-50 tubing, to a Gould Stathem P23 ID pressure transducer (Ohmeda Inc., NJ, U.S.A.). The pressure signal was recorded on a Grass 79D Physiograph (Grass Instrument Co., MA, U.S.A.) over the 0 – 50 mmHg range. NTG (Schwarz Pharma, Monheim, Germany, 1 mg ml-1) was delivered via a 250 μl-syringe through a PE-10 tubing inserted 7 mm into the anus. The balloon and the tubes were positioned in the anus using small smooth-ended surgical forceps as a speculum to gently dilate the orifice. After insertion of the balloon assembly, the system was allowed to reach a stable baseline (generally between 1 – 1.5 h) before initiation of the experiment. A scheme of the animal preparation is shown in Figure 1.
Figure 1.
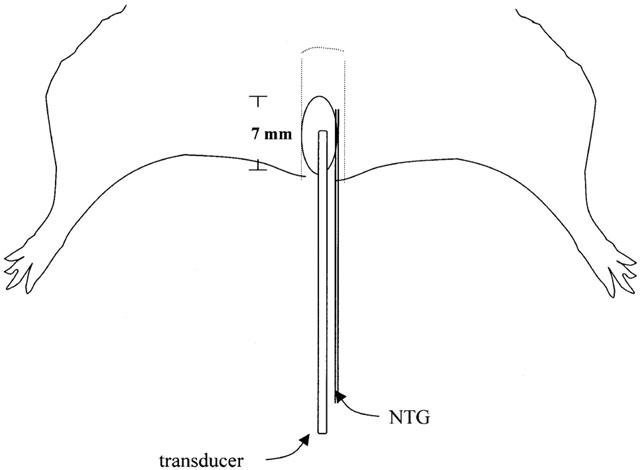
A schematic representation of the experimental setup. A water-filled balloon, along with a PE-10 tubing, were inserted 7 mm into the anus. Balloon pressure was monitored via PE-50 tubing connected from the balloon to the transducer.
Drug administration
In separate experiments, NTG was administered intra-rectally either as consecutive bolus doses or as a continuous infusion to the anaesthetized rat. In the bolus dose experiment, each rat (n=6) was given one of the bolus doses (2.5, 5.0, 10.0, 15.0 and 25.0 μg) over 10 s, with each escalating dose separated by a 30-min interval. Anorectal pressure was measured every 5 min for 3 consecutive min after dosing. In the experiment involving continuous NTG infusion into the anorectal cavity, three separate animal groups were infused, either with control (5% dextrose, D5W, n=4), 67 ng min−1 NTG (n=5), or 333 ng min−1 NTG (n=3). The infusion volume (333 nl min−1) was identical for all groups, and was controlled by an electronic infusion pump (Harvard Apparatus, MA, U.S.A.). Anorectal pressure was measured every 30 min during the infusion. It was observed that these infusion volumes did not lead to visible leakage of the dosing solution outside the anus. In additional experiments, animals received continuous i.v. infusion of either 10 μg min−1 NTG (n=4) or D5W (n=5) for 2 h, and the blood pressure and anorectal pressure were monitored simultaneously. To determine the presence of haemodynamic and anorectal NTG tolerance, the mean arterial blood pressure (MAP) and anorectal responses to a 30 μg i.v. NTG bolus were compared before and after NTG infusion. The 30 μg i.v. NTG bolus was chosen because this dose of NTG has been shown to cause near maximal reduction in MAP in rats (E.Q. Wang and H.L. Fung, unpublished data).
