Abstract
Vasorelaxant properties of three nitric oxide (NO) donor drugs (glyceryl trinitrate, sodium nitroprusside and spermine NONOate) in mouse aorta (phenylephrine pre-contracted) were compared with those of endothelium-derived NO (generated with acetylcholine), NO free radical (NO·; NO gas solution) and nitroxyl ion (NO−; from Angeli's salt).
The soluble guanylate cyclase inhibitor, ODQ (1H-(1,2,4-)oxadiazolo(4,3-a)-quinoxalin-1-one; 0.3, 1 and 10 μM), concentration-dependently inhibited responses to all agents. 10 μM ODQ abolished responses to acetylcholine and glyceryl trinitrate, almost abolished responses to sodium nitroprusside but produced parallel shifts (to a higher concentration range; no depression in maxima) in the concentration-response curves for NO gas solution, Angeli's salt and spermine NONOate.
The NO· scavengers, carboxy-PTIO, (2-(4-carboxyphenyl)-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide; 100 μM) and hydroxocobalamin (100 μM), both inhibited responses to NO gas solution and to the three NO donor drugs, but not Angeli's salt. Hydroxocobalamin, but not carboxy-PTIO, also inhibited responses to acetylcholine.
The NO− inhibitor, L-cysteine (3 mM), inhibited responses to Angeli's salt, acetylcholine and the three NO donor drugs, but not NO gas solution.
The data suggest that, in mouse aorta, responses to all three NO donors involve (i) activation of soluble guanylate cyclase, but to differing degrees and (ii) generation of both NO· and NO−. Glyceryl trinitrate and sodium nitroprusside, which generate NO following tissue bioactivation, have profiles resembling the profile of endothelium-derived NO more than that of exogenous NO. Spermine NONOate, which generates NO spontaneously outside the tissue, was the drug that most closely resembled (but was not identical to) exogenous NO.
Keywords: Nitric oxide, nitroxyl ion, nitric oxide donors, guanylate cyclase inhibitor, endothelium-dependent relaxation, spermine NONOate, glyceryl trinitrate, sodium nitroprusside, mouse aorta
Introduction
Endogenous nitric oxide (NO) is an important physiological modulator of vascular tone. Its synthesis and release can be stimulated by acetylcholine and by other endothelium-dependent vasodilators. The vasorelaxation induced by NO involves, at least in part, activation of soluble guanylate cyclase (Waldman & Murad, 1988; McDonald & Murad, 1995). NO donor drugs are thought to mimic the cellular effects of endogenously synthesized NO. However, several studies have suggested that the mechanisms for vascular relaxation vary for different NO donors, particularly with respect to the degree of involvement of soluble guanylate cyclase (van der zypp & Majewski, 1998; Tseng et al., 2000; Homer et al., 1999). Mechanisms that are independent of soluble guanylate cyclase have been reported in a number of studies, not only for NO donors (Homer & Wanstall, 2000; Lovren & Triggle, 2000) but also for authentic NO (Bolotina et al., 1994; Lovren & Triggle, 2000; Trottier et al., 1998) and endogenous NO synthesized from inducible NO synthase (Laurent et al., 1996). At present it is not known which NO donors have pharmacological profiles that most closely resemble endogenous, endothelium-derived NO.
NO can exist in a variety of forms, viz. as free radical (NO·) or as the ions nitroxyl (NO−) or nitrosonium (NO+), and the form(s) predominating may vary depending on the source of the NO. Thus (i) NO from the endothelium may differ from NO generated by NO donor drugs and (ii) various NO donors can be expected to differ from one another. Furthermore, the degree of involvement of soluble guanylate cyclase may depend on which of the forms of NO predominates. For example it has been claimed that NO· is the only form that can activate soluble guanylate cyclase (Dierks & Burstyn, 1996) although this view has been disputed, at least for NO− (Li et al., 1999). Hence, the form of NO present may contribute to the heterogeneity in the mechanisms of action of different NO donors. Although it is generally accepted that endothelium-derived NO is most likely NO· (Feelisch et al., 1994), several studies have indicated that another species of NO may also be produced. It has also been suggested that the NO generated by the endothelium may exist as a nitrosylated compound such as the nitrosothiol, S-nitrosocysteine, which subsequently releases NO (Myers et al., 1990).
