Abstract
Antiplatelet drugs have been demonstrated to reduce the incidence of recurrent events in patients with symptomatic vascular disease. However, there is no experimental data indicating the effects of these agents when given together on platelets and leukocytes. We investigated the ability of aspirin (an inhibitor of cyclo-oxygenase), dipyridamole (an inhibitor of phospodiesterases and adenosine uptake) and AR-C69931 (a direct acting P2T antagonist with effects similar to those of clopidogrel which can be used in vitro) when used alone or in combination to inhibit platelet and leukocyte function.
Measurements of platelet and leukocyte function were performed in blood taken from normal volunteers, and the inhibitory effects of aspirin (100 μmol l−1), dipyridamole (10 μmol l−1) and AR-C66931 (100 nmol l−1) were determined. Platelet aggregation was induced by stirring blood with and without adenosine diphosphate (ADP) or platelet activating factor (PAF) and measured by platelet counting. Platelet P-selectin expression, platelet-leukocyte conjugate formation, and leukocyte activation were determined by flow cytometry.
Dipyridamole, AR-C69931, dipyridamole and AR-C69931, dipyridamole and aspirin, AR-C69931 and aspirin, and all three agents together inhibited platelet aggregation induced by stirring, ADP and PAF (P<0.01). However, it was only the combination of all three agents inhibited P-selectin expression (P<0.01). Similarly, it was the combination of all three antiplatelet agents that most consistently inhibited platelet-monocyte and platelet-neutrophil conjugate formation and monocyte and neutrophil activation.
Since both platelets and leukocytes are thought to contribute to arterial thrombosis and atherosclerosis, it is possible that combinations of different antiplatelet agents with different mechanisms of action may afford better protection than individual or pairs of agents used on their own.
Keywords: Platelet, monocyte, neutrophil, aspirin, dipyridamole, clopidogrel
Introduction
The use of antiplatelet agents is now a routine part of secondary prevention following ischaemic stroke and myocardial infarction (Antiplatelet Trialists, 1994; Lees et al., 2000). The three agents that are used most are aspirin, dipyridamole and clopidogrel, all of which have a different mechanism of action. Aspirin is an inhibitor of cyclo-oxygenase, clopidogrel is an ADP P2T receptor antagonist whilst dipyridamole inhibits adenosine uptake and guanosine 3′5′ cyclic monophosphate (cyclic GMP) and adenosine cyclic monophosphate (cyclic AMP) phosphodiesterases. Since all three drugs, used individually, have been shown to be effective as anti-thrombotic agents (Diener et al., 1996; Muller et al., 2000), it is possible that combinations of these drugs might be more effective. The Second European Stroke Prevention Study (ESPS II) indicated that the combined use of aspirin and dipyridamole has an additive effect in patients with transient ischaemic attack or ischaemic stroke as compared with either drug alone on reducing recurrent vascular events (Diener et al., 1996). Combined aspirin and clopidogrel are frequently used in the prevention of subacute thrombosis following coronary stent implantation, and appear to be a safe and effective therapy (Muller et al., 2000; Kolansky et al., 2000).
Increased platelet activation is well-documented in acute vascular syndromes including ischaemic stroke and myocardial infarction (Kolansky et al., 2000; Joseph et al., 1989; Butterworth & Bath, 1998; Smith et al., 1999), where platelets act as the first responsive elements triggering thrombosis. There is a growing interest in the role of leukocytes in thrombosis. Studies have shown that platelet activation can lead to the formation of platelet-leukocyte conjugates and leukocyte activation (Mickelson et al., 1996; Serrano et al., 1997; Rinder et al., 1994; Gawaz et al., 1996; Ott et al., 1996; Furman et al., 1997). The process of the interaction of platelets with leukocytes involves a P-selectin-dependent recognition step followed by an adhesion-strengthening interaction mediated by the β2 integrin Mac-1 (CD11b) (de gaetano et al., 1999). Stimulated platelets adhere to leukocytes triggering their activation, leukocytes then modulate platelet responsiveness thereby contributing to the pathogenesis of thrombotic and inflammatory processes (Marcus, 1990; Bazzoni et al., 1991).
