Abstract
In rat aortic smooth muscle cells (RASMC), exposure to lipopolysaccharide (LPS) resulted in NF-κB-DNA binding, degradation of IκB-α, -β and -ε and increased activity of both α and β isoforms of inhibitory kappa B kinase (IKK).
Expression of dominant-negative (DN)-IKK-α, IKK-β and NF-κB-inducing kinase (NIK) abolished LPS-stimulated NF-κB reporter activity, suggesting that activation of a NIK/IKK-dependent pathway is indispensable for NF-κB activation by LPS in this cell type.
The tyrosine phosphatase inhibitor, pervanadate, abolished LPS-stimulated NF-κB-DNA-binding activity. However, the effect of pervanadate was shown to be mediated by excess hydrogen peroxide (H2O2) present in the reaction mix. Preincubation of RASMC with H2O2 inhibited LPS-stimulated IKK kinase activity and downstream NF-κB-DNA binding activity.
H2O2 also strongly stimulated p38 MAP kinase activity in RASMCs. Effective inhibition of this pathway using SB203580 did not reverse the effects of H2O2 on LPS-stimulated IKK/NF-κB signalling.
These studies show that hydrogen peroxide-mediated inhibition of LPS-stimulated NF-κB activation in RASMC occurs upstream of IKK. The inhibitory effect of H2O2 is not due to tyrosine phosphatase inhibition, it is mediated by H2O2 through a mechanism which is independent of any cross-talk involving MAP kinase homologues.
Keywords: Hydrogen peroxide, lipopolysaccharide, inhibitory kappa B kinase, rat aortic smooth muscle cells
Introduction
Cellular exposure to lipopolysaccharide (LPS), the active component of bacterial endotoxin, stimulates systemic shock, manifest as profound hypotension and organ failure (Julou-Schaeffer et al., 1990; Loegering et al., 1995). This occurs through release of inflammatory mediators from both macrophages and vascular smooth muscle cells and the induction of a number of proteins such as inducible nitric oxide synthase (iNOS) and cyclo-oxygenase-2 (COX-2) (Meng et al., 1996; Akarasereenont et al., 1994; Xie et al., 1992; Lyons et al., 1992). Studies using pharmacological or molecular approaches have implicated a number of important signal transduction cascades in the cellular actions of LPS including the classical mitogen-activated protein kinases (MAP kinases) or extracellular signal related kinases (ERK kinases) and the stress-activated protein kinases, c-Jun N terminal kinase (JNK) and p38 MAP kinase (Feng et al., 1999, Caivano & Cohen, 2000).
In addition to these signalling pathways evidence has accumulated recently indicating an important role for the NF-κB transcription factor pathway in the cellular effects of LPS (Lenardo & Baltimore, 1989; Baeuerle & Baltimore, 1991; Cordle et al., 1993; Hawiger et al., 1999; Fischer et al., 1999; Shimada et al., 1999). Nuclear factor kappa B (NF-κB) comprises a family of homo- and heterodimeric transcription factors that are activated in response to stress or inflammatory mediators (Baeuerle & Baltimore, 1996). At rest NF-κB is held within the cytosol bound to isoforms of inhibitory kappa B (IκB). Upon activation of the cell, IκB proteins are phosphorylated on critical serine residues facilitating their ubiquitination and subsequent 26S proteosomal degradation (Beg & Baltimore, 1996; Baldwin, 1996). Freed NF-κB can then translocate to the nucleus and bind to the promoter region of its target genes (Didonato et al., 1997). Phosphorylation of IκB is in turn regulated by both α and β isoforms of inhibitory kappa B kinase (IKK). Recent studies have indicated that these kinases and additional upstream intermediates such as NF-κB-inducing kinase (NIK) may represent a novel site for pharmacological intervention in a number of inflammatory conditions (Regnier et al., 1997; Karin & Delhase, 1998; Medzhitov et al., 1998; Muzio et al., 1998; Irie et al., 2000).
Protein tyrosine phosphatases (PTPases) have also been implicated as having a role in NF-κB signalling. Evidence shows that IκB-α contains a tyrosine phosphorylation site (Devi Menon et al., 1995) and recently a PTPase called PTP-BAS has also been shown to associate with the ankyrin repeats of IκB-α (Maekawa et al., 1999). This putative phosphatase may thus represent a novel site of pharmacological intervention. However, controversy has surrounded the evidence presented utilizing the PTPase inhibitors which have been shown to inhibit activation of NF-κB depending on the cell type involved (Devi Menon et al., 1995) suggesting different sites and mechanisms of action of these compounds. In addition, since tyrosine phosphatase inhibition can activate a number of tyrosine kinase-dependent signalling pathways, it is possible that cross-talk between these pathways and NF-κB can occur. This has been described for p38 MAP kinase which has been shown to mediate an inhibition of NF-κB signalling in HT-29 human colon adenocarcinoma cells and FS-4 fibroblasts (Alpert et al., 1999).
In this study, we sought to provide evidence for the presence of a tyrosine phosphatase in the regulation of LPS-stimulated NF-κB signalling pathway in rat aortic smooth muscle cells (RASMC) and to determine the site of action. LPS stimulated an increase in NF-κB-DNA binding and transcriptional activity as well as the degradation of IκB-α -β and -ε which were dependent upon activation of IKK-α and -β kinase activity and the upstream kinase NIK. However, we found that the effect of the tyrosine phosphatase inhibitor pervanadate was mediated via excess H2O2 which also inhibits LPS-stimulated NF-κB activation at the level or upstream of IKK. Whilst H2O2 was found to be able to strongly activate p38 MAP kinase this was shown not to mediate the inhibitory effect on NF-κB signalling.
Methods
Materials
The plasmids used in this study were kindly donated as follows: the plasmid containing the cDNA encoding the GST-tagged truncated N-terminus of c-Jun (GST-c-Jun5 – 89) was donated by Prof J.R. Woodgett (Ontario Cancer Institute, Princess Margaret Hospital, Toronto, Canada). The GST-tagged MAPKAP kinase-2 vector was donated by Prof C.J. Marshall (Chester Beatty Laboratories, Institute of Cancer Research, London, U.K.) and the MAPKAP kinase-2 antibody and the KKLNRTSSVA peptide substrate were gifts from Prof P. Cohen (MRC Protein Phosphorylation Unit, University of Dundee, U.K.). The dominant negative IKK-α and -β plasmids and that for dominant negative NIK were kind gifts from Dr D. Goeddel (Tularik Inc., CA, U.S.A.). The 3×NF-κB.enh.luciferase reporter plasmid was a gift from Prof R.T. Hay, (University of St. Andrews, U.K.) as was the plasmid containing the cDNA encoding the GST-tagged truncated N-terminus of IκB (GST-IκBN). The lipofect AMINE used in all transfections was purchased from Life Technologies (Paisley, U.K.). The antibodies for IκB-α, -β and -ε, IKK-α and -β, p42/44 MAP kinase were purchased from Insight Biotechnology (Santa Cruz, Wembley, UK), SB203580 and PD098059 from Calbiochem-Novabiochem (Nottinghamshire, U.K.), LPS from Sigma Aldrich (Dorset, U.K.) and [γ-32P]-ATP (3000 Ci/mmol) from NEN Du-Pont (Hertfordshire, U.K.). All other chemicals were of the highest commercial grade available.
Cell culture
Rat Aortic Smooth Muscle primary cell cultures (RASMC) were established from thoracic aortae from 180 – 200 g Wistar rats by the method of Berk et al. (1989).
