Abstract
This study examined the effects of trifluoromethylphenylimidazole (TRIM) on tone, and calcium entry, in mouse anococcygeus stimulated by either thapsigargin (Tg; 100 nM) which activates capacitative calcium entry (CCE), or high K (60 mM) which activates voltage-operated calcium channels. TRIM (1 – 333 μM) produced concentration-related relaxation of Tg-induced tone (EC50, 42 μM) but was much less effective against high K. In single smooth muscle cells loaded with FURA-2, TRIM reduced the increase in fluorescence ratio produced by Tg but had no effect on that produced by high K. The relaxations of Tg-induced tone, and reduction in fluorescence ratio, were obtained in the presence of L-NG-nitroarginine and were thus independent of nitric oxide synthase inhibition; further, TRIM had no discernible effect on nitrergic responses. TRIM provides a novel drug for the selective inhibition of CCE and a template for the development of more potent inhibitors.
Keywords: Anococcygeus (mouse), capacitative calcium entry, potassium, smooth muscle, thapsigargin, TRIM
Introduction
In many cell types, including smooth muscle (Gibson et al., 1998), depletion of the calcium stores within the endoplasmic/sarcoplasmic reticulum (ER/SR) activates calcium-permeable, store-operated channels (SOCs) in the plasma membrane, initiating so-called capacitative calcium entry (CCE; Putney, 1990; Berridge, 1995; Parekh & Penner, 1997; Putney & McKay, 1999). There is currently intense interest in CCE directed mainly towards identifying the signal between the ER/SR and the plasma membrane, the molecular identity of the SOC proteins, and the cellular functions dependent on calcium entry via this route (Putney & McKay, 1999; Harteneck et al., 2000). However, although drugs are available for the selective activation of CCE, including blockers of the ER/SR Ca-ATPase such as thapsigargin (Tg), research is hindered by a paucity of selective inhibitors of CCE. The most commonly used drugs, including SKF96365 (Merritt et al., 1990), inhibit calcium entry through both voltage-dependent and voltage-independent channels at similar concentrations (Clementi & Meldolesi, 1996). There is therefore a pressing need for the identification of more selective inhibitors of CCE. Here, we report that trifluoromethylphenylimidazole (TRIM) produces selective inhibition of Tg-induced CCE in the mouse anococcygeus muscle and that this action of TRIM is independent of its previously reported ability to inhibit nitric oxide synthase (Handy et al., 1995; Moore & Handy, 1997).
Methods
Tension studies
Male mice (LACA; 25 – 35 g) were killed by stunning and exsanguination. Anococcygeus muscles were dissected and set up for the recording of isometric tension responses to drugs and nitrergic field stimulation (10 Hz; 10 s trains every 100 s) as described previously (Gibson et al., 1994). The composition of the Krebs solution was (mM): NaCl 118.1, KCl 4.7, MgSO4 1.0, KH2PO4 1.0, glucose 11.1, NaHCO3 25.0, CaCl2 2.5; the solution was maintained at 37°C and gassed continuously with 95%O2:5%CO2. High K (60 mM) Krebs solution was prepared by increasing the amount of KCl and reducing NaCl appropriately to maintain isotonicity. In all experiments, the Krebs solution contained 1 μM phentolamine and 50 μM L-NG-nitroarginine (L-NOARG), with the exception that the latter was omitted in experiments involving nitrergic stimulation. When Tg was used to raise tone the Krebs solution also contained 10 μM verapamil to block Ltype voltage-operated calcium channels; at this concentration, verapamil completely prevented contraction to 60 mM K solution (not shown). To measure the effects of relaxant drugs (TRIM, papaverine, SKF96365, miconazole), muscle tone was raised with either 100 nM Tg or 60 mM K; once a stable increase in tension had been established, the relaxants were applied and the per cent relaxation of induced tone calculated after 4 min.
