Abstract
Extracellularly added P1,P3-di(adenosine-5′) triphosphate (Ap3A), P1,P4-di(adenosine-5′) tetraphosphate (Ap4A), ATP, ADP, AMP and adenosine are growth inhibitory for rat C6 glioma cells. Analysis of nucleotide hydrolysis and the use of nucleotidase inhibitors demonstrated that the latter inhibition is due to hydrolysis of the nucleotides to adenosine.
Agonists of the P2YAC−-receptor enhance the growth of C6 cells if their hydrolysis to adenosine is inhibited by pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS). In these conditions, the potency to stimulate cell growth parallels the ranking of the receptor agonists, i.e. 2-methylthioadenosine-5′-diphosphate (2MeSADP)>Ap3A>Ap4A. ATP and ADP are still hydrolysed in the presence of PPADS and have no proliferative effect on C6 cells.
The enhanced growth is due to a P2YAC−-receptor-mediated activation of p42/44 mitogen-activated protein kinase (MAPK) as shown by immunoblotting and protein kinase assays for active MAPK and the use of the MAPK/extracellular signal-regulated kinase kinase (MEK) inhibitor PD98059.
The UTP-induced enhancement of the growth of C6 cells is due to activation of MAPK by a PPADS sensitive nucleotide receptor.
In conclusion, the effect of nucleotides on the growth of C6 cells is determined by ecto-nucleotidases and by activation of nucleotide receptors. Hydrolysis of nucleotides to adenosine induces growth inhibition while inhibition of the hydrolysis of agonists of the P2YAC−-receptor enhances cell growth by activation of MAPK.
Keywords: Ap3A, Ap4A, C6 cells, growth inhibition, MAPK, NPPase, nucleotide receptor, proliferation, purinoceptor, P2YAC−-receptor
Introduction
Nucleotides, and in particular ATP and adenosine, have been reported to affect the proliferation of different cell types. The effects are not always unequivocal, e.g. ATP stimulates the proliferation of primary cultures of astrocytes and rat aortic smooth muscle cells (Abbracchio et al., 1994; Harper et al., 1998), is ineffective on the growth of transformed mouse fibroblasts (Weisman et al., 1988) and has an inhibitory effect on the growth of several human tumour cell lines (Seetulsingh-Goorah & Stewart, 1998; Rapaport et al., 1983). Adenosine has a growth inhibitory effect on most of the examined cell types (Seetulsingh-Goorah & Stewart, 1998; Fishman et al., 1998), although a growth stimulation is also reported (Lelievre et al., 1998). This apparent discrepancy may be explained by the existence of several mechanisms that take place at the cell surface: firstly, extracellular nucleotides are degraded by a cascade of cell surface-bound enzymes, i.e. ecto-ATPase, ecto-apyrase, ecto-nucleotide pyrophosphatase/phosphodiesterase I (NPPase) and ecto-5′-nucleotidase, hydrolysing ATP into ADP, AMP and adenosine (Zimmermann, 1996). Cellular uptake of the latter by adenosine transporters can induce an adenosine-dependent pyrimidine starvation resulting in inhibition of proliferation (Lasso de la Vega et al., 1994). Secondly, the added nucleotides and their breakdown products affect cell proliferation by activation of nucleotide receptors, coupled to phospolipase C (PLC) or adenylate cyclase (AC). In addition, ATP may activate ecto-protein kinases that modulate the activity of autocrine growth factors and growth inhibitors (Vilgrain & Baird, 1991; Friedberg et al., 1995). The inhibitory effect of ATP and other adenosine phosphates on cell proliferation may be explained by one or a combination of these mechanisms.
Two main classes of nucleotide receptors, P1 and P2, have been described (Burnstock, 1978). The P1-receptors are mainly responsive to adenosine. The P1-receptor subtypes A1 and A3 are negatively, and A2 is positively coupled to AC. The P2-receptors, activated by ATP/ADP or UTP/UDP, are subdivided in metabotropic P2Y- and ionotropic P2X- receptors. P2Y-receptors are coupled to a PLC-dependent Ca2+ mobilization and activation of protein kinase C (PKC) or to AC (P2Y11, P2Y12), while activation of the P2X-receptors, ligand gated ion-channels, generate a Ca2+-influx (Fredholm et al., 1994; Communi et al., 1997; Boeynaems et al., 2000; Hollopeter et al., 2001).
We investigated the effect of adenosine and its mono- and dinucleotides on the proliferation of rat C6 glioma cells. The latter is a bipotential cell line often used as a model system for astrocytes. Several purinergic receptors are reported to be present on C6 cells: an A2b-receptor, a ‘nucleotide receptor' coupled to PLC and activated by all nucleoside triphosphates, a PLC-coupled P2Y6-receptor activated by UDP, and a P2Y-receptor negatively coupled to AC presumed to be the P2Y12-receptor (Ohkubo et al., 2001; Lin & Chuang, 1994; Nicholas et al., 1996; Boyer et al., 1993; Hollopeter et al., 2001). The agonist potency of the latter AC-coupled receptor is more or less the same as that of the P2Y1-receptor and has hitherto been called the P2Y1-like receptor (Albert et al., 1997; Grobben et al., 2000). However, in contrast to the P2Y1-receptor, this receptor is not coupled to PLC and it is not sensitive to the antagonists PPADS (pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid) and 5′-phosphoadenosine 3′-phosphate (Boyer et al., 1994). Although Webb et al. (1996) concluded that the P2Y1-like- and the P2Y1-receptor are identical but coupled to a different second messenger system, Schachter et al. (1997) disproved this finding. To describe more accurately the pharmacological properties of the P2Y1-like receptor we propose the name P2YAC− for future reference to this receptor. The recently cloned P2Y12-receptor has been proposed to be present on C6 glioma cells (Hollopeter et al., 2001). However, ATP, an antagonist for the P2Y12-receptor, is an agonist for the P2YAC−-receptor (Grobben et al., 2000), suggesting that P2Y12 and P2YAC− are different receptors. When the P2YAC−-receptor is cloned and appears to be a unique receptor, and not the P2Y1-receptor coupled to a different second messenger system, the P2Yn nomenclature can be used.
