Abstract
In this study we administered nociceptin/orphanin FQ (NC) ionotophoretically onto neurons located in functionally distinct thalamic structures of urethane-anesthetized rats. Extracellular single unit recordings were made in the medial and lateral ventroposterior nucleus, posterior thalamic nucleus, zona incerta, lateral posterior nucleus, laterodorsal nucleus, ventrolateral nucleus and reticular nucleus.
NC decreased the firing rate in 60% of thalamic neurons. This decrease in firing rate was accompanied by a significant reduction in the number of high threshold bursts.
In about 20% of the neurons NC increased the firing rate. In most cells NC-induced increases in discharge rate could be blocked by the GABAA receptor antagonists bicuculline and SR 95531.
The NC receptor ligands [Phe1Ψ(CH2-NH)Gly2] nociceptin(1-13)NH2, Ac-RYYRIK-NH2 and [Nphe1]NC(1-13)NH2 were also evaluated. All these peptides inhibited NC-induced changes in firing rate. In addition, in some neurons where NC inhibited firing, [Nphe1]NC(1-13)NH2 and Ac-RYYRIK-NH2 elicited per se an increase in firing rate, suggesting the existence of tonic innervation of thalamic neurons by NC-containing fibres.
In NC-inhibited neurons nocistatin induced a significant increase in firing rate.
The present study demonstrated that NC regulates various thalamic nuclei related not only to somatosensory, but also to the visual and motor functions.
Keywords: Thalamus, nociceptin/orphanin F/Q, [Phe1Ψ(CH2-NH)Gly2]Nociceptin(1-13)NH2, Ac-RYYRIK-NH2, [Nphe1]NC(1-13)NH2, bicuculline, iontophoresis, urethane
Introduction
Over the last few years, a new G-protein-coupled receptor (ORL1) that shows high homology with opioid receptors, but is not activated by the known opioid ligands, has been identified and cloned. In 1995 its endogenous ligand was simultaneously identified by two groups (Meunier et al., 1995; Reinscheid et al., 1995). Despite consistent inhibitory actions of NC at the cellular level in various brain sites (Moran et al., 2000), the effect of NC on pain behaviour remains controversial.
The thalamus, especially the somatosensory thalamus, is involved in pain perception (Willis, 1997). Somatic sensory information is relayed to the cortex through major thalamic nuclei, the medial ventroposterior nucleus (VPM), the lateral ventroposterior nucleus (VPL), and the posterior thalamic nucleus (PO). In the rat the former two nuclei are also referred to as the ventrobasal complex. Whereas the VPL receives inputs from dorsal column nuclei and the spinal cord, the VPM receives information from trigeminal nuclei (Price, 1995). The zona incerta (ZI) is also involved in somatosensory processing (Nicolelis et al., 1992) in addition to other functions (Kim et al., 1992; Spencer et al., 1988). The lateral posterior nucleus (LP) as well as the laterodorsal nucleus (LD) receives fibres from several vision-related structures. The ventrolateral nucleus (VL) is thought to be a motor nucleus, relaying activity from the cerebellum (Price, 1995). Each of these nuclei are connected to a restricted sector of the reticular nucleus (Rt), which is composed of GABAergic cells that receive synapses from both thalamocortical and corticothalamic fibres (Jones, 1985). In situ hybridization analysis in rats has revealed that NC receptor mRNA is expressed in all these different thalamic nuclei (Neal et al., 1999).
In the present study we have evaluated the effects of NC on spontaneous firing rates and burst patterns in these thalamic nuclei. Furthermore, we have attempted a pharmacological characterization of the receptor mediating the effects of NC using the ORL-1 receptor ligands [Phe1Ψ(CH2-NH)Gly2]Nociceptin(1-13)NH2 (F/G) (Guerrini et al., 1998) and Acetyl-Arg-Tyr-Tyr-Arg-Ile-Lys-NH2 (AcRNH2) (Dooley et al., 1997; Berger et al., 1999; 2000a), which have been shown to exert both agonist and antagonist activity in different in vitro and in vivo systems (Calo' et al., 2000a), and the recently identified selective and pure ORL-1 receptor antagonist, [Nphe1]NC(1-13)NH2 (Nphe) (Guerrini et al., 2000). In addition, the actions of nocistatin, a biologically active peptide derived from the same precursor as NC (Okuda Ashitaka et al., 1998) were evaluated on thalamic firing rate and compared with NC-induced effects.
Methods
Maintenance and preparation of animals
All procedures performed on adult male rats (Wistar, 300 – 400 g) were carried out according to the standards of animal welfare and were approved by the Berlin regional animal ethics committee (G 0056/97). Rats were housed five to a cage with food and water available ad libitum and on a diurnal light – dark cycle. All testing took place during daylight hours.
Rats were anaesthetized with urethane (1.2 g kg−1, i.p.) and placed in a stereotaxic frame. Throughout the recording sessions, depth of anaesthesia was confirmed by the lack of withdrawal reflex to pinching of the hindlimbs and by the absence of corneal reflexes. Further injections of urethane were administered as required (for detail see Albrecht & Davidowa, 1989). Rectal temperature was maintained at 37 – 38°C using a heating pad. Electrocardiogram as well as EEG from the visual cortex was monitored. A small hole was drilled into the skull at a site 2.5 mm lateral to the midline suture and 5.0 mm anterior to the lambdoid suture. An electrode was lowered 3.5 to 6 mm with a microdrive through the hole to the level of the ventrobasal thalamus.
Recording
Glass microelectrodes for extracellular recording were filled with saturated Trypan blue solution (tip resistance 10 – 30 MΩ). Recorded action potentials displayed on an oscilloscope were analysed, after passing a window discriminator (World Precision Instruments, Sarasota, U.S.A.), with custom-made software (Spike2 from Cambridge Electronic Design, U.K.) running on a personal computer. Standardized pulses corresponding to individual action potentials from a single neuron were used for computing frequency time histograms, which were displayed on-line during sampling. Data were stored on disk for subsequent analysis.
Drugs and iontophoresis
Electrodes for iontophoresis were prepared from 5- or 7-barrel micropipettes (World Precision Instruments, Sarasota, U.S.A.) with a horizontal puller (P-87, Sutter Instr. Co.), and the tips were broken under microscopic visualization (tip diameter 5 – 7 μm). Micropipette assembly was glued to the recording electrode with a tip separation of 20 – 40 μm.