Fura-2 fluorescence
Single smooth muscle cells were isolated from the mouse anococcygeus by enzymic digestion, loaded with FURA-2, and fluorescence recorded by a method fully described elsewhere (Wallace et al., 1999). In all experiments, the perfusion fluid contained 50 μM L-NOARG, and 10 μM verapamil was present in experiments involving Tg. To determine the effect of TRIM, the cells were initially perfused with calcium-free medium prior to the addition of either 100 nM Tg or 60 mM K. After 10 min in the presence of these agents, 2.5 mM calcium was then applied; once a stable increase in fluorescence ratio had been established, TRIM was added and the per cent change in fluorescence ratio calculated after 4 min. Because of the recognized uncertainties inherent in the measurement of absolute intracellular calcium concentrations, the results throughout this study are expressed as the ratio of fluorescence at 340 and 380 nm (R340/380). However, in some cells, the absolute values of cytosolic calcium concentration were obtained using the equation of Grynkiewicz et al. (1985); cells were permeabilized with ionomycin (50 μM) and exposed to bathing medium containing 10 mM calcium followed by medium containing zero added calcium plus 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraaecetic acid (BAPTA; 1 mM). Using this procedure, the resting calcium concentration was estimated as 54±11 nM (n=6).
Statistics
Results are expressed as mean±s.e.mean and statistical analysis was by Student's t-test with a P value of 0.05 or less taken as significant.
Drugs used
All drugs were obtained from Sigma, except TRIM (Lancaster Synthesis); they were dissolved in distilled water, except Tg (1 mM stock solution in dimethylsulphoxide) and miconazole (10 mM stock solution in dimethylsulphoxide).
Results
The initial aim of this study was to investigate the effects of TRIM, an inhibitor of neuronal nitric oxide synthase (Handy et al., 1995; Moore & Handy, 1997), on nitrergic relaxations of the mouse anococcygeus; tone was raised with 100 nM Tg, which (in the presence of 10 μM verapamil) contracts the mouse anococcygeus by activating CCE (Wallace et al., 1999). Under these conditions, relaxation to field stimulation is completely blocked by the general nitric oxide synthase inhibitor L-NOARG (Ayman et al., 2001). As shown in Figure 1, TRIM had no detectable effect on nitrergic relaxations; rather, it caused clear, concentration-related relaxation of Tg-induced tone. To determine the selectivity of this relaxation, we compared the effects of TRIM against tone induced by either 100 nM Tg (via CCE) or high K (60 mM; via voltage-operated calcium channels). In these experiments, field stimulation was not applied, and 50 μM L-NOARG was included in the bathing medium to inhibit nitric oxide synthase. Sustained contractions to Tg and high K were similar in magnitude (550±50 mg and 440±40 mg tension respectively; n=8 in both cases) and were equally sensitive to relaxation by the general smooth muscle relaxant papaverine (pEC50 M, 5.55±0.07 and 5.75±0.09 respectively; n=6 in both cases). As observed in the initial experiments with field stimulation, TRIM relaxed Tg-induced tone (pEC50 M, 4.38±0.07; n=6; Figure 2) but it was much less effective against high K, producing only a small, but significant, relaxation at the highest concentration used.
Figure 1.
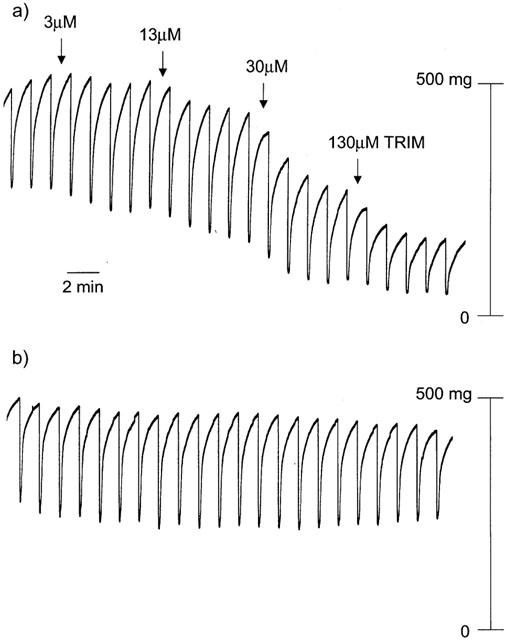
(a) Trace showing the effect of TRIM on tone and nitrergic relaxations (10 Hz; 10 s trains every 100 s) of the mouse anococcygeus following contraction by 100 nM thapsigargin (in the presence of 10 μM verapamil). TRIM was added at the times indicated by the arrows. Transient decreases in tone are in response to activation of nitrergic nerves by electrical field stimulation. (b) Control experiment for the above, showing that Tg-induced tone and nitrergic relaxations remained relatively constant in the absence of TRIM.