Preparation of nuclear extracts
Cells were stimulated with vehicle or agonist as required, harvested in ice-cold PBS (centrifugation 13,000×g, 1 min), and resuspended in 400 μl buffer 1 (in mM): HEPES 10, pH 7.9, KCl 10, EDTA 0.1, EGTA 0.1, DTT 1, PMSF 0.5; (in μg ml−1): leupeptin 10, aprotinin 10, pepstatin 10, then incubated on ice (15 min). Samples were then vortexed at full speed for 10 s following the addition of 25 μl of 10% (v v−1) NP-40 in buffer 1 and cells pelleted by centrifugation (13,000×g, 30 s). The crude nuclear material was then resuspended in 50 μl buffer 2 (in mM): HEPES 20, pH 7.9, (25% (v v−1) glycerol, 0.4 M NaCl), EDTA 1, EGTA 1, DTT 1, PMSF 0.5; (in μg ml−1): leupeptin 10, aprotinin 10, pepstatin 10. Following incubation on ice (15 min) tubes were sonicated (2×30 s) in a bath-type sonicator and extracted nuclear proteins recovered by centrifugation (13,000×g, 15 min at 4°C). The soluble nuclear extract was collected and stored at −80°C until use.
Electrophoretic mobility shift assay
Protein concentrations in the nuclear extracts were determined by the Bradford method and 5 μg incubated with 2 μl of gel-shift binding buffer (5×) (in mM): Tris-HCl 50, pH 7.5, (20% (v v−1) glycerol) MgCl2 5, EDTA 2.5, DTT 2.5, NaCl 250 (0.25 mg/ml poly(dI-dC).poly(dI-dC)), for 15 min at room temperature prior to the addition of 1 μl [γ-32P]-labelled consensus oligonucleotide (approx. 50,000 c.p.m.). Samples were incubated for a further 20 min at room temperature before the addition of 1 μl 10×gel loading buffer (250 mM Tris-HCl, pH 7.5, 0.2% (w v−1) bromophenol blue, 40% (v v−1 glycerol). Samples were subject to non-denaturing electrophoresis on pre-run 5% polyacrylamide gels, dried and protein-DNA complexes detected by autoradiography.
Western-blot analysis
Western blotting of IκB proteins using SDS – PAGE was performed as outlined previously (Paul et al., 1995). All antibodies were titred for optimum conditions.
IKK immunocomplex kinase assay
Cells were incubated with vehicle or agonist as appropriate, washed twice in ice-cold PBS and then lysed with solubilization buffer (in mM): Tris-HCl 20 (pH 7.6), EDTA 1, EGTA 0.5, NaCl 150, (0.1% (w v−1) Brij 35, 1% (w v−1) Triton X-100) sodium fluoride 20, sodium orthovanadate 0.5, β-glycerophosphate 20; (in μg ml−1): aprotinin 10, pepstatin A 10, leupeptin 10, and PMSF 1). Clarified extracts were incubated with 1.5 μg of either IKK-α or IKKβ-specific antisera (Insight Biotechnology, U.S.A.) pre-coupled to protein G agarose (2 h, 4°C) with rotation. Immunocomplexes were collected by centrifugation (13,000×g, 1 min), washed once with solubilization buffer and once with HEPES buffer 25 mM, pH 7.6 containing (mM): β-glycerophosphate 25, NaF 25, MgCl2 15 and DTT 1, before incubation in the same buffer containing 25 μM/5 μCi [γ-32P] ATP and 1 μg of a recombinant GST-fusion protein of the N-terminus of IκB-α (final volume 30 μl, 30 min) at 30°C. Samples were boiled with 4×sample buffer (5 min). Aliquots of each sample were then subjected to electrophoresis on 10% SDS – PAGE gels and fixed in 20% (v v−1) methanol/10% (v v−1) acetic acid for 30 min. After drying phosphorylated IκB was visualized by autoradiography.
JNK, p38 MAP kinase and MAPKAP kinase-2 activity assay
Measurement of JNK p38 MAP kinase and MAPKAP kinase-2 activity was determined by measuring incorporation of [γ-32P]-phosphate into the respective substrates (c-Jun, MAPKAP kinase-2 and substrate peptide KKLNRTLSVA) as outlined previously (Paul et al., 2000).
Luciferase reporter assay
RASMC were transiently transfected according to Gibco manufacturers instructions using lipofectAMINE, with 1 μg well−1 luciferase reporter plasmid (containing three NF-κB binding sites) either alone or co-transfected with dominant negative (DN) IKK-α, -β or NIK. Amounts of DNA were normalized with pRK plasmid. Cells were then serum-starved for 24 h prior to stimulation with either vehicle or 100 μg ml−1 LPS for 8 h. Cells were washed twice with ice-cold PBS and harvested in 200 μl lysis buffer (25 mM Tris phosphate, pH 7.8, 8 mM magnesium chloride, 1 mM DTT, 1% (v v−1) Triton X-100, 15% (v v−1) glycerol). Clarified extracts were then subject to Bradford protein assay and stored at −80°C. Lysis buffer (100 μl) containing 1 mM ATP, 0.25 mM luciferin substrate and 1% (w v−1) BSA was then added to an equal volume of cell lysate and relative light units were measured by a Berthold luminometer over 60 s with a 1 s delay.
Statistical analysis
Graphical data was analysed using a one-way ANOVA followed by a Dunnets post test. *Significantly different from control value (P<0.05).
Results
LPS-stimulated IKK/NF-κB signalling in RASMC
RASMC were exposed to 100 μg ml−1 LPS and NF-κB-DNA binding measured by EMSA (Figure 1A). An increase in DNA-binding was apparent 15 min after LPS stimulation, reaching a maximum by 1 h that was sustained up to 6 h. The expression of the inhibitory proteins IκB-α and -β were also examined over a similar time frame (Figure 1B,C). Results showed that IκB-α was degraded to 38.2±20.3% of control (n=3) at 15 min and maximally by 30 min to 9.6±8.3% of control (n=3), returning to basal values by 90 min. In contrast to this transient loss, IκB-β degradation was not apparent until 30 min post LPS stimulation and did not return fully to basal levels by 8 h. High expression of the ε isoform of IκB was also observed in RASMCs (Figure 1D) and examination of the kinetics of degradation in response to LPS showed initial degradation at 30 min, maximal by 2 h with a return to basal levels by 8 h.
Figure 1.
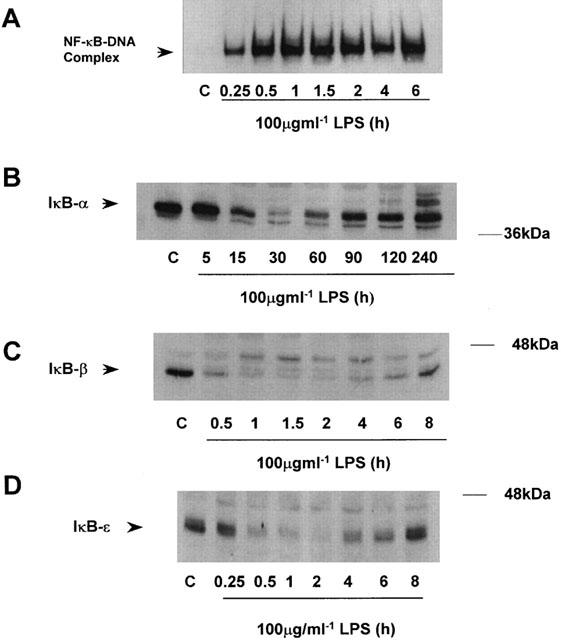
Time course of LPS-stimulated NFκB-DNA binding and IκB degradation in RASMC. Serum starved RASMC were exposed to vehicle (C) or 100 μg ml−1 LPS (L) for the times indicated. NF-κB-DNA binding (A), IκB-α, (B), IκB-β (C) and IκB-ε (D) protein expression were measured as described in the Methods section. The autoradiographs and blots shown are representative of three individual experiments.