Drugs were ejected with positive currents by iontophoresis: NC (100 μM, pH 4; Bachem; ORL-1 agonist); F/G (1 mM, pH 4; Tocris; ORL-1 antagonist); AcRNH2 (1 mM, pH 4; from our institutes; ORL-1 antagonist), and Nphe (3 mM; pH 4; from our institutes; ORL-1 antagonist); nocistatin (100 μM, pH 4; Bachem); (−)-bicuculline methiodide (5 mM, pH 3; Research Biochemicals International, GABAA antagonist); SR 95531 (5 mM in 150 mM NaCl, pH 3.5; Tocris, GABAA antagonist), CGP 35348 (CGP, 1 mM, pH 3.0; a gift of Ciba-Geigy, GABAB antagonist). Retaining currents (4 – 10 nA) of opposite polarity were applied to the pipettes between drug ejections.
In a number of experiments a barrel filled with sodium chloride (165 mM, pH 4.0) was used for current balance. In control experiments no significant contribution from current or pH was detected.
Experimental protocols
Iontophoretic ejection of NC was used repeatedly during continuous recording of baseline activity. Following determination of NC-induced effects, the action of different receptor antagonists (in most cases 180 s before the co-administration with NC) was tested. To evaluate the possible involvement of GABAergic transmission in NC actions the GABA receptor antagonists bicuculline or SR95531 (GABAA) and CGP 35348 (GABAB) were tested against NC. Finally, we investigated the effect of nocistatin on neuronal firing rate. Since not all neurons received all drug combinations experimental numbers vary in the results section.
Analysis of neuronal responses
Drug responses were compared with control firing frequency recorded immediately before drug administration. From continuously recorded rate meter counts, the average discharge rate of each neuron was evaluated for 100 s prior to iontophoresis. This value (referred to as ‘control') was subtracted from all subsequent changes in firing rate and results were expressed as ‘% change of control'. If the average change in discharge rate during the entire response time was larger than ±40%, the neuron was considered as being sensitive to the applied substance. This criterion was used before co-application of NC with GABA receptor antagonists to divide the neurons into ‘responders' and ‘non-responders'.
In addition, we analysed the pattern of discharge (i) of spontaneous activity, (ii) during drug administration and (iii) during recovery. In addition to what extent spikes occurred singularly or in groups was quantified. Sequences of spikes with an interspike interval ⩽4 ms were regarded as bursts as recommended by Lo et al. (1991) and Lu et al. (1992) and low-threshold bursts were differentiated from high-threshold bursts (high threshold spike, HTS) by analysis of the pre-burst interval. The absolute requirement for hyperpolarization prior to the occurrence of a low-threshold Ca2+ spike (LTS) has the practical implication that these events may be detected using extracellular recordings as a high-frequency burst of action potentials that is preceded by a period of inhibition lasting longer than 100 ms (Lo et al., 1991; Lu et al., 1992; Guido & Weyand, 1995). As in our previous investigation (Davidowa et al., 1995) several parameters were determined: the absolute number of bursts per 100 s, the percentage of spikes involved in bursts, and the number of spikes per burst.
Localization of recording sites
At the end of recording, a small amount of Trypan blue was iontophoretically deposited in the brain by passing a 10 μA negative current through the recording electrode for approximately 10 min. The rat was then killed with an overdose of urethane, decapitated and the brain fixed with 10% formaldehyde. Frozen frontal sections were stained with nuclear red and the location of blue spots within the thalamus was determined. The location of each neuron studied was mapped according to the atlas of Paxinos & Watson (1998).
Data analysis
Data are presented in the text and in Table 1 as the mean±s.d., in the bar histograms as the mean±s.e.mean of the number (n) of neurons. Wilcoxon Rank sum test (two tailed) or paired t-test was used to determine the predominating effect of a drug in a neuronal population. Mann – Whitney test and χ2 test were used for statistical analysis of differences between two samples of neurons (software GraphPad Prism (San Diego, CA, U.S.A.) and SPSS).
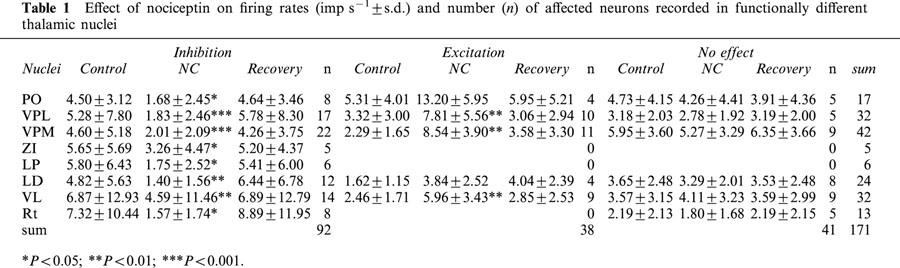
Table 1.
Effect of nociceptin on firing rates (imp s−1±s.d.) and number (n) of affected neurons recorded in functionally different thalamic nuclei
Results
Effect of NC on firing rate in functionally different thalamic neurons
Extracellular recordings were made from a total of 171 spontaneously active neurons localized in functionally different thalamic nuclei. In almost all thalamic nuclei the responsiveness to NC was high (mean: 76%) (Table 1). In the whole sample of neurons, NC induced a significant decrease in firing rate (P=0.001, Wilcoxon test; n=171). For all neurons in the different thalamic nuclei examined, significant decreases in firing rate were found in LP (P=0.027), LD (P=0.018), ZI (P=0.043) and Rt (P=0.013). The dose-dependent inhibitory response of a representative example is illustrated in Figure 1A. Higher ejection currents caused greater decreases in firing rate of single neurons. A decrease in firing rate occurred in 54% of neurons (92/171), whereas an increase was obtained in 22% (38/171). The mean used iontophoretic current and the duration of NC-ejection did not differ significantly in both samples (NC-inhibited neurons: 38±9.5 nA and 73±21 s versus NC-excited neurons: 39±6 nA and 75±15 s). Similar results were obtained concerning the mean duration of NC-responses (NC-inhibited neurons: 277±181 s versus NC-excited neurons: 312±179 s). Most of the NC-excited neurons were localized in the ventrobasal complex and the VL. In neurons excited by NC, these increases in firing rate were also dose-dependent (Figure 1B). Baseline activity was significantly higher in NC-inhibited neurons when compared to NC-excited neurons (P=0.043, Mann – Whitney test, n=130).
Figure 1.