In this study we examined the effect of adenosine nucleotides on the growth of C6 cells. Both growth inhibitory and proliferative effects were observed, depending on whether the added adenosine nucleotides were hydrolysed by ecto-nucleotidases or not. Hydrolysis to adenosine proved to be essential for growth inhibition. We previously studied the hydrolysis of extracellular nucleotides, and identified NPPase as the main ATP hydrolysing ecto-enzyme of C6 cells (Grobben et al., 1999). When nucleotide hydrolysis was inhibited by PPADS, an enhanced proliferation was observed for all potent agonists of the P2YAC−-receptor, except for ADP and to a lesser extent ATP, which are hydrolysed even in the presence of PPADS. The enhanced proliferation is due to a P2YAC−-receptor-mediated activation of p42/44 MAPK.
Methods
Materials
Nucleotides, nucleotide derivatives and (−)-isoproterenol were from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), and Reactive Blue 2 (RB2) were from RBI (Köln, Germany). [3H]-myo-inositol (21 Ci mmol−1) was from NEN (Boston, MA, U.S.A.). PD98059 was from Calbiochem (La Jolla, CA, U.S.A.).
Cell culture
Rat C6 glioma cells (ATCC CCL 107) were obtained from ATCC (Manassas, VA, U.S.A.) and maintained in monolayer culture as described previously (Slegers & Joniau, 1996). Experiments were performed in 96-well plates or 34 mm petri dishes on cells cultured in serum-free chemically defined medium containing Ham's F10/minimal essential medium (MEM, 1 : 1 v v−1), 2 mM L-glutamine, 1% (v v−1), MEM vitamines (100×), 1% (v v−1) MEM non-essential amino acids (100×), 100 U ml−1 penicillin, 100 μg ml−1 streptomycin (GIBCO, Paisley, Scotland), and 30 nM sodium selenite (Sigma Chemical Co.). Cell numbers were measured in a haemocytometer after cell detachment with trypsin/EDTA in Dulbecco's phosphate-buffered saline (1 : 50).
Measurement of intracellular cyclic AMP concentration
C6 cells were cultivated in 96-well plates in serum-free chemically defined medium up to a cell density of 2.5×105 cells cm−2. Cyclic AMP synthesis was induced by addition of 5 μM (−)-isoproterenol. The effect of extracellular nucleotides on the activity of adenylate cyclase was determined by simultaneous addition of the nucleotide and (−)-isoproterenol. After a 30 min incubation at 37°C, the intracellular cyclic AMP concentration was determined with a cyclic AMP-enzyme immunoassay kit [Amersham Pharmacia Biotech, Buckinghamshire, England] according to the manufacturer's instructions.
Measurement of phosphatidylinositol hydrolysis
C6 cells were grown in 35 mm petri dishes. At a density of 1×105 cells cm−2, cells were labelled overnight with 2 μCi ml−1 of [3H]-myo-inositol in serum-free chemically defined medium. Subsequently, cells were washed with 2×2 ml chemically defined medium supplemented with 10 mM LiCl and incubated for 30 min. After addition of the indicated effectors, cells were incubated for another 30 min. The reactions were terminated by aspiration of the medium and addition of 1 ml of 5% (w v−1) trichloroacetic acid (TCA). Dishes were shaken for 15 min and the supernatant was collected. TCA was removed by washing with 3×1 ml diethylether. The aqueous fraction was applied on a column containing 0.5 g dowex AG 1-X8 resin (100 – 200 mesh) in formate form (BioRad, Hertfordshire, U.K.). Free inositol was eluted with 2×10 ml 50 mM ammoniumformate. Inositol mono-, di- and triphosphates were eluted with 10 ml 1 M ammoniumformate, 0.1 M formic acid. The remaining radioactivity was eluted with 4 M ammoniumformate, 0.1 M formic acid. Radioactivity was measured in a liquid scintillation counter (Tri-Carb 1600 TR, Packard, Meriden, U.S.A.).
Measurement of nucleotide hydrolysis
Assays were performed in the logarithmic growth phase and at a cell density of approximately 1.35×105 cells cm−2. Cells were incubated for 15 min with PPADS or RB2 prior to addition of the indicated nucleotides. The medium was aspirated at the indicated time points. Samples were diluted 1 : 1 with buffer A [200 mM potassium phosphate (pH 6.0), 8 mM tetrabutylammonium hydrogen sulphate (Fluka, Bornem, Belgium)] and 100 μl was injected onto a reverse phase C18 column (Vydac, Hesperia, CA, U.S.A.). Bound nucleotides were eluted with a gradient from buffer A to buffer B [100 mM potassium phosphate (pH 6.0), 8 mM tetrabutylammonium hydrogen sulphate, 30% (v v−1) methanol]: 0 min 100% A, 0% B; 9 min 100% A, 0% B; 15 min 75% A, 25% B; 22.5 min 10% A, 90% B; 25 min 0% A, 100% B; 30 min 100% A, 0% B. Nucleotides were detected by absorption measurement at 254 nm and eluted with a retention time of 8.5 min (AMP), 9.6 min (adenosine), 16.1 min (ADP) and 24.1 min (ATP).
Detection of p42/44 MAPK activation
C6 cells were cultivated in 96-well plates in serum-free chemically defined medium up to a cell density of 1.2×105 cells cm−2. Cells were incubated for 15 min with nucleotide receptor antagonists before stimulation with nucleotides. Incubation was at 37°C for 10 min. The reaction was stopped by aspiration of the medium and addition of 1 μl 10−3 cells−1 SDS – PAGE sample buffer (6 mM Tris/HCl (pH 6.8), 0.5% (w v−1) SDS, 10% (v v−1) glycerol, 0.5% (v v−1) 2-mercaptoethanol]. Samples were boiled for 5 min, and 20 μl was analysed by SDS – PAGE on a 12.5% (w v−1) polyacrylamide gel. Proteins were electroblotted overnight onto a nitrocellulose membrane (Hybond-C pure, Amersham Pharmacia Biotech, Buckinghamshire, U.K.). Immunodetection was performed using pAbs (1:5×103) raised against p44 (ERK1) and p42 (ERK2) (Anti-ACTIVE® MAPK, Promega, Leiden, The Netherlands). The nitrocellulose membrane was incubated with horseradish peroxidase conjugated donkey anti-rabbit antibody (1:4×104) and active MAPK was visualized on film (X-OMAT blue, Kodak, Rochester, N.Y., U.S.A.) by enhanced chemiluminescence (ECL, Amersham Pharmacia Biotech, Buckinghamshire, U.K.).