With the introduction of the putatively heme site selective and specific inhibitor for soluble guanylate cyclase, 1H-(1,2,4-)oxadiazolo(4,3-a)quinoxalin-1-one (ODQ; Schrammel et al., 1996) it has been possible to explore the contribution of both soluble guanylate cyclase-dependent and -independent mechanisms to the cellular actions of NO, whether derived from the endothelium or from NO donors. It is also claimed that it is possible to distinguish between the various forms of NO produced using a variety of pharmacological tools. For example, the free radical scavengers, carboxy-PTIO (2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide) and hydroxocobalamin, scavenge and thus inhibit NO· (Rand & Li, 1993; Li & Rand, 1996; Li et al., 1999). On the other hand, L-cysteine, at millimolar concentrations, inhibits NO− (Zamora & Feelisch, 1994) but not NO· (Arvola et al., 1992; Feelisch et al., 1994; Pino & Feelisch, 1994). The inhibition of NO− occurs by a two-stage reaction in which hydroxylamine is formed and cysteine is oxidized to cystine (Doyle et al., 1988); other mechanisms, enzymatic or non-enzymatic, may also be involved (Zamora & Feelisch, 1994).
In the present study we have used these various pharmacological tools to characterize the vasorelaxant properties of three different NO donors (glyceryl trinitrate, sodium nitroprusside and spermine NONOate) and to compare their properties with those of endothelium-derived NO (generated with acetylcholine), NO· (aqueous solution of NO gas) and NO− (derived from Angeli's salt). The vascular preparation used was the mouse descending thoracic aorta. The overall objective was to elucidate which of these different NO donors had properties closest to those of endothelium-derived NO and/or ‘authentic' NO. In this study, solutions of NO gas have been used to represent ‘authentic' NO. A comparison with the effects of NO+ was not included as this ion has been reported to be very unstable under the conditions in which vascular contractility was recorded (i.e. in an aqueous physiological salt solution; Bonner & Stedman, 1996).
A preliminary report of these findings has been presented to a meeting of the Australasian Society of Clinical and Experimental Pharmacologists and Toxicologists, Newcastle, NSW, December 2000 (Wanstall et al., 2000).
Methods
This study conforms to the Code of Practice for Animal Experiments issued by the National Health and Medical Research Council of Australia.
Mouse aorta preparations
Male outbred mice (Quackenbush or Charles River; 35±0.38 g, n=101) on the day of the experiment, were anaesthetized with pentobarbitone (120 mg kg−1, i.p.), the thorax was opened and heparin (100 IU) injected into the left ventricle to prevent clotting of blood. The descending thoracic aorta was removed, cleared of adhering connective tissue and from it two or three ring preparations, 2 mm in length, were obtained using a specially designed cutting device.
Aorta preparations (with the endothelium intact) were mounted on 40 μm diameter stainless steel wires in a small vessel myograph (Mulvany-Halpern type: Models 410A and 610M; JP Trading, Aarhus, Denmark). The tissue chamber of the myograph contained physiological salt solution (PSS) at 37°C bubbled with 95% O2/5% CO2. The composition of the PSS was (mM): NaCl 119, KCl 4.7, KH2PO4 1.18, CaCl2 2.5; MgSO4 1.17, NaHCO3 25, glucose 11.1 and EDTA 0.026. The preparations were set up at a resting force of 6 mN. Active force was recorded isometrically via the transducer incorporated in the myograph.