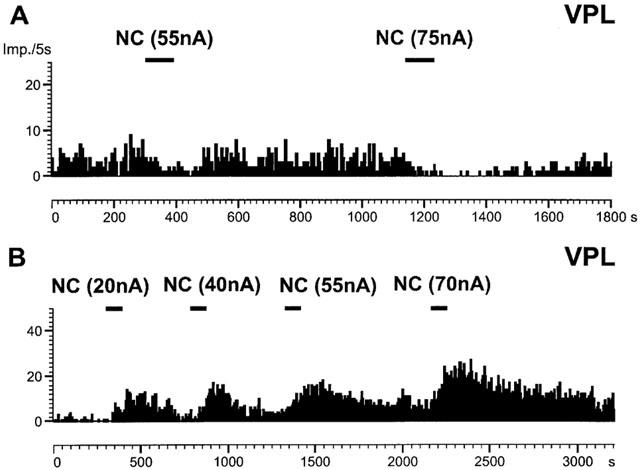
Effects of nociceptin (NC) on discharge rate. Frequency time histograms show dose-dependent decreases (A) or increases (B) in the firing rate of two neurons located in lateral ventroposterior nucleus (VPL). The y-axes indicate the number of spikes per 5 s, the x-axes indicate the time in seconds. The bars represent time and duration of ejection, the numbers show current intensity.
In this study, using urethane-anaesthetized rats, LTS bursts were most often found (152 out of the 171 thalamic neurons), whereas HTS bursts were only found in 77 out of 171 neurons. For baseline activity of the whole sample, there was a significant difference in the percentage of spikes involved in bursts of the LTS- and the HTS-type (LTS-type: mean=47.41±32.52% versus HTS-type: mean=6.03±8.27%; P=0.0001, Mann – Whitney test). With the exception of Rt (4 – 8 spikes within a burst), the bursts consisted of 2 – 3 spikes.
NC induced a significant increase in the percentage of spikes involved in bursts of the LTS-type in NC-excited neurons (p=0.0004, Wilcoxon test). In NC-inhibited neurons no significant changes were observed (Figure 2A). As shown in Figure 2B, the percentage of spikes involved in bursts of the HTS-type was significantly increased in NC-excited neurons (P=0.0021) and significantly decreased in NC-inhibited neurons (P=0.0132). The number of spikes within a burst did not change significantly following NC ejection.
Figure 2.
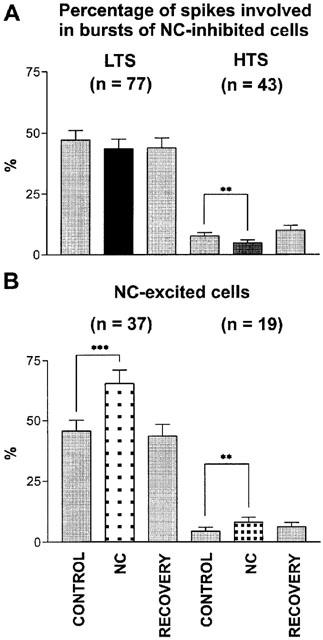
Effects of NC on appearance of burst discharges. Bar histogram shows the percentage of spikes involved in bursts of low threshold-type (LTS) and high threshold-type (HTS). Bars represent the percentage (±s.e.mean) before NC (control), during (NC) and after NC administration (recovery). The asterisks show significant differences (**P<0.001, ***P<0.0001); n=number of neurons.
However, when considering the anatomical localization of functionally different thalamic nuclei, the distribution of neurons inhibited, excited or unaffected by NC did not differ significantly (P>0.05; χ2 test). Therefore, for detailed analysis of the effects of other drugs (GABA, ORL1 receptor antagonists and nocistatin) all thalamic neurons were considered regardless of their localization.
Involvement of GABAergic signalling in NC-induced effects
Low-ejection currents (29±6.85 nA; n=41) of the GABAA receptor antagonists caused frequently an increase in discharge rate (for example see Figure 3A). In 34 neurons bicuculline methiodide was used, in seven neurons SR 95531. For NC-inhibited as well as for NC-excited neurons this increase in firing rate was significant (Figure 3C). Whereas GABAA receptor antagonists increased the firing rate, they did not change significantly the percentage of spikes involved in high or low threshold bursts. In some cases, as shown in Figure 3B (a ‘non-responder' neuron), a clear response to the iontophoretic ejection of NC was only observed when bicuculline was co-administered. These examples also show that the effects of co-administration of GABAA receptor antagonists and NC were not additive. It can be seen in Figure 3c that bicuculline or SR 95531 significantly reduced the excitatory effect of NC. A GABAA receptor antagonist-evoked reduction of the NC-induced excitation was also found in eight neurons located in the ventrobasal complex. The remaining neurons which were located in the ventrobasal complex and were investigated by co-administration of NC and GABAA antagonists responded with a decrease of activity in response to the application of NC. In contrast to the results obtained in NC-excited neurons, the NC-induced decrease in firing rate could not be blocked by GABAA receptor antagonists. In NC-inhibited neurons co-administration of bicuculline or SR 95531 together with NC did not significantly differ from the effect of NC alone on firing rate.
Figure 3.
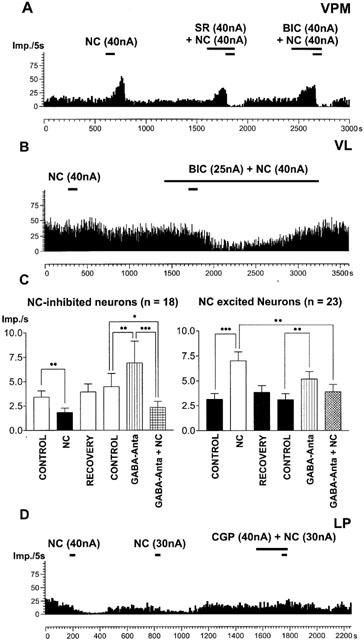
Effects of GABA receptor antagonists. (A, B) Frequency time histograms showing that SR 95531 (SR) and bicuculline (BIC) blocked NC-induced increases in firing rate in two neurons located in the lateral ventroposterior (VPM) and ventrolateral (VL) nuclei. With the exception of some neurons (B), bicuculline or SR 95531 alone induced strong increases in firing rate (A). (C) Histogram demonstrating the effect of co-administration of bicuculline or SR 95531 and NC on mean firing rate (Imp s−1±s.e.mean). In both samples of neurons GABAA receptor antagonists (GABA-Anta) significantly enhanced activity (*P<0.05, **P<0.001, ***P<0.0001). In NC-excited neurons NC-induced discharge rate differed significantly from that activity induced by the co-ejection of GABAA antagonists and NC. In contrast, in NC-inhibited neurons GABAA antagonists did not influence the inhibitory effect of NC. Co-administration of GABAA antagonists and NC did differ significantly from baseline recorded immediately prior to GABAA antagonists ejection (control) (n=number of neurons). (D) The GABAB antagonist CGP35348 reduced NC-induced decrease in firing rate in a neuron located in the lateral posterior nucleus (LP). In (A, B, D) the y-axes indicate the number of spikes per 5 s, the x-axes indicate the time in seconds. The bars represent time and duration of ejection and numbers show current intensity.