Here we looked at the in vitro effects of aspirin, AR-C69931 and dipyridamole used singly, in pairwise combinations and all together, on platelet aggregation and platelet P-selectin expression (CD62P, a marker of platelet activation), platelet-leukocyte conjugate formation and leukocyte CD11b expression (a marker of leukocyte activation). AR-C69931, a direct acting P2T receptor antagonist was used since clopidogrel is a pro drug requiring hepatic metabolism.
All studies were performed in whole blood containing hirudin as the anticoagulant. Hirudin was used because, unlike citrate, plasma divalent cation concentrations are maintained at physiological levels (Evans et al., 1997). Platelet aggregation was assessed directly in the whole blood using a platelet counting technique which provides a very sensitive means of monitoring the aggregation process and the results of which correlate well with aggregation measured using other approaches (Story et al., 1999). Other assays were performed by flow cytometry.
Methods
Subjects
Forty-two non-fasted volunteers who denied taking any antiplatelet drugs in the previous 2 weeks were recruited in this study. The study protocol was reviewed and approved by the local ethics committee.
Blood preparation
Blood (16 ml) from each subject was collected into two plastic tubes. One tube contained hirudin provided by Rhone-Poulenc-Rorer (Kent, U.K.) and stored at a concentration of 5 mg ml−1 in 150 mmol l−1 NaCl (final concentration (fc) 50 μg ml−1) to anticoagulate the blood. The other tube contained hirudin together with one of the drugs or drug combinations that was to be investigated. This was aspirin from Sigma (Poole, fc 100 μmol l−1), AR-C69931, a gift from AstraZeneca (Charnwood, fc 100 nmol l−1), dipyridamole from Boehringer Ingelheim Ltd (Germany, fc 10 μmol l−1) or the various combinations of these. The first tube contained 150 mmol l−1 NaCl in place of the drug being tested. The blood samples were then incubated at 37°C for 30 min. Each drug or drug combination was tested using blood from six different volunteers.
Platelet aggregation
Aliquots of blood (500 μl) were stirred in a Multi-Sample Agitator without an agonist, and following addition of ADP (fc 3 μmol l−1) or PAF (fc 1 μmol l−1), both from Sigma (Poole). Platelet aggregation was measured at 10 min after adding the agonist. This was achieved by removing and fixing 15 μl of the samples in fixing solution that was 150 mmol l−1 NaCl that contained 0.16% w v−1 formaldehyde, 4.6 mmol l−1 disodium EDTA, 4.5 mmol l−1 Na2HPO4 and 1.6 mmol l−1 KH2PO4, pH 7.4, and counting the number of single platelets using an Ultra-Flo 100 Whole Blood Platelet Counter (Fox et al., 1982). The percentage fall in number of single platelets compared with the initial platelet count was used to indicate the percentage of platelet aggregation that had occurred.
Platelet activation
Separate aliquots (500 μl) of blood were used to determine the effects of the drug(s) on platelet CD62P expression. In this case the samples were incubated unstirred for 10 min with or without ADP (fc 3 μmol l−1) or PAF (fc 1 μmol l−1). These samples were then fixed, centrifuged at 380 × g for 10 min and the cell pellets resuspended in PBS that was Dulbecco's A tablets dissolved in distilled water purchased from Unipath Ltd (Bedford), Aliquots (30 μl) was then incubated with a saturating concentration of anti-CD62P:FITC or mouse IgG1:FITC control antibody, both purchased from Serotec Ltd (Oxford).