The role of IKK and NIK in the regulation of LPS-stimulated NF-κB activation was also assessed. We found that in response to LPS, both IKK-α and -β kinase activities were substantially increased (Figure 2A,B). Both kinases were activated within 15 min of LPS treatment, observed to be maximal activation at 30 min, for IKK-α, and 1 h for IKK-β. Correspondingly, over-expression of DN forms of IKK inhibited LPS-stimulated NF-κB reporter activity (Figure 3A). Alone LPS stimulated a 30 – 40 fold increase in luciferase activity in control transfected cells, however, this stimulation was reduced in a dose dependent manner by (1 – 10 μg) of either DN-IKK-α or DN-IKK-β but not control pRK plasmid. Indeed, LPS stimulated activity was almost completely abolished following co-transfection with 10 μg of either construct. A similar effect was also observed following transfection with DN-NIK (Figure 3B). LPS-stimulated NF-κB reporter activity was inhibited by transfection of increasing amounts of DN-NIK and substantially inhibited (∼80%) following transfection of 10 μg of construct. Similarly, TNF-α-stimulated luciferase activity was also inhibited confirming that the effects of NIK are independent of receptor. Taken together these results suggest that in RASMC, LPS stimulates NF-κB activity through an IKK/NIK-dependent pathway.
Figure 2.
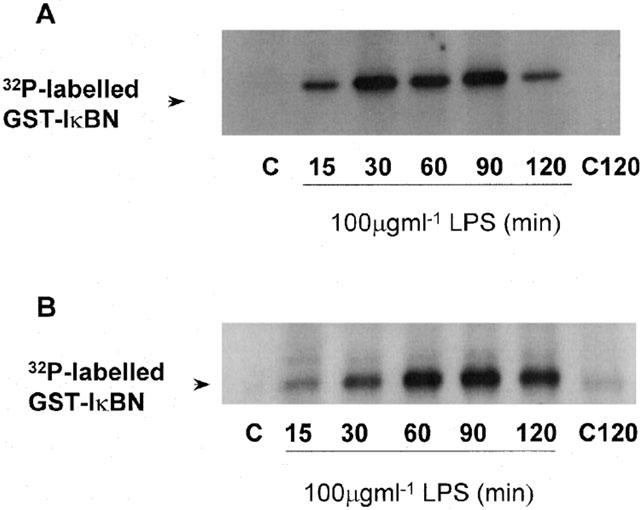
LPS-stimulated kinase activity of IKK-α and -β in RASMC. Serum starved cells were incubated with vehicle (C) or 100 μg ml−1 LPS (L) for the times indicated. IKK-α kinase activity (A) or IKK-β kinase activity (B) was assayed as described in the Methods section. The autoradiographs shown are representative of three individual experiments.
Figure 3.
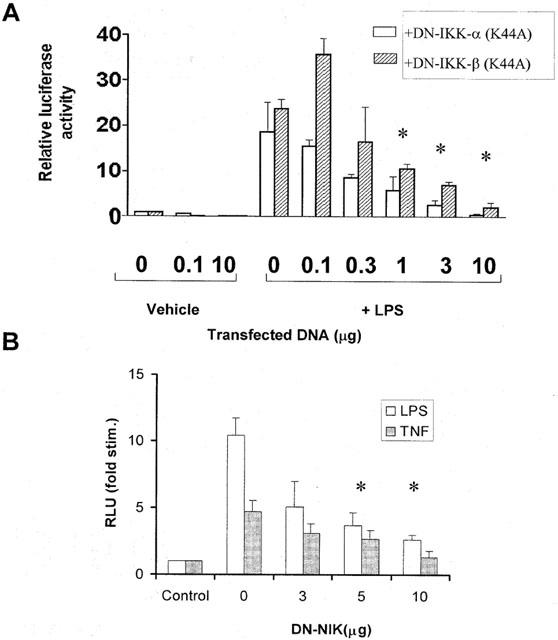
Effect of DN-IKK-α and -β and DN-NIK on LPS-stimulated NF-κB reporter activity. RASMC were co-transfected with 1 μg luciferase reporter plasmid with increasing concentrations of DN-IKK-α or DN-IKK-β (A) or DN-NIK (B) made up to 10 μg total DNA with pRK plasmid as described in the Methods section. Serum starved cells were then incubated with vehicle (C), 100 μg ml−1 LPS (L) or 30 ng ml−1 TNF-α for 8 h. and NF-κB reporter activity measured as described in the Methods section. Data is representative of three individual experiments±s.e.mean. *Statistically significant from stimulation with no DN-DNA P<0.05.
Pervanadate inhibits LPS-stimulated NF-κB-DNA binding via an H2O2-dependent mechanism
In order to confirm the role of a PTPase in the NF-κB pathway cells were preincubated for 30 min with 100 μM of the PTPase inhibitor pervanadate prior to stimulation with LPS (Figure 4). Pervanadate in a manner similar to pretreatment with PAO, another PTPase inhibitor, reversed LPS-stimulated NF-κB-DNA binding. However, when each component of pervanadate H2O2 (1 mM) and orthovanadate (1 mM) was tested alone, H2O2 also reversed LPS-stimulated NF-κB-DNA binding. To investigate whether excess H2O2 present in the reaction mix may have been responsible for the reversal effect of pervanadate, 100 μg ml−1 catalase was added to the orthovanadate and H2O2 mix to degrade any excess H2O2. Preincubation with catalase alone did not have any effect but when added to pervanadate the reversal of LPS-stimulated NF-κB-DNA binding was abrogated (Figure 4).
Figure 4.
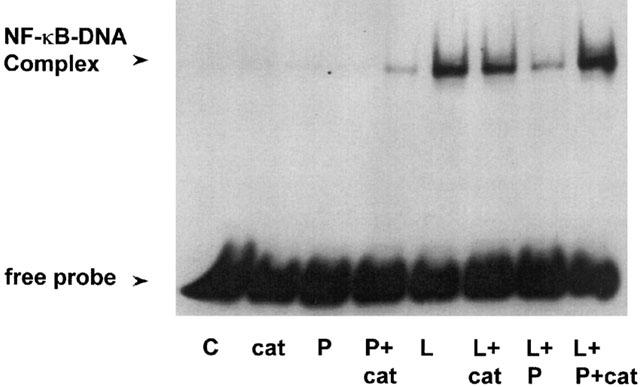
Effect of pervanadate and catalase on LPS-stimulated NF-κB-DNA binding in RASMC. Serum starved cells were incubated with vehicle (C), 100 μM catalase (cat), pervanadate (P) or pervanadate in combination with 100 μg ml−1 catalase (P+cat) alone or 30 min prior to stimulation with 100 μg ml−1 LPS (L). NF-κB-DNA binding was measured as described in the Methods section. The autoradiograph shown represents three separate experiments.
H2O2 reverses LPS-stimulated NF-κB signalling at the level of IKK
The site and mechanism of H2O2-mediated inhibition of LPS-stimulated NF-κB-DNA binding was further examined in Figure 5. Approximately 50% (56.2±8.16%, n=3) reversal of LPS-stimulated DNA-binding was observed at 0.5 mM H2O2 with full reversal of DNA-binding apparent following pre-treatment with 1 mM (Figure 5A). Preincubation of RASMC with 0.5 mM H2O2 also reversed LPS-stimulated IκB-α, -β and -ε degradation by 45.2±26.69%, 16.67±16.12% and 90.6±46.5% respectively, with full reversal observed following pre-treatment with 1 mM H2O2 (Figure 5B and C for IκB-α, and -ε, IκB-β not shown). Similar effects were observed when IKK-α and -β kinase activities were assayed in vitro. Pre-incubation with 0.3 mM or 1 mM H2O2 for 30 min resulted in complete inhibition of LPS-stimulated IKK-α or -β kinase activity (Figure 5D,E).