In gel assay for p42/44 MAP kinase
The assay was performed as described by Kameshita & Fujisawa (1989) with slight modifications. Cell lysates were prepared as described in the previous paragraph, and proteins were seperated on a SDS-polyacrylamide gel consisting of a stacking gel and a 12.5% (w v−1) separation gel containing 0.5 mg ml−1 myelin basic protein (MBP) added prior to polymerization. After electrophoresis, SDS was removed by incubation of the gel at room temperature in 2×100 ml of 20% (v v−1) 2-propanol, 50 mM Tris-HCl (pH 8.0) for 1 h, and incubation in 250 ml of 50 mM Tris-HCl (pH 8.0), 5 mM 2-mercaptoethanol for 1 h. Subsequently, proteins were denatured by incubation of the gel in 2×100 ml of 6M guanidine-HCl, 50 mM Tris-HCl (pH 8.0), 5 mM 2-mercaptoethanol for 1 h, and renatured by addition of 6×250 ml 50 mM Tris-HCl (pH 8.0), 5 mM 2-mercaptoethanol, 0.04% (v v−1) Tween 40 at 4°C for 16 h. After renaturation of the proteins, the gel was preincubated at room temperature for 30 min with 100 ml 40 mM HEPES – NaOH (pH 8.0), 2 mM dithiotreitol, 0.1 mM EGTA, 5 mM MgCl2. The in gel kinase assay was initiated by addition of 25 μM [γ-32P]ATP (25 μCi) in the same buffer. After incubation for 1 h, the non-incorporated radioactivity was removed by repeated washes with 5% (w v−1) TCA for 4 h. The gel was dried and the incorporated radioactivity was detected using a phospho-imager (PhosphoImager SI, Molecular Dynamics, Amersham Pharmacia Biotech, Buckinghamshire, U.K.).
Statistical analysis
Results are represented as the means±s.e.mean calculated from at least three independent experiments. Statistically significant differences were calculated using the Student's t-test.
Results
Hydrolysis of nucleotides to adenosine determines the growth inhibition of C6 cells
Adenosine, AMP, ADP, ATP, Ap3A and Ap4A inhibit the growth of C6 cells (Figure 1). Previously we demonstrated that adenosine nucleotides, as well as diadenosine polyphosphates (ApnA), are rapidly hydrolysed to adenosine by this cell line (Grobben et al., 1999, 2000). To investigate whether hydrolysis of the added nucleotides is essential for growth inhibition, several experiments were conducted. To inhibit ecto-NPPase, the main mono- and dinucleotide hydrolysing ecto-enzyme of C6 cells (Grobben et al., 1999), we used inhibitors of this enzyme, i.e. Reactive Blue 2 (RB2) and pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS). PPADS as well as RB2 blocked the growth inhibitory effect of ATP and ApnA, while the effect of ADP was only blocked by RB2. No effect was observed on the AMP- and adenosine-induced growth inhibition. Except for the result of ADP, these data indicated that the hydrolysis by ecto-nucleotidases is required for inhibition of the cell growth induced by adenine nucleotides. In contrast, UTP, a rapidly hydrolysed nucleotide (Grobben et al., 1999) and 2-methylthioADP (2MeSADP), a non-hydrolysable ADP analogue, did not inhibit but even enhanced the growth of C6 cells (Figure 1).
Figure 1.
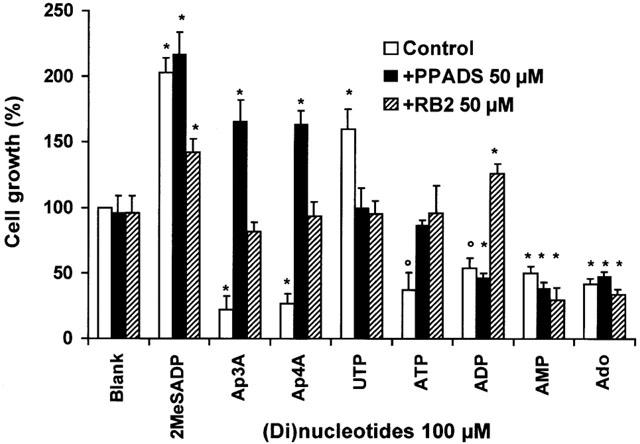
Effect of mono- and dinucleotides on cell proliferation in the presence and absence of purinergic receptor antagonists. Cells were grown in serum-free chemically defined medium in 96-well plates. At a density of 1.0 – 1.4×105 cells cm−2 nucleotides (100 μM) were added to the cells, preceded by a 15 min incubation with PPADS or RB2 (50 μM) as indicated. After 48 h of cultivation, cell numbers were counted in a haemocytometer. The number of cells grown without stimulation was taken as 100%. Data are the means±s.e.mean of three independent experiments. The statistically significant difference from control is indicated (*P<0.01; °P<0.02).
To investigate further the growth inhibitory effect of ADP even in the presence of PPADS, we compared the hydrolysis rate of 100 μM ADP (1.28 fmol min−1 cell−1) and ATP (1.10 fmol min−1 cell−1) in the presence of 50 μM PPADS or RB2 (Figure 2). The hydrolysis of ADP was reduced 5.4 fold in the presence of RB2 (0.24 fmol min−1 cell−1) but only 1.7 fold in the presence of PPADS (0.75 fmol min−1 cell−1). The hydrolysis of ADP in the presence of PPADS was 1.8 fold faster than that of ATP in the presence of PPADS (0.42 fmol min−1 cell−1) and explains the adenosine-mediated growth inhibition by ADP in the presence of PPADS. RB2 almost completely inhibited the hydrolysis of ADP and reversed growth inhibition into a moderate growth enhancement (Figure 1). These data demonstrated that hydrolysis of nucleotides into AMP and/or adenosine is a prerequisite for the growth inhibition of C6 cells.
Figure 2.
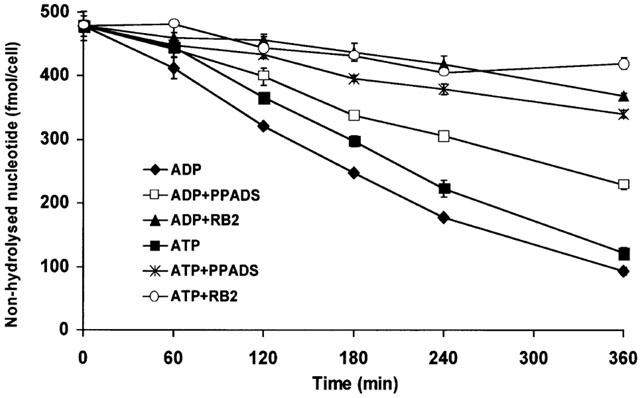
Hydrolysis of ADP and ATP in the presence of PPADS and RB2. Cells were grown in serum-free chemically defined medium in 96-well plates. At a density of 1.35×105 cells cm−2 nucleotides (100 μM) were added to the cells, preceded by a 15 min incubation with PPADS or RB2 (50 μM) as indicated. The medium was aspirated at the indicated time points and the remaining amount of ATP or ADP (fmol cell−1) determined by hydrophobic ion-pairing chromatography. Data are the means±s.e.mean of three independent experiments.