In vitro cyclic GMP measurement
The thoracic aorta and the anal canal were collected from anaesthetized untreated rats. The aorta was cleaned of extraneous tissue, and the endothelium was removed by gently rubbing the interior of the lumen with a thin wire. The anal canal was separated into the anal sphincter and the anorectal tissue by visual inspection. The anal sphincter was identified as a muscular strip of pinkish colour, which situated at about 2 mm from the end of the anus. All tissues were incubated with either 0.22 mM NTG or D5W control in Krebs buffer at 37°C for 1 h in the presence of 0.5 mM 3-isobutyl-1-methylxanthine (IBMX, Sigma Chemical Co., MO, U.S.A.), while being aerated with constant bubbling of 5% CO2 in O2. To lessen the contribution of degraded NTG in solution, the incubation media was changed every 30 min. Following the 1-h NTG incubation, all tissues were rinsed with Krebs buffer several times and challenged with 1 μM NTG at 37°C for 30 s in the presence of 0.5 mM IBMX, according to the methods of Hasegawa et al. (1999). Tissues were then immediately frozen in liquid nitrogen and stored at −80°C until cyclic GMP analysis. The frozen tissues were homogenized in 1 ml of ice-cold 6% (w v−1) trichloroacetic acid and centrifuged at 1500×g for 15 min. The supernatant was extracted four times with 5×volume of water-saturated ether and the aqueous extract was then lyophilized. cyclic GMP content in lyophilized samples was determined using a commercially available radioimmunoassay kit (Biomedical Technologies Inc., MA, U.S.A.).
Data treatment
Hard copies of the anorectal pressure tracings at various times after drug treatment were transferred electronically into a graphic computer file with a scanner. The area-under-the-contraction-waveforms vs time curve (AUEC, see Figure 2) was obtained from these tracings using the NIH free software entitled ‘Data Thief'. The AUEC per min was recorded over 3 consecutive min, and these triplicate values were averaged to yield a single value of AUEC. To correct for inter-animal variability, AUEC was expressed as per cent baseline AUEC observed in each animal. Correction in baseline anorectal pressure was also carried out, if needed, by connecting the flat parts of the waveforms between each group of rhythmic contraction. When i.v. NTG was administered, the anorectal pressure effect of the 30 μg i.v. NTG bolus was compared to that just before the bolus dose. MAP was calculated as diastolic blood pressure+1/3 (systolic blood pressure – diastolic blood pressure).
Figure 2.
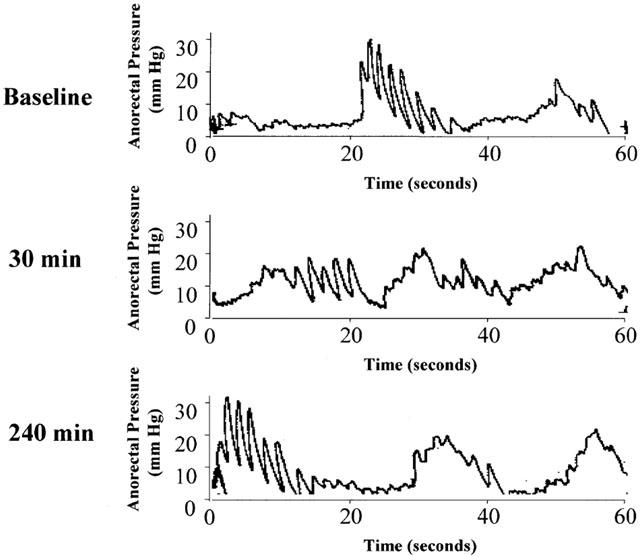
Representative anorectal pressure tracings measured over 1 min in a rat. Top panel: baseline; middle and lower panels: after 30 and 240 min of infusion of D5W at 333 nl min−1, respectively.
Statistical analysis
One-way ANOVA was used in the anorectal bolus dose study, followed by the Student-Newman-Keuls post-hoc test. Two-way ANOVA was used in the anorectal infusion study to determine statistical differences between treatment groups (i.e., vehicle control, 66 ng min−1 NTG, and 333 ng min−1 NTG). One-way ANOVA was used to determine, for each treatment, the statistical difference of the AUEC at various time points vs its own baseline value. Paired Student t-test was used to determine the MAP and anorectal pressure effects of the 30 μg i.v. NTG bolus before and after 10 μg min−1 i.v. NTG infusion. The difference in cyclic GMP accumulation between D5W and NTG pre-treated tissues was analysed using unpaired Student t-test. Statistical significance was denoted when P<0.05.