Figure 5.
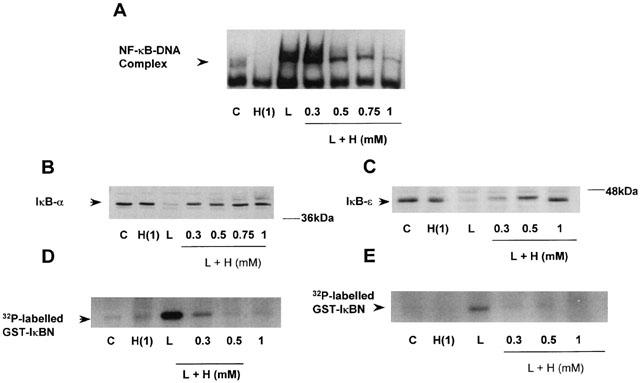
Effect of H2O2 on LPS-stimulated IKK/NF-κB signalling Serum starved cells were pretreated with vehicle (C) or increasing concentrations (as indicated) of H2O2 (H) for 30 min prior to exposure to 100 μg ml−1 LPS (L). NF-κB-DNA binding (A), protein expression of IκB-α (B), and IκB-ε (C) and kinase activity of IKK-α (D) and IKK-β (E) were measured as described in the Methods section. The autoradiographs and blots shown are representative of three individual experiments.
Inhibitory effects of H2O2 are independent of JNK, p38 and p42/44 MAP kinases
Previously, H2O2 has been shown to activate MAP kinase homologues in a number of cell types (Yoshizumi et al., 2000; Abe et al., 2000; Yin et al., 2000). We therefore examined the potential of these kinase cascades to participate in the negative regulation of LPS-stimulated IKK/NF-κB signalling. Figure 6 shows that H2O2 stimulated a rapid increase in p38 MAP kinase activity. A maximal, 10.15±1.62 fold increase (n=3) in kinase activity was observed 30 min post-H2O2 stimulation returning towards basal levels by 2 h H2O2 (Figure 6A). This activity was concentration dependent reaching a maximum between 0.5 – 1 mM (result not shown). In contrast, H2O2-stimulated JNK kinase activity was weak and comparatively slow in onset, with activation only observed at concentrations of 1 mM or above (Figure 6B). Activation of p42/44 MAP kinase by H2O2 was moderate in comparison to 10% (v v−1) foetal calf serum (FCS), generating a partial electrophoretic mobility shift in the retention of either isoform on SDS – PAGE (Figure 6C). However, activation of this signalling pathway was delayed relative to p38 MAP kinase, reaching a peak between 30 and 60 min.
Figure 6.
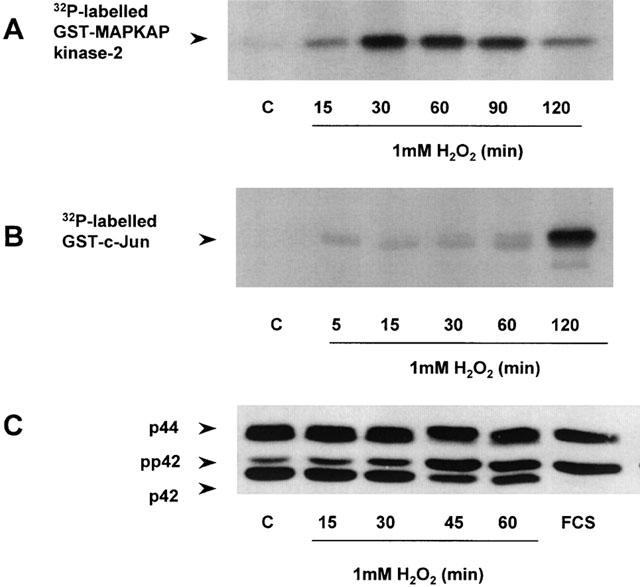
Effect of H2O2 on p38, JNK and p42/44 MAP kinases Serum-starved RASMC were exposed to vehicle (C), FCS (10% v v−1, 5 min) or 1 mM H2O2 for the times indicated. p38 MAP kinase (A) and JNK (B) kinase activities and phosphorylation of p42/44 MAP kinases (C) were determined as outlined in the Methods section. The autoradiographs and blots shown represent three individual experiments.
H2O2 also stimulated activity of the downstream substrate of p38 MAP kinase, MAPKAP kinase-2 (Figure 7) giving maximum 14.69±6.7 fold increase (n=3) in kinase activity at 1 mM. This response was completely inhibited by pre-incubation with 10 or 20 μM of SB203580, a p38 MAP kinase inhibitor, for 30 min (Figure 8). However, this intervention failed to prevent the inhibitory effects of H2O2 at the level of NF-κB-DNA binding (Figure 8A), IκB degradation (Figure 8B,C) or IKK activity (Figure 8D). Similarly, pre-incubation with the MEK kinase inhibitor PD098059 abolished H2O2-stimulated MAP kinase activity but did not reverse LPS-stimulated NF-κB activation (results not shown).
Figure 7.
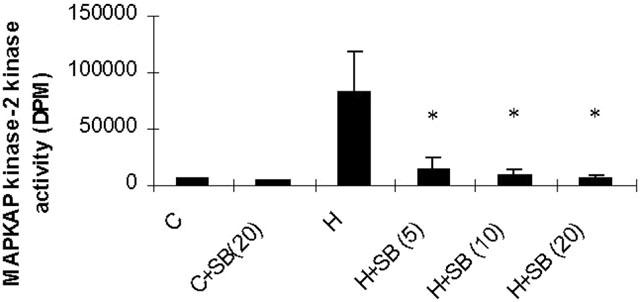
Effect of SB203580 on H2O2-stimulated MAPKAP kinase-2 activity. Serum-starved cells were pre-incubated with vehicle (C) or increasing concentrations of SB203580 (SB) (as indicated in μM) for 30 min prior to exposure to vehicle (C) or 1 mM H2O2. MAPKAP kinase-2 kinase activity was then determined as described in the Methods section. Data shown is representative of three individual experiments and expressed as mean±s.e.mean *Statistically significant from control P<0.05.
Figure 8.
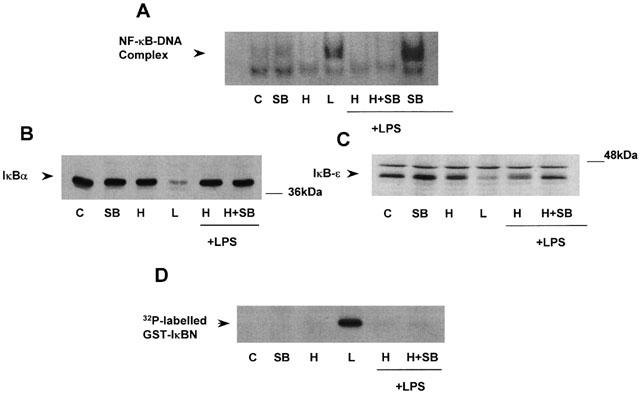
Effect of SB203580 on H2O2 inhibition of LPS-stimulated NF-κB signalling Serum starved cells were exposed to vehicle, (C), 20 μM SB203580, 1 mM hydrogen peroxide (H), 100 μg ml−1 LPS (L) or pre-incubated for 30 min with SB203580 at the concentrations indicated (μM) prior to exposure to vehicle (C), 1 mM hydrogen peroxide (H), 100 μg ml−1 LPS (L). NF-κB-DNA binding (A), IκB-α (B) and -ε (C) degradation and kinase activity of IKK-α (D) were determined as described in the Methods section. The autoradiographs shown are representative of three individual experiments.