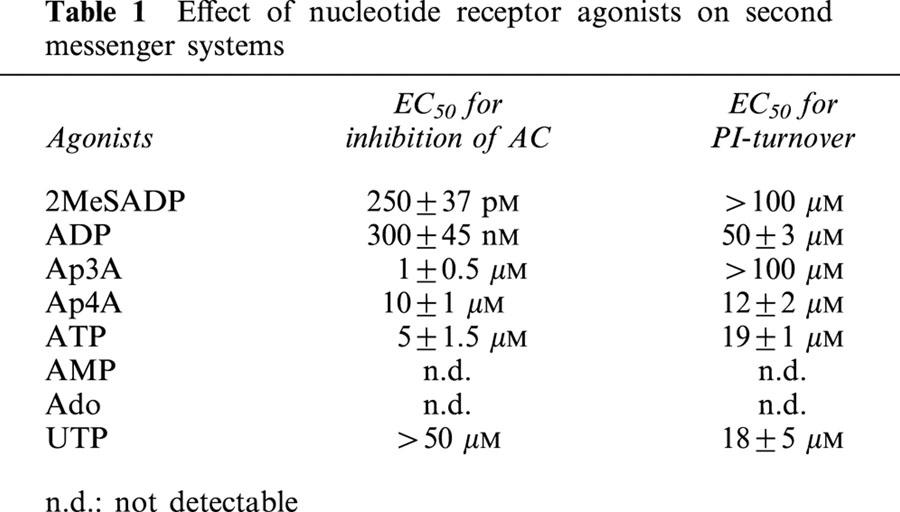
PPADS and RB2 are widely used P2Y-receptor antagonists, and it is not excluded that the effect of these compounds on the nucleotide-mediated growth inhibition is due to their antagonizing effect on nucleotide receptors. To demonstrate that P2Y-receptor activation is not involved in the induction of growth inhibition, we tested whether the growth inhibition is correlated with the nucleotide potency to activate P2Y-receptors. Therefore we determined the potency of the adenosine nucleotides and UTP to activate PLC- or AC-coupled receptors in C6 cells (Table 1). Ap4A (EC50 12±2 μM) and ATP (EC50 19±1 μM) appeared to be the most potent P2 receptor-agonist for PLC activation and stimulated PI-turnover more potently than ADP (EC50 50±3 μM). Since the PLC-coupled P2Y2-receptor was recently reported to enhance the proliferation of C6 cells (Tu et al., 2000), we also tested the effect of the P2Y2 agonist UTP. The EC50 for PLC activation by UTP was 18±5 μM. Agonists of the P2YAC−-receptor inhibit the (−)-isoproterenol-induced activation of AC. The obtained EC50 values for P2YAC−-activation by adenosine mono- and dinucleotides are compiled in Table 1. Adenosine and AMP had no measurable effect on PI-turnover and on AC activity, excluding the involvement of PLC- and AC-coupled P2Y-receptors in the mechanism of growth inhibition of C6 cells.
Table 1.
Effect of nucleotide receptor agonists on second messenger systems
Activation of the P2YAC−-receptor enhances cell proliferation
Based on inhibition of the (−)-isoproterenol-induced AC activation, the agonist potency for the P2YAC−-receptor was determined to be 2MeSADP>ADP>Ap3A>ATP>Ap4A (Table 1). With the exception of ATP and ADP, all these agonists enhanced the growth of C6 cells when their hydrolysis was blocked with PPADS. 2MeSADP had a strong proliferative effect in the absence and presence of PPADS (210±20%) and in the presence of RB2 (142±11%) (Figure 1). In the case of Ap3A and Ap4A, growth inhibition was inverted into growth stimulation when the cells were preincubated with PPADS, but not with RB2. In the presence of RB2, ATP and ADP had no effect and a minor growth stimulatory effect respectively. Activation of MAPK is correlated with cell proliferation. Therefore, we measured the effect of P2YAC−-receptor agonists on p42/44 MAPK activation in the absence and presence of PPADS and RB2 (50 μM). We determined this activation by immunoblotting against phosphorylated MAPK, and also demonstrated that the phosphorylation was correlated with the enzymatic activity by an in gel kinase assay using MBP as a substrate (Figure 3).
Figure 3.
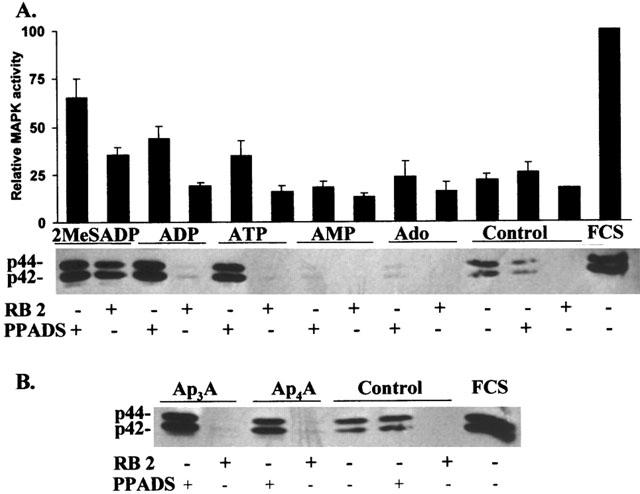
Nucleotide-mediated activation of p42/44 MAPK. (A) Cells were grown in serum-free chemically defined medium in 96-well plates. At a density of 1.0 – 1.4×105 cells cm−2 mononucleotides (100 μM) were added to the cells, preceded by a 15 min incubation with PPADS or RB2 (50 μM) as indicated. After 10 min, the medium was removed, the cells were dissolved in SDS – PAGE buffer and analysed for MAPK activation by immunoblotting. The blot shown is representative for three independent experiments. Samples of the blot were analysed for MAPK activation by an in gel kinase assay using MBP as a substrate as described in Methods. Stimulation of the cells with 10% (v v−1) foetal calf serum was used as a positive control and was taken as 100% for the in gel kinase assay. Data are the mean±s.e.mean of three independent experiments. (B) Cells were grown as described in A. Dinucleotides (100 μM) were added to the cells preceded by a 15 min incubation with PPADS or RB2 (50 μM) as indicated. Immunoblotting was performed as described in A.