Experimental protocol
Preparations were allowed to equilibrate for 1 h with the PSS being replaced at 15 min intervals. A cumulative concentration-response (contraction) curve to phenylephrine was obtained. Once the contraction to the final concentration of phenylephrine was stable, acetylcholine (10 μM) was added. A relaxant response indicated the endothelial integrity of the preparation (mean acetylcholine response, 70±0.94% reversal of this maximal phenylephrine contraction, n=225; range, 23 – 95%). After washing with PSS the vessel was pre-contracted with phenylephrine at a concentration (0.25 – 2.5 μM) corresponding to 75 – 85% of the maximum contraction determined from the phenylephrine curve and a cumulative concentration-response (relaxation) curve to one of the following vasorelaxant drugs was determined (control curve): acetylcholine, sodium nitroprusside, glyceryl trinitrate, spermine NONOate, Angeli's salt or nitric oxide (aqueous solution of NO gas). Following this control curve, the tissue was washed thoroughly and one of the following inhibitor drugs was added to the bath: Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME; 100 μM), ODQ (0.3, 1 or 10 μM), carboxy-PTIO (100 μM), hydroxocobalamin (100 μM), L-cysteine (3 mM), superoxide dismutase (150 u ml−1) or bathocuproinedisulfonic acid (bathocuproine; 10 μM). 30 min after the addition of L-NAME or ODQ (Homer et al., 1999) and 3 min after the addition of each of the other inhibitors (Ellis et al., 2000; Wanstall et al., 1997), preparations were again pre-contracted with phenylephrine and a second concentration-response curve to the vasorelaxant drug was determined. It was usually necessary to adjust the concentration of phenylephrine in order to obtain a contraction that was ±15% of that obtained for the preceding control curve (i.e. in the absence of inhibitor). No relaxant drug/inhibitor combination was examined on more than one preparation from any one animal. Repeated concentration-response curves, in the absence of inhibitor, were reproducible for all drugs except Angeli's salt where there was a small shift in the curve to a higher concentration range (shift in neg log IC50=0.49±0.07 log units; response to 1 μM reduced to 51±10.1% of the control value; n=4).
Data analysis
Contractions to phenylephrine were measured in mN. Relaxant responses to the vasorelaxant drugs were measured from the plateau of the phenylephrine contraction and were expressed as ‘per cent reversal' of the phenylephrine contraction. The IC50 (concentration giving 50% reversal of the phenylephrine contraction) was interpolated from the plot of relaxant-response versus log molar concentration of vasorelaxant drug. The potency of these drugs was expressed as negative log IC50. The magnitude of any parallel shift in the vasorelaxant concentration-response curve by ODQ was measured as the ‘log unit shift', defined as (negative log IC50 (control) – negative log IC50 (inhibitor present)). For Angeli's salt, individual values of ‘log unit shift' were corrected for the time-dependent shift in the absence of inhibitor referred to above, i.e. 0.49 log units was subtracted from each of the experimentally determined values of ‘log unit shift'. For visual purposes only, the second curve from the time control experiments has been reproduced on the graphs for Angeli's salt and is shown by a broken line. Also in the experiments with carboxy-PTIO, hydroxocobalamin and L-cysteine, individual responses to 1 μM Angeli's salt in the presence of each of the inhibitors were corrected for the time-dependent shift in the absence of inhibitor by dividing by 0.51.
Drugs and solutions
Acetylcholine chloride (Sigma); Angeli's salt (sodium trioxodinitrate; Cayman); bathocuproinedisulfonic acid disodium salt (bathocuproine; Sigma); carboxy-PTIO potassium salt [2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide] (Cayman); L-cysteine (free base; Sigma); glyceryl trinitrate (GTN-POHL; Rhone-Poulenc Rorer and David Bull Laboratories; ampoules); heparin sodium (Fisons); hydroxocobalamin hydrochloride (Sigma); nitric oxide gas (Linde Gas Pty. Ltd., Melbourne, Australia); Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME; Sigma); ODQ (Sigma); pentobarbitone sodium (Rhone Merieux); phenylephrine hydrochloride (Sigma); sodium nitroprusside (Sigma); superoxide dismutase (Sigma) spermine NONOate (Z-1-{N-[3-aminopropyl]-N-{4-(3-aminopropylammonio)butyl]-amino}diazen-1-ium-1,2-dioloate; Cayman).
Solutions of drugs were prepared as follows (mM): ACh 10, carboxy-PTIO 100, L-cysteine 100, hydroxocobalamin 100, L-NAME 10 and superoxide dismutase (10,000 u ml−1) in deionized water; sodium nitroprusside 10 in PSS; bathocuproine 10, ODQ 10 in dimethylsulphoxide; Angeli's salt 100, spermine NONOate 100 in 10 mM NaOH; nitric oxide (saturated solution; 2 mM) in deoxygenated distilled water as described by Rajanayagam et al. (1993). Ampoules of glyceryl trinitrate contained 22 mM in ethanol. Dilutions, when required, were made in PSS except spermine NONOate and Angeli's salt which were diluted in 10 mM NaOH and solutions of nitric oxide gas, which were diluted in water that had been deoxygenated by gassing it with argon for 1 h.