Discussion
In this study we investigated the site and mechanism of action of the PTPase inhibitor pervanadate on LPS-stimulated NF-κB activation in rat aortic smooth muscle cells. In initial experiments, LPS was found to stimulate a time-dependent increase in NF-κB-DNA binding and a corresponding increase in NF-κB transcriptional activity. LPS also induced the degradation of both IκB-α and -β proteins. The kinetics of these activities are consistent with that observed in other cytokine receptor systems (Ghosh & Baltimore, 1990; Beg & Baldwin, 1993; Hoshi et al., 2000).
Interestingly, we found, for the first time, high expression of the ε isoform of IκB in RASMC and this isoform was observed to be degraded also following LPS stimulation, with kinetics similar to that of IκB-β degradation. These data concur with the functional characterisation of IκB-ε which has reported its upregulation by known activators of NF-κB, in a manner similar to that of IκB-α. In contrast to IκB-α and -β, the functional activity of IκB-ε has previously been thought to be restricted to the cytoplasm due to its inefficiency in entering the nucleus and is, therefore, thought to mainly sequester p65 homodimers. Therefore, IκB-ε degradation may indicate activation of p65 homodimers of the NF-κB family in addition to p50/p65 (Simeonidis et al., 1997). Indeed, preliminary experiments using supershift analysis show the presence of p65 but not p50 subunits within the nucleus of LPS-stimulated RASMCs, despite p50 and p65 members of the Rel family being expressed in vascular smooth muscle cells (Bellas et al., 1995; Bourcier et al., 1997).
Activation of signalling events upstream of IκB have not been assessed in RASMC in response to LPS. This study revealed activation of both IKK-α and -β kinase activities upon LPS stimulation in a concentration and time-dependant manner. Commonly, characterization of NF-κB in other cell types has revealed IKK isoforms, in particular -α and -β, to be responsible for catalysing phosphorylation of the IκB proteins (Mercurio et al., 1997; Zandi et al., 1997; Geleziunas et al., 1998; Li et al., 1998; O'connell et al., 1998). Recent studies have shown that IKK does not always mediate NF-κB activation. For example, in the case of short-wavelength UV (UV-C), IκB degradation is observed in the absence of IKK activation (Bender et al., 1998; Li & Karin, 1999). An IKK-independent mechanism has also been shown for the opportunistic pathogen Pseudomonas aeruginosa, which utilises a c-Src-Ras-MEK1/2-MAP kinase-pp90rsk pathway to activate NF-κB (Li et al., 1998). However, in this study, co-transfection with DN-IKK-α and DN-IKK-β alone or in combination (data not shown) reduced LPS-stimulated NF-κB reporter activity in a concentration-dependant manner showing for the first time that LPS-stimulated NF-κB activation in RASMC is mediated via IKK-α and -β. Although 0.1 μg IKK-β DNA showed a significant increase in reporter activity this could be due to the mechanisms by which IKK is regulated. The IKKs form both homo and heterodimers and in unstimulated cells IKK-α inhibits constitutive IKK-β activity and in activated cells IKK-α kinase activity must be present to induce IKK-β kinase activity (O'mahony et al., 2000). Thus it is possible that an imbalance in the α/β ratio by addition of 0.1 μg IKK-β DNA may have resulted in enhanced activation of the complex and hence activation of NF-κB-dependent luciferase reporter activity. Furthermore, we also confirmed that LPS is likely to stimulate IKK activity via a NIK-dependent cascade as LPS-stimulated NF-κB reporter activity was abolished by transfection with increasing concentrations of DN-NIK (Figure 3). This suggests that the receptor for LPS in RASMC is also likely to involve similar intermediates to those observed in macrophages such as IRAK, MyD88 and TRAFs, which link cytokine receptors to IKK through intermediate kinases such as NIK (Hultmark, 1994; Muzio et al., 1998; Yang et al., 1998). These possibilities are currently being examined in our laboratory.
The tyrosine phosphatase inhibitor, pervanadate, which prevents conversion of an essential cysteine residue at the catalytic site of PTPases to a thiol phosphate intermediate within the PTPase (Guan & Dixon, 1991) was also found to cause inhibition of LPS-stimulated NF-κB activity. This is in agreement with a number of recent studies. However, as the inhibitory effect of pervanadate was abolished by increasing concentrations of catalase it would appear that the effect could be attributed to excess H2O2 in the reaction mixture. Many studies have neglected to investigate this H2O2-mediated action of pervanadate with only a few studies using catalase (Huyer et al., 1997), however, our studies suggest major limitations in it's use as a PTPase inhibitor in RASMC. Combining orthovanadate and H2O2 forms a mix containing not only various peroxovanadium complexes which are themselves potent oxidizing agents but also unreacted vanadate and H2O2. It is likely that these components can mediate both stimulatory and inhibitory effects upon NF-κB signalling independent of any effects upon tyrosine phosphatase activity.
Despite the complexities of using pervanadate our present study clearly demonstrates for the first time, in vascular smooth muscle cells, H2O2-mediated inhibition of LPS-stimulated NF-κB activation, manifest at the level of IKK or further upstream. Furthermore, as TNF-α-stimulated NF-κB signalling was affected in a similar manner by pervanadate, this may indicate its mode of action at a site upstream of IKK that is common to both receptors. These findings contrast with a large number of studies which show H2O2 to induce NF-κB activation in several cell lines including HeLa cells, cardiac myocytes and Jurkat T-lymphocytes (Schreck et al., 1991; Meyer et al., 1993; Peng et al., 1995; Manna & Aggarwal, 1999; Bowie & O'neill, 2000). More recent evidence has however, suggested that the effect of H2O2 may be cell-type specific; H2O2 does not activate NF-κB in thyoma, epidermal or various bronchial epithelial cell types (Das et al., 1995; Brennan & O'neill, 1995; Warner et al., 1996), whilst studies in mouse mesangial cells have shown that O2− but not H2O2 stimulates NF-κB activation (Satriano & Schlondroff, 1994). Reasons for this emerging cell-type specificity of H2O2 may be varying levels of intracellular catalase or other ROI scavengers such as GSH or NAC (Meister & Anderson, 1983; Aruoma et al., 1989). Alternatively, it may suggest the presence of diverse H2O2-sensitive signalling components between cell types.
An important consideration regarding the effects of H2O2 was the potential for cross-talk with other pathways which can regulate NF-κB activity. In a number of cell types H2O2 is a strong activator of the classical MAP kinase homologues p42/p44 as well as the stress-activated protein kinases, p38 MAP kinase and JNK (Clerk et al., 1998; Bae et al., 1999; Abe et al., 2000). Additionally, it is well recognized that JNK and p38 MAP kinases are activated in response to a variety of cellular stresses including LPS. It is, therefore, conceivable that MAP kinase homologues may be involved in mediating the H2O2-induced inhibition of the LPS response. This concept has been investigated in a recent study showing that H2O2 inhibited TNF-induced IκB phosphorylation and degradation in HT-29 cells via p38 MAP kinase activation (Alpert et al., 1999).