In 11 neurons the GABAB receptor antagonist CGP 35348 was tested. This antagonist did not affect NC-induced increases in firing rate (n=6). In neurons that were inhibited by NC, CGP 35348 was able to reduce the inhibitory effect of NC in four out of five neurons. An example is shown in Figure 3D.
Pharmacological characterization of the receptor mediating the effects of NC
In order to pharmacologically characterize the effects of NC on thalamic neurons several ORL-1 receptor ligands were used. In the first experiments, we used F/G (mean current: 37±5 nA) to block NC-induced effects. An example of the pure antagonistic effect of F/G is shown in Figure 4A. However, F/G alone induced effects comparable to NC in 7/41 neurons as shown, for example, in Figure 4B. In the whole sample of neurons, co-administration of F/G and NC significantly reduced neuronal activity when compared to the ejection of F/G alone and to control drug free conditions in neurons which are inhibited by NC (Figure 5A). Nevertheless, F/G blocked the action of NC in 24 out of 41 neurons (59%).
Figure 4.
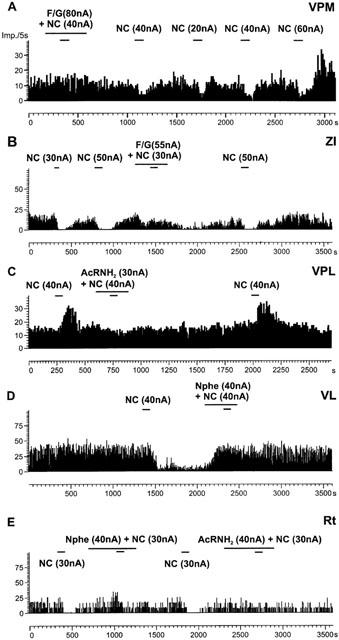
Representative examples of the action of NC receptor antagonists. Frequency time histograms show NC-induced decreases or increases in firing rate of different neurons located in the medial ventroposterior nucleus (VPM), the zona incerta (ZI), the lateral ventroposterior (VPL), the ventrolateral (VL) and the reticular nucleus (Rt). In (A) the NC receptor antagonist F/G blocked NC-induced inhibition. An example of an agonist effect of F/G is shown in (B). The NC receptor antagonists AcRNH2 and Nphe effectively blocked NC-induced effects on discharge rate (C – E). The y-axes indicate the number of spikes per 5 s, the x-axes indicate the time in seconds. The bars represent time and duration of ejection and numbers show current intensity.
Figure 5.
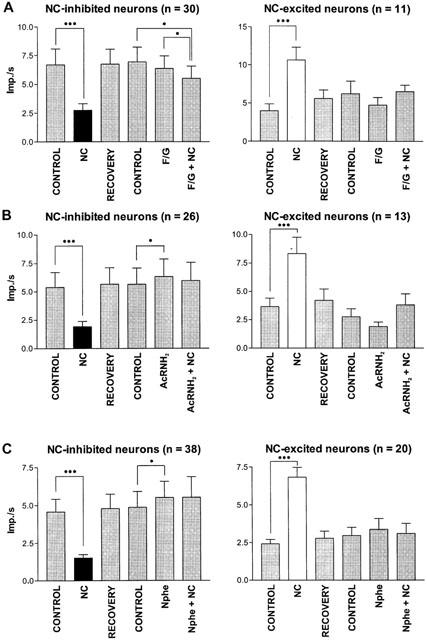
Effects of NC receptor antagonists. Bar histograms demonstrate the changes in mean firing rates±s.e.mean induced by NC, by the NC-receptor antagonists (F/G, AcRNH2, Nphe) and by co-administration of antagonists and NC. Co-administration of F/G and NC caused a significant decrease in firing rate when compared to baseline activity recorded immediately prior to F/G ejection (control) in the sample of NC-inhibited neurons. In addition, there was a significant difference between the firing rate during F/G ejection and that during co-administration of F/G and NC. AcRNH2 and Nphe alone induced a significant increase in firing rate. Co-administration of NC antagonists and NC blocked the NC-induced effects. *P<0.05, ***P<0.0001, n=number of neurons.
AcRNH2 was tested in 39 neurons (mean current: 57±15 nA). Examples of the inhibitory effects of AcRNH2 on NC-induced changes in discharge rate are shown in Figure 4C,E. As shown in Figure 5B for NC-inhibited neurons iontophoretic administration of AcRNH2 alone significantly increased discharge rate. Co-administration of AcRNH2 and NC did not significantly modify this activity when compared to control, either in NC-inhibited neurons or NC-excited neurons. Therefore, AcRNH2 efficiently blocked the NC-induced effects on discharge rate considering the whole sample of neurons, although AcRNH2 only blocked efficiently the NC-induced effects in 26 neurons (67%).
Using a concentration of Nphe 30 times higher than NC (mean current 64±9 nA), NC-induced effects were blocked in nearly all neurons tested (51/58, 88%). Examples of the inhibitory action of Nphe on NC-induced decreases in firing rate in single cells are shown in Figure 4D,E. In neurons inhibited by NC, the ejection of Nphe alone induced a significant increase in firing rate (Figure 5C) comparable to the effects of AcRNH2.
Effects of nocistatin
Nocistatin (51±17 nA, 75±17 s) significantly increased the discharge rate of thalamic neurons (P=0.009, n=35, Wilcoxon test). An example of the effect of nocistatin is shown in Figure 6C. No significant changes in burst firing were observed. Responsiveness of thalamic neurons to nocistatin (11/35 (40%)) was lower than that to NC (25/35 (71%)). In NC-inhibited neurons only (n=16), nocistatin induced a significant increase in firing rates (Figure 6A), whereas nocistatin did not significantly change firing rate in NC-excited neurons (n=9, Figure 6B).
Figure 6.
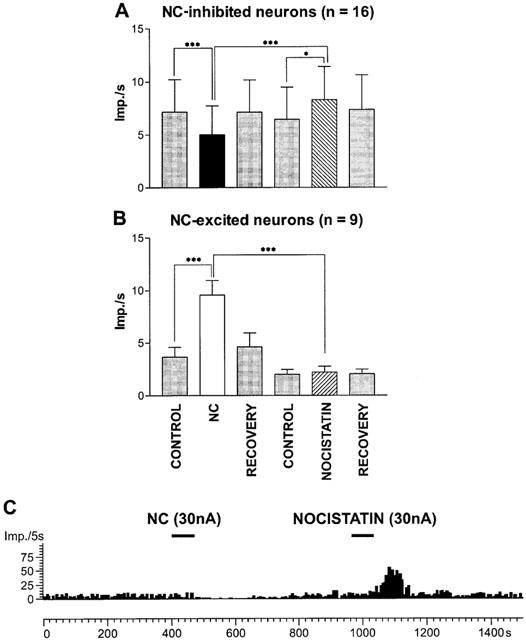
Effects of nocistatin on mean firing rate (±s.e.mean) in the sample of nociceptin-inhibited (A) and NC-excited neurons (B). Significant NC-induced decreases in firing rates were accompanied by significant increases in firing rate induced by nocistatin (A). There were no significant changes induced by nocistatin (B). *P<0.05, ***P<0.0001, n=number of neurons. (C) Frequency time histogram shows a representative example of a neuronal response to nocistatin.
