Abstract
Recent studies have shown beneficial effects of an adenosine A2A receptor agonist in dtsz mutant hamsters, an animal model of paroxysmal dystonia, in which stress and consumption of coffee can precipitate dystonic attacks. This prompted us to examine the effects of adenosine receptor agonists and antagonists on severity of dystonia in dtsz hamsters in more detail.
The non-selective adenosine A1/A2A receptor antagonists, caffeine (10 – 20 mg kg−1 i.p.) and theophylline (10 – 30 mg kg−1 s.c.), worsened the dystonia in dtsz hamsters.
Aggravation of dystonia was also caused by the selective adenosine A1/A2A antagonist CGS 15943 (9-chloro2-2-furyl)[1,2,4]triazolo[1,5-c]quinazolin-5-amine) at a dose of 30 mg kg−1 i.p. and by the adenosine A1 antagonist DPCPX (8-cyclopentyl-1,3-dipropylxanthine; 20 – 30 mg kg−1 i.p.), while the A2 antagonist DMPX (3,7-dimethyl-1-propargylxanthine; 2 – 4 mg kg−1 i.p.) and the highly selective A2A antagonist ZM 241385 (4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol; 2 – 5 mg kg−1 i.p.) failed to exert any effects on dystonia.
In contrast to the antagonists, both the adenosine A1 receptor agonist CPA (N6-cyclopentyladenosine; 0.1 – 1.0 mg kg−1 i.p.) and the A2A agonist CGS 21680 (2p-(2carboxyethylphen-ethylamino-5′-N-ethylcarboxamindoadenosine; 0.1 – 2.0 mg kg−1 i.p.) exerted a striking improvement of dystonia.
These data suggest that the precipitating effects of methylxanthines are, at least in part, related to their adenosine receptor antagonistic action.
Although adenosine receptor agonists can be regarded as interesting candidates for the therapy of paroxysmal dystonia, adverse effects may limit the therapeutic potential of adenosine A1 agonists, while beneficial effects of the adenosine A2A agonist CGS 21680 were already found at well tolerated doses.
Keywords: Adenosine, choreoathetosis, dystonia, dyskinesia, movement disorders, basal ganglia
Introduction
Dystonia is a movement disorder characterized by involuntary, sustained, patterned, and often repetitive muscle contractions of opposing muscles, frequently causing twisting movements or abnormal postures (Jankovic & Fahn, 1998). The pathophysiology of different types of idiopathic dystonia is not well understood, but there is evidence that basal ganglia dysfunctions can cause dystonic symptoms (Bhatia & Marsden, 1994; Fahn et al., 1998). The existence of various phenotypic and genotypic subtypes indicates that the pathogenesis of idiopathic dystonias is heterogeneous (Fahn et al., 1998). In a type of idiopathic paroxysmal dystonia (paroxysmal non-kinesiogenic dystonic choreoathetosis), attacks of generalized dystonia can be provoked by stress and by consumption of coffee or tea (Demirkiran & Jankovic, 1995; Fahn, 1994), i.e., by factors which are known to increase dopaminergic activity (Abercrombie et al., 1989; Fredholm, 1995). However, the mechanism of the precipitating effects of the methylxanthines in paroxysmal dystonia has not yet been examined. Methylxanthines are known to inhibit adenosine A1/A2A receptors and, at higher concentrations, also cyclic nucleotide phosphodiesterases and the direct release of intracellular calcium (Fredholm, 1995). Blockade of adenosine receptors, which is known to interact with the dopaminergic activity in the striatum (see below), is possibly important for the induction of dystonic attacks by methylxanthines, since there is evidence that idiopathic dystonias are related to striatal dopaminergic dysfunctions (Todd & Perlmutter, 1998).
As shown in several previous studies, mutant dystonic hamsters (gene symbol dtsz), which show the phenotypic characteristics of this type of idiopathic paroxysmal dystonia in humans (Demirkiran & Jankovic, 1995; Richter & Löscher, 1998) are useful for preclinical drug testing and for giving insights into the underlying mechanism of this movement disorder. Previous studies have shown that striatal dopaminergic overactivity plays a crucial role in the manifestation of dystonic attacks in dtsz hamsters, probably based on GABAergic disinhibition (Gernert et al., 2000; Rehders et al., 2000; Richter & Löscher, 1998). Preliminary data indicated that caffeine aggravates dystonia in mutant dystonic hamsters (Richter & Löscher, 1998), allowing an examination of the mechanism of the precipitating effects of methylxanthines. This is important because knowledge about the mechanisms of the triggers of paroxysmal dystonia can contribute to our understanding of the pathophysiology of this movement disorder.
Adenosine, an endogenous nucleoside, modulates motor activity by interactions with the dopaminergic system within the basal ganglia (Fuxe et al., 1998). Four different subtypes of G protein-coupled adenosine receptors have been described in the brain, namely A1, A2A, A2B and A3 receptors, all of which have been cloned from several species (Fredholm et al., 1994; Ongini & Fredholm, 1996). Specific antagonistic interactions between adenosine and dopamine receptors in the striatum are known between adenosine A1 and dopamine D1 receptors localized on GABAergic striatonigral/-entopeduncular neurons, and between adenosine A2A and dopamine D2 receptors on GABAergic striatopallidal projections (Ferre et al., 1997). Adenosine receptor antagonists, such as the methylxanthines, caffeine and theophylline, potentiate and adenosine receptor agonists inhibit the motor activating effects of dopamine receptor agonists (Ferre et al., 1992).
Adenosine A2A antagonists exert anti-parkinsonian action (Kanda et al., 1998; Müller, 2000), while adenosine receptor agonists have been suggested as new therapeutics for hyperkinetic basal ganglia disorders (Ferre et al., 1997). However, examination of the therapeutic potential of adenosine receptor agonists for the treatment of hyperkinesias, such as paroxysmal dystonia, is still rare. Since permanent or stress-inducible paroxysmal dystonias/dyskinesias in humans are often intractable there is a need for new therapeutics. The observation that the intake of low doses of caffeine, which exerts adenosine antagonistic action, can provoke dystonic episodes, suggests that adenosine receptor agonists may exert beneficial effects also against stress-induced dystonia. Recently, first investigations of the effect of relatively high doses (0.5 – 2.0 mg kg−1) of the adenosine A2A receptor agonist CGS 21680 have shown beneficial effects in dtsz mutant hamsters (Richter et al., 2000). Therefore, the therapeutic potential of the highly selective A1 adenosine receptor against CPA (Jacobson & Van Rhee, 1997) and of the highly selective A2A agonist CGS 21680 (Fredholm et al., 1994; Ongini & Fredholm, 1996) for the treatment of paroxysmal dystonia was investigated in more detail in the present study. In order to examine whether the precipitating effects of methylxanthines may be related to their adenosine antagonistic action, the effects of the non-selective adenosine receptor antagonists caffeine and theophylline (Fredholm, 1995; Müller, 2000) on severity of dystonia were compared to those of selective adenosine receptor antagonists. CGS 15943 has high affinity for A2A receptors but it also potently blocks A1 receptors (Ongini & Fredholm, 1996). The A1 antagonist DPCPX displays about 700 fold selectivity for A1 versus the A2A receptor (Jacobson & Van Rhee, 1997). DPMX, introduced as the first A2A-selective antagonist, also inhibits A2B receptors and at higher doses A1 receptors, while ZM 241385 fulfill the criteria of a highly selective A2A antagonist (Müller, 2000; Ongini & Fredholm, 1996).