Agonists of the P2YAC−-receptor activated p42/44 MAPK in the presence of PPADS. The observed activation also correlated with the observed growth stimulation by 2MeSADP and the minor growth stimulation by ADP in the presence of the antagonist RB2. Indeed 2MeSADP, the most potent agonist of the P2YAC−-receptor, is still partially activating MAPK in the presence of RB2 (Figure 3), while the effect of the less potent P2YAC−-agonists ADP, ATP, Ap3A and Ap4A was blocked by RB2.
Since 2MeSADP does not induce PI-turnover in C6 cells and thus is a specific and potent agonist of the P2YAC−-receptor of these cells, we determined the concentration-response of 2MeSADP on cell growth (Figure 4). An EC50 of 250 – 500 pM was measured for the activation of MAPK. This value corresponds with the EC50 for the inhibition of AC by P2YAC−-activation (Table 1), resulting in an EC50 of 10 nM for the stimulation of proliferation measured after 48 h. The MEK inhibitor PD98059 was used to demonstrate that the activation of p42/44 MAPK is necessary for the growth enhancement (Figure 5). The enhanced proliferation induced by 2MeSADP was reduced to the basal level when C6 cells were stimulated in the presence of PD98059, proving that the P2YAC−-mediated growth enhancement is p42/44 MAPK dependent.
Figure 4.
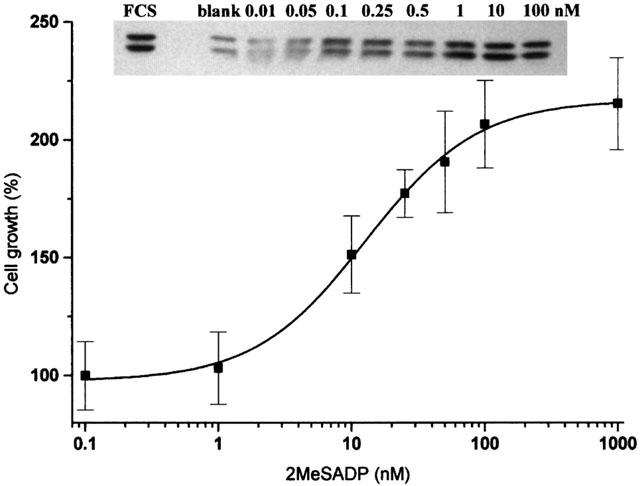
Concentration-response of 2MeSADP on cell proliferation and p42/44 MAPK activation. Cells were grown in serum-free chemically defined medium in 96-well plates. At a density of 1.0×105 cells cm−2 a varying concentration of 2MeSADP was added to the cells. After 10 min the medium was removed, the cells were dissolved in SDS – PAGE buffer and analysed for MAPK activation by immunoblotting (insert). Stimulation of the cells with 10% (v v−1) foetal calf serum was used as a positive control. The blot shown is representative for three independent experiments. In parallel, cells were grown for 48 h and cell numbers were counted in a haemocytometer. The number of cells grown without stimulation was taken as 100%. Data are the means±s.e.mean of three independent experiments.
Figure 5.
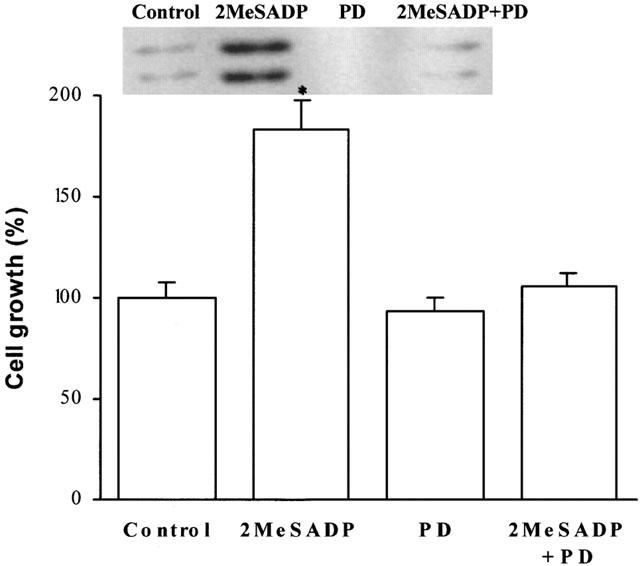
The nucleotide-mediated growth enhancement is p42/44 MAPK dependent. Cells were grown in serum-free chemically defined medium in 96-well plates. At a density of 1.0 – 1.4×105 cells cm−2, 2MeSADP (100 nM) was added to the cells, preceded by a 3 h incubation with the MEK inhibitor PD98059 (5 μM). After a 48 h incubation, cell numbers were counted in a haemocytometer. The number of cells grown without stimulation was taken as 100%. Data are the means±s.e.mean of three independent experiments. The statistically significant difference from control is indicated (*P<0.05). Insert: In parallel, cells were dissolved in SDS – PAGE buffer 10 min after stimulation with 2MeSADP and analysed for p42/44 MAPK activation by immunoblotting.
Recently, Tu et al. (2000) have reported that MAPK is activated by ATP and UTP in C6 cells through activation of the P2Y2-receptor. In agreement with these data, UTP had a stimulatory effect on cell proliferation. This effect was blocked by RB2 and PPADS (Figure 1), in contrast to the growth enhancement induced by activation of the PPADS insensitive P2YAC−-receptor.
Discussion
Hydrolysable adenosine mono- and dinucleotides have a growth inhibitory effect on C6 cells. The inhibition is due to hydrolysis of the added nucleotides to AMP and adenosine. All growth inhibitory nucleotides have been shown to be substrates of the ecto-NPPase, the main nucleotide hydrolysing ecto-enzyme of C6 cells. This enzyme is potently inhibited by RB2 and PPADS (Grobben et al., 1999; 2000), and RB2 abolished the growth inhibition of all hydrolysable nucleotides tested. With the exception of ADP, addition of PPADS also blocked the growth inhibition of all nucleotides. Determination of the hydrolysis rate of ATP and ADP in the absence or presence of PPADS and RB2 demonstrated that ADP is still hydrolysed in the presence of PPADS. This indicated the presence of a RB2 sensitive ADP hydrolysing enzyme on the plasma membrane of C6 cells that is only weakly inhibited by PPADS. This enzyme could be an ADPase or an ATPDase preferentially hydrolysing ADP. In this context, it has already been reported that ATPDase is more potently inhibited by Evans Blue than by PPADS (Heine et al., 1999).