Discussion
This study provides the first electrophysiological description of the actions of NC in the rat thalamus. These data showed that a strong responsiveness to NC was not only obtained in somatosensory neurons but also in visual- and motor-related thalamic nuclei. It has been shown that only a small fraction (10%) of neurons in the VPL, VPM and PO can be activated by noxious cutaneous stimuli (Willis, 1997). In contrast to these observations we obtained an 80% NC response rate in the thalamus. NC-responsible neurons were also found in visual and motor-related nuclei. Therefore, it seems unlikely that NC has actions only related to pain perception in the thalamus.
In all thalamic nuclei investigated NC mainly produced a decrease in firing rate. In line with these results NC profoundly inhibited spontaneous discharges of neurons in the rostral ventrolateral medulla (Chu et al., 1998), in the supraoptic nucleus (Doi et al., 1998) and in ventromedial hypothalamic neurons (Lee et al., 1997) of rat brain slices. The NC receptor has been shown to be coupled to a G-protein-activated inwardly rectifying K+ (GIRK) conductance in several neuronal populations (Connor et al., 1996; Ikeda et al., 1997; Madamba et al., 1999). This conductance seems to be involved in the mediation of NC-induced effects in the ventrobasal thalamus and the Rt (S. Meis, personal communication). In addition, NC has been reported to inhibit low and high-threshold calcium currents in dorsal root ganglion neurons (Abdulla & Smith, 1997). In acutely dissociated rat periaqueductal grey neurons NC inhibited (predominantly) the N- and P/Q-type Ca2+ channel. L- and R-type currents were relatively unaffected (Connor & Christie, 1998). Similar results were described in the hippocampus (Knoflach et al., 1996). In agreement with these data we obtained a significant decrease in the number of bursts of the HTS-type following NC ejection in NC-inhibited neurons. In NC-excited neurons the percentage of spikes involved in bursts of both types significantly increased. Collectively, the inhibitory effect of NC in thalamic neurons might be due to either the opening of a potassium conductance and/or the closing of voltage sensitive Ca2+ channels. It is worthy of mention that we have shown that the appearance of burst discharges is higher in urethane-anaesthetized rats when compared to freely moving rats (Albrecht et al., 1998).
Our results also show that GABAA receptor antagonists blocked most of the NC-induced excitatory effects in the thalamus. Therefore, we suggest that GABAergic signalling is involved in the mediation of NC-induced increases in firing rate. In the hippocampus it has been shown that opioids excite pyramidal neurons indirectly by inhibition of neighbouring inhibitory interneurons (Zieglgänsberger et al., 1979; Nicoll & Alger, 1981). Because CGP 35348 did not influence NC-induced excitatory effects it can be concluded that NC-mediated excitation is exclusively via the GABAA receptor. In contrast to other thalamic relay nuclei, the rat ventrobasal complex can be characterized by a virtual absence of GABAergic interneurons (Harris & Hendrickson, 1987). Thus, with the exception of that complex (VPL, VPM) the excitatory effects of NC could be mediated by GABaergic interneurons. A NC-induced hyperpolarization of the local circuit neuron would cause a disinhibition of thalamic projection neurons. If NC receptors are localized presynaptically on GABAergic afferents, then a reduced release of GABA might depolarize thalamic projection neurons. A possible presynaptic location of NC receptors is also supported by our results with GABAA receptor antagonists in the ventrobasal complex. GABAA receptor antagonists also reduced the excitatory effect of NC in VPM and VPL neurons. It has been shown that GABAergic transmission is also involved in somatosensory processing within the ventrobasal complex by using either bicuculline methiodide (Salt, 1989; Vahle Hinz et al., 1994) or the GABAA antagonist SR95531 (Roberts et al., 1992). NC could increase the discharge rate via a presynaptic inhibition of afferents from the Rt. Such a presynaptic inhibitory action of NC has been reported in substantia gelatinosa (Lai et al., 1997; Liebel et al., 1997).
Blocking the GABAB receptor by CGP 35348 reduced the inhibitory effects of NC. It has been shown that the GABAB agonist, baclofen, acts on calcium channels (Connor & Christie, 1998) and also couples to increases in an inwardly rectifying K+ conductance (Connor et al., 1996) similar to NC. Thus, these results can be interpreted assuming that ORL-1 and GABAB receptors act synergistically in reducing neuronal excitability, and the block of the GABAB reduces the response to ORL1 activation. However, this hypothesis needs further studies to be validated. In summary, our study favours the existence of both postsynaptic and presynaptic actions of NC within the thalamus.
The synthetic analogue of NC, F/G, was reported to fully antagonize the inhibitory effects of NC in amygdaloid (Meis & Pape, 1998) as well as ventrolateral medullary neurons (Chu et al., 1999). Moreover, F/G activated a small outward current and significantly reduced the amplitude of the NC-stimulated current in suprachiasmatic nucleus neurons (Allen et al., 1999). In contrast, in hypothalamic neurons F/G acted as a full agonist at the ORL-1 receptor (Yakimova & Pierau, 1999). In locus coeruleus and periaqueductal grey neurons, F/G exhibited partial agonist activity for inhibition of calcium channel currents and opening of K+ channels (Chiou, 1999; Connor et al., 1999). In our study, the pseudopeptide did not effectively block NC-induced effects in all cells tested. In agreement with our results, F/G has also been shown to exert partial agonist activity, as well as NC antagonist actions in hippocampal CA1 neurons (Madamba et al., 1999). The number of receptors expressed on a given NC-responsive cell appears to be an important factor in determining the pharmacological behaviour of ORL-1 receptor ligands. In cells transfected with the human ORL1 receptor it was found that partial agonists demonstrated pure antagonistic activity at low levels of expression (Toll et al., 1998). The spectrum of activity of F/G in different thalamic neurons may possibly be explained by differences in coupling reserve between different neurons (Berger et al., 2000a). We have recently shown that AcRNH2 and Nphe antagonized the NC stimulation of [35S]-GTPγS binding to G proteins in membranes and sections of rat brain (Berger et al., 1999; 2000b). In the present study AcRNH2 and Nphe completely blocked NC-induced effects in most cells tested. For the whole sample of neurons tested both AcRNH2 and Nphe increased the discharge rate when administered alone. Therefore, it can be suggested that NC-containing fibres terminate in the thalamus and exert a tonic inhibitory influence on neuronal activity which was antagonized when AcRNH2 or Nphe were ejected. A similar antagonistic effect of F/G might be hypothesized. However because of some residual agonist activity such receptor antagonist-induced increases in firing rate were missed. The data presented in our study support previous findings (Calo' et al., 2000a; Guerrini et al., 2000) that Nphe represents a pure ORL-1 receptor antagonist. However, a limitation of Nphe is represented by its low potency (pA2 6.0 – 6.5 in different in vitro assays) and its chemical (peptide) nature. Therefore, further studies should involve new non-peptide ORL-1 receptor antagonist recently identified (Kawamoto et al., 1999).