Our data did not, however, support such a proposal. Whilst H2O2 strongly activated the kinase activity of p38 MAP kinase and correspondingly MAPKAP kinase-2, abrogation of this pathway using SB203580 failed to modulate the inhibitory effects of H2O2 on LPS-stimulated IKK/IκB/NF-κB signalling. Furthermore, other agents which have been shown previously to activate p38 MAP kinase, such as PDGF and angiotensin-II (A-II) (Ushio-Fukai et al., 1998; Meloche et al., 2000), did not mimic this inhibition, despite A-II also being able to cause the generation of reactive oxygen intermediates. Whilst H2O2 stimulated JNK activation, this was very weak and as such was unlikely to mediate the effects of these agents. In addition, whilst we also found that H2O2 activated p42/44 MAP kinase the response was moderate and somewhat delayed relative to the rapid effects upon NF-κB signalling. Finally, pharmacological inhibition of MAP kinase activation using PD098059 did not result in the reversal of the inhibitory effects of H2O2 (results not shown). This clearly excludes a role for any homologues of MAP kinase in this effect and indicates a role for another pathway or indeed a direct pharmacological effect upon one of the components higher upstream in the cascade.
The data presented in this study may have some pathophysiological relevance as in a variety of disease states reactive oxygen species are generated. It has been proposed that these types of intermediate act as second messengers to induce proinflammatory or growth-stimulatory signals. This has led to the current dogma suggesting oxidative stress to be linked to chronic inflammation which underlies a number of disease states including atherosclerosis (Speir et al., 1996; Fei et al., 2000), renal toxicity (Zhang et al., 2000) and septic shock. Our studies suggest that in certain cases, for example in RASMC, ROS may inhibit the expression of inflammatory genes thus aiding in the recovery of an inflammatory-based disease.
In conclusion we have demonstrated for the first time a role for both IKK and NIK in the regulation of NF-κB activation in response to LPS in cultures of rat aortic smooth muscle cells (RASMCs). We have also established a novel mechanism of inhibition of this pathway by hydrogen peroxide which involves a direct pharmacological effect rather than any cross-talk mechanism involving other signal transduction pathways.
Acknowledgments
The authors wish to acknowledge the kind gifts of reagents from various laboratories. This work was sponsored by the British Heart Foundation. Lindsay J. Torrie is a recipient of an A.J. Clark British Pharmacological Society Prize studentship.
Abbreviations
- COX-2
cyclo-oxygenase-2
- ERK
extracellular signal regulated kinase
- FCS
foetal calf serum
- GSH
glutathione
- HEK cells
Human Embyonic Kidney cells
- H2O2
hydrogen peroxide
- IκB
inhibitory kappa B
- IKK
inhibitory kappa B kinase
- iNOS
inducible nitric oxide synthase
- IRAK
interleukin-1 receptor-associated kinase
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- MAP kinase
mitogen-activated protein kinase
- MAPKAP kinase-2
mitogen-activated protein kinase-activated protein kinase-2
- MEK kinase
mitogen-activated protein kinase kinase kinase
- NAC
N-acetylcysteine
- NF-κB
nuclear factor kappa B
- NIK
NF-κB-inducing kinase
- PDGF
platelet-derived growth factor
- PAO
phenylarsine oxide
- PTPase
protein tyrosine phosphatase
- RASMC
rat aortic smooth muscle cells
- TAK-1
TGF-β-activated kinase
- TNF-α
tumour necrosis factor-α
- TRAF
TNF-receptor-associated factor
References
- ABE J., OKUDA M., HUANG Q., YOSHIZUMI M., BERK B.C. Reactive oxygen species activate p90 ribosomal S6 kinase via Fyn and Ras. J. Biol. Chem. 2000;275:1739–1748. doi: 10.1074/jbc.275.3.1739. [DOI] [PubMed] [Google Scholar]
- AKARASEREENONT P., MITCHELL J.A., APPLETON I., THIEMERMANN C., VANE J.R. Involvement of tyrosine kinase in the induction of cyclo-oxygenase and nitric oxide synthase by endotoxin in cultured cells. Br. J. Pharmacol. 1994;114:1522–1528. doi: 10.1111/j.1476-5381.1994.tb17169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALPERT D., SCHWENGER P., HAN J., VILCEK J. Cell stress and MKK6b-mediated p38 MAP kinase activation inhibit tumor necrosis factor-induced IκB phosphorylation and NF-κB activation. J. Biol. Chem. 1999;274:22176–22183. doi: 10.1074/jbc.274.32.22176. [DOI] [PubMed] [Google Scholar]
- ARUOMA O.I., HALLIWELL B., HOEY B.M., BUTLER J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Rad. Biol. Med. 1989;6:593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- BAE G.-U., SEO D.-W., KWON H.-K., LEE H.Y., HONG S., LEE Z.-W., HA K.-S., LEE H.-W., HAN J.-W. Hydrogen peroxide activates p70(S6k) signaling pathway. J. Biol. Chem. 1999;274:32596–32602. doi: 10.1074/jbc.274.46.32596. [DOI] [PubMed] [Google Scholar]
- BAEUERLE P.A., BALTIMORE D. Molecular Aspects of Cellular Regulation: Hormonal Control Regulation of Gene Transcription 1991Amsterdam: Elsevier/North Holland Biomed; 409–432.ed. Cohen, P. & Foulkes, J.G. pp [Google Scholar]
- BAEUERLE P.A., BALTIMORE D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- BALDWIN A.S. The NF-kappa B and I kappa B proteins: new discoveries and insights. Ann. Rev. Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- BEG A.A., BALDWIN A.S. , JR The IκB proteins: multifunctional regulators of the Rel/NF-κB transcription factors. Genes Devel. 1993;7:2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- BEG A.A., BALTIMORE D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- BELLAS R.E., LEE J.S., SONENSHEIN G.E. Expression of a constitutive NF-κB-like activity is essential for proliferation of cultured bovine vascular smooth muscle cells. J. Clin. Invest. 1995;96:2521–2527. doi: 10.1172/JCI118313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENDER K., GOTTLICHER M., WHITESIDE S., RAHMSDORF H.J., HERRLICH P. Sequential DNA damage-independent and -dependent activation of NF-kappaB by UV. EMBO J. 1998;17:5170–5181. doi: 10.1093/emboj/17.17.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERK B.C., VEKSHTEIN V., GORDON H.M., TSUDA T. Angiotensin II-stimulated protein synthesis in cultured vascular smooth muscle cells. Hypertension. 1989;13:305–314. doi: 10.1161/01.hyp.13.4.305. [DOI] [PubMed] [Google Scholar]
- BOURCIER T., SUKHOVA G., LIBBY P. The Nuclear Factor κ-B Signalling pathway participates in dysregulation of vascular smooth muscle cells in vitro and in human atherosclerosis. J. Biol. Chem. 1997;272:15817–15824. doi: 10.1074/jbc.272.25.15817. [DOI] [PubMed] [Google Scholar]