The observation that both the ADP-induced growth inhibition as well as the ADP hydrolysis are more potently blocked by RB2 than by PPADS, supports the proposition that hydrolysis of adenosine nucleotides to AMP and adenosine is the key mechanism for the nucleotide-induced growth inhibition. As AMP is rapidly converted into adenosine by 5′-nucleotidase, and PPADS as well as RB2 do not inhibit the latter enzyme, adenosine is the active compound in the nucleotide-mediated growth inhibition of C6 cells. Adenosine has been reported to inhibit cell proliferation by two different mechanisms. Firstly, adenosine uptake by adenosine transporters may cause an adenosine-dependent pyrimidine starvation (Weisman et al., 1988; Lasso de la Vega et al., 1994). Secondly, adenosine activates A2-receptors present on the plasma membrane (Jackson & Carlson, 1992), inducing growth inhibition by inactivation of the MAPK cascade (Hirano et al., 1996). It remains to be determined which mechanism is responsible for the observed growth inhibition of C6 cells.
PPADS inhibits the hydrolysis of diadenosine polyphosphates to such an extent that growth inhibition was converted into an enhanced proliferation comparable to the one observed with the nonhydrolysable nucleotide 2MeSADP. We have recently demonstrated that diadenosine polyphosphates are P2YAC−-agonists, and that this PPADS insensitive receptor activates p42/44 MAPK. Since 2MeSADP is the most potent agonist of the P2YAC−-receptor (Boyer et al., 1994), the presented data demonstrate that the enhanced growth is correlated with an activation of this receptor. In addition 50 μM RB2, an antagonist of the P2YAC−-receptor, is not sufficient to inhibit the nucleotide receptor-mediated p42/44 MAPK-activation of 100 μM 2MeSADP completely. We thus proved that activation of the P2Y-receptor, negatively coupled to AC, enhances cell growth by activation of p42/44 MAPK. Also, a basal level of p42/44 MAPK activity was observed in the cells. Since inhibition of this basal activity by PD98059 did not affect the growth rate of C6 cells (Figure 5), p42/44 MAPK activity appears to be non-essential for cell growth under normal conditions. In addition, RB2 blocks the basal activity of p42/44 MAPK, suggesting that C6 cells are permanently stimulated by an efflux of nucleotides. Indeed, a release of nucleotides by C6 cells has been reported (Lazarowski & Harden, 1999; Lazarowski et al., 2000). As is the case for PD98059, inhibition by RB2 of the basal autocrine p42/44 MAPK stimulation does not affect the normal growth of C6. Therefore, it is unlikely that the inhibition of basal p42/44 MAPK activity by AMP and adenosine as observed in Figure 4 is the cause of the growth inhibition.
Activation of the MAPK-signal transduction cascade by nucleotide receptors has been reported previously (Harper et al., 1998; Neary et al., 1998; 1999; Lenz et al., 2000). P2Y2 has been identified as the receptor through which the mitogenic signal of ATP and UTP is transduced into the cell (Miyagi et al., 1996; Soltoff et al., 1998; Tu et al., 2000). PPADS, an antagonist for several P2Y-receptors, blocked the growth stimulatory effect of UTP in C6 cells. Furthermore, Tu et al. (2000) reported that the UTP-mediated p42/44 MAPK activation is pertussis toxin insensitive. Since the P2Y2-receptor is PLC-coupled through the pertussis toxin sensitive Gi protein (Lustig et al., 1996; Soltoff et al., 1998), and the P2YAC−-receptor is PPADS insensitive, UTP activates MAPK by a different still unknown mechanism. As UTP in the absence of inhibitors of ecto-nucleotidases is still capable of enhancing cell proliferation, we may conclude that UMP and uridine, UTP-hydrolysis products generated by NPPase and 5′-nucleotidase, are not growth inhibitory for C6 cells. ATP is an agonist of both the PPADS sensitive P2Y2-receptor and the PPADS insensitive P2YAC−-receptor, and can thus activate MAPK by binding to both receptors. Notwithstanding this activation, no growth enhancement was observed after stimulation of C6 cells with ATP in the absence or presence of PPADS. In contrast to Ap3A and Ap4A, ATP is hydrolysed by a PPADS insensitive ecto-ATPase present on the plasma membrane of C6 cells (Grobben et al., 1999). Hydrolysis of ATP results in the formation of adenosine, a growth inhibitor of C6 cells, which explains the lack of growth enhancement by ATP.
In conclusion, the presented data demonstrated that P2YAC−-receptor agonists induce an enhanced growth of C6 cells. If these nucleotides are hydrolysed into the growth inhibitory adenosine, the growth enhancement is neutralized. In addition, the nucleotide-mediated cell growth is due to a P2YAC−-induced p42/44 MAPK activation.
Acknowledgments
This work is supported by the Concerted Research Action ‘96-‘00 ‘Control mechanisms of cell proliferation in eukaryotes', by the Fund for Scientific Research Flanders (H. Slegers). P. Claes is a fellow of the ‘Concerted Research Action'. K. Van Kolen is a fellow of the ‘Institute of Scientific Technology' (IWT), B. Grobben is a fellow of the ‘Emmanuel Van der Schueren foundation'.