We have shown that nocistatin per se has some biological activity. In contrast to the NC-induced decreases in firing rate, our results show that nocistatin mainly induced an increase in the discharge rate of thalamic neurons. However, the responsiveness of neurons to nocistatin was lower than that to NC. Nocistatin binds to membranes of mouse brain and of spinal cord in vitro (Okuda Ashitaka et al., 1998). However, nocistatin does not displace bound [3H]-NC and does not prevent NC mediated inhibition of forskolin-induced cyclic AMP accumulation in Chinese hamster ovary cells transfected with the ORL1 receptor (Okuda Ashitaka et al., 1998). We did not find any significant changes in the appearance of burst discharges and this result is in agreement with the findings of Connor et al. (1999) in locus coeruleus neurons where nocistatin did not affect the calcium conductance. A possible mechanism might be a nocistatin-mediated reduction of GABAergic activity in the thalamus as Zeilhofer et al. (2000) have recently shown that nocistatin reduces inhibitory glycinergic and GABAergic transmission.
In summary, NC has been implicated in many behavioural and physiological processes, including nociception, allodynia, locomotion, learning, stress responses, sexual behaviour, feeding, cardiovascular control, and sodium balance (Darland et al., 1998; Taylor & Dickenson, 1998; Calo' et al., 2000b). Although the main effect of NC within functionally different structures in the thalamus is inhibitory, with excitatory effects mediated by an involvement of GABAergic neurons, the functional role of NC needs to be analysed in further experiments using sensory stimulation.
Acknowledgments
The authors thank Roland Schneider and Ursula Seider for their excellent technical assistance and D.G. Lambert (University of Leicester, U.K.) for proofreading this paper. This work was supported by the Deutsche Forschungsgemeinschaft (AI 342/7-1).
Abbreviations
- AcRNH2
Acetyl-Arg-Tyr-Tyr-Arg-Ile-Lys-NH2
- F/G
[Phe1Ψ(CH2-NH)Gly2]Nociceptin(1-13)NH2
- LD
laterodorsal nucleus
- LP
lateral posterior nucleus
- NC
nociceptin/orphanin FQ
- Nphe
[Nphe1]NC(1-13)NH2
- PO
posterior thalamic nucleus
- Rt
reticular nucleus
- VL
ventrolateral nucleus
- VPL
lateral ventroposterior nucleus
- VPM
medial ventroposterior nucleus
- ZI
zona incerta
References
- ABDULLA F.A., SMITH P.A. Nociceptin inhibits T-type Ca2+ channel current in rat sensory neurons by a G-protein-independent mechanism. J. Neurosci. 1997;17:8721–8728. doi: 10.1523/JNEUROSCI.17-22-08721.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALBRECHT D., DAVIDOWA H. Action of urethane on dorsal lateral geniculate neurons. Brain Res. Bull. 1989;22:923–927. doi: 10.1016/0361-9230(89)90001-4. [DOI] [PubMed] [Google Scholar]
- ALBRECHT D., ROYL G., KANEOKE Y. Very slow oscillatory activity in lateral geniculate neurons of freely moving and anesthetized rats. Neurosci. Res. 1998;32:209–220. doi: 10.1016/s0168-0102(98)00087-x. [DOI] [PubMed] [Google Scholar]
- ALLEN C.N., JIANG Z.G., TESHIMA K., DARLAND R., IKEDA M., NELSON C.S., QUIGLEY D.I., YOSHIOKA T., ALLEN R.G., REA M.A., GRANDY D.S. Orphanin-FQ/nociceptin (OFQ/N) modulates the activity of suprachiasmatic nucleus neurons. J. Neurosci. 1999;19:2152–2160. doi: 10.1523/JNEUROSCI.19-06-02152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGER H., ALBRECHT E., WALLUKAT G., BIENERT M. Antagonism by acetyl-RYYRIK-NH2 of G-protein activation in rat brain preparations and of chronotropic effect on rat cardiomyocytes evoked by nociceptin/orphanin FQ. Br. J. Pharmacol. 1999;126:555–558. doi: 10.1038/sj.bjp.0702353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGER H., BIGONI R., ALBRECHT E., RICHTER R.M., KRAUSE E., BIENERT M., CALO' G. The nociceptin/orphanin FQ receptor ligand acetyl-RYYRIK-amide exhibits antagonist and agonist properties. Peptides. 2000a;21:1131–1139. doi: 10.1016/s0196-9781(00)00251-5. [DOI] [PubMed] [Google Scholar]
- BERGER H., CALO' G., ALBRECHT E., GUERRINI R., BIENERT M. [Nphe1]NC(1-13]NH2] selectively antagonizes nociceptin/orphanin FQ-stimulated G-protein activation in rat brain. J. Pharmacol. Exp. Ther. 2000b;294:428–433. [PubMed] [Google Scholar]
- CALO' G., GUERRINI R., BIGONI R., RIZZI A., MARZOLA G., OKAWA H., BIANCHI C., LAMBERT D.G., SALVADORI S., REGOLI D. Characterization of [Nphe1]nociceptin(1-13)NH2, a new selective nociceptin receptor antagonist. Br. J. Pharmacol. 2000a;129:1183–1193. doi: 10.1038/sj.bjp.0703169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALO' G., GUERRINI R., RIZZI A., SALVADORI S. , REGOLI D. Pharmacology of nociceptin and its receptor: a novel therapeutic target. Br. J. Pharmacol. 2000b;129:1261–1283. doi: 10.1038/sj.bjp.0703219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIOU L.C. [Phe1psi(CH2-NH)Gly2]nociceptin-(1-13)-NH2 activation of an inward rectifier as a partial agonist of ORL1 receptors in rat periaqueductal gray. Br. J. Pharmacol. 1999;128:103–107. doi: 10.1038/sj.bjp.0702746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHU X., XU N., LI P., WANG J.Q. Profound inhibition of cardiomotor neurons in the rat rostral ventrolateral medulla by nociceptin (orphanin FQ) Neuroreport. 1998;9:1081–1084. doi: 10.1097/00001756-199804200-00022. [DOI] [PubMed] [Google Scholar]