Statistical analyses
Mean values were calculated from data obtained in preparations from a number (n) of different animals and are quoted with their s.e.mean. Differences between pairs of (i) mean negative log IC50 values and (ii) responses to individual concentrations of vasorelaxant drugs, in the absence and presence of inhibitor, have been assessed by paired t-test. For each vasorelaxant drug, the significance of varying the concentration of ODQ was determined by carrying out a one-way analysis of variance (ANOVA), with a post test for linear trend, on values for ‘log unit shift'. At each concentration of ODQ a one-way ANOVA with a Tukey-Kramer post hoc test was used to compare the log unit shifts obtained for the various vasorelaxant drugs.
Results
Relaxation responses in mouse aorta
In endothelium-intact mouse aorta, pre-contracted submaximally with phenylephrine, acetylcholine caused concentration-dependent relaxation. The potency (negative log IC50) was 6.20±0.07, n=37 and the maximum relaxation was 81±1.9%, n=38. The NO synthase inhibitor, L-NAME (100 μM) abolished the responses (n=3; data not shown) indicating that the responses could be completely accounted for by NO generation.
NO gas solution, Angeli's salt (reputedly a source of NO−) and three different NO donor drugs (sodium nitroprusside, glyceryl trinitrate and spermine NONOate) also caused concentration-dependent relaxation of mouse aorta pre-contracted with phenylephrine. At the highest concentrations used each of these agents was able to fully reverse the phenylephrine contraction (Figures 1 and 2). The curves for glyceryl trinitrate were biphasic (Figure 2). NO gas solution and Angeli's salt were equipotent (negative log IC50 values, 5.91±0.04, n=30 and 5.99±0.03, n=36, respectively; P>0.05). The order of potency of the three NO donor drugs was sodium nitroprusside>glyceryl trinitrate>spermine NONOate (negative log IC50 values, 7.76±0.05, n=32; 6.82±0.09, n=37; 6.02±0.02, n=31, respectively; P<0.001).
Figure 1.
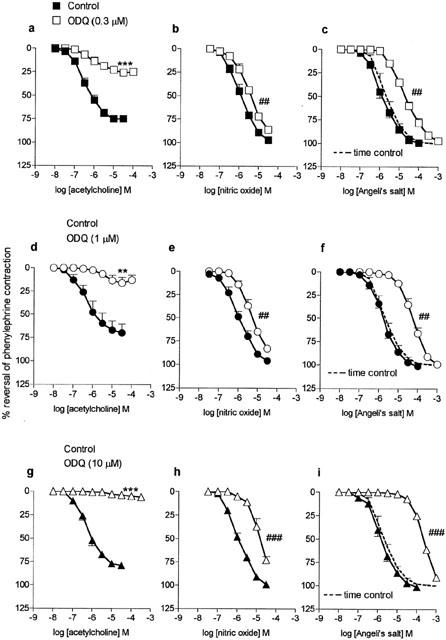
Mean concentration-response curves to acetylcholine (a, d and g; n=4 – 7), nitric oxide gas solution (b, e and h; n=4) and Angeli's salt, (c, f and i; n=4) on mouse aortae pre-contracted with phenylephrine in the absence (control; closed symbols) and then in the presence (open symbols) of ODQ (0.3 μM, a, b and c; 1 μM, d, e and f; 10 μM, g, h and i; pre-incubation 30 min). The broken line represents the time control values for Angeli's salt (i.e second curve in the absence of ODQ; n=4). Relaxation responses are expressed as per cent reversal of the phenylephrine-induced contraction. Points are mean values with s.e.mean shown by vertical bars except when smaller than the size of the symbols. **0.01>P>0.001, ***P<0.001 Response significantly less than corresponding response in the absence of ODQ. ## 0.01>P>0.001, ### P<0.001 Significant parallel shift in curve based on a decrease in negative log IC50 (see ‘log unit shifts' in Table 1).
Figure 2.