- BOWIE A., O'NEILL L.A.J. Oxidative stress and nuclear factor-κB activation - a reassessment of the evidence in the light of recent discoveries. Biochem. Pharmacol. 2000;59:13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- BRENNAN P., O'NEILL L.A.J. Effects of oxidants and antioxidants on nuclear factor kappa β activation in three different cell lines: Evidence against a universal hypothesis involving oxygen radicals. Biochim. Biophys. Acta. 1995;1260:167–175. doi: 10.1016/0167-4781(94)00186-7. [DOI] [PubMed] [Google Scholar]
- CAIVANO M., COHEN P. Role of mitogen-activated protein kinase cascades in mediating lipopolysaccharide-stimulated induction of cyclooxygenase-2 and IL-1 beta in RAW264 macrophages. J. Immunol. 2000;164:3018–3025. doi: 10.4049/jimmunol.164.6.3018. [DOI] [PubMed] [Google Scholar]
- CLERK A., FULLER S.J., MICHAEL A., SUGDEN P.H. Stimulation of “stress-regulated” mitogen-activated protein kinases (stress-activated protein kinases/c-Jun N-terminal kinases and p38-mitogen-activated protein kinases) in perfused rat hearts by oxidative and other stresses. J. Biol. Chem. 1998;273:7228–7234. doi: 10.1074/jbc.273.13.7228. [DOI] [PubMed] [Google Scholar]
- CORDLE S.R., DONALD R., READ M.A., HAWIGER J. Lipopolysaccharide induces phosphorylation of MAD-3 and activation of c-rel and related NF-κB proteins in human THP-1 cells. J. Biol. Chem. 1993;268:11803–11810. [PubMed] [Google Scholar]
- DAS K.C., LEWIS-MOLOCK Y., WHITE C.W. Activation of NF-κB and elevation of MNSOD gene-expression by thiol reducing agents in lung adenocarcinoma (A549) cells. Am. J. Physiol. 1995;269:L588–L602. doi: 10.1152/ajplung.1995.269.5.L588. [DOI] [PubMed] [Google Scholar]
- DEVI MENON S., GUY G.R., TAN Y.H. Involvement of a putative protein-tyrosine phosphatase and IκBα Serine phosphorylation in nuclear factor κB activation by tumor necrosis factor. J. Biol. Chem. 1995;270:18881–18887. doi: 10.1074/jbc.270.32.18881. [DOI] [PubMed] [Google Scholar]
- DIDONATO J.A., HAYAKAWA M., ROTHWARF D.M., ZANDI E., KARIN M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- FEI J., VIEDT C., SOTO U., ELSING C., JAHN L., KREUZER J. Endothelin-1 and smooth muscle cells: induction of jun amino-terminal kinase through an oxygen radical-sensitive mechanism. Arterioscl. Thromb. Vasc. Biol. 2000;20:1244–1249. doi: 10.1161/01.atv.20.5.1244. [DOI] [PubMed] [Google Scholar]
- FENG G.J., GOODRIDGE H.S., HARNETT M.M., WEI X.Q., NIKOLAEV A.V., HIGSON A.P., LIEW F.Y. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages. Leishmania phosphoglycons subvert macrophage IL-12 production by targeting ERK MAP kinase. J. Immunol. 1999;163:6403–6412. [PubMed] [Google Scholar]
- FISCHER C., PAGE S., WEBER M., EISELE T., NEUMEIER D., BRAND K. Differential effects of lipopolysaccharide and tumor necrosis factor on monocytic IκB kinase signalsome activation and IκB proteolysis. J. Biol. Chem. 1999;274:24625–24632. doi: 10.1074/jbc.274.35.24625. [DOI] [PubMed] [Google Scholar]
- GELEZIUNAS R., FERRELL S., LIN X., MU Y., CUNNINGHAM E.J., GRANT M., CONNELLY M.A., HAMBOR J.E., MARCU K.B., GREENE W.C. Human T-cell leukaemia virus type 1 Tax induction of NF-κB involves activation of the IκB kinase alpha (IKKα) and IKKβ cellular kinases. Mol. Cell. Biol. 1998;18:5157–5165. doi: 10.1128/mcb.18.9.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHOSH S., BALTIMORE D. Activation in vitro of NF-κB by phosphorylation of its inhibitor IκB. Nature (London) 1990;344:678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- GUAN K., DIXON J.E. Evidence for protein-tyrosine-phosphatase catalysis proceeding via a cysteine-phosphate intermediate. J. Biol. Chem. 1991;266:17026–17030. [PubMed] [Google Scholar]
- HAWIGER J., VEACH R.A., LIU X.Y., TIMMONS S., BALLARD D.W. IkappaB kinase complex is an intracellular target for endotoxic lipopolysaccharide in human monocytic cells. Blood. 1999;94:1711–1716. [PubMed] [Google Scholar]
- HOSHI S., GOTO M., KOYAMA N., NOMOTO K., TANAKA H. Regulation of vascular smooth muscle cell proliferation by nuclear factor kappa β and it's inhibitor, IκB. J. Biol. Chem. 2000;275:883–889. doi: 10.1074/jbc.275.2.883. [DOI] [PubMed] [Google Scholar]
- HULTMARK D. Macrophage differentiation marker MyD88 is a member of the Toll/IL-1 receptor family. Biochem. Biophys. Res. Commun. 1994;199:144–146. doi: 10.1006/bbrc.1994.1206. [DOI] [PubMed] [Google Scholar]
- HUYER G., LIU S., KELLY J., MOFFAT J., PAYETTE P., KENNEDY B., TSAPRAILIS G., GRESSER M.J., RAMACHANDRAN C. Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate. J. Biol. Chem. 1997;272:843–851. doi: 10.1074/jbc.272.2.843. [DOI] [PubMed] [Google Scholar]
- IRIE T., MUTA T., TAKESHIGE K. TAK1 mediates an activation signal from toll-like receptor(s) to nuclear factor-kappaB in lipopolysaccharide-stimulated macrophages. FEBS Lett. 2000;467:160–164. doi: 10.1016/s0014-5793(00)01146-7. [DOI] [PubMed] [Google Scholar]
- JULOU-SCHAEFFER G., GRAY G.A., FLEMING E., SCHOTT C., PARRAT J.R., STOCLET J.-C. Loss of vascular responsiveness induced by endotoxin involves L-arginine pathway. Am. J. Physiol. 1990;259:H1038–H1043. doi: 10.1152/ajpheart.1990.259.4.H1038. [DOI] [PubMed] [Google Scholar]
- KARIN M., DELHASE M. JNK or IKK, AP-1 or NF-κB, which are targets for MEK kinase 1 action. Proc. Natl. Acad. Sci. U.S.A. 1998;95:9067–9069. doi: 10.1073/pnas.95.16.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENARDO M.J., BALTIMORE D. NF-κB: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989;58:227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- LI J.-D., FENG W., GALLUP M., KIM J.-H., GUM J., KIM Y., BASBAUM C. Activation of NF-κB via a Src-dependent Ras-MAPK-pp90rsk pathway is required for Pseudomonas aeruginosa-induced mucin overproduction in epithelial cells. Proc. Natl. Acad. Sci. 1998;95:5718–5723. doi: 10.1073/pnas.95.10.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI N., KARIN M. Is NF-kappaB the sensor of oxidative stress. FASEB J. 1999;13:1137–1143. [PubMed] [Google Scholar]
- LOEGERING D.J., RICHARD C.A.H., DAVIDSON C.B., WIRTH G.A. Diethyldithiocarbamate amerliorates the effect of lipopolysaccharide on both increased nitrite production by vascular smooth muscle cells and decreased contractile response of aortic rings. Life Sci. 1995;57:169–176. doi: 10.1016/0024-3205(95)00257-7. [DOI] [PubMed] [Google Scholar]
- LYONS C.R., ORLOFF G.J., CUNNINGHAM J.M. Molecular cloning and functional expression of an inducible nitric oxide synthase from a murine macrophage cell line. J. Biol. Chem. 1992;267:6370–6374. [PubMed] [Google Scholar]