Figure 2.
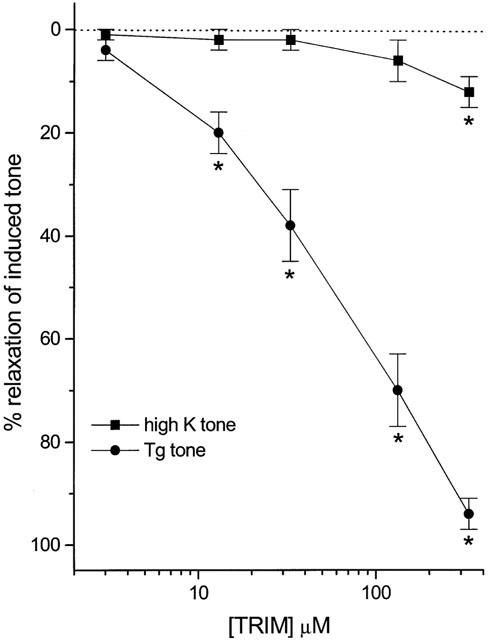
Concentration-response curves for relaxations of the mouse anococcygeus muscle in response to TRIM when tone is raised by either 100 nM thapsigargin (Tg; in the presence of 10 μM verapamil) or high K (60 mM). Measurements were taken 4 min after addition of TRIM to the bath. Each point is the mean±s.e.mean from a minimum of five individual muscle preparations. *P<0.05, significant relaxation.
For comparison, the effects of SKF96365 and miconazole were examined. 20 μM SKF96365, which we have used in previous studies to inhibit CCE in the mouse anococcygeus (Gibson et al., 1994; Wayman et al., 1996; Wallace et al., 1999), relaxed Tg-induced tone by 88±4% (n=7) but also relaxed high K-induced tone to the same extent (79±4%; n=7; P>0.05). Similarly, 100 μM miconazole was equi-effective against Tg (52±7% relaxation; n=6) and high K (46±6% relaxation; n=6; P>0.05).
In single smooth muscle cells, loaded with FURA-2 and bathed in calcium-free medium, Tg (in the presence of 10 μM verapamil) caused an initial transient increase in R340/380 reflecting depletion of calcium from the SR; subsequent addition of 2.5 mM calcium resulted in a sustained increase in fluorescence ratio as a result of CCE (Figure 3; Wallace et al., 1999). As expected, high K (in the absence of verapamil) did not produce an initial transient increase in fluorescence ratio, but the subsequent addition of 2.5 mM calcium resulted in a sustained increase similar to that observed with Tg (Figure 3; increase in R340/380 of 0.27±0.02 with high K, n=7, and 0.23±0.02 with Tg, n=6). Whilst it is perhaps surprising that the increase in fluorescence ratio was the same when calcium influx was activated by either CCE (Tg) or voltage-operated calcium channels (high K), TRIM produced a clear differentiation between the two pathways. Thus, application of 200 μM TRIM during the sustained phase caused a significant reduction of fluorescence ratio in the case of Tg, but had no effect with high K (Figure 3).
Figure 3.
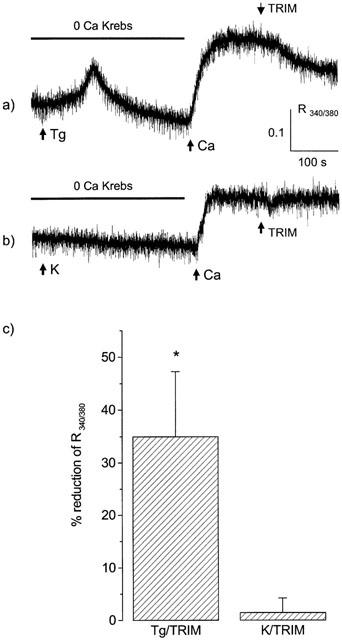
(a and b) Traces showing the fluorescence ratio from single smooth muscle cells freshly dispersed from the mouse anococcygeus and loaded with FURA-2. The cells were bathed initially in calcium-free medium and exposed to either 100 nM thapsigargin (Tg; a) or 60 mM K (b) followed by 2.5 mM calcium and then 200 μM TRIM. Verapamil (10 μM) was present throughout in the experiments with Tg, but omitted in those with high K. The combined results from at least five such experiments are contained in the histogram (c) which shows the mean±s.e.mean reduction in fluorescence ratio obtained in each case 4 min after addition of TRIM. *P<0.05, significant reduction.