Platelet-leukocyte conjugates and leukocyte activation
Measurement of platelet-leukocyte conjugate formation and leukocyte activation was also performed at 10 min after adding the agonist. A further 100 μl of the stirred blood (from the same sample in which platelet aggregation was measured) was transferred to 2 ml Erythrolyse solution (Serotec Ltd) and left for 10 min at room temperature. The lysed samples were then centrifuged at 380 × g for 10 min, the pellet washed with PBS, and then re-centrifuged. The pellet was resuspended in 100 μl PBS containing 10% (v v−1) new born calf serum from Gibco (Middlesex, U.S.A.). Aliquots of the resuspended material (30 μl) was then incubated with a saturating concentration of anti-CD14:PE (Becton Dickinson) which recognizes monocytes and either anti-CD42a:FITC (for platelet-leukocyte conjugates) or CD11b:FITC (for activated leukocytes), both from Serotec Ltd. Non-specific binding was determined using a FITC conjugated control antibody (Serotec Ltd).
Flow cytometry
Platelet-leukocyte conjugates, leukocyte activation (CD11b expression) and platelet activation (CD62P expression) were measured using a FACScan flow cytometry (Becton Dickinson) equipped with a 5 W laser operating at 15 mW power and a wavelength of 488 nm, and attached to an Apple Mac G3 computer. The method was as previously described (Sanderson et al., 1998). To determine platelet-leukocyte conjugates, a total of 5000 leukocyte events were collected using a combination of forward scatter (approximating to cell size) and side scatter (approximating to cell granularity) with linear amplification. All fluorescence parameters were acquired with logarithmic amplification. Leukocyte subsets were distinguished from one another on the basis of their differential light scatter characteristics and positive anti-CD14 fluorescence. Platelet-leukocyte conjugates were identified as the percentage of leukocytes with CD42a:FITC fluorescence greater than non-specific antibody control. In addition, the median platelet fluorescence of whole monocyte and neutrophil population was determined (CD42a). The measurement of leukocyte activation was performed using anti-CD11b antibody, a marker of monocyte and neutrophil activation. Activated monocytes and neutrophils were quantified as the median CD11b fluorescent intensity of the whole monocyte and neutrophil populations. For determination of the percentage of platelet expression CD62P, forward light scatter and side scatter and FITC fluorescence were acquired using logarithmic amplification. A total of 2000 platelet events were collected. An isotype-matched control antibody was used to set a threshold (99% of events below threshold) for CD62P positivity.
Statistical analyses
Data were analysed by comparing the mean (±s.d.) of data from each of the six samples for each antagonist with their control (saline, Table 1). These results are presented in Figures 1, 2, 3, 4 and 5. Statistical analysis was via Student's t-test for paired values. In view of the number of comparisons that were performed P<0.01 was considered to be significant.
Table 1.
Platelet aggregation, P-selectin expression, platelet-leukocyte conjugate formation and leukocyte activation following addition of saline, ADP or PAF
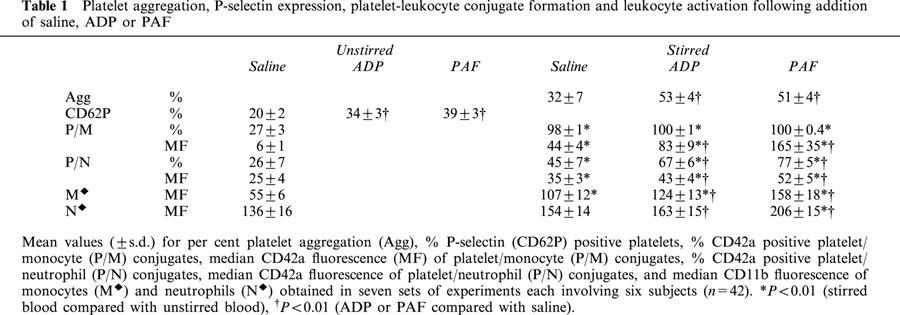
Figure 1.
Inhibition of platelet aggregation in whole blood in vitro by AR-C69931 (ARC 100 nmol l−1), dipyridamole (dip 10 μmol l−1), aspirin (ASA 100 μmol l−1) and combinations of these. (a) Spontaneous aggregation; (b) with 3 μmol l−1 ADP; (c) with 1 μmol l−1 PAF. Percentage of platelet aggregation is shown (mean±s.d.), n=6, *P<0.01.