Results
Figure 2 shows representative 1-min anorectal pressure tracings from a control animal. The top panel presents baseline tracings prior to D5W vehicle infusion, while the second and third panels show tracings after the animal had been infused with D5W (at 333 nl min−1) for 30 and 240 min, respectively. It is seen that the pressure waves were somewhat rhythmic but irregular in nature. Typically, the anorectal pressure tracings exhibited 2 – 3 groups of contractions per min, with each group composed of several waves of contractions. Because of their irregular nature, quantification of the composite contractions was achieved by measuring the area-under-the contraction waveforms over a fixed time (1 min). This parameter, abbreviated as AUEC, therefore represents an integrated index that reflected both the amplitude and frequency of contraction. Thus, the larger the AUEC, the more intense the contraction. The relaxation effect of NTG on anorectal pressure was therefore determined by its ability to lower AUEC.
Figure 3 shows representative tracings of anorectal pressure in a rat at baseline (A), 30 min after an intra-rectal NTG bolus dose of 2.5 μg (B, representing a low dose), 10 μg (C, a medium dose) and 25 μg (D, a high dose). It is seen that both the frequency and amplitude of these tracings were substantially reduced with increasing doses of NTG. At the 25 μg NTG bolus dose, the rhythmic anorectal contractions were essentially abolished, even after 30 min.
Figure 3.
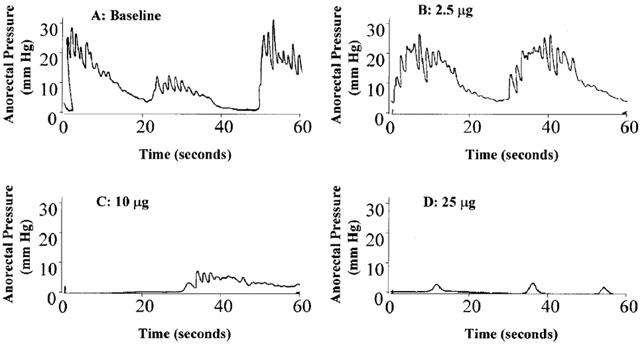
Representative anorectal pressure tracings measured over 1 min in a rat, at baseline, and 30 min after bolus NTG doses at 2.5, 10, and 25 μg, respectively.
Figure 4 shows the time course of NTG effect on the anorectal pressure after different bolus doses of NTG. In the bolus dosing study, five of the six animals responded significantly to NTG dosing, while one animal did not respond to NTG dosing. Since data from the non-responding animal were more than two standard deviations away from the rest of the treatment group, results from this animal were not included in subsequent data analysis. It is evident that at doses above 5 μg rat−1, NTG exerted immediate and sustained decreases in AUEC, indicative of reduction of in vivo anorectal pressure. Although data from the 2.5 μg dose did not exhibit statistical significance from baseline, there were clear trends that even at this dose, NTG could lead to some reduction in anorectal pressure. At the highest dose of 25 μg, NTG still produced 81±10% reduction in anorectal pressure 30 min after dosing. These results strongly indicated that bolus doses of NTG potently reduced anorectal pressure when the drug was introduced into the anorectal cavity. These effects were both immediate and relatively sustained, as well as dose- and time-dependent, at least within the dose-range studied. In addition, it was also observed that intra-rectal NTG bolus caused a dose-dependent but transient decrease in MAP which quickly returned to baseline within 2 – 3 min. At the highest dose of 25 μg, a 33.5±2.5% decrease in MAP was observed (data not shown).
Figure 4.