- CHU X.P., XU N.S., LI P., WANG J.Q. The nociceptin receptor-mediated inhibition of the rat rostral ventrolateral medulla neurons in vitro. Eur. J. Pharmacol. 1999;364:49–53. doi: 10.1016/s0014-2999(98)00816-4. [DOI] [PubMed] [Google Scholar]
- CONNOR M., CHRISTIE M.J. Modulation of Ca2+ channel currents of acutely dissociated rat periaqueductal grey neurons. J. Physiol. Lond. 1998;509:47–58. doi: 10.1111/j.1469-7793.1998.047bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONNOR M., VAUGHAN C.W., CHIENG B., CHRISTIE M.J. Nociceptin receptor coupling to a potassium conductance in rat locus coeruleus neurones in vitro. Br. J. Pharmacol. 1996;119:1614–1618. doi: 10.1111/j.1476-5381.1996.tb16080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONNOR M., VAUGHAN C.W., JENNINGS E.A., ALLEN R.G., CHRISTIE M.J. Nociceptin, Phe1psi-nociceptin1-13, nocistatin and prepro-nociceptin154-181 effects on calcium channel currents and a potassium current in rat locus coeruleus in vitro. Br. J. Pharmacol. 1999;128:1779–1787. doi: 10.1038/sj.bjp.0702971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARLAND T., HEINRICHER M.M., GRANDY D.K. Orphanin FQ/nociceptin: a role in pain and analgesia, but so much more. Trends Neurosci. 1998;21:215–221. doi: 10.1016/s0166-2236(97)01204-6. [DOI] [PubMed] [Google Scholar]
- DAVIDOWA H., ALBRECHT D., GABRIEL H.J., ZIPPEL U. Cholecystokinin affects the neuronal discharge mode in the rat lateral geniculate body. Brain Res. Bull. 1995;36:533–537. doi: 10.1016/0361-9230(94)00238-v. [DOI] [PubMed] [Google Scholar]
- DOI N., DUTIA M.B., BROWN C.H., LENG G., RUSSELL J.A. Inhibitory actions of nociceptin (orphanin FQ) on rat supraoptic nucleus oxytocin and vasopressin neurones in vitro. Adv. Exp. Med. Biol. 1998;449:147–151. doi: 10.1007/978-1-4615-4871-3_17. [DOI] [PubMed] [Google Scholar]
- DOOLEY C.T., SPAETH C.G., BERZETEI GURSKE I.P., CRAYMER K., ADAPA I.D., BRANDT S.R., HOUGHTEN R.A., TOLL L. Binding and in vitro activities of peptides with high affinity for the nociceptin/orphanin FQ receptor, ORL1. J. Pharmacol. Exp. Ther. 1997;283:735–741. [PubMed] [Google Scholar]
- GUERRINI R., CALO G., BIGONI R., RIZZI A., VARANI K., TOTH G., GESSI S., HASHIBA E., HASHIMOTO Y., LAMBERT D.G., BOREA P.A., TOMATIS R., SALVADORI S., REGOLI D. Further studies on nociceptin-related peptides: discovery of a new chemical template with antagonist activity on the nociceptin receptor. J. Med. Chem. 2000;43:2805–2813. doi: 10.1021/jm990075h. [DOI] [PubMed] [Google Scholar]
- GUERRINI R., CALO G., RIZZI A., BIGONI R., BIANCHI C., SALVADORI S., REGOLI D. A new selective antagonist of the nociceptin receptor. Br. J. Pharmacol. 1998;123:163–165. doi: 10.1038/sj.bjp.0701640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUIDO W., WEYAND T. Burst responses in thalamic relay cells of the awake behaving cat. J. Neurophysiol. 1995;74:1782–1786. doi: 10.1152/jn.1995.74.4.1782. [DOI] [PubMed] [Google Scholar]
- HARRIS R.M., HENDRICKSON A.E. Local circuit neurons in the rat ventrobasal thalamus – a GABA immunocytochemical study. Neuroscience. 1987;21:229–236. doi: 10.1016/0306-4522(87)90335-6. [DOI] [PubMed] [Google Scholar]
- IKEDA K., KOBAYASHI K., KOBAYASHI T., ICHIKAWA T., KUMANISHI T., KISHIDA H., YANO R., MANABE T. Functional coupling of the nociceptin/orphanin FQ receptor with the G-protein-activated K+ (GIRK) channel. Mol. Brain Res. 1997;45:117–126. doi: 10.1016/s0169-328x(96)00252-5. [DOI] [PubMed] [Google Scholar]
- JONES E.G. The Thalamus. New York: Plenum; 1985. [Google Scholar]
- KAWAMOTO H., OZAKI S., ITOH Y., MIYAJI M., ARAI S., NAKASHIMA H., KATO T., OHTA H., IWASAWA Y. Discovery of the first potent and selective small molecule opioid receptor-like (ORL1) antagonist: 1-[(3R,4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1, 3-dihydro-2H-benzimidazol-2-one (J-113397) J. Med. Chem. 1999;42:5061–5063. doi: 10.1021/jm990517p. [DOI] [PubMed] [Google Scholar]
- KIM U., GREGORY E., HALL W.C. Pathway from the zona incerta to the superior colliculus in the rat. J. Comp. Neurol. 1992;321:555–575. doi: 10.1002/cne.903210405. [DOI] [PubMed] [Google Scholar]
- KNOFLACH F., REINSCHEID R.K., CIVELLI O., KEMP J.A. Modulation of voltage-gated calcium channels by orphanin FQ in freshly dissociated hippocampal neurons. J. Neurosci. 1996;16:6657–6664. doi: 10.1523/JNEUROSCI.16-21-06657.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAI C.C., WU S.Y., DUN S.L., DUN N.J. Nociceptin-like immunoreactivity in the rat dorsal horn and inhibition of substantia gelatinosa neurons. Neuroscience. 1997;81:887–891. doi: 10.1016/s0306-4522(97)00251-0. [DOI] [PubMed] [Google Scholar]
- LEE K., NICHOLSON J.R., MCKNIGHT A.T. Nociceptin hyperpolarises neurones in the rat ventromedial hypothalamus. Neurosci. Lett. 1997;239:37–40. doi: 10.1016/s0304-3940(97)00875-6. [DOI] [PubMed] [Google Scholar]