Figure 2.
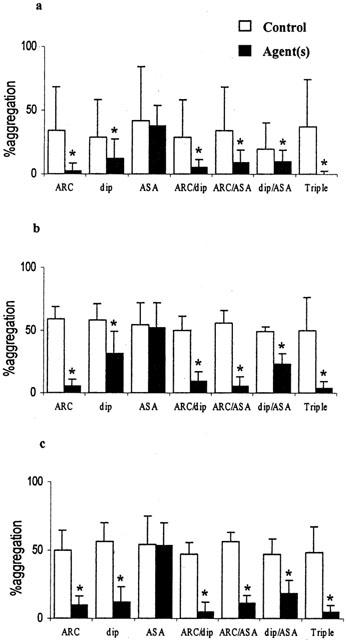
Inhibition of platelet-monocyte conjugate formation in whole blood in vitro by AR-C69931 (ARC 100 nmol l−1), dipyridamole (dip 10 μmol l−1), aspirin (ASA 100 μmol l−1) and combinations of these. (a) Spontaneous conjugates; (b) with 3 μmol l−1 ADP; (c) with 1 μmol l−1 PAF. The median fluorescence of CD42a positive platelets is shown (mean±s.d.), n=6, zP<0.01.
Figure 3.
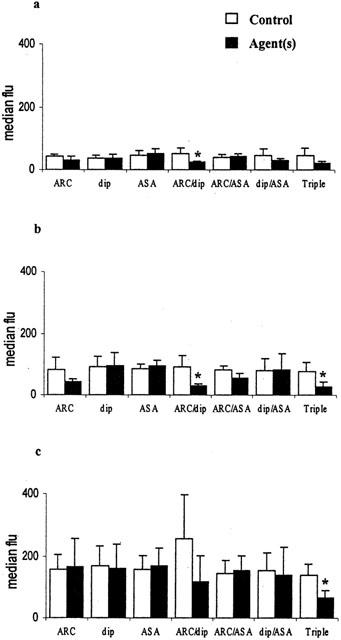
Inhibition of platelet-neutrophil conjugate formation in whole blood in vitro by AR-C69931 (ARC 100 nmol l−1), dipyridamole (dip 10 μmol l−1), aspirin (ASA 100 μmol l−1) and combinations of these. (a) Spontaneous conjugates; (b) with 3 μmol l−1 ADP; (c) with 1 μmol l−1 PAF. The median fluorescence of CD42a positive platelets is shown (mean±s.d.), n=6, *P<0.01.
Figure 4.
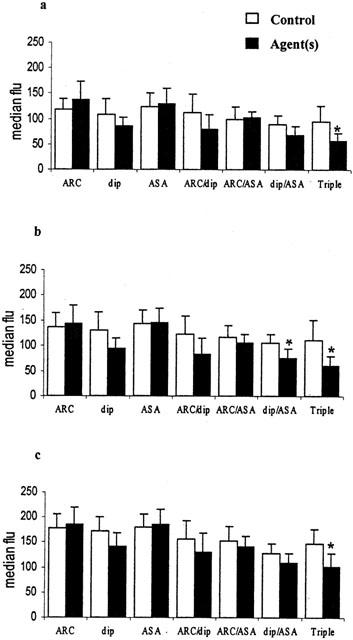
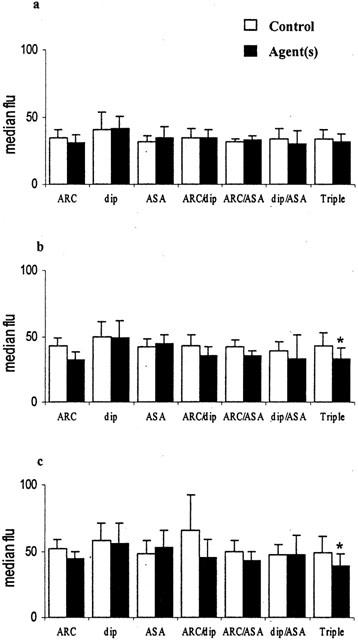
Inhibition of monocyte activation in whole blood in vitro by AR-C69931 (ARC 100 nmol l−1), dipyridamole (dip 10 μmol l−1), aspirin (ASA 100 μmol l−1) and combinations of these. (a) Spontaneous activation; (b) with 3 μmol l−1 ADP; (c) with 1 μmol l−1 PAF. The median fluorescence of CD11b positive monocytes is shown (mean±s.d.), n=6, *P<0.01.