- MAEKAWA K., IMAGAWA N., NAITO A., HARADA S., YOSHIE O., TAKAGI S. Association of protein-tyrosine phosphatase PTP-BAS with the transcripton factor-inhibitory protein IκBα through interaction between the PDZ1 domain and ankyrin repeats. Biochem. J. 1999;337:179–184. [PMC free article] [PubMed] [Google Scholar]
- MANNA S.K., AGGARWAL B.B. Lipopolysaccharide inhibits TNF-induced apoptosis: Role of nuclear factor-κB activation and reactive oxygen intermediates. J. Immunol. 1999;162:1510–1518. [PubMed] [Google Scholar]
- MEDZHITOV R., PRESTON-HURLBURT P., KOPP E., STADLEN A., CHEN C., GHOSH S., JANEWAY C.A. , JR MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- MEISTER A., ANDERSON M.E. Glutathione. Ann. Rev. Biochem. 1983;5:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- MELOCHE S., LANDRY J., HUOT J., HOULE F., MARCEAU F., GAISSON E. p38 MAP kinase pathway regulates angiotensin II-induced contraction of rat vascular smooth muscle. Am. J. Physiol. 2000;279:H741–H751. doi: 10.1152/ajpheart.2000.279.2.H741. [DOI] [PubMed] [Google Scholar]
- MENG X., BROWN J.M., AO L., NORDEEN S.K., FRANKLIN W., HARDEN A.H., BANERJEE A. Endotoxin induces cardiac HSP70 and resistance to endotoxemic myocardial depression in rats. Am. J. Physiol. 1996;271:C1316–C1324. doi: 10.1152/ajpcell.1996.271.4.C1316. [DOI] [PubMed] [Google Scholar]
- MERCURIO F., ZHU H., MURRAY B.W., SHEVCHENKO A., BENNET B., LI J.W., YOUNG D., BARBOSA M., MANN M., MANNING A., RAO A. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;281:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- MEYER M., SCHRECK R., BAEUERLE P.A. H202 and antioxidants have opposite effects on activation of NF-κB and AP-1 in intact cells - AP-1 as secondary antioxidant-responsive factor. EMBO J. 1993;12:2005–2015. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUZIO M., NATOLI G., SACCANI S., LEVRERO M., MANTOVANI A. The human toll signalling pathway: divergence of nuclear factor kappa-β and JNK/SAPK activation upstream of tumour necrosis factor receptor-associated factor 6 (TRAF6) J. Exp. Med. 1998;187:2097–2101. doi: 10.1084/jem.187.12.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'CONNELL M.A., BENNET B.L., MERCURIO F., MANNING A.M., MACKMAN N. Role of IKK1 and IKK2 in lipopolysaccharide signalling in human monocytic cells. J. Biol. Chem. 1998;273:30410–30414. doi: 10.1074/jbc.273.46.30410. [DOI] [PubMed] [Google Scholar]
- O'MAHONY A., LIN X., GELEZIUNAS R., GREENE W.C. Activation of the heterodimeric IκB kinase-α (IKK-α)-IKK-β complex is directional: IKK-α regulates IKK-β under both basal and stimulated conditions. Mol. Cell. Biol. 2000;20:1170–1178. doi: 10.1128/mcb.20.4.1170-1178.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAUL A., PENDREIGH R.H., PLEVIN R. Protein kinase C and tyrosine kinase pathways regulate lipopolysaccharide-induced nitric oxide synthase activity in RAW 264.7 murine macrophages. Br. J. Pharmacol. 1995;114:482–488. doi: 10.1111/j.1476-5381.1995.tb13252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAUL A., TORRIE L.J., MCLAREN G.J., KENNEDY C., GOULD G.W., PLEVIN R. P2Y Receptor-mediated inhibition of tumor necrosis factor-α stimulated stress-activated protein kinase activity in EAhy926 endothelial cells. J. Biol. Chem. 2000;275:13243–13249. doi: 10.1074/jbc.275.18.13243. [DOI] [PubMed] [Google Scholar]
- PENG M., HUANG L.Y., XIE Z.J., HUANG W.H., ASKARI A. Oxidant-induced activations of nuclear factor-kappa-B and activator protein-1 in cardiac myocytes. Cell. Mol. Biol. Res. 1995;41:189–197. [PubMed] [Google Scholar]
- REGNIER C.H., YEONG SONG H., GAO X., GOEDDEL D.V. Identification and characterisation of an IκB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- SATRIANO J., SCHLONDROFF D. Activation and attenuation of transcription factor NF-κB in mouse glomerular mesangial cells in response to tumour necrosis factor-alpha, immnoglobulin G, and adenosine 3′:5′-cyclic monophosphate evidence for involvement of reactive oxygen species. J. Clin. Invest. 1994;94:1629–1636. doi: 10.1172/JCI117505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHRECK R., RIEBER P., BAEUERLE P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMADA T., KAWAI T., TAKEDA K., MATSUMOTO M., INOUE J., TATSUMI Y., KANAMARU A., AKIRA S. IKK-i, a novel lipopolysaccharide-inducible kinase that is related to IkappaB kinases. Int. Immunol. 1999;11:1357–1362. doi: 10.1093/intimm/11.8.1357. [DOI] [PubMed] [Google Scholar]
- SIMEONIDIS S., LIANG S., CHEN G., THANOS D. Cloning and functional characterisation of mouse IκBε. Proc. Nat. Acad. Sci. U.S.A. 1997;26:14372–14377. doi: 10.1073/pnas.94.26.14372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPEIR E., SHIBUTANI T., YU Z.X., FERRANS V., EPSTEIN E. Role of reactive oxygen intermediates in cytomegalovirus gene expression and in the response of human smooth muscle cells to viral infection. Circ. Res. 1996;6:1143–1152. doi: 10.1161/01.res.79.6.1143. [DOI] [PubMed] [Google Scholar]
- USHIO-FUKAI M., ALEXANDER R.W., AKERS M., GRIENDLING K.K. p38 Mitogen-activated protein kinase is a critical component of the redox-sensitive signalling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J. Biol. Chem. 1998;273:15022–15029. doi: 10.1074/jbc.273.24.15022. [DOI] [PubMed] [Google Scholar]
- WARNER B.B., GEBB S.L., WISPE J.R. Redox regulation of manganese superoxide dismutase. Am. J. Physiol. 1996;271:L150–L158. doi: 10.1152/ajplung.1996.271.1.L150. [DOI] [PubMed] [Google Scholar]
- XIE Q.-W., CHO H.J., CALAYCAY J., MUMFORD R.A., SWIDEREK K.M., LEE T.D., DING A., TROSO T., NATHAN C. Cloning and characterisation of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256:225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- YANG R.B., MARK M.R., GRAY A., HUANG A., XIE M.H., ZHANG M., GODDARD A., WOOD W.I., GURNEY A.L., GODOWSKI P.J. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;395:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- YIN Z., IVANOV V.N., HABELHAH H., TEW K., RONAI Z. Glutathione S-transferase p elicits protection against H2O2-induced cell death via coordinated regulation of stress kinases. Cancer Res. 2000;60:4053–4057. [PubMed] [Google Scholar]
- YOSHIZUMI M., ABE J., HAENDELER J., HUANG Q., BERK B.C. Src and Cas mediate JNK activation but not ERK1/2 and p38 kinases by reactive oxygen species. J. Biol. Chem. 2000;275:11706–11712. doi: 10.1074/jbc.275.16.11706. [DOI] [PubMed] [Google Scholar]
- ZANDI E., ROTHWARF D.M., DELHASE M., MAYAKAWA M., KARIN M. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- ZHANG C., WALKER L.M., MAYRUEX P.R. Role of nitric oxide in lipopolysaccharide-induced oxidant stress in the rat kidney. Biochem. Pharmacol. 2000;59:203–209. doi: 10.1016/s0006-2952(99)00324-x. [DOI] [PubMed] [Google Scholar]