Methods
Animals
The present experiments were carried out in male and female dtsz mutant Syrian golden hamsters which were obtained by selective breeding as described in detail elsewhere (Fredow & Löscher, 1991). In mutant hamsters the motor disturbances are transmitted by a recessive gene (Löscher et al., 1989). All dystonic hamsters were born and kept under the same controlled and constant environmental conditions. All experiments were done in compliance with the German Animal Welfare Act.
Induction of dystonic attacks and severity-score of dystonia
As reported previously, motor impairments in dtsz hamsters show several features in common with human primary paroxysmal non-kinesigenic dystonia (paroxysmal dystonic choreoathetosis), characterized by long-lasting dystonic attacks (Demirkiran & Jankovic, 1995; Richter & Löscher, 1998). In mutant hamsters, dystonic attacks can be reproducibly induced by a triple stimulation technique (Löscher et al., 1989; Richter & Löscher, 1998), i.e., stressful stimuli consisting of (1) taking the animal from its home cage and placing it on a balance, (2) injection of saline/vehicle (or of drugs), and (3) placement of the animal in a new plastic cage. After this procedure, dtsz hamsters develop a sequence of abnormal movements and postures. Therefore, the severity of dystonia can be rated by following score-system (Löscher et al., 1989; Richter & Löscher, 1998): stage 1, flat body posture; stage 2, facial contortions, rearing with forelimbs crossing, disturbed gait with hyperextended forepaws; stage 3, hyperextended hindlimbs so that the animals appear to walk on tiptoes; stage 4, twisting movements and loss of balance; stage 5, hindlimbs hyperextended caudally; stage 6, immobilization in a twisted, hunched posture with hind- and forelimbs tonically extended forward. After reaching the individual maximum stage the hamsters recover within 2 – 5 h. The individual maximum stage of dystonia is usually reached within 3 h after the hamsters were placed in the new cage. As described in several previous studies in detail (e.g., Richter & Löscher, 1993; 1998; Richter et al., 2000), the dystonic syndrome in dtsz mutants shows an age-dependent time-course. The severity of dystonia reaches a maximum at an age of about 32 – 42 days. Thereafter, the severity slowly declines until complete remission occurs at an age of about 10 weeks. In the present study, all animals were examined for the presence of dystonia after weaning at the age of 21 days by the triple stimulation procedure three times per week until the animals exhibited constant individual severity scores and latencies to onset of unequivocal dystonic symptoms (stage 2). The present drug experiments were done during the life-period of maximum expression of dystonia. Not all hamsters reach stage 6, but the individual maximum severity and the latency to onset is usually reproducible during this period (Richter & Löscher, 1998). To obtain reproducible latencies and avoid onset of dystonia prior or during the triple stimulation technique it was important to keep in time from taking the animals out of their home cage to placing them in a new cage (duration: 25 – 35 s). Animals that exhibited dystonic symptoms before injections of drug or vehicle were omitted from evaluation.
Drug treatments
The effects of adenosine receptor agonists and antagonists on the severity of dystonia were examined in groups of 7 – 12 dystonic hamsters. Each group was used for one to two doses. In cases of repeated testing of drugs, the drug-free interval was 4 to 5 days. Dystonic attacks were induced by the procedure of triple stimulation, as described above. Since the individual maximum stage of dystonia (score rating system see above) is usually reached within 3 h, the hamsters were observed for 3 h after triple stimulation. For drug testing, a control trial was undertaken with the triple stimulation technique, injecting the vehicle used for drug administration (see below) by the same route of administration, i.e., i.p. or for control trial of theophylline s.c., and the latencies and severity of the dystonic attacks were noted after placing the animals in the new cage (pre-drug control). Two days later, the drug was administered in the same group of animals and the latency and severity were noted. Furthermore, animals were observed for central adverse effects. As described for pre-drug-controls, a control trial with vehicle was done 2 days after drug treatment (post-drug control). Hamsters that differed in the maximum severity of dystonia in the pre-drug and post-drug control trials by more than two stages (about 4%) were omitted from the drug evaluation. All control and drug trials were done at the same time of the day between 0900 and 1200 h.
The examiner rating the severity of dystonia was blind to the treatment condition of the animals or in cases of unequivocal side effects at least unaware of the drugs used in the present experiments. Apart from examination of the effects of adenosine receptor agonists and antagonists on dystonia, the side effects (in part not quantified) were observed by a second examiner in order to assure that sufficient doses, i.e., central acting doses, were chosen (see below). Animal locomotor activity was determined by a score system, as used in previous studies (e.g., Richter & Löscher, 1995). In cases of a marked reduction of locomotor activity after drug administration, the catalepsy response was determined by quantifying descent latency (in seconds) in a block test in comparison to the post-drug vehicle control. The hamsters were placed with the forelimbs on a block (6 cm high) and the descent latency, i.e., the time over which the animals maintained this position, was noted. The effects of adenosine receptor agonists on body temperature were determined by rectal measurements in separate groups of 5 – 6 dtsz hamsters.
Drugs
The non-selective adenosine receptor antagonists caffeine and theophylline (Sigma, Deisenhofen, Germany) were dissolved in isotonic saline. The adenosine A1/2A receptor antagonist 9-chloro2-(2-furyl) [1,2,4]triazolo[1,5-c]quinazolin-5-amine (CGS 15943), kindly provided by Novartis (Summit, NJ, U.S.A.), the A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX; Biotrend, Cologne, Germany) and the A2A receptor antagonist 4-(2-[7-amiono-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol (ZM 241385; Biotrend, Cologne, Germany) were suspended in 0.3% Tween 80. The adenosine A2 receptor antagonist 3,7-dimethyl-1-propargylxanthine (DMPX; Biotrend, Cologne, Germany) was dissolved in water. The adenosine A1 agonist N6-cyclopentyladenosine (CPA, Biotrend, Cologne, Germany) and the adenosine A2A agonist 2p-(2carboxyethylphenethylamino-5′-N-ethylcarboxamindoadenosine) (CGS 21680; Biotrend, Cologne, Germany) were dissolved in 0.3% Tween 80. All solutions and suspensions were freshly prepared. For all drug administrations, injection volume was 5 ml kg−1. For pre- and post-drug control recordings the animals received the same volume of vehicle.
The doses of these drugs and routes of administration were chosen on the basis of several previous experiments in which central effects of adenosine receptor ligands were investigated in rodents (e.g, Klitgaard et al., 1993; Pinna et al., 1996; Yacoubi et al., 2000). The doses of these drugs were increased until unequivocal behavioural effects could be observed in mutant hamsters. This criterion was fulfilled for all compounds at least for the highest doses. All drugs were injected intraperitoneally. In addition, theophylline was administered subcutaneously in order to obtain a longer duration of action than observed after i.p. injections.