Figure 5.
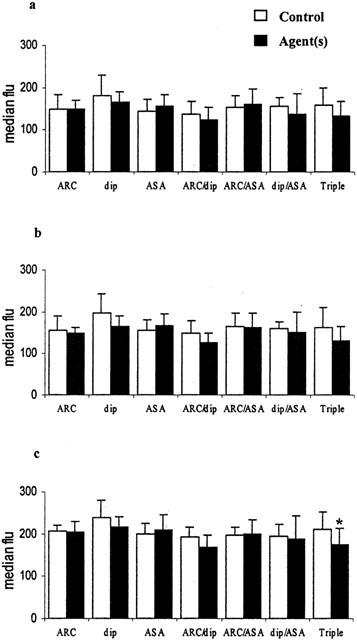
Inhibition of neutrophil activation in whole blood in vitro by AR-C69931 (ARC 100 nmol l−1), dipyridamole (dip 10 μmol l−1), aspirin (ASA 100 μmol l−1) and combinations of these. (a) Spontaneous activation; (b) with 3 μmol l−1 ADP; (c) with 1 μmol l−1 PAF. The median fluorescence of CD11b positive monocytes is shown (mean±s.d.), n=6, *P<0.01.
Results
Platelet aggregation
Platelet aggregation occurred as a result of stirring whole blood both in the absence and presence of a platelet agonist. The extents of the aggregation that occurred in the absence of any antiplatelet agents are shown in Table 1. Overall it can be seen that ADP and PAF enhanced the extent of aggregation in the absence of drug(s) in comparison with blood without agonist (P<0.01 for both ADP and PAF, n=42). The effects of the various antiplatelet agents on the aggregation are shown in Figure 1. In each case the aggregation obtained in the presence of a drug (hatched histogram) is compared with the aggregation obtained in the absence of the drug (open histogram), n=6. AR-C69931 (100 nmol l−1), dipyridamole (10 μmol l−1), the combination of AR-C69931 and dipyridamole, the combination of AR-C69931 and aspirin (100 μmol l−1), the combination of dipyridamole and aspirin, and the combination of all three drugs all significantly (P<0.01) reduced the extent of the platelet aggregation. This was the case whether the blood was stirred with or without an agonist. Aspirin alone did not reduce the extent of the platelet aggregation.
Platelet P-selectin expression
α-Granule secretion occurs following platelet activation and results in increased expression of CD62P on the platelet surface. CD62P expression was enhanced (Table 1) by ADP and by PAF (P<0.01, n=42). In these experiments the blood was left unstirred so as to avoid any platelet aggregation so that the CD62P expression on single platelets could be determined. On average 20% of platelets expressed CD62P before addition of any agonist (Table 1) and this was not significantly reduced in the presence of AR-C69931 (100 nmol l−1), aspirin (100 μmol l−1) and dipyridamole (10 μmol l−1) singly or in any combination. In contrast, 34% and 39% of platelets were CD62P positive following incubation with ADP or PAF (Table 1). Only the combination of all three drugs significantly (P<0.01) inhibited the increased CD62P expression that occurred following incubation with ADP or PAF.