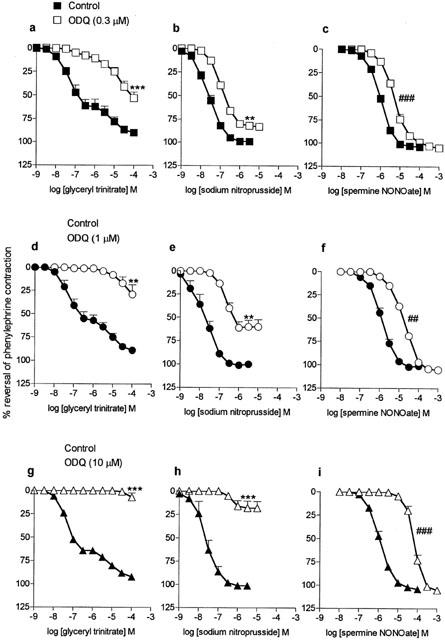
Mean concentration-response curves to glyceryl trinitrate (a, d and g; n=4 – 7), sodium nitroprusside (b, e and h; n=4) and spermine NONOate (c, f and i; n=4) on mouse aortae pre-contracted with phenylephrine in the absence (control; closed symbols) and then in the presence (open symbols) of ODQ (0.3 μM, a, b and c; 1 μM, d, e and f; 10 μM, g, h and i; pre-incubation 30 min). Relaxation responses are expressed as per cent reversal of the phenylephrine-induced contraction. Points are mean values with s.e.mean shown by vertical bars except when smaller than the size of the symbols. **0.01>P>0.001, ***P<0.001 Response significantly less than corresponding response in the absence of ODQ. ## 0.01>P>0.001, ### P<0.001 Significant parallel shift in curve based on a decrease in negative log IC50 (see ‘log unit shifts' in Table 1).
Effects of ODQ (soluble guanylate cyclase inhibitor)
ODQ (0.3, 1 and 10 μM) caused concentration-dependent inhibition of responses to all of the agents studied. It caused a non-parallel shift in the concentration-response curve to acetylcholine, together with a depression in maximum; at the highest concentration of ODQ (10 μM) responses were abolished (Figure 1). In contrast, ODQ caused parallel shifts in the curves for NO gas solution and Angeli's salt and even with 10 μM ODQ there was no depression in maximum (Figure 1).
The effects of ODQ on responses to the NO donors are shown in Figure 2. Responses to all three drugs were inhibited by ODQ but the patterns of inhibition varied. Responses to sodium nitroprusside were significantly depressed by each of the concentrations of ODQ, i.e. there were significant reductions in maximum response rather than parallel shifts in the curves (Figure 2). These data for sodium nitroprusside were in marked contrast to those for spermine NONOate where ODQ caused parallel shifts in the concentration-response curves with no depression in maxima (Figure 2). The maximal response for glyceryl trinitrate could not be established because of the effects of the vehicle at concentrations of glyceryl trinitrate >100 μM. However, within the range of concentrations studied, responses to glyceryl trinitrate, like those to acetylcholine, were virtually abolished by 10 μM ODQ (Figure 2). With the two lower concentrations of ODQ (0.3 and 1 μM) the curves were shifted by approximately 2 and 2.5 orders of magnitude, respectively (Figure 2; shifts assessed at the level of the IC30).
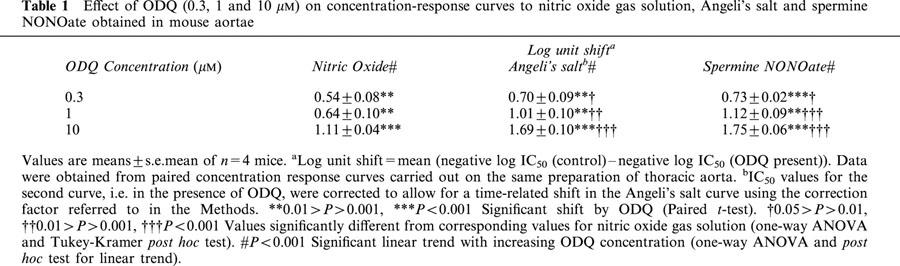
For the three agents where ODQ caused parallel shifts in the curves with no depression in maxima, i.e. NO gas solution, Angeli's salt and spermine NONOate, the magnitude of the shifts (expressed in log units) are summarized in Table 1. For each drug, the magnitude of the shift was dependent on the concentration of ODQ (post hoc test for linear trend) and at each concentration of ODQ the shifts for Angeli's salt and spermine NONOate were greater than that for NO gas solution. The shifts obtained with the highest concentration of ODQ (10 μM; Table 1) were all markedly less than the estimated shifts for glyceryl trinitrate obtained with lower concentrations of ODQ (0.3 or 1 μM; see above).