Statistics
The significance of differences (severity of dystonia, latencies to onset of dystonia, adverse effects) between control trials (pre- and post-drug) and drug trial in the same group of animals was calculated by the Friedman test and, if a significant difference (at least P<0.05) was found, the Wilcoxon signed rank test for paired replicates was used post hoc to determine which pairs differed. The significance of differences between different groups (i.e. effects of adenosine receptor agonists on body temperature vs vehicle, effects on dystonia of caffeine or adenosine receptor agonists administered alone vs co-administrations) was evaluated by the Kruska – Wallis test and, if a significant difference (at least P<0.05) was found, the Mann – Whitney U-test was used to analyse which groups differed.
Results
Effects of adenosine receptor antagonists
Methylxanthines (non-selective adenosine A1/A2A receptor antagonists)
In dtsz hamsters, caffeine significantly accelerated the progression of dystonia at doses of 10 and 20 mg kg−1 i.p., i.e., the severity of dystonia was significantly increased during the first hour of observation (Figure 1) and the latency to onset of dystonia was significantly decreased (Table 1). Furthermore, the latency to stage 6 was significantly reduced to 60.0±6.8 min vs 128.0±18.2 min (pre-drug control, P<0.01) or 122.5±18.7 min (post-drug control, P<0.05) after administration of 10 mg kg−1 i.p. and to 29,6±4.5 min vs 102.2±18.6 min (pre-drug control, P<0.05) or 120.2±9.2 min (post-drug control, P<0.05) after i.p. injection of 20 mg kg−1. At a lower dose of 5 mg kg−1 i.p. (not illustrated) caffeine did not exert any prodystonic effects. Behavioural effects were moderate hyperlocomotion during the first hour after administration of 20 mg kg−1 i.p., while no side effects could be observed at the lower doses.
Figure 1.
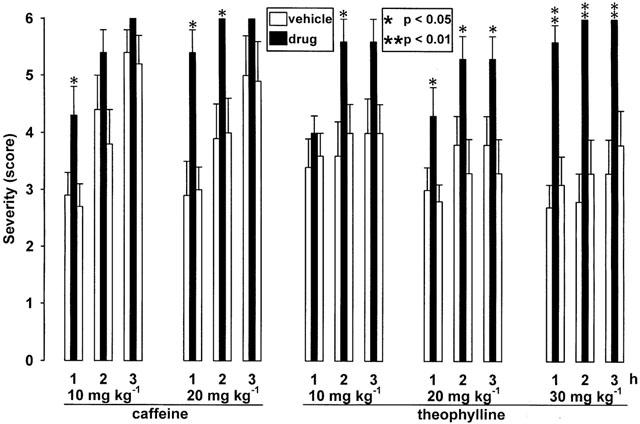
Effects of the methylxanthines caffeine (10.0 and 20.0 mg kg−1 i.p.) and theophylline (10.0, 20.0 and 30.0 mg kg−1 s.c.) on severity of dystonia in mutant hamsters. Usually, the individual maximum severity of dystonia is reached within 3 h after induction of dystonia by triple stimulation including the injection of drugs (black bars) or vehicle for pre- and post-drug controls (open bars). The figure shows the average of the maximum individual severity scores of dystonia reached within the first, second and third hour after administration of the methylxanthine or vehicle, reflecting the progression of dystonia in dtsz hamsters after treatment with the active compound and during control recordings. Control recordings were undertaken 2 days before (pre-drug control) and 2 days after (post-drug control) the drug trial. Asterisks indicate significant aggravation of dystonia in comparison to the pre- and post-drug control (*P<0.05, **P<0.01). Data are shown as means±s.e. of seven (20 mg kg−1 caffeine), eight (10 and 20 mg kg−1 theophylline), nine (30 mg kg−1 theophylline) or 10 (10 mg kg−1 caffeine) dystonic hamsters. Absence of s.e. bars indicates that all hamsters had reached the same severity.
Table 1.
Drug effects on latency to onset of dystonia in dtsz mutant hamsters
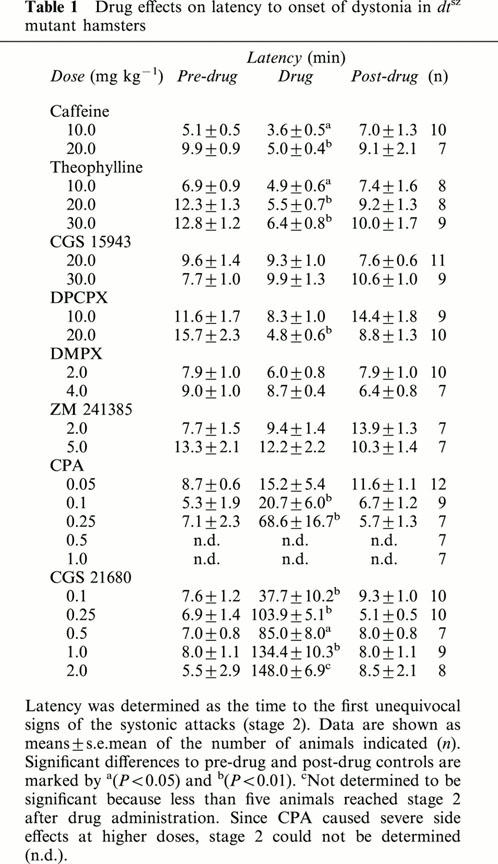
Theophylline caused a marked aggravation of dystonia after subcutaneous injections of 10, 20 and 30 mg kg−1 (Figure 1), but not at a dose of 5 mg kg−1 (not illustrated). By using animal groups with lower expression of dystonia (see Figure 1, pre- and post-drug control data) than those used for the experiments with caffeine, not only an acceleration of the progression of dystonia, but also a significant increase of the maximum severity reached during the 3-h observation period became evident after treatment with 20 and 30 mg kg−1 s.c. As shown in Table 1, theophylline significantly decreased the latency to onset of dystonia. Intraperitoneal injections of 20 mg kg−1 of theophylline exerted only short-lasting effects, but accelerated the progression of dystonia, i.e., a significant increase of the severity was observed during the first hour after administration (not illustrated) and the latency to onset of dystonia was significantly reduced. At low doses of 5 and 10 mg kg−1 s.c. theophylline did not cause any adverse effects. Twenty mg kg−1 s.c. moderately increased the locomotor activity only during the first 20 min after administration, while 30 mg kg−1 s.c. caused an increased urinary output and more marked hyperlocomotion, which lasted up to 60 min after injection.
The selective adenosine A1/2A receptor antagonist
CGS 15943 significantly increased the individual maximum severity of dystonia (i.e., the severity reached during the third hour of observation) at a dose of 30 mg kg−1 i.p., but not at a lower dose of 20 mg kg−1 (Figure 2). At both tested doses, CGS 15943 tended to increase the severity also during the first 2 h after administration. CGS 15943 did not reduce the latency to onset of dystonia (Table 1), probably because of a delayed onset of action as indicated by the relatively late occurrence of behavioural effects. About 15 min after i.p. administration of 20 or 30 mg kg−1 the hamsters showed a moderate hyperactivity which lasted for about 60 min.
Figure 2.