Discussion
The important finding of the present study is that TRIM produced marked inhibition of contractions and calcium entry induced by Tg, which are mediated via CCE in the mouse anococcygeus (Wallace et al., 1999), in concentrations which had no effect on responses to high K, which are mediated via voltage-operated calcium channels (Gibson et al., 1994). Thus, TRIM represents a novel agent for the selective inhibition of store-operated, as opposed to voltage-operated, calcium entry. TRIM is an N1-substituted imidazole and several of the agents that have been employed to date as inhibitors of CCE also fall into this class, including SKF96365 and miconazole (Clementi & Meldolesi, 1996). However, these latter drugs also inhibit voltage-operated calcium entry in similar concentrations and are therefore non-selective. Although TRIM was less potent than SKF96365 against Tg-induced tone, its degree of selectivity represents an important advance. As noted previously with SKF96365 (Wallace et al., 1999), the inhibitory effect of TRIM on Tg-induced calcium influx was relatively small compared with its effect on Tg-induced tone; two possible explanations for this are, (i) there is a threshold concentration above which cytoplasmic calcium must rise in order to initiate contraction and, (ii) a fraction of the increase in fluorescence ratio observed on calcium re-admission is due to the small non-CCE ‘leak' observed in control cells not exposed to Tg (Wallace et al., 1999).
TRIM was initially shown to be an inhibitor of neuronal nitric oxide synthase (Handy et al., 1995; Moore & Handy, 1997). However, this action cannot explain the effects observed here. First, TRIM did not produce a discernible inhibition of nitrergic relaxations; whilst interpretation of these results was complicated by the marked loss of tone in the presence of TRIM, they agree with a similar lack of effect on nitrergic relaxations reported in rabbit corpus cavernosum (Teixeira et al., 1998). Secondly, relaxations of Tg-induced tone were obtained in the presence of the nitric oxide synthase inhibitor L-NOARG which completely blocks nitrergic relaxations of mouse anococcygeus contracted by Tg (Ayman et al., 2001). Thirdly, nitric oxide itself has been found to inhibit, not activate, CCE in smooth muscle (Cohen et al., 1999; Ayman et al., 2001). Thus, inhibition of CCE represents an additional action of TRIM, independent of its ability to inhibit nitric oxide synthase.
It is not yet possible to determine whether TRIM inhibits CCE by a direct effect on the SOC or by interference with the signal generated by depletion of the SR. There is mounting evidence that SOCs may be comprised of protein subunits similar to the transient receptor potential (TRP) proteins first detected in Drosophila. Several mammalian TRP homologues have been identified to date (Harteneck et al., 2000) raising the possibility that the subunit composition and functional characteristics of SOCs may vary widely among tissues. In preliminary studies, using the polymerase chain reaction in whole mouse anococcygeus, we have detected expression of TRPs 1, 2, 3 and 6 (communicated to the Joint British/Canadian Pharmacological Societies Meeting, Vancouver, 2001); more definitive studies using single smooth muscle cells, freshly dispersed from the tissue, are now in progress. Given this diversity of TRP proteins, it will be important to determine whether the inhibitory action of TRIM reported here is a general effect or whether it is tissue specific; if the latter, then TRIM may prove an important tool for the characterization of CCE in different cell types.
In conclusion, TRIM provides a novel, selective inhibitor of CCE and should be useful in improving our understanding of this ubiquitous calcium entry pathway. In addition, it may act as a template for the development of analogues which are both more potent and free from the complication of nitric oxide synthase inhibition.
Acknowledgments
The authors thank the Wellcome Trust for support.