Table 1.
Effect of ODQ (0.3, 1 and 10 μM) on concentration-response curves to nitric oxide gas solution, Angeli's salt and spermine NONOate obtained in mouse aortae
Effects of carboxy-PTIO, hydroxocobalamin, L-cysteine, superoxide dismutase and bathocuproine
Responses to each of the NO-generating agents (at concentrations giving close to 50% reversal of the phenylephrine contraction) in the absence and presence of the inhibitors, carboxy-PTIO (100 μM), hydroxocobalamin (100 μM) and L-cysteine (3 mM), are shown in Figure 3. Carboxy-PTIO and hydroxocobalamin caused significant reductions in the responses to NO gas solution but had no effect on responses to Angeli's salt. In contrast, L-cysteine significantly inhibited responses to Angeli's salt but not those to NO gas solution. Responses to acetylcholine were inhibited by both L-cysteine and hydroxocobalamin but not by carboxy-PTIO (Figure 3).
Figure 3.
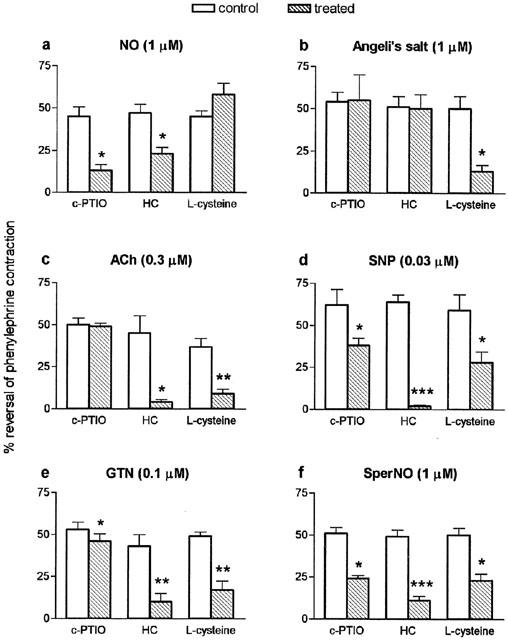
Mean responses to (a) nitric oxide gas solution (NO; 1 μM; n=4 – 6), (b) Angeli's salt (1 μM; n=4), (c) acetylcholine (ACh; 0.3 μM n=4), (d) sodium nitroprusside (SNP; 0.03 μM; n=4), (e) glyceryl trinitrate (GTN; 0.1 μM; n=4 – 6 (f) spermine NONOate (SperNO; 1 μM; n=3 – 4) on mouse aortae in the absence (control) and presence (treated) of carboxy-PTIO (c-PTIO; 100 μM), hydroxocobalamin (HC; 100 μM) or L-cysteine (3 mM); pre-incubation time 3 min. Relaxation responses are expressed as per cent reversal of the phenylephrine-induced contraction. Responses to Angeli's salt in the presence of inhibitors have been corrected for the time-related reduction in responses to this agent seen in the absence of inhibitors, using the correction factor referred to in the Methods. Points are mean values with s.e.mean shown by vertical bars. *0.05>P>0.01, **0.01>P>0.001, ***P<0.001 when compared with corresponding control value.
Responses to all three of the NO donor drugs were inhibited not only by carboxy-PTIO and hydroxocobalamin (thus resembling NO gas solution) but also by L-cysteine (thus resembling Angeli's salt) (Figure 3). It was noted that, for each of the NO donors, the inhibition by carboxy-PTIO was consistently less than the inhibition by hydroxocobalamin; the inhibition of glyceryl trinitrate by carboxy-PTIO, though significant, was particularly small (i.e. only 13% inhibition).
Neither superoxide dismutase (150 u ml−1) nor bathocuproine (10 μM) had any effect on responses to any of the relaxant drugs tested (n=4 for all groups; data not shown).
Discussion
The vasorelaxant properties of three different NO donors, sodium nitroprusside, glyceryl trinitrate and spermine NONOate, on mouse aorta have been compared. The drugs were chosen because they generate NO in different ways, viz, spermine NONOate has been shown to release NO spontaneously (Keefer et al., 1996; Homer & Wanstall, 1998; Murphy, 1999) whereas glyceryl trinitrate and sodium nitroprusside both require enzymatic or non-enzymatic bioactivation in the tissue (intracellularly and/or in the cell membrane) in order to generate NO (Marks et al., 1991; Kleschyov et al., 1994; Feelisch & Stamler, 1996). The three drugs were found to differ in the involvement of soluble guanylate cyclase in their respective responses, but not in the species of NO produced.