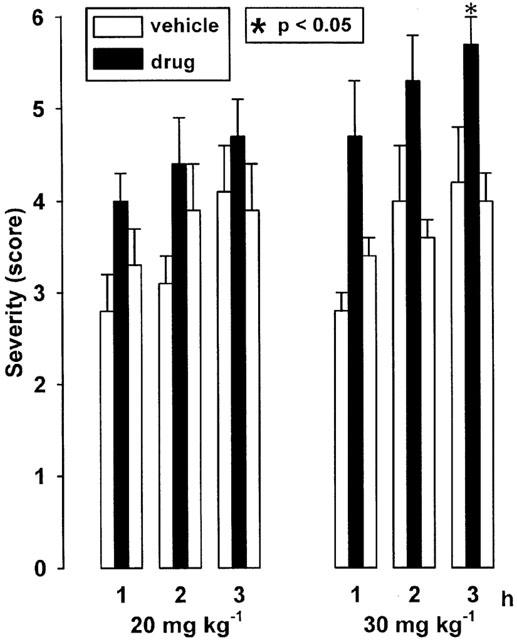
Effect of the adenosine A1/2A receptor antagonist CGS 15943 on severity of dystonia in mutant hamsters after administration of 20 and 30 mg kg−1 i.p. The figure shows the average of the maximum individual severity scores of dystonia reached within the first, second and third hour after administration of CGS 15943. Control recordings were taken 2 days before (pre-drug control) and 2 days after (post-drug control) the drug trial. Asterisks indicate significant aggravation of dystonia in comparison to the pre- and post-drug control (*P<0.05). Data are shown as means±s.e. of 11 (20 mg kg−1) or nine (30 mg kg−1) hamsters. For further explanation see Figure 1 legend.
The selective adenosine A1 receptor antagonist
DPCPX significantly increased the severity of dystonia at doses of 10 and 20 mg kg−1 (Figure 3). The latency to onset of dystonia was significantly reduced after administration of 20 mg kg−1 i.p. DPCPX (Table 1). The hamsters exhibited a moderate hyperactivity 10 – 40 min after i.p. injection of 10 mg kg−1. At a dose of 20 mg kg−1 i.p. a more marked hyperlocomotion was observed already after 3 min and lasted for about 1 h.
Figure 3.
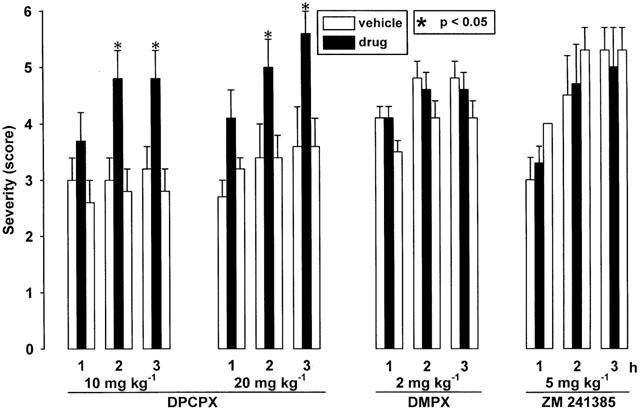
Effect of the adenosine A1 receptor antagonist DPCPX (10 and 20 mg kg−1 i.p.), of the A2 receptor antagonist DMPX (2 mg kg−1 i.p.) and of the A2A antagonist ZM 241385 (5 mg kg−1 i.p.) on severity of dystonia in mutant hamsters. The figure shows the average of the maximum individual severity scores of dystonia reached within the first, second and third hour after drug administration. Control recordings were taken 2 days before (pre-drug control) and 2 days after (post-drug control) the drug trial. Asterisks indicate significant aggravation of dystonia in comparison to the pre- and post-drug control (*P<0.05). Data are shown as means±s.e. of seven (ZM 241385), nine (10 mg kg−1 DPCPX) or 10 (2 mg kg−1 DMPX, 20 mg kg−1 DPCPX) mutant hamsters. For further explanation see Figure 1 legend.
In contrast to DPCPX, the adenosine A2 receptor antagonist DMPX at a dose of 2 mg i.p. (Figure 3) or at a higher dose of 4 mg kg−1 i.p. (not illustrated) and the selective A2A receptor antagonist ZM 241385 at doses of 2 (not illustrated) and 5 mg kg−1 i.p. (Figure 3) failed to exert any effects on the severity of dystonia and on latency to onset (Table 1). Both compounds caused a moderate hyperlocomotion at the tested doses during the first 30 – 60 min after administration.
Effects of adenosine receptor agonists
As shown in Figure 4, the adenosine A1 receptor agonist CPA exerted enormous antidystonic effects. At a dose of 1.0 mg kg−1 i.p., CPA completely prevented the occurrence of dystonia during the first 2 h after the induction of an attack by the triple stimulation. A delayed progression of dystonia and a dose-dependent decrease of the individual maximum severity of dystonia, reached at the end of the 3-h observation period, was also observed after i.p. administration of 0.1, 0.25 and 0.5 mg kg−1 (Figure 4), while 0.05 mg kg−1 did not exert significant effects on the severity or latency to onset of dystonia (not illustrated). A significant increase of the latency to onset of dystonia could be observed at doses of 0.1 and 0.25 mg kg−1 i.p. (Table 1), while central side effects exerted at higher doses disturbed the evaluation of stage 2. Striking antidystonic effects after treatment with 1.0 mg kg−1 i.p. were accompanied by severe adverse effects, i.e., a marked ataxia, sedation, piloerection and a loss of spontaneous locomotor activity. However, determinations of the descent latency in a block test, i.e. the time in which the hamsters maintained the forelimbs on a block (6 cm high), did not reveal any catalepsy. The reduced locomotor activity observed 5 min after treatment lasted for about 2 h, followed by short periods of head tremor. Ataxia and hypolocomotion were also intensive after i.p. injections of 0.5 mg kg−1 CPA. At a dose of 0.25 mg kg−1 i.p., the depressant effect on motor activity was still present and the animals showed a moderate ataxia within the first 2 h, while no behavioural effects could be observed at lower doses of 0.1 and 0.05 mg kg−1 i.p.
Figure 4.
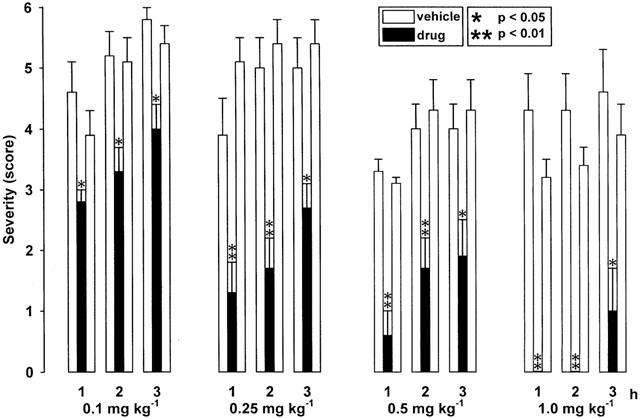
Effect of the adenosine A1 receptor agonist CPA on severity of dystonia in mutant hamsters after administration of 0.1, 0.25, 0.5 and 1.0 mg kg−1 i.p. The figure shows the average of the maximum individual severity scores of dystonia reached within the first, second and third hour after administration of CPA. Control recordings were taken 2 days before (pre-drug control) and 2 days after (post-drug control) the drug trial. Asterisks indicate significant reduction of dystonia in comparison to the pre- and post-drug control (*P<0.05; **P<0.01). Data are shown as means±s.e. of seven (0.25 – 1.0 mg kg−1) or nine (0.1 mg kg−1) dtsz hamsters. For further explanation see Figure 1.