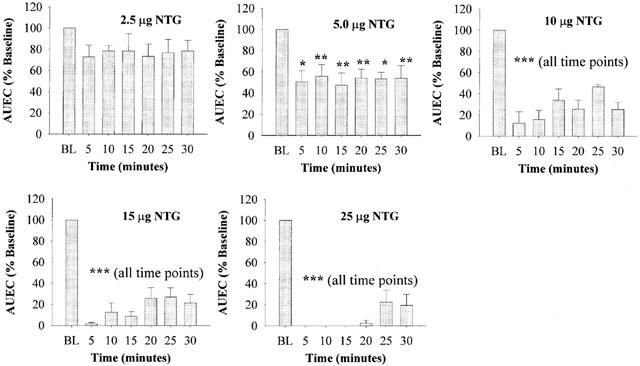
Time-course of the relative anorectal relaxation effects of consecutive bolus doses of NTG, as examined by per cent AUEC changes over 30 min. Mean baseline value (BL) for each animal before drug dosing was set at 100%. Data are expressed as mean±s.e.mean, n=5. *P<0.05; **P<0.01; ***P<0.001 vs BL, by ANOVA.
Figure 5 shows the results of the experiment involving continuous NTG infusion into the anorectal cavity. Continuous infusion of vehicle (D5W) did not cause any change in the AUEC parameter. Infusion of NTG, either at 67 or 333 ng min−1 for 240 min (equivalent to total doses of 16.1 and 79.9 μg, respectively), significantly reduced the AUEC parameter. The effects of NTG were essentially constant from the first point of measurement (30 min) to the end of the experiment (4 h). Two-way ANOVA analysis indicated that both NTG doses produced effects significantly different from the vehicle control and from each other (P<0.05). It was also observed that intra-rectal NTG infusion did not cause any apparent changes in MAP (data not shown).
Figure 5.
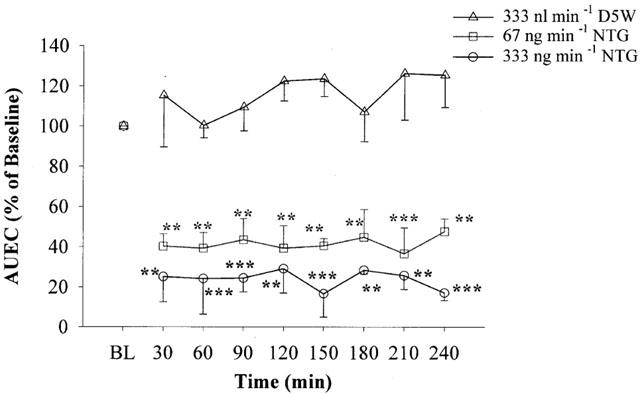
Effects of intra-rectal NTG infusion on the relative AUEC values of anorectal pressure over 4 h. Data are expressed as mean±s.e.mean, n=3 – 5. Two-way ANOVA indicated that the treatments are different from D5W vehicle infusion and from each other (P<0.05). **P<0.01; ***P<0.001 vs BL for each individual treatment by one-way ANOVA.
Figure 6 shows the effects of a 30 μg NTG i.v. bolus challenge dose on MAP and anorectal pressure, before and after 2 h of i.v. NTG infusion (10 μg min−1) in the anaesthetized rat. Prior to NTG infusion, the challenge dose produced a substantial decrease (47.2±3.3%) in MAP vs baseline (P<0.0001). The peak MAP effect was immediate (within 2 min), but not sustained, and MAP returned to baseline values after 3 – 5 min (data not shown). However, after 2 h of continuous NTG infusion, the same challenge dose of NTG produced a smaller MAP effect (25.7±3.6%), which was significantly reduced vs pre-infusion values (P<0.01).
Figure 6.
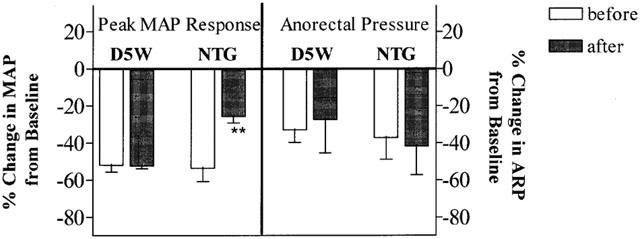
Effect of a 30 μg NTG i.v. bolus challenge on MAP and anorectal pressure before and after a 2-h i.v. NTG infusion at 10 μg min−1 in anaesthetized rats. Data are expressed as mean±s.e.mean, n=4 – 5. **P<0.01 vs corresponding pre-infusion (before) effect, paired Student t-test.