One of the purposes of this study was to determine which of the three NO donors studied most closely resembled endothelium-derived NO. This was achieved by comparing them with the endothelium-dependent vasodilator, acetylcholine. It was valid to use acetylcholine for this purpose because, in mouse aorta, relaxant responses to this vasodilator can be completely accounted for by NO generation, i.e. do not involve endothelium-derived hyperpolarizing factor. The evidence for this is that responses were (i) abolished by the NO synthase inhibitor, L-NAME, (present study) and (ii) lost in aortae from eNOS ‘knockout' (−/−) mice (Huang et al., 1995; Waldron et al., 1999).
The two approaches used to characterize the various agents were (i) quantification of the inhibitory effects of the soluble guanylate cyclase inhibitor, ODQ and (ii) the use of pharmacological probes in an attempt to identify the species of NO produced.
The inhibitory effect of ODQ on responses to acetylcholine was more pronounced than the inhibition of either NO gas solution or Angeli's salt. Furthermore, ODQ blocked Angeli's salt more than NO gas solution; this was unexpected but is in agreement with data obtained by Ellis et al. (2000) on rat aorta. ODQ also inhibited responses to each of the NO donors, including both phases of the biphasic curve to glyceryl trinitrate. However, the key observation was that the effects of ODQ were not identical for each of the drugs. This confirmed previous findings with these three NO donors on rat pulmonary artery (Homer et al., 1999) and suggested that the involvement of soluble guanylate cyclase in the response varied for the different drugs. The pattern of inhibition by ODQ of spermine NONOate was comparable to that for NO gas solution and Angeli's salt in that there was a parallel shift in the curves with no depression in maximum response. A different pattern of inhibition was seen with glyceryl trinitrate and sodium nitroprusside; for these two drugs the data were more like those for acetylcholine than NO gas solution, with the highest concentration of ODQ almost abolishing the responses. A differential effect of ODQ on responses to NO gas solution compared with sodium nitroprusside has previously been noted in human umbilical artery (Lovren & Triggle, 2000).
The ODQ data suggest that responses to sodium nitroprusside, glyceryl trinitrate and acetylcholine are mediated almost exclusively via soluble guanylate cyclase/cyclic GMP. However for NO gas solution, Angeli's salt and spermine NONOate, where responses were not abolished by ODQ, there may be a component that is independent of cyclic GMP, particularly with the higher concentrations of these vasodilator agents. Various cyclic GMP-independent mechanisms of action of NO have previously been described, including the direct activation of potassium channels (Bolotina et al., 1994; Trottier et al., 1998; Homer & Wanstall, 2000; Lovren & Triggle, 2000) as well as the activation of Na+-K+-ATPase (Gupta et al., 1994; Homer & Wanstall, 2000) and sarco-endoplasmic reticulum Ca2+-ATPase (Trepakova et al., 1999; Homer & Wanstall, 2000). We cannot exclude the possibility that the more pronounced effect of ODQ on responses to glyceryl trinitrate and nitroprusside, compared with spermine NONOate, may reflect inhibition of enzymes responsible for the bioactivation of these NO donors (Feelisch et al., 1999). However, we consider this unlikely since differences between spermine NONOate and the other two NO donors were seen with concentrations of ODQ as low as 0.3 μM, i.e. 30 – 100 fold lower than the concentrations reported to inhibit bioactivation (Feelisch et al., 1999).