The adenosine A2A receptor agonist CGS 21680 significantly decreased the severity of dystonia (Figure 5) and increased the latency to onset (Table 1) not only at the higher doses of 0.5 – 2 mg kg−1 i.p., as shown by recent examinations (Richter et al., 2000). Significant antidystonic effects could be already observed at doses of 0.1 mg kg−1 i.p., which did not provoke any observable side effects, and of 0.25 mg kg−1 i.p. which caused a moderate reduction of the spontaneous activity 5 – 70 min after administration. As indicated by the early disappearance of adverse effects, the duration of action of CGS 21680 was shorter than this of CPA at a same dose of 0.25 mg kg−1 i.p. Even at a high dose of 2.0 mg kg−1 i.p., CGS 21680 exerted less marked adverse effects than CPA at doses of 0.5 and 1 mg kg−1 i.p.
Figure 5.
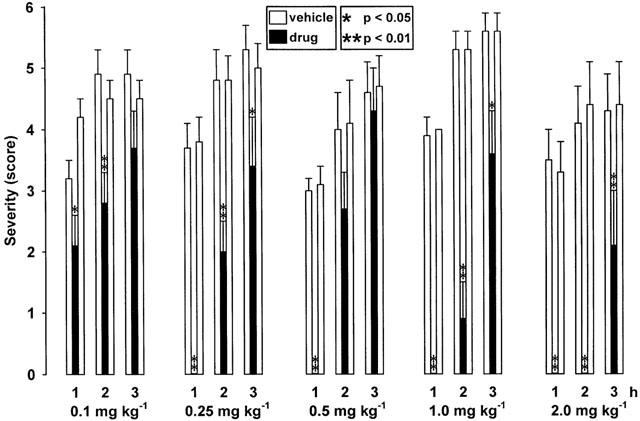
Effect of the adenosine A2A receptor agonist CGS 21680 on severity of dystonia in mutant hamsters after administration of 0.1, 0.25, 0.5, 1.0 and 2.0 mg kg−1 i.p. Note that CGS 21680 already exerts antidystonic effects at lower doses of 0.1 and 0.25 mg kg−1 than at those described recently (0.5 – 2.0 mg kg−1; Richter et al., 2000). The figure shows the average of the maximum individual severity scores of dystonia reached within the first, second and third hour after administration of CGS 21680. Control recordings were taken 2 days before (pre-drug control) and 2 days after (post-drug control) the drug trial. Asterisks indicate a significant reduction of dystonia in comparison to the pre- and post-drug control (*P<0.05; **P<0.01). Data are shown as means±s.e. of eight (2 mg kg−1), nine (1 mg kg−1) or 10 (0.1, 0.25 mg kg−1) hamsters. For further explanation see Figure 1 legend.
As determined in an additional group of five mutant hamsters, CGS 21680 and CPA decreased the rectal body temperature at a dose of 0.5 mg kg−1 i.p. in comparison to the vehicle control group (Figure 6). CPA, but not CGS 21680, caused a significant hypothermia 30 and 60 min after administration in comparison to animals of the same group prior treatment, while the body temperature increased after injection of the vehicle Tween 80, probably caused by an enhanced muscle activity during dystonic attacks.
Figure 6.
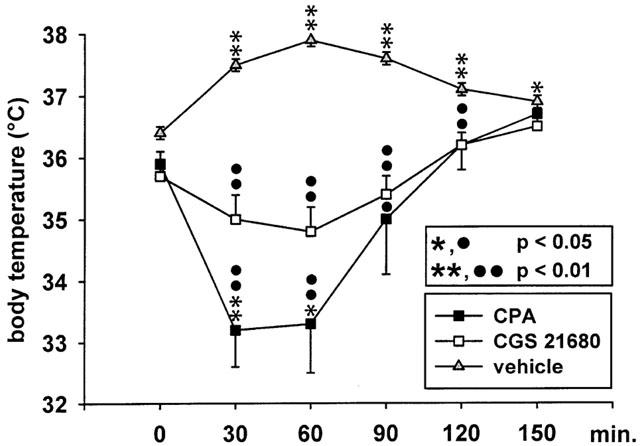
Effect of the adenosine A1 receptor agonist CPA (0.5 mg kg−1 i.p.) and of the adenosine A2A receptor agonist CGS 21680 (0.5 mg kg−1 i.p.) on rectal temperature in a group of dtsz mutant hamsters. The figure shows the average body temperature prior (0 min) and after drug administrations (30 – 150 min) in comparison to a control group treated with the vehicle (Tween 80). Data are shown as means±s.e. of five (CPA and CGS 21680) or six (vehicle control) mutant hamsters. Asterisks indicate significant differences after injections of vehicle or CPA versus prior treatment in the same group of animals (*P<0.05, *P<0.01). Circles indicate a significant decrease in comparison to the vehicle control group (•P<0.05; ••P<0.01).
As shown in Figure 7, i.p. injections of antidystonic effective doses (0.25 and 0.5 mg kg−1) of the A1 receptor agonist CPA 10 min prior administration of 20 mg kg−1 i.p. caffeine could counteract the prodystonic effects of caffeine. Otherwise, caffeine significantly reduced the antidystonic efficacy of CPA (compare Figure 4), obtained after single administrations of 0.25 mg kg−1 i.p. (first to second hour, P<0.01) and of 0.5 mg kg−1 i.p. (first hour not significant; second hour P<0.01; third hour P<0.05). Pretreatments with the A2A receptor agonist CGS 21680 at doses of 0.25 and 0.5 mg kg−1 i.p. failed to prevent the caffeine-provoked aggravation of dystonia in mutant hamsters (Figure 7). The antidystonic efficacy of CGS 21680, observed after single administrations (see Figure 5), completely disappeared after treatment with caffeine (P<0.01 vs single injections of 0.25 or 0.5 mg kg−1 i.p. during the first and second hour; P<0.05 vs both doses during the third hour after single administrations).
Figure 7.