Platelet-monocyte and platelet-neutrophil conjugates
Platelet-leukocyte conjugate formation was quantitated both by determining the percentage of monocytes and of neutrophils positive for the platelet marker CD42a and as the median CD42a:FITC fluorescence of monocytes and neutrophils. Mean results obtained for unstirred samples of blood after stirring with and without ADP and PAF are shown in Table 1. It can be seen that all the monocytes were positive for the platelet marker following stirring with or without agonist, and that 45 – 77% of the neutrophils were adherent to platelets depending on the precise conditions used. There were also significant increases in the median fluorescence values for the monocytes and neutrophils after stirring the blood and further significant increases induced by ADP and PAF (all P<0.01, n=42). It was possible to inhibit the extent of platelet-monocyte (Figure 2) and platelet-neutrophil conjugate formation (Figure 3) using some drug combination but not by any of the drugs used singly. In Figure 2 it can be seen that the combination of AR-C69931 (100 nmol l−1) and dipyridamole (10 μmol l−1) and/or the combination of AR-C69931, dipyridamole and aspirin (100 μmol l−1) inhibited platelet-monocyte conjugate formation. In Figure 3 it can be seen that the combination of all three drugs was required to inhibit ADP- and PAF-induced platelet-neutrophil conjugate formation.
Monocyte and neutrophil CD11b expression
Leukocyte activation results in increased expression of CD11b on the leukocyte surface and can be quantitated by determining the median CD11b:FITC fluorescence of monocytes and neutrophils. Table 1 shows the mean results obtained overall in unstirred samples and following stirring with and without ADP and PAF. Simply stirring blood significantly increased monocyte activation and adding ADP or PAF increased CD11b expression on monocytes and neutrophils further (all P<0.01, n=42). The presence of ADP or PAF was required to bring about a significant increase in neutrophil activation. Monocyte activation was inhibited using the combination of dipyridamole (10 μmol l−1) and aspirin (100 μmol l−1) and/or the combination of AR-C69931 (100 nmol l−1), dipyridamole and aspirin (Figure 4). Neutrophil activation induced by PAF was inhibited by the combination of AR-C69931, dipyridamole and aspirin only (Figure 5).
Discussion
Overall the aggregation induced by stirring whole blood and in response to ADP or PAF was inhibited by AR-C69931, dipyridamole and the combination of these drugs but not by aspirin. Aspirin did not interfere with the inhibitory effects of AR-C69931 and of dipyridamole. AR-C69931 is a direct acting P2T antagonist and interferes specifically with ADP-induced aggregation, thus inhibition of the aggregation induced by stirring and PAF by AR-C6993 confirms a role for ADP in these processes (Ishii-Watabe et al., 2000; Kuanpuli et al., 1999). Dipyridamole provided an alternative means of inhibiting platelet aggregation. Presumably dipyridamole inhibited aggregation through a mechanism involving cyclic AMP (Fitzgerald, 1987) The lack of effect of aspirin, an inhibitor of thromboxane A2 (TXA2) synthesis in platelets suggests that TXA2 did not contribute to the platelet aggregation in these experiments.
Interestingly, it was only the combination of AR-C69931, dipyridamole and aspirin that significantly inhibited ADP- and PAF-induced P-selectin expression on platelets in these experiments. Clearly individual drugs were not as effective as the three drugs together. The same was true for platelet-leukocyte conjugate formation, with the combination of all three drugs providing the most effective inhibition of conjugate formation. These results are entirely consistent with platelet-leukocyte conjugate formation being mainly mediated by P-selectin with several different processes (ADP, cyclic AMP and TXA2) contributing to α-granule release and promotion of platelet-leukocyte adhesion.
Since the combination of all three drugs was also most effective in inhibiting monocyte and neutrophil activation (CD11b expression), it would appear that the more completely platelet activation is inhibited, the more likely it is that leukocyte activation is inhibited also.