Prior to NTG infusion, the 30 μg NTG i.v. bolus challenge dose also produced a significant reduction (34.8±5.6%) in rectal rhythmic contractions, as measured by AUEC (P<0.05 vs baseline). After NTG infusion for 2 h, the 30 μg NTG challenge dose produced a similar decrease in AUEC (41.9±15.3%, P>0.05 vs AUEC before NTG infusion). Thus, in the same animal, 2-h i.v. infusion of NTG produced a diminished MAP response toward a challenge dose of NTG. In contrast, the anorectal response was maintained under the same conditions (Figure 6). D5W vehicle infusion had no apparent effect on the MAP and anorectal relaxation responses of the 30 μg NTG i.v. bolus challenge.
Figure 7 shows the results from the in vitro cyclic GMP experiment. NTG pre-treatment of rat aorta for 1 h significantly decreased cyclic GMP accumulation upon NTG challenge, when compared to D5W control (P<0.01), consistent with the development of vascular NTG tolerance. Interestingly, in both the anal sphincter and anorectal tissue, NTG pre-treatment not only did not decrease cyclic GMP accumulation upon NTG challenge, but rather slightly increased it when compared to the D5W control. Student t-test revealed that in the anal sphincter, cyclic GMP accumulation from the NTG pre-treated group was significantly higher than the D5W control (P<0.01).
Figure 7.
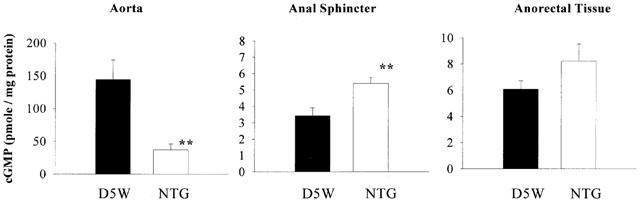
Effect of 1-h control (D5W) or NTG (0.22 mM) pre-incubation on cyclic GMP accumulation upon 1 μM NTG challenge. Data are expressed as mean±s.e.mean, n=8. **P<0.01 vs corresponding D5W control, unpaired Student t-test.
Discussion
Our results demonstrated that NTG potently inhibited contraction of the smooth muscle lining the anorectal canal in the anaesthetized rat. The effects were immediate, sustained and dose-dependent. A threshold bolus dose of about 5 μg NTG, when administered intra-rectally to a 300 g rat−1 (about 16.5 μg kg−1 of body weight), produced a sustained 50% reduction in AUEC over the entire 30-min observation period. The relaxation effects on the anorectum of a bolus intra-rectal dose of NTG, however, did dissipate with time (Figure 4). This time-dependent loss of effect is likely due to the clearance of the drug from the anorectal site (e.g., from metabolism or distribution of NTG into the systemic circulation). The existence of pharmacological tolerance cannot therefore be answered from the bolus dose experiments (Figure 4), but from experiments involving continuous intra-rectal dosing of NTG (Figure 5), where a constant input dose was applied.
Figure 5 shows that two different infusion regimens of NTG were able to reduce anorectal contractions continuously, over the 4-h study period. We only carried out our studies to 4 h, since the anaesthetized preparation usually began to show instability after this duration. The continuous relaxation effects produced by NTG in the anorectal region contrasted markedly with the rapid development of haemodynamic tolerance produced by i.v. NTG infusion. Haemodynamic tolerance to the MAP lowering effect of a 30 μg NTG i.v. bolus was observed within 2 h of NTG infusion, while a similar degree of reduction in anorectal pressure was maintained in the same rat (Figure 6). It is therefore likely that the mechanisms which govern haemodynamic nitrate tolerance for the cardiovascular system may not be operative in the anorectum, or at least to a much lesser degree.