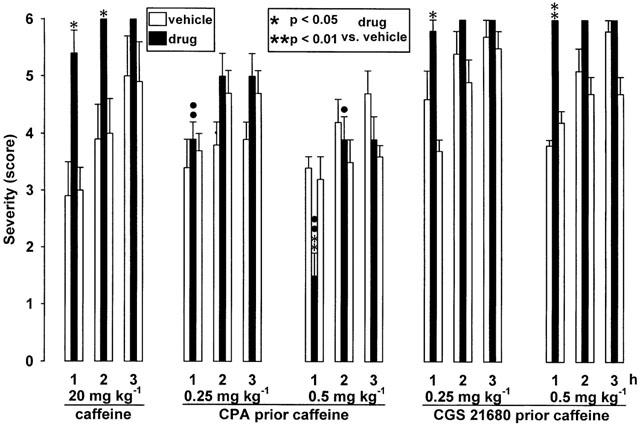
Pretreatments with the adenosine A2A receptor agonist CGS 21680 (0.25 mg and 0.5 kg−1 i.p.) or with the A1 receptor agonist CPA (0.25 and 0.5 mg kg−1 i.p.) 10 min prior i.p. injections of 20 mg kg−1 caffeine. The figure shows the average of the maximum individual severity scores of dystonia reached within the first, second and third hour after caffeine alone and after pretreatments. Asterisks indicate significant differences of the severity of dystonia in comparison to the pre- and post-drug control (*P<0.05). Circles indicate a significant reduction of the differences of severity score (caffeine vs control data) in animal groups pretreated with CPA (•P<0.05; ••P<0.01). Data are shown as means±s.e. of seven (20 mg kg−1 caffeine), nine (caffeine after pretreatment with 0.5 mg kg−1 CPA or CGS 21680) or 10 (caffeine after 0.25 mg kg−1 CPA or CGS 21680) mutant hamsters. For further explanation see Figure 1 legend.
Discussion
The present data showing significant prodystonic effects of the methylxanthines caffeine and theophylline substantiate the dtsz mutant hamster as a valid animal model of hereditary paroxysmal dystonic choreoathetosis (PDC) because ingestion of coffee and tea are (apart from stress and exercise) the most common precipitating factors of dystonic episodes in patients (Demirkiran & Jankovic, 1995; Fahn, 1994). Caffeine and theophylline worsened dystonia in dtsz hamsters already at doses which did not provoke hyperlocomotion, i.e., the prodystonic effects are not merely due to an increase in locomotor activity. Considering a shorter half-life time of methylxanthines in rodents than in man (Fredholm, 1995), prodystonic effective levels in dtsz hamsters are probably comparable to those reached after coffee intake in humans. Matsuo et al. (1999) reported that patients with PDC developed severe attacks within 40 min after oral intake of 300 mg caffeine, an amount known to be contained in about three cups of coffee.
Methylxanthines inhibit adenosine A1 and A2A receptors at low concentrations, while inhibition of phosphodiesterase and of intracellular calcium release occurs at much higher brain levels than those attained by usual caffeine consumption (Fredholm, 1995). In fact, the prodystonic effects of the selective adenosine A1/A2A receptor antagonist CGS 15943 and of the A1 antagonist DPCPX found in this study indicate that the induction of dystonic episodes by methylxanthines is, at least in part, related to their adenosine receptor antagonist action. The prodystonic effects of caffeine and theophylline were, however, more pronounced than those of CGS 15943, an antagonist with a higher affinity for A2A than A1 receptors (Ongini & Fredholm, 1996) and A2/2A receptor antagonists failed to exert any effects on the severity of dystonia in dtsz hamsters. In view of the behavioural effects caused by both A1 and A2 antagonists, it seems unlikely that the lack of prodystonic effects of A2 receptor inhibitors is related to insufficient penetration of these drugs into the brain. Therefore, the precipitating effects of methylxanthines seem to be predominantly mediated by an adenosine A1 receptor blockade. This is substantiated by the finding that pretreatment with the A1 receptor agonist CPA, but not with the A2A agonist CGS 21680, counteracted the prodystonic effect of caffeine.
Several studies have shown that methylxanthines and inhibitors of A1 receptors increase striatal dopamine release, while A2A receptor blockers do not cause any changes in extracellular dopamine levels in the rodent striatum (Ferre et al., 1993; Okada et al., 1997; Zetterström & Fillenz, 1990). There is strong evidence that striatal dopaminergic overactivity plays an important role in the manifestation of paroxysmal dystonia in mutant hamsters (Nobrega et al., 1996; Rehders et al., 2000). Thus, a further increase in dopamine release in the striatum provoked by A1 receptor blockade, is probably relevant for the prodystonic effects of methylxanthines and DPCPX in dtsz mutants. Ongoing microdialysis studies in mutant hamsters have to clarify whether the aggravation of dystonia caused by caffeine is accompanied by a rise of extracellular dopamine levels.
Recent investigations suggested that the striato-entopeduncular activity is enhanced in dtsz mutants (Gernert et al., 1999; 2000). The activity of striato-entopeduncular neurons is regulated via interacting adenosine A1 and D1 receptors (Ferre et al., 1996; 1997). A further increase of striato-entopeduncular activity by A1 receptor blockers can therefore also be important for the prodystonic effects of methylxanthines and DPCPX in dystonic hamsters. In contrast to A1 receptor blockade, adenosine A1 receptor agonists are known to reduce striatal dopamine release and striato-entopeduncular activity (Ferre et al., 1996; Okada et al., 1997), which may explain the striking antidystonic efficacy of CPA in dtsz mutants.
Apart from methylxanthines, known as triggers of dystonic attacks in patients with PDC (Demirkiran & Jankovic, 1995; Fahn, 1994), the present data suggest that antagonists of A1 receptors, but not of A2 receptors, may carry a risk of inducing dyskinetic episodes in predisposed individuals. Interestingly, adenosine A2A receptor antagonists have been reported to exert anti-parkinsonian efficacy in parkinsonian monkeys without leading to dyskinesias (Kanda et al., 1998). This observation is in line with the present finding that A2A receptor inhibitors did not worsen paroxysmal dyskinesia in mutant hamsters.
Stimulation of A2A receptors, which are co-localized with D2 receptors on striatopallidal neurons, completely counteracts a D2 receptor agonist-induced inhibition of these GABAergic projection neurons (Ferre et al., 1997). This mechanism may provide an explanation for the pronounced beneficial effects of the A2A receptor agonist CGS 21680 in dystonic hamsters, because previous intrastriatal injections of the dopamine D2 receptor agonist quinpirole worsened dystonia in dtsz hamsters (Rehders et al., 2000). However, adenosine also modulates other neurotransmitters, such as GABA release from interneurons, which is increased by A2A receptor agonists (Sebastiao & Ribeiro, 2000). Since dopaminergic overactivity is probably secondary to an inborn deficit of striatal GABAergic interneurons in dtsz hamsters (Gernert et al., 2000), the antidystonic efficacy of CGS 21680 could also be related to increased GABA release. Thus, further striatal microinjections of adenosine and dopamine receptor ligands in mutant hamsters have to clarify if the present findings with adenosine receptor agonists and antagonists are related to their interaction with the dopaminergic system.
As suggested by Ferre et al. (1997), the present results indicate that adenosine receptor agonists could be interesting candidates for the treatment of movement disorders which are related to striatal dopaminergic overactivity. Paroxysmal dyskinesias are usually worsened by L-dopa, while benzodiazepines and neuroleptics exert beneficial effects in PDC (Fahn, 1994; Przuntek & Monninger, 1983). Fink et al. (1997) proposed that dystonic episodes occur during a period of striatal dopamine depletion after caffeine-induced dopamine release. Methylxanthines provoke an increase in extracellular dopamine levels in the rat striatum within 1 h of administration (Okada et al., 1997). The fast onset of prodystonic effects of methylxanthines in mutant hamsters, shown in the present study, and the rapid occurrence of dystonic episodes in patients with PDC after oral caffeine intake (Matsuo et al., 1999), suggest that dopaminergic overactivity is essential for the manifestation of this type of paroxysmal dystonia.