In summary, the overall message of our work is that combinations of three antiplatelet drugs which work through different mechanism are superior to any single agent alone, or pairs of agents, in modifying platelet activity and heterotypic cell adhesion and leukocyte activation. Combining three different antiplatelet agents may be a new mechanistic strategy for antiplatelet therapy, specifically in the secondary prevention of vascular disease. We are now studying the effect of combining aspirin, dipyridamole and clopidogrel on platelet and leukocyte activity measured ex vivo in normal volunteers and patients with prior ischaemic stroke.
Acknowledgments
We thank Dr HM Sanderson in the University of Nottingham for assistance with methodology development. Lian Zhao was funded in part by the University of Nottingham (international office), BGWG Charitable Foundation and Henry Lester Trust Limited. Philip Bath is Stroke Association Professor of Stroke Medicine.
Abbreviations
- ADP
adenosine diphosphate
- cyclic AMP
cyclic adenosine monophosphate
- cyclic GMP
guanosine 3′5′ cyclic monophosphate
- EDTA
ethylenediaminetetraacetic acid
- PAF
platelet activating factor
- TXA2
thromboxane A2
References
- ANTIPLATELET TRIALISTS' COLLABORATION Collaborative overview of randomised trials of antiplatelet therapy. Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Br. Med. J. 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- BAZZONI G., DEJANA E., MASCHIO A.D. Platelet-neutrophil interactions. Possible relevance in the pathogenesis of thrombosis and inflammation. Haematologica. 1991;76:491–499. [PubMed] [Google Scholar]
- BUTTERWORTH R.J., BATH P.M.W. The relationship between mean platelet volume stroke subtype and clinical outcome. Platelet. 1998;9:359–364. doi: 10.1080/09537109876429. [DOI] [PubMed] [Google Scholar]
- DE GAETANO G., CERLETTI C., EVANGELISTA V. Recent advances in platelet-polymorphonuclear leukocyte interaction. Haemostasis. 1999;29:41–49. doi: 10.1159/000022459. [DOI] [PubMed] [Google Scholar]
- DIENER H.C., CUNHA L., FORBES L., SIVENIUS J., SMETS P., LOWENTHAL A. European stroke prevention study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J. Neurol. Sci. 1996;143:1–13. doi: 10.1016/s0022-510x(96)00308-5. [DOI] [PubMed] [Google Scholar]
- EVANS P.A., MADIRA W., RIYATT M.S., ERRINGTON M., HEPTINSTALL S. Changes in plasma ionised calcium within 24 hours of trauma in patients infused with the calcium containing colloid haemaccel during fluid resuscitation. J. Accid. Emerg. Med. 1997;14:73–75. doi: 10.1136/emj.14.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZGERALD G.A. Dipyridamole. N. Eng. J. Med. 1987;316:1247. doi: 10.1056/NEJM198705143162005. [DOI] [PubMed] [Google Scholar]
- FOX S.C., BURGESS-WILSON M., HEPTINSTALL S. Platelet aggregation in whole blood determined using the Ultra-Flo 100 platelet counter. Thromb Haemostas. 1982;48:327–329. [PubMed] [Google Scholar]
- FURMAN M.I., BENOIT S.E., BORBONE M., BECKER R.C., MICHELSON A.D. Patients with stable coronary artery disease have increased platelet reactivity and circulation monocyte-platelet aggregates. Thromb. Haemostas. 1997. p. 62. [DOI] [PubMed]
- GAWAZ M., REINNINGER A., NEUMANN F.-J. Platelet function and platelet-leukocyte adhesion in symptomatic coronary heart disease. Effects of intravenous magnesiom. Thromb. Res. 1996;83:341–349. doi: 10.1016/0049-3848(96)00144-2. [DOI] [PubMed] [Google Scholar]