Unlike the intra-rectal NTG bolus, the anorectal pressure effect produced by an i.v. NTG bolus only lasted for ∼1 min. This is likely due to a much lower NTG concentration in the anorectum after i.v. administration as compared to the locally applied intra-rectal NTG dose. Newman et al. (1990) reported that in anaesthetized rats, tolerance toward the hypotensive effect of bolus NTG was observed within 1 h of 40 μg kg−1 min−1 NTG infusion. We have shown previously (Bauer & Fung, 1994) that in rats with congestive heart failure, continuous i.v. NTG infusion at 10 μg min−1 produced significant haemodynamic tolerance, as measured by the left ventricular end-diastolic pressure. In normal conscious rats, i.v. NTG infusion at 10 μg min−1 also produced rapid tolerance development to the MAP lowering effect of a 30 μg NTG i.v. bolus dose within 1 h of infusion (E.Q. Wang and H.L. Fung unpublished results).
In order to measure anorectal relaxation in vivo, the use of anaesthetics was unavoidable in our animal preparation. The effects of ketamine and xylazine on anorectal activity have not been explicitly examined. Paskins et al. (1984) have shown that anaesthesia induced by ketamine did not affect the frequency or amplitude of rhythmical activity of the internal anal sphincter. Xylazine is a known α-adrenoceptor agonist (Docherty & Hyland, 1986; Mizobe & Maze, 1995), so it is possible that anorectal relaxation may be affected by this anaesthetic. We found in this study, however, that administration of a second or third dose of anaesthetics to control animals (which received intra-rectal D5W infusion) did not significantly change the anorectal contraction pattern. Thus, the effects of ketamine/xylazine on anorectal activity are likely to be insignificant under our experimental conditions.
Our in vitro data were also consistent with the in vivo results. As expected, prior NTG incubation of the rat aorta significantly reduced cyclic GMP accumulation upon subsequent NTG challenge, consistent with many reports in the literature (Dikalov et al., 1998; Hasegawa et al., 1999). However, in the anal sphincter as well as the anorectal tissue, prior in vitro NTG exposure did not decrease cyclic GMP accumulation upon NTG challenge. The use of 1 μM NTG as the challenge concentration was based on a previous report by Hasegawa et al. (1999) who showed that this concentration of NTG produces maximal cyclic GMP accumulation in the rat aorta.
We observed an unexpected increase in cyclic GMP accumulation in the anal sphincter after NTG pre-incubation. The mechanism for this observation is currently unknown, but may relate to the experimental design. Since the tissues were incubated in the presence of the phosphodiesterase inhibitor, IBMX, during the 1 h NTG pre-incubation time, the amount of cyclic GMP measured may be a composite of that produced from NTG pre-incubation as well as from the NTG challenge dose. Although cyclic GMP has a very short half-life under normal conditions, its half-life in the presence of a phosphodiesterase inhibitor such as IBMX may be substantially prolonged. The time used to wash tissues with Krebs buffer before NTG challenge may not be sufficient to remove all the cyclic GMP accumulated during the NTG pre-incubation.
It is noted that the changes in cyclic GMP accumulation in the anorectal tissue and the anal sphincter were comparatively small when compared to that observed for the aorta in the in vitro study (Figure 7). It remains to be determined whether cyclic GMP is primarily responsible for mediating the NTG-induced relaxation of anorectal smooth muscle, as it has been extensively demonstrated for the vascular smooth muscle (Fung et al., 1992; Kukovetz & Holzmann, 1983). It is presently unknown whether other dilative mediators such as cyclic AMP may be additionally involved in mediating NTG-induced anorectal relaxation. Further studies involving determining cyclic AMP accumulation and specific inhibition of guanylate cyclase with ODQ, 1H-[1,2,4]oxadiazolo [4,3-α]-quinoxalin-1-one, may be warranted. In recent studies (Tseng et al., 2000), we showed that NTG-induced relaxation in the rat aorta was significantly reduced by the guanylyl cyclase inhibitor, ODQ. This ODQ inhibition, however, was not reversed by the phosphodiesterase inhibitor zaprinast. In contrast, the ODQ-mediated inhibition of vascular relaxation induced by other NO donors such as sodium nitroprusside and S-nitroso-N-acetylpenicillamine were reversible by zaprinast.