The striking antidystonic efficacy of CPA and CGS21680, demonstrated in the present study, suggests that adenosine A1 and A2A receptor agonists may provide new approaches for the treatment of paroxysmal dystonia, but adverse effects may limit their therapeutic use. As observed in the present study in dtsz hamsters, both the A1 agonist CPA and the A2A agonist CGS 21680 exert hypothermia and depressant effects on locomotor activity similar to neuroleptics (Fuxe et al., 1998; Richter & Löscher, 1993). Hypotensive effects due to vasodilatation can be provoked by A1 and A2A agonists as well as by neuroleptics (Nyce, 1999; Ongini & Fredholm, 1996). While long-term treatment with neuroleptics, however, is associated with a risk of tardive dyskinesia (Marsden & Quinn, 1990), adenosine A2A receptor agonists may attenuate tardive dyskinesias (Fuxe et al., 1998). Therefore, in types of dystonia in which neuroleptics exert beneficial effects, such as in paroxysmal dystonia (Fahn, 1994; Richter & Löscher, 1993), adenosine A2A receptor agonists may prove advantageous. Furthermore, in dtsz hamsters the antidystonic efficacy of CGS21680 was more marked than that of neuroleptics (Richter & Löscher, 1993). In contrast to CPA, which is also known to cause a depression of cardiac function and bronchoconstriction (Nyce, 1999), CGS 21680 exerted marked antidystonic action not only at high doses, as shown in a recent study in dtsz mutants (Richter et al., 2000), but also at well tolerated doses.
In summary, the present data indicates that induction of dystonic attacks by methylxanthines in PDC is, at least in part, related to their adenosine antagonistic effects. The pronounced antidystonic efficacy of adenosine receptor agonists in dtsz mutants indicates that these compounds could provide new approaches for the treatment of hyperkinetic basal ganglia disorders. In view of the antidystonic efficacy of CGS 21680 at well tolerated doses, particularly adenosine A2A receptor agonists are interesting candidates for the treatment of paroxysmal dystonia.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (Ri 845/1-1). We thank Mrs C. Bartling for her excellent technical assistance.
Abbreviations
- CGS 15943
9-chloro2-2-furyl[1,2,4]triazolo[1,5-c]quinazolin-5-amine
- CGS 21680
2p-(2carboxyethylphen-ethylamino-5′-N-ethylcarboxamindoadenosine
- CPA
N6-cyclopentyladenosine
- DMPX
3,7-dimethyl-1-propargylxanthine
- DPCPX
8-cyclopentyl-1,3-dipropylxanthine
- PDC
paroxysmal dystonic choreoathetosis
- ZM 241385
4-(2-[7-amino-2-(2-furyl)]1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol
References
- ABERCROMBIE E.D., KEEFE K.A., DIFRISCHIA D.F., ZIGMOND M.J. Differential effects of stress on in vivo dopamine release in striatum, nucleus accumbens and medial frontal cortex. J. Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- BHATIA K.P., MARSDEN C.D. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain. 1994;117:859–876. doi: 10.1093/brain/117.4.859. [DOI] [PubMed] [Google Scholar]
- DEMIRKIRAN M., JANKOVIC J. Paroxysmal diskinesias: clinical features and classification. Ann. Neurol. 1995;38:571–579. doi: 10.1002/ana.410380405. [DOI] [PubMed] [Google Scholar]
- FAHN S.The paroxysmal dyskinesias Movement Disorders 3 1994Oxford: Butterworth-Heinemann; 311–345.eds. Marsden, C.D. & Fahn, S. pp [Google Scholar]
- FAHN S., BRESSMAN S.B., MARSDEN C.D.Classification of dystonia Advances in Neurology, vol. 78, Dystonia 3 1998New York: Lippincott-Raven; 1–10.eds. Fahn, S., Marsden, C.D. & DeLong, M.R [PubMed] [Google Scholar]
- FERRE S., O'CONNOR W.T., FUXE K., UNGERSTEDT U. The striatopallidal neuron: A main locus for adenosine-dopamine interactions in the brain. J. Neurosci. 1993;13:5402–5406. doi: 10.1523/JNEUROSCI.13-12-05402.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRE S., O'CONNOR W.T., SVENNINGSSON P., BJÖRKLUND L., LINDBERG J., TINNER B., STRÖMBERG I., GOLDSTEIN M., ÖGREN S., UNGERSTEDT U., FREDHOLM B., FUXE K. Dopamine D1 receptor-mediated facilitation of GABAergic neurotransmission in the rat striatoentopeduncular pathway and its modulation of adenosine A1 receptor-mediated mechanism. Eur. J. Neurosci. 1996;8:1545–1553. doi: 10.1111/j.1460-9568.1996.tb01617.x. [DOI] [PubMed] [Google Scholar]
- FERRE S., FREDHOLM B.B., MORELLI M., POPOLI P., FUXE K. Adenosine-dopamine receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- FERRE S., FUXE K., VON EULER G., JOHANNSON B., FREDHOLM B.B. Adenosine-dopamine interactions in the brain. Neuroscience. 1992;51:501–512. doi: 10.1016/0306-4522(92)90291-9. [DOI] [PubMed] [Google Scholar]
- FINK J.K., HEDERA P., MATHAY J.G., ALBIN R.L. Paroxysmal dystonic choreoathetosis linked to chromosome 2q: Clinical analysis and proposed pathophysiology. Neurology. 1997;49:177–183. doi: 10.1212/wnl.49.1.177. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B. Adenosine, adenosine receptors and the actions of caffeine. Pharmacol. Toxicol. 1995;76:93–101. doi: 10.1111/j.1600-0773.1995.tb00111.x. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B., ABBRACCHIO M.P., BURNSTOCK G., DALY J.W., HARDEN T.K., JACOBSEB K.A., LEFF P., WILLIAMS M. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- FREDOW G., LÖSCHER W. Effects of pharmacological manipulation of GABAergic neurotransmission in a new mutant hamster model of paroxysmal dystonia. Eur. J. Pharmacol. 1991;192:207–219. doi: 10.1016/0014-2999(91)90045-r. [DOI] [PubMed] [Google Scholar]
- FUXE K., FERRE S., ZOLI M., AGNATI L.F. Integrated events in central dopamine transmission as analysed at multiple levels. Evidence for intramembrane adenosine A2A/dopamine D2 and adenosine A1/dopamine D1 receptor interactions in the basal ganglia. Brain Res. Rev. 1998;26:258–273. doi: 10.1016/s0165-0173(97)00049-0. [DOI] [PubMed] [Google Scholar]