Abbreviations
- Ap3A
P1,P3-di(adenosine-5′) triphosphate
- Ap4A
P1,P4-di(adenosine-5′) tetraphosphate
- ATPase
adenosine triphosphatase
- ATPDase
adenosine triphosphate diphosphohydrolase
- MAPK
mitogen-activated protein kinase
- MBP
myelin basic protein
- MEK
MAPK/extracellular-signal regulated kinase kinase
- MEM
minimal essential medium
- 2MeSADP
2-methylthioadenosine-5′-diphosphate
- NPPase
ecto-nucleotide pyrophosphatase/phosphodiesterase I
- PPADS
pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid
- RB2
reactive blue 2
- TCA
trichloroacetic acid
References
- ABBRACCHIO M.P., SAFFREY M.J., HÖPKER V., BURNSTOCK G. Modulation of astroglial cell proliferation by analogues of adenosine and ATP in primary cultures of rat striatum. Neuroscience. 1994;59:67–76. doi: 10.1016/0306-4522(94)90099-x. [DOI] [PubMed] [Google Scholar]
- ALBERT J.L., BOYLE J.P., ROBERTS J.A., CHALLIS R.A., GUBBY S.E., BOARDER M.R. Regulation of brain capillary endothelial cells by P2Y receptors coupled to Ca2+, phospholipase C and mitogen activated protein kinase. Br. J. Pharmacol. 1997;122:935–941. doi: 10.1038/sj.bjp.0701453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOEYNAEMS J.M., COMMUNI D., SAVI P., HERBERT J.M. P2Y receptors: in the middle of the road. TiPS. 2000;21:1–3. doi: 10.1016/s0165-6147(99)01415-7. [DOI] [PubMed] [Google Scholar]
- BOYER J.L., LAZAROWSKI E.R., CHEN X., HARDEN T.K. Identification of a P2Y-purinergic receptor that inhibits adenylyl cyclase. J. Pharmacol. Exp. Ther. 1993;276:1140–1146. [PubMed] [Google Scholar]
- BOYER J.L., ZOHN I.E., JACOBSON K.A., HARDEN T.K. Differential effects of P2-purinoceptor antagonists on phospholipase C- and adenylyl cyclase-coupled P2Y-purinoceptors. Br. J. Pharmacol. 1994;113:614–620. doi: 10.1111/j.1476-5381.1994.tb17034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G.A basis for distinguishing two types of purinergic receptors Cell Membrane Receptors for Drugs and Hormones: A Multidisciplinary Approach 1978New York: Raven Press; 107–118.ed. Straub, R.W. & Bolis L. pp [Google Scholar]
- COMMUNI D., GOVAERTS C., PARMENTIER M., BOEYNAEMS J.M. Cloning of a human purinergic P2Y receptor coupled to phospholipase C and adenylyl cyclase. J. Biol. Chem. 1997;272:31969–31973. doi: 10.1074/jbc.272.51.31969. [DOI] [PubMed] [Google Scholar]
- FISHMAN P., BARYEHUDA S.B., VAGMAN L. Adenosine and low molecular weight factors released by muscle cells inhibit tumor cell growth. Cancer Res. 1998;58:3181–3187. [PubMed] [Google Scholar]
- FREDHOLM B.B., ABBRACCHIO M., BURNSTOCK G., DALY J., KENDALL HARDEN T., JACOBSON K., LEFF P., WILLIAMS M. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 1994;146:143–156. [PMC free article] [PubMed] [Google Scholar]
- FRIEDBERG I., BELZER I., OGEDPLESZ O., KUEBLERF D. Activation of cell growth inhibitor by ectoprotein kinase-mediated phosphorylation in transformed mouse fibroblasts. J. Biol. Chem. 1995;270:20560–20567. doi: 10.1074/jbc.270.35.20560. [DOI] [PubMed] [Google Scholar]
- GROBBEN B., ANCIAUX K., ROYMANS D., STEFAN C., BOLLEN M., ESMANS E., SLEGERS H. An ecto-nucleotide pyrophosphatase is one of the main enzymes involved in the extracellular metabolism of ATP in rat C6 glioma. J. Neurochem. 1999;72:826–834. doi: 10.1046/j.1471-4159.1999.0720826.x. [DOI] [PubMed] [Google Scholar]
- GROBBEN B., CLAES P., ROYMANS D., ESMANS E.L., VAN ONCKELEN H., SLEGERS H. Ecto-nucleotide pyrophosphatase modulates the purinoceptor-mediated signal transduction and is inhibited by purinoceptor antagonists. Br. J. Pharmacol. 2000;130:139–145. doi: 10.1038/sj.bjp.0703289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARPER S., WEBB T.E., CHARLTON S.J., NG L.L., BOARDER M.R. Evidence that P2Y4 nucleotide receptors are involved in the regulation of rat aortic smooth muscle cells by UTP and ATP. Br. J. Pharmacol. 1998;124:703–710. doi: 10.1038/sj.bjp.0701895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEINE P., BRAUN N., HEILBRONN A., ZIMMERMANN H. Functional characterization of rat ecto-ATPase and ecto-ATP diphosphohydrolase after heterologous expression in CHO cells. Eur. J. Biochem. 1999;262:102–107. doi: 10.1046/j.1432-1327.1999.00347.x. [DOI] [PubMed] [Google Scholar]
- HIRANO D., AOKI Y., OGASAWARA H., KODAMA H., WAGA I., SAKANAKA C., SHIMIZU T., NAKAMURA M. Functional coupling of adenosine A2a receptor to inhibition of the mitogen-activated protein kinase cascade in chinese hamster ovary cells. Biochem. J. 1996;316:81–86. doi: 10.1042/bj3160081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLOPETER G., JANTZEN H.M., VINCENT D., LI G., ENGLAND L., RAMAKRISHNAN V., YANG R.B., NURDEN P., JULIUS D., CONLEY P.B. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–206. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- JACKSON J.A., CARLSON E.C. Inhibition of bovine retinal microvascular pericyte proliferation in vitro by adenosine. Am. J. Physiol. 1992;263:H634–H640. doi: 10.1152/ajpheart.1992.263.2.H634. [DOI] [PubMed] [Google Scholar]
- KAMESHITA I., FUJISAWA H. A sensitive method for detection of calmodulin-dependent protein kinase II activity in sodium dodecyl sulfate-polyacrylamide gel. Anal. Biochem. 1989;183:139–143. doi: 10.1016/0003-2697(89)90181-4. [DOI] [PubMed] [Google Scholar]
- LASSO DE LA VEGA M.C., TERRADEZ P., OBRADOR E., NAVARRO J., PELLICER J.A., ESTRELA J.M. Inhibition of cancer growth and selective glutathione depletion in Ehrlich tumour cells in vivo by extracellular ATP. Biochem. J. 1994;298:99–105. doi: 10.1042/bj2980099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAZAROWSKI E.R., HARDEN T.K. Quantitation of extracellular UTP using a sensitive enzymatic assay. Br. J. Pharmacol. 1999;127:1272–1278. doi: 10.1038/sj.bjp.0702654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAZAROWSKI E.R., BOUCHER R.C., HARDEN T.K. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J. Biol. Chem. 2000;275:31061–31068. doi: 10.1074/jbc.M003255200. [DOI] [PubMed] [Google Scholar]