Abbreviations
- CCE
capacitative calcium entry
- ER/SR
endoplasmic/sarcoplasmic reticulum
- L-NOARG
L-NG-nitroarginine
- SOC
store-operated channel
- Tg
thapsigargin
- TRIM
trifluoromethylphenylimidazole
- TRP
transient receptor potential
References
- AYMAN S., GIBSON A., MCFADZEAN I., REYNOLDS M., WALLACE P. Inhibition of capacitative calcium entry is not obligatory for relaxation of the mouse anococcygeus by the NO/cyclic GMP signalling pathway. Br. J. Pharmacol. 2001;132:807–814. doi: 10.1038/sj.bjp.0703888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERRIDGE M.J. Capacitative calcium entry. Biochem. J. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLEMENTI E., MELDOLESI J. Pharmacological and functional properties of voltage-independent Ca2+ channels. Cell. Calcium. 1996;19:269–279. doi: 10.1016/s0143-4160(96)90068-8. [DOI] [PubMed] [Google Scholar]
- COHEN R.A., WEISBROD R.M., GERICKE M., YAGHOUBI M., BIERL C., BOLOTINA V. Mechanism of nitric oxide-induced vasodilatation. Circ. Res. 1999;84:210–219. doi: 10.1161/01.res.84.2.210. [DOI] [PubMed] [Google Scholar]
- GIBSON A., MCFADZEAN I., TUCKER J.F., WAYMAN C.P. Variable potency of nitrergic-nitrovasodilator relaxations of the mouse anococcygeus against different forms of induced tone. Br. J. Pharmacol. 1994;113:1494–1500. doi: 10.1111/j.1476-5381.1994.tb17165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON A., MCFADZEAN I., WALLACE P., WAYMAN C.P. Capacitative calcium entry and the regulation of smooth muscle tone. TiPS. 1998;19:266–269. doi: 10.1016/s0165-6147(98)01222-x. [DOI] [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- HANDY R.L.C., WALLACE P., GAFFEN Z., WHITEHEAD K.J., MOORE P.K. 1-(2-Trifluoromethylphenyl)imidazole (TRIM): a potent inhibitor of neuronal nitric oxide synthase in vitro is antinociceptive in the mouse. Br. J. Pharmacol. 1995;116:2349–2350. doi: 10.1111/j.1476-5381.1995.tb15078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTENECK C., PLANT T.D., SCHULTZ G. From worm to man: three subfamilies of TRP channels. TINS. 2000;23:159–166. doi: 10.1016/s0166-2236(99)01532-5. [DOI] [PubMed] [Google Scholar]
- MERRITT J.E., ARMSTRONG W.P., BENHAM C.D., HALLAM T.J., JACOB R., JAXA-CHAMIEC A., LEIGH B.K., MCCARTHY S.E., MOORES R., RINK T.J. SKF96365, a novel inhibitor of receptor-mediated calcium entry. Biochem. J. 1990;271:515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE P.K., HANDY R.L.C. Selective inhibitors of neuronal nitric oxide synthase - is no NOS really good NOS for the nervous system. TiPS. 1997;18:204–211. doi: 10.1016/s0165-6147(97)01064-x. [DOI] [PubMed] [Google Scholar]
- PAREKH A.B., PENNER R. Store-depletion and calcium influx. Physiol. Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- PUTNEY J.W. Capacitative calcium entry revisited. Cell. Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- PUTNEY J.W., MCKAY R.R. Capacitative calcium entry channels. BioEssays. 1999;21:38–46. doi: 10.1002/(SICI)1521-1878(199901)21:1<38::AID-BIES5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- TEIXEIRA C.E., BENTO A.C., LOPES-MARTINS S.A., TEIXEIRA V., VON EICKESTEDT M.N., MUSCARA M.N., ARANTES E.C., GIGLIO J.R., ANTUNES E., DE NUCCI G. Effects of Tityus serrulatus scorpion venom on the rabbit isolated corpus cavernosum and the involvement of NANC nitrergic nerve fibres. Br. J. Pharmacol. 1998;123:435–442. doi: 10.1038/sj.bjp.0701623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLACE P., AYMAN S., MCFADZEAN I., GIBSON A. Thapsigargin-induced tone and capacitative calcium influx in mouse anococcygeus smooth muscle. N.S. Arch. Pharmacol. 1999;360:368–375. doi: 10.1007/s002109900100. [DOI] [PubMed] [Google Scholar]
- WAYMAN C.P., MCFADZEAN I., GIBSON A., TUCKER J.F. Two distinct membrane currents activated by cyclopiazonic acid-induced calcium store depletion in single smooth muscle cells from mouse anococcygeus. Br. J. Pharmacol. 1996;117:566–572. doi: 10.1111/j.1476-5381.1996.tb15228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