The mechanisms for the apparent lack of tolerance in NTG-induced anorectal relaxation are at present unknown. This tissue may not possess many of the counter-regulatory mechanisms that regulate systemic blood pressure and vascular relaxation, such as the baroreceptor reflex or the vascular angiotensin, endothelin and catecholamine systems, which are all thought to contribute to haemodynamic NTG tolerance (Munzel et al., 1996). The redox state of the anorectal tissue may also be different from that of the vasculature and increased oxidative stress induced by NTG (Munzel et al., 1995) may not be operative in this tissue. Further studies are necessary to delineate the apparent difference in anorectal vs vascular responses to NTG and other organic nitrates.
A limitation of the present study was that the fluid-filled balloon assembly for pressure measurement was relatively large, and its lateral apex was placed approximately 5 mm from the anus. Thus, the pressure tracings obtained were unlikely to be derived exclusively from the anal sphincter, and the effects of NTG on in vivo anal sphincter relaxation could not be directly obtained.
In conclusion, our studies have demonstrated that NTG potently inhibited anorectal contractions in the anaesthetized rat, without apparent tolerance development over at least 4 h. These findings are consistent with clinical observations that NTG can be beneficially used as a first-line therapy in the treatment of anorectal disorders such as anal fissures and haemorrhoids.
Acknowledgments
This work was supported in part by a grant from Cellegy Pharmaceuticals, Inc.
Abbreviations
- AUEC
area-under-the-contraction-waveforms vs time curve
- BL
baseline
- D5W
5% dextrose
- IBMX
3-isobutyl-1-methylxanthine
- MAP
mean arterial blood pressure
- NO
nitric oxide
- NTG
nitroglycerin
- ODQ
1H-[1,2,4]oxadiazolo[4,3-α]quinoxalin-1-one
References
- BAUER J.A., FUNG H.L. Pharmacodynamic models of nitroglycerin-induced hemodynamic tolerance in experimental heart failure. Pharm. Res. 1994;11:816–823. doi: 10.1023/a:1018917522072. [DOI] [PubMed] [Google Scholar]
- BOOTH B.P., JACOB S., BAUER J.A., FUNG H.L. Sustained antiplatelet properties of nitroglycerin during hemodynamic tolerance in rats. J. Cardiovasc. Pharmacol. 1996;28:432–438. doi: 10.1097/00005344-199609000-00013. [DOI] [PubMed] [Google Scholar]
- DIKALOV S., FINK B., SKATCHKOV M., STALLEICKEN D., BASSENGE E. Formation of reactive oxygen species by pentaerithrityltetranitrate and glyceryl trinitrate in vitro and development of nitrate tolerance. J. Pharmacol. Exp. Ther. 1998;286:938–944. [PubMed] [Google Scholar]
- DOCHERTY J.R., HYLAND L. Alpha-adrenoceptor responsiveness in the aged rat. Eur. J. Pharmacol. 1986;126:75–80. doi: 10.1016/0014-2999(86)90740-5. [DOI] [PubMed] [Google Scholar]
- FUNG H.L., BAUER J.A. Mechanisms of nitrate tolerance. Cardiovasc. Drugs Ther. 1994;8:489–499. doi: 10.1007/BF00877927. [DOI] [PubMed] [Google Scholar]
- FUNG H.L., CHUNG S.J., BAUER J.A., CHONG S., KOWALUK E.A. Biochemical mechanism of organic nitrate action. Am. J. Cardiol. 1992;70:4B–10B. doi: 10.1016/0002-9149(92)90588-p. [DOI] [PubMed] [Google Scholar]