- GERNERT M., HAMANN M., BENNAY M., LÖSCHER W., RICHTER A. Deficit of striatal parvalbumin-reactive GABAergic interneurons and decreased basal ganglia output in a genetic rodent model of idiopathic paroxysmal dystonia. J. Neurosci. 2000;20:7052–7058. doi: 10.1523/JNEUROSCI.20-18-07052.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERNERT M., RICHTER A., LÖSCHER W. In vivo extracellular electrophysiology of pallidal neurons in dystonic and nondystonic hamsters. J. Neurosci. Res. 1999;57:894–905. [PubMed] [Google Scholar]
- JACOBSON K.E., VAN RHEE M.Development of selective purinoceptor agonists and antagonists Purinergic approaches in experimental therapeutics 1997New York: Wiley-Liss; 101–128.eds. Jacobson, K.A. & Jarvis, M.F. pp [Google Scholar]
- JANKOVIC J., FAHN S.Dystonic disorders Parkinson's Disease and Movement Disorders 1998New York: Lippincott-Williams & Wilkins; 513–552.eds. Jankovic, J. & Tolosa, E. pp [Google Scholar]
- KANDA T., JACKSON M.J., SMITH L.A., PEARCE R.K.B., NAKAMURA J., KASE H., KUWANA Y., JENNER P. Adenosine A2A antagonist: A novel antiparkinsonian agent that does not provoke dyskinesia in parkinsonian monkeys. Ann. Neurol. 1998;43:507–513. doi: 10.1002/ana.410430415. [DOI] [PubMed] [Google Scholar]
- KLITGAARD H., KNUTSEN L.J.S., THOMSEN C. Contrasting effects of adenosine A1 and A2 receptor ligands in different chemoconvulsive rodent models. Eur. J. Pharmacol. 1993;242:221–228. doi: 10.1016/0014-2999(93)90245-d. [DOI] [PubMed] [Google Scholar]
- LÖSCHER W., FISHER J.E., JR, SCHMIDT D., FREDOW G., HÖNACK D., ITURRIAN W.B. The sz mutant hamster: a genetic model of epilepsy or of paroxysmal dystonia. Movement Dis. 1989;4:219–232. doi: 10.1002/mds.870040304. [DOI] [PubMed] [Google Scholar]
- MARSDEN C.D., QUINN N.P. The dystonias. Br. Med. J. 1990;300:139–144. doi: 10.1136/bmj.300.6718.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUO H., KAMAKURA K., SAITO M., OKANO M., NAGASE T., TADANO Y., KAIDA K., HIRATA A., MIYAMOTO N., MASAKI T., NAKAMURA R., MOTOYOSHI K., TANAKA H., TSUJI S. Familial paroxysmal dystonic choreoathetosis. Clinical findings in a large Japanese Family and genetic linkage to 2q. Arch. Neurol. 1999;56:721–726. doi: 10.1001/archneur.56.6.721. [DOI] [PubMed] [Google Scholar]
- MÜLLER C.E. A2A adenosine receptor antagonists – future drugs for Parkinson's disease. Drug Future. 2000;25:1043–1052. [Google Scholar]
- NOBREGA J.N., RICHTER A., TOZMAN N., JIWA D., LÖSCHER W. Quantitative autoradiography reveals regionally selective changes in dopamine D1 and D2 receptor binding in the genetically dystonic hamster. Neuroscience. 1996;71:927–936. doi: 10.1016/0306-4522(95)00511-0. [DOI] [PubMed] [Google Scholar]
- NYCE J.W. Insight into adenosine receptor function using antisense and gene-knockout approaches. Trends Pharmacol. Sci. 1999;20:79–83. doi: 10.1016/s0165-6147(99)01305-x. [DOI] [PubMed] [Google Scholar]
- OKADA M., KIRYU K., KAWATA Y., MIZUNO K., WADA K., TASAKI H., KANEKO S. Determination of the effects of caffeine and carbamazepine on striatal dopamine by in vivo microdialysis. Eur. J. Pharmacol. 1997;321:181–188. doi: 10.1016/s0014-2999(96)00938-7. [DOI] [PubMed] [Google Scholar]
- ONGINI E., FREDHOLM B.B. Pharmacology of adenosine A2A receptors. Trends Pharmacol. Sci. 1996;17:364–372. [PubMed] [Google Scholar]
- PINNA A., DI CHIARA G., WARDAS J., MORELLI M. Blockade of A2A adenosine receptors positively modulates turning behaviour and c-Fos expression induced by D1 agonists in dopamine denervated rats. Eur. J. Neurosci. 1996;8:1176–1181. doi: 10.1111/j.1460-9568.1996.tb01285.x. [DOI] [PubMed] [Google Scholar]
- PRZUNTEK H., MONNINGER P. Therapeutic aspects of kinesigenic paroxysmal choreoathesosis and familial paroxysmal choreoathetosis of the Mount and Reback type. J. Neurol. 1983;230:163–169. doi: 10.1007/BF00313627. [DOI] [PubMed] [Google Scholar]
- REHDERS J.H., LÖSCHER W., RICHTER A. Evidence for striatal dopaminergic overactivity in paroxysmal dystonia indicated by microinjections in a genetic rodent model. Neuroscience. 2000;97:267–277. doi: 10.1016/s0306-4522(00)00073-7. [DOI] [PubMed] [Google Scholar]
- RICHTER A., HAMANN M., BARTLING C. CGS 21680 exerts marked antidystonic effects in a genetic model of paroxysmal dyskinesia. Eur. J. Pharmacol. 2000;404:299–302. doi: 10.1016/s0014-2999(00)00627-0. [DOI] [PubMed] [Google Scholar]
- RICHTER A., LÖSCHER W. The atypical neuroleptic, clozapine, exerts antidystonic activity in a mutant hamster model. Comparison with haloperidol. Eur. J. Pharmacol. 1993;242:309–312. doi: 10.1016/0014-2999(93)90256-h. [DOI] [PubMed] [Google Scholar]
- RICHTER A., LÖSCHER W. Behavioural response to pharmacologic manipulation of serotonin receptors in the genetically dystonic hamster. Pharmacol. Biochem. Behav. 1995;52:655–665. doi: 10.1016/0091-3057(95)00162-p. [DOI] [PubMed] [Google Scholar]
- RICHTER A., LÖSCHER W. Pathology of idiopathic dystonia: findings from genetic animal model. Prog. Neurobiol. 1998;54:633–677. doi: 10.1016/s0301-0082(97)00089-0. [DOI] [PubMed] [Google Scholar]
- SEBASTIAO A.M., RIBEIRO J.A. Fine-tuning neuromodulation by adenosine. Trends Pharmacol. Sci. 2000;21:341–346. doi: 10.1016/s0165-6147(00)01517-0. [DOI] [PubMed] [Google Scholar]
- TODD R.D., PERLMUTTER J.S. Mutational and biochemical analysis of dopamine in dystonia. Mol. Neurobiol. 1998;16:135–147. doi: 10.1007/BF02740641. [DOI] [PubMed] [Google Scholar]
- YACOUBI M.E., LEDENT C., MENARD J.-F., PARMENTIER M., COSTENTIN J., VAUGEOIS J.-M. The stimulant effects on locomotor behaviour in mice are mediated through its blockade of adenosine A2A receptors. Br. J. Pharmacol. 2000;129:1465–1473. doi: 10.1038/sj.bjp.0703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZETTERSTRÖM T., FILLENZ M. Adenosine agonists can both inhibit and enhance in vivo striatal dopamine release. Eur. J. Pharmacol. 1990;180:137–141. doi: 10.1016/0014-2999(90)90601-2. [DOI] [PubMed] [Google Scholar]