- ISHII-WATABE A., UCHIDA E., MIZUGUCHI H., HAYAKAWA T. On the mechanism of plasmin-induced platelet aggregation- implications of the dual role of granule ADP. Biochem. Pharmacol. 2000;59:1345–1355. doi: 10.1016/s0006-2952(00)00279-3. [DOI] [PubMed] [Google Scholar]
- JOSEPH R., D'ANDREA G., OSTER S.B., WELCH K.M. Whole Blood Platelet Function in Acute Ischemic Stroke. Stroke. 1989;20:38–44. doi: 10.1161/01.str.20.1.38. [DOI] [PubMed] [Google Scholar]
- KOLANSKY D.M., KLUGHERZ B.D., CURRAN S.C., HERRMANN H.C., MAGNESS K., WILENSKY R.L., HIRSHFELD J.W. , JR Combination therapy with clopidogrel and aspirin after coronary stenting. Catheter. Cardio. Interven. 2000;50:276–279. doi: 10.1002/1522-726x(200007)50:3<276::aid-ccd2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- KUANPULI S.P., DANGELMAIER C., JIN J.G., KIM Y.B., KUNAPULI S.P. Role of intracellular signalling events in ADP-induced platelet aggregation. Thromb. Haemostas. 1999. p. 2256. [PubMed]
- LEES K.R., BATH P.M.W., NAYLOR A.R. ABC of arterial and venous disease - Secondary prevention of TIA and stroke. Br. Med. J. 2000;173:254–258. [Google Scholar]
- MARCUS A.J. Thrombosis and inflammation as multicellular processes: pathophysiologic significance of transcellular metabolism. Blood. 1990;76:1903–1907. [PubMed] [Google Scholar]
- MICKELSON J.K., LAKKIS M., VILARREAL-LEVY G., HUGHES B.J., SMITH C.W. Leukocyte activation with platelet adhesion after coronary angioplasty: mechanism for recurrent disease. J. Am. Coll. Cardiol. 1996;28:345–353. doi: 10.1016/0735-1097(96)00164-7. [DOI] [PubMed] [Google Scholar]
- MULLER C., BUTTENER H.J., PETERSEN J., ROSKAMM H. A randomized comparison of clopidogrel and aspirin versus ticlopidine and aspirin after the placement of coronary-artery stents. Circulation. 2000;101:590–593. doi: 10.1161/01.cir.101.6.590. [DOI] [PubMed] [Google Scholar]
- OTT I., NEUMANN F.-J., GAWAZ M., SCHMITT M., SCHOMIG A. Increased neutrophil-platelet adhesion in patients with unstable angina. Circulation. 1996;94:1239–1246. doi: 10.1161/01.cir.94.6.1239. [DOI] [PubMed] [Google Scholar]
- RINDER C., GALL D., STUDENT L.A., SMITH B.R. Platelet-leukocyte activation and modulation of adhesion receptors in pediatric patients with congental heart disease undergoing cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 1994;107:280–288. [PubMed] [Google Scholar]
- SANDERSON H.M., FOX S.C., ROBBINS R.A. Role of GPIIb-IIIa in platelet-monocyte and platelet-neutrophil conjugate formation in whole blood. Platelet. 1998;9:245–250. doi: 10.1080/09537109876780. [DOI] [PubMed] [Google Scholar]
- SERRANO C.V., RAMIRES J.A., VENTURINELLA M., ARIE S., D'AMICO E., ZWEIER J.L., PILEGGI F., DA-LUZ P.L. Coronary angioplasty results in leukocyte and platelet activation with adhesion molecules expression. Evidence of inflammatory responses in coronary angioplasty. J. Am. Coll. Cardiol. 1997;27:1276–1283. doi: 10.1016/s0735-1097(97)00070-3. [DOI] [PubMed] [Google Scholar]
- SMITH N.M., PATHANSALI R., BATH P.M.W. Platelet and stroke. Vasc. Med. 1999;4:1–8. doi: 10.1177/1358836X9900400307. [DOI] [PubMed] [Google Scholar]
- STORY R.F., MAY J.A., WILCOX R.G., HEPTINSTALL S. A whole blood assay of inhibition of platelet aggregation by glycoprotein IIb/IIIa antagonists: comparison with other aggregation methodologies. Thromb. Haemostas. 1999;82:1307–1311. [PubMed] [Google Scholar]