- GORFINE S.R. Topical nitroglycerin therapy for anal fissures and ulcers [letter] N. Engl. J. Med. 1995a;333:1156–1157. doi: 10.1056/NEJM199510263331718. [DOI] [PubMed] [Google Scholar]
- GORFINE S.R. Treatment of benign anal disease with topical nitroglycerin Dis. Colon Rectum. 1995b38456–457.453-6; Discussion [DOI] [PubMed] [Google Scholar]
- HASEGAWA K., TANIGUCHI T., TAKAKURA K., GOTO Y., MURAMATSU I. Possible involvement of nitroglycerin converting step in nitroglycerin tolerance. Life Sci. 1999;64:2199–2206. doi: 10.1016/s0024-3205(99)00171-x. [DOI] [PubMed] [Google Scholar]
- KUKOVETZ W.R., HOLZMANN S. Mechanism of nitrate-induced vasodilation and tolerance. Z. Kardiol. 1983;72:14–19. [PubMed] [Google Scholar]
- LYSY J., ISRAELIT-YATZKAN Y., SESTIERE-ITTAH M., KERET D., GOLDIN E. Treatment of chronic anal fissure with isosorbide dinitrate: long-term results and dose determination. Dis. Colon Rectum. 1998;41:1406–1410. doi: 10.1007/BF02237057. [DOI] [PubMed] [Google Scholar]
- MIZOBE T., MAZE M. Alpha 2-adrenoceptor agonists and anesthesia. Int. Anesthesiol. Clin. 1995;33:81–102. doi: 10.1097/00004311-199500000-00005. [DOI] [PubMed] [Google Scholar]
- MUNZEL T., KURZ S., HEITZER T., HARRISON D.G. New insights into mechanisms underlying nitrate tolerance. Am. J. Cardiol. 1996;77:24C–30C. doi: 10.1016/s0002-9149(96)00185-3. [DOI] [PubMed] [Google Scholar]
- MUNZEL T., SAYEGH H., FREEMAN B.A., TARPEY M.M., HARRISON D.G. Evidence for enhanced vascular superoxide anion production in nitrate tolerance. A novel mechanism underlying tolerance and cross-tolerance. J. Clin. Invest. 1995;95:187–194. doi: 10.1172/JCI117637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEEDLEMAN P., JOHNSON E.M. , JR Mechanism of tolerance development to organic nitrates. J. Pharmacol. Exp. Ther. 1973;184:709–715. [PubMed] [Google Scholar]
- NEWMAN C.M., WARREN J.B., TAYLOR G.W., BOOBIS A.R., DAVIES D.S. Rapid tolerance to the hypotensive effects of glyceryl trinitrate in the rat: prevention by N-acetyl-L- but not N-acetyl-D-cysteine. Br. J. Pharmacol. 1990;99:825–829. doi: 10.1111/j.1476-5381.1990.tb13014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASKINS J.R., LAWSON J.O., CLAYDEN G.S. The effect of ketamine anesthesia on anorectal manometry. J. Pediatr. Surg. 1984;19:289–291. doi: 10.1016/s0022-3468(84)80189-x. [DOI] [PubMed] [Google Scholar]
- RATTAN S., CHAKDER S. L-citrulline recycling in opossum internal anal sphincter relaxation by nonadrenergic, noncholinergic nerve stimulation. Gastroenterology. 1997;112:1250–1259. doi: 10.1016/s0016-5085(97)70137-9. [DOI] [PubMed] [Google Scholar]
- STEBBING J.F. Nitric oxide synthase neurones and neuromuscular behaviour of the anorectum. Ann. R. Coll. Surg. Engl. 1998;80:137–145. [PMC free article] [PubMed] [Google Scholar]
- TSENG C.M., TABRIZI-FARD M.A., FUNG H.L. Differential sensitivity among nitric oxide donors toward ODQ-mediated inhibition of vascular relaxation. J. Pharmacol. Exp. Ther. 2000;292:737–742. [PubMed] [Google Scholar]