- LIEBEL J.T., SWANDULLA D., ZEILHOFER H.U. Modulation of excitatory synaptic transmission by nociceptin in superficial dorsal horn neurones of the neonatal rat spinal cord. Br. J. Pharmacol. 1997;121:425–432. doi: 10.1038/sj.bjp.0701149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LO F.S., LU S.M., SHERMAN S.M. Intracellular and extracellular in vivo recording of different response modes for relay cells of the cat's lateral geniculate nucleus. Exp. Brain Res. 1991;83:317–328. doi: 10.1007/BF00231155. [DOI] [PubMed] [Google Scholar]
- LU S.M., GUIDO W., SHERMAN S.M. Effects of membrane voltage on receptive field properties of lateral geniculate neurons in the cat: contributions of the low-threshold Ca2+ conductance. J. Neurophysiol. 1992;68:2185–2198. doi: 10.1152/jn.1992.68.6.2185. [DOI] [PubMed] [Google Scholar]
- MADAMBA S.G., SCHWEITZER P., SIGGINS G.R. Nociceptin augments K+ currents in hippocampal CA1 neurons by both ORL-1 and opiate receptor mechanisms. J. Neurophysiol. 1999;82:1776–1785. doi: 10.1152/jn.1999.82.4.1776. [DOI] [PubMed] [Google Scholar]
- MEIS S., PAPE H.C. Postsynaptic mechanisms underlying responsiveness of amygdaloid neurons to nociceptin/orphanin FQ. J. Neurosci. 1998;18:8133–8144. doi: 10.1523/JNEUROSCI.18-20-08133.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEUNIER J.C., MOLLEREAU C., TOLL L., SUAUDEAU C., MOISAND C., ALVINERIE P., BUTOUR J.L., GUILLEMOT J.-C., FERRARA P., MONSARRAT B., MAZARGUIL H., VASSART G., PARMENTIER M., COSTENTIN J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- MORAN T.D., ABDULLA F.A., SMITH P.A. Cellular neurophysiological actions of nociceptin/orphanin FQ. Peptides. 2000;21:969–976. doi: 10.1016/s0196-9781(00)00235-7. [DOI] [PubMed] [Google Scholar]
- NEAL-CR J., MANSOUR A., REINSCHEID R., NOTHACKER H.P., CIVELLI O., WATSON-SJ J. Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J. Comp. Neurol. 1999;406:503–547. [PubMed] [Google Scholar]
- NICOLELIS M.A., CHAPIN J.K., LIN R.C. Somatotopic maps within the zona incerta relay parallel GABAergic somatosensory pathways to the neocortex, superior colliculus, and brainstem. Brain Res. 1992;577:131–141. doi: 10.1016/0006-8993(92)90546-l. [DOI] [PubMed] [Google Scholar]
- NICOLL R.A., ALGER B.E. Synaptic excitation may activate a calcium-dependent potassium conductance in hippocampal pyramidal cells. Science. 1981;212:957–959. doi: 10.1126/science.6262912. [DOI] [PubMed] [Google Scholar]
- OKUDA ASHITAKA E., MINAMI T., TACHIBANA S., YOSHIHARA Y., NISHIUCHI Y., KIMURA T., ITO S. Nocistatin, a peptide that blocks nociceptin action in pain transmission. Nature. 1998;392:286–289. doi: 10.1038/32660. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Coordinates 1998New York, Academic Press; 4th edn [Google Scholar]
- PRICE J.L.Thalamus The rat nervous system 1995San Diego, New York, Boston, London, Sydney, Tokyo, Toronto: Academic Press; 629–648.2nd edn. ed. Paxinos, G [Google Scholar]
- REINSCHEID R.K., NOTHACKER H.P., BOURSON A., ARDATI A., HENNINGSEN R.A., BUNZOW J.R., GRANDY D.K., LANGEN H., MONSMA F.J., JR, CIVELLI O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- ROBERTS W.A., EATON S.A., SALT T.E. Widely distributed GABA-mediated afferent inhibition processes within the ventrobasal thalamus of rat and their possible relevance to pathological pain states and somatotopic plasticity. Exp. Brain Res. 1992;89:363–372. doi: 10.1007/BF00228252. [DOI] [PubMed] [Google Scholar]
- SALT T.E. Gamma-aminobutyric acid and afferent inhibition in the cat and rat ventrobasal thalamus. Neuroscience. 1989;28:17–26. doi: 10.1016/0306-4522(89)90228-5. [DOI] [PubMed] [Google Scholar]
- SPENCER S.E., SAWYER W.B., LOEWY A.D. L-glutamate stimulation of the zona incerta in the rat decreases heart rate and blood pressure. Brain Res. 1988;458:72–81. doi: 10.1016/0006-8993(88)90497-0. [DOI] [PubMed] [Google Scholar]
- TAYLOR F., DICKENSON A. Nociceptin/orphanin FQ. A new opioid, a new analgesic. Neuroreport. 1998;9:R65–R70. [PubMed] [Google Scholar]
- TOLL L., BURNSIDE J., BERZETEI GURSKE I.P.Agonist activity of ORL1 antagonists is dependent upon receptor number INRC Conference 1998A89Garmisch-Partenkirchen
- VAHLE HINZ C., HICKS T.P., GOTTSCHALDT K.M. Amino acids modify thalamo-cortical response transformation expressed by neurons of the ventrobasal complex. Brain Res. 1994;637:139–155. doi: 10.1016/0006-8993(94)91227-0. [DOI] [PubMed] [Google Scholar]
- WILLIS W.D.Nociceptive functions of thalamic neurons Thalamus 1997Amsterdam, Lausanne, New York, Oxford, Shannon, Tokyo: Elsevier Science Ltd; 373–424.ed. Steriade, M., Jones, E.G. & McCormick, D.A [Google Scholar]
- YAKIMOVA K.S., PIERAU F.K. Effect of nociceptin and [Phe]1Psi(CH2-NH)Gly2]-nociceptin-(1-13)-NH2 on tonic activity of rat hypothalamic neurons. Neurosci. Lett. 1999;274:87–90. doi: 10.1016/s0304-3940(99)00684-9. [DOI] [PubMed] [Google Scholar]
- ZEILHOFER H.U., MUTH-SELBACH U., GUEHRING H., ERB K., AHMADI S. Selective suppression of inhibitory synaptic transmission by nocistatin in the rat spinal cord dorsal horn. J. Neurosci. 2000;20:4922–4929. doi: 10.1523/JNEUROSCI.20-13-04922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIEGLGÄNSBERGER W., FRENCH E.D., SIGGINS G.R., BLOOM F.E. Opioid peptides may excite hippocampal pyramidal neurons by inhibition of adjacent inhibitory interneurons. Science. 1979;405:415–417. doi: 10.1126/science.451610. [DOI] [PubMed] [Google Scholar]