- LELIEVRE V., MULLER J.M., FALCON J. Adenosine modulates cell proliferation in human colonic adenocarcinoma. I. Possible involvement of adenosine A1 receptor subtypes in HT29 cells. Eur. J. Pharmacol. 1998;341:289–297. doi: 10.1016/s0014-2999(97)01462-3. [DOI] [PubMed] [Google Scholar]
- LENZ G., GOTTFRIED C., LUO Z., AVRUCH J., RODNIGHT R., NIE W.-J., KANG Y., NEARY J.T. P2Y purinoceptor subtypes recruit different Mek activators in astrocytes. Br. J. Pharmacol. 2000;129:927–936. doi: 10.1038/sj.bjp.0703138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN W., CHUANG D. Different signal transduction pathways are coupled to the nucleotide receptor and the P2Y receptor in C6 glioma cells. J. Pharmacol. Exp. Ther. 1994;269:926–931. [PubMed] [Google Scholar]
- LUSTIG K.D., WEISMAN G.A., TURNER J.T., GARRAD R., SHIAU A.K., ERB L.P2U purinoceptors: cDNA cloning, signal transduction mechanisms and structure-function analysis P2 purinoceptors: localization, function and transduction mechanisms 1996198Chichester: John Wiley & Sons; 193–204.ed. Chadwick D.J., Goode J.Adiscussion 204-7 [DOI] [PubMed] [Google Scholar]
- MIYAGI Y., KOBAYASHI S., AHMED A., NISHIMURA J., FUKUI M., KANAIDE H. P2U purinergic activation leads to the cell cycle progression from the G1 to the S and M phases but not from the G0 to G1 phase in vascular smooth muscle cells in primary culture. Biochem. Biophys. Res. Commun. 1996;222:652–658. doi: 10.1006/bbrc.1996.0750. [DOI] [PubMed] [Google Scholar]
- NEARY J.T., KANG Y., BU Y., YU E., AKONG K., PETERS C.M. Mitogenic signaling by ATP/P2Y purinergic receptors in astrocytes: involvement of a calciumin-dependent protein kinase C, extracellular signal-regulated protein kinase pathway distinct from the phosphatidylinositol-specific phospholipase C/calcium pathway. J. Neurosci. 1999;19:4211–4220. doi: 10.1523/JNEUROSCI.19-11-04211.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEARY J.T., MCCARTHY M., KANG Y., ZUNIGA S. Mitogenic signalling from P1 and P2 purinergic receptors to mitogen-activated protein kinase in human fetal astrocyte cultures. Neurosci. Lett. 1998;242:159–162. doi: 10.1016/s0304-3940(98)00067-6. [DOI] [PubMed] [Google Scholar]
- NICHOLAS R.A., LAZAROWSKI E.R., WATT W.C., LI Q., BOYER J., HARDEN T.K. Pharmacological and second messenger signaling selectivities of cloned P2Y receptors. J. Autonomic Pharmacol. 1996;16:319–323. doi: 10.1111/j.1474-8673.1996.tb00044.x. [DOI] [PubMed] [Google Scholar]
- OHKUBO S., KUMAZAWA K., SAGAWA K., KIMURA J., MATSUAKO I. â,ã-methylene ATP-induced cAMP formation in C6Bu-1 cells: involvement of local metabolism and subsequent stimulation of adenosine A2B receptor. J. Neurochem. 2001;76:872–880. doi: 10.1046/j.1471-4159.2001.00098.x. [DOI] [PubMed] [Google Scholar]
- RAPAPORT E., FISHMAN R.F., GERCEL C. Growth inhibition of human tumor cells in soft-agar cultures by treatment with low levels of adenosine 5′-triphosphate. Cancer Res. 1983;43:4402–4406. [PubMed] [Google Scholar]
- SCHACHTER J.B., BOYER J.L., LI Q., NICHOLAS R.A., HARDEN T.K. Fidelity in functional coupling of the rat P2Y1 receptor to phospholipase C. Br. J. Pharmacol. 1997;122:1021–1024. doi: 10.1038/sj.bjp.0701479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEETULSINGH-GOORAH S.P., STEWART B.W. Growth inhibition of HL-60 cells by extracellular ATP: concentration-dependent involvement of a P2 receptor and adenosine generation. Bioch. Biophys. Res. Comm. 1998;250:390–396. doi: 10.1006/bbrc.1998.9329. [DOI] [PubMed] [Google Scholar]
- SLEGERS H., JONIAU M. Lipopolysaccharide-enhanced expression of interleukin-6 in dibutyryl cyclic AMP-differentiated rat C6 glioma. J. Neurochem. 1996;66:466–473. doi: 10.1046/j.1471-4159.1996.66020466.x. [DOI] [PubMed] [Google Scholar]
- SOLTOFF S.P., AVRAHAM H., AVRAHAM S., CANTLEY L.C. Activation of P2Y2 receptors by UTP and ATP stimulates mitogenactivated kinase activity through a pathway that involves related adhesion focal tyrosine kinase and protein kinase C. J. Biol. Chem. 1998;273:2653–2660. doi: 10.1074/jbc.273.5.2653. [DOI] [PubMed] [Google Scholar]
- TU M.-T., LUO S.-F., WANG C.-C., CHIEN C.-S., CHIU C.-T., LIN C.-C., YANG C.-M. P2Y2 receptor-mediated proliferation of C6 glioma cells via activation of Ras/Raf/MEK/MAPK pathway. Br. J. Pharmacol. 2000;129:1481–1489. doi: 10.1038/sj.bjp.0703182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VILGRAIN I., BAIRD A. Phosphorylation of basic fibroblast growth factor by a protein kinase associated with the outer surface of a target cell. Mol. Endocrinol. 1991;5:1003–1012. doi: 10.1210/mend-5-7-1003. [DOI] [PubMed] [Google Scholar]
- WEBB T., FEOLDE E., VIGNE P., NEARY J., RUNBERG A., FRELIN C., BARNARD E. The P2Y purinoceptor in rat brain microvascular endothelial cells couple to inhibition of adenylate cyclase. Br. J. Pharmacol. 1996;119:1385–1392. doi: 10.1111/j.1476-5381.1996.tb16050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISMAN G.A., LUSTIG K.D., LANE E., HUANG N., BELZER I., FRIEDBERG I. Growth inhibition of transformed mouse fibroblasts by adenine nucleotides occurs via generation of extracellular adenosine. J. Biol. Chem. 1988;263:12367–12372. [PubMed] [Google Scholar]
- ZIMMERMANN H. Biochemistry, localization and functional roles of ecto-nucleotidases in the nervous system. Prog. Neurobiol. 1996;49:589–618. doi: 10.1016/0301-0082(96)00026-3. [DOI] [PubMed] [Google Scholar]