The second approach to characterizing the various agents was to use various pharmacological tools to obtain information on the species of NO involved in the responses to each of the agents studied. Carboxy-PTIO, hydroxocobalamin and L-cysteine effectively distinguished between NO gas solution (NO·) and Angeli's salt (a source of NO−; Feelisch & Stamler, 1996). As predicted, the NO· scavengers, carboxy-PTIO and hydroxocobalamin, inhibited responses to NO gas solution but not Angeli's salt, whereas L-cysteine inhibited Angeli's salt but not NO gas solution. These findings are in agreement with other studies in both vascular and non-vascular tissues (Li et al., 1999; Ellis et al., 2000). Interestingly, the three NO donor drugs, as well as acetylcholine, were inhibited not only by the NO scavengers but also by L-cysteine. The simplest explanation for this observation is that both NO· and NO− are produced by each of these agents. With acetylcholine, additional support for this view was obtained from the findings that a combination of hydroxocobalamin and L-cysteine caused a greater inhibition than either inhibitor alone (T.K. Jeffery; unpublished). If this conclusion is correct, the variation in the effects of ODQ on the different NO donors and acetylcholine, described above, is unlikely to be due to differences in the species of NO produced. Admittedly, we cannot rule out the possible influence of NO+ especially since it is claimed that responses to this cation are inhibited by ODQ. However we could not test this directly in our experiments in PSS because in aqueous solutions NO+ is rapidly (i.e. within nanoseconds) converted to nitrite (Bonner & Stedman, 1996).
One unexpected observation from this study was the marked inhibition of Angeli's salt by ODQ; in fact the inhibition of Angeli's salt was significantly greater than that of NO gas solution. Although this finding was in agreement with data reported by Li et al. (1999) and Ellis et al. (2000), it appears incompatible with the view that NO· is the only form of NO that can activate soluble guanylate cyclase (Dierks & Burstyn, 1996). Our data suggest that either (i) the NO− generated by Angeli's salt is rapidly converted to NO· or (ii) NO− is able to activate soluble guanylate cyclase in mouse aorta (in contrast to the view of Dierks & Burstyn, 1996). If the former is the explanation, it is unlikely that this conversion to NO· occurred in the bathing solution for two reasons. Firstly, no inhibition of responses to Angeli's salt was seen with either of the free radical scavengers. Secondly, conversion of NO− to NO· is reported to require copper (Nelli et al., 2000) and the PSS used in our experiments included the copper chelator, EDTA, at a concentration (26 μM) that would have chelated any copper present in the PSS and, thus, inhibited the formation of NO· from NO− (Nelli et al., 2000). Furthermore, the addition of the copper chelator, bathocuproine, to the PSS had no effect on responses to Angeli's salt. However we cannot exclude the possibility that the NO− from Angeli's salt is converted into NO· within the tissue at a site where it is not scavenged by either carboxy-PTIO or hydroxocobalamin but has access to soluble guanylate cyclase.
Although both carboxy-PTIO and hydroxocobalamin clearly differentiated between NO gas solution (NO·) and Angeli's salt (NO−), they had divergent effects on responses to acetylcholine (inhibited by hydroxocobalamin but not by carboxy-PTIO). The lack of effect of carboxy-PTIO on responses to acetylcholine reflects previous findings in mouse anococcygeus muscle where, unlike hydroxocobalamin, carboxy-PTIO failed to inhibit responses to nitrergic stimulation (endogenous NO) even though it inhibited responses to NO gas solution (Lilley & Gibson, 1996). The explanation given was that the effect of carboxy-PTIO may have been prevented by the presence of tissue-derived anti-oxidants, such as ascorbate, which would prevent it scavenging tissue-derived (endogenous) NO but not exogenous NO (Lilley & Gibson, 1996); the reduced form of carboxy-PTIO (hydroxy-carboxy-PTIO) does not scavenge NO (Tsunoda et al., 1994). Hence some caution is required in interpreting any negative results with carboxy-PTIO; this has been overcome in the present study by obtaining data with a second scavenger of NO, namely hydroxocobalamin. The other agent that was tested with a view to distinguishing NO· from NO− was superoxide dismutase. However this agent had no effect on responses to any of the drugs suggesting that, in our experiments, there was either minimal superoxide production or excess endogenous superoxide dismutase.
The data in this study have highlighted the heterogeneity in the pharmacological profiles of each of the six NO-producing agents. Of the NO donors studied, glyceryl trinitrate and sodium nitroprusside were the ones that most resembled acetylcholine, both with respect to the nature of the inhibition by ODQ and the apparent involvement of both NO· and NO−. In relation to the effects of ODQ, it is tempting to speculate that the similarity between these three agents reflects the fact that they each generate NO within the tissue (endothelium or smooth muscle cell) and hence relatively small amounts of NO may be sufficient to produce relaxation. The concept that responses to NO that is generated within the tissue may be particularly sensitive to the inhibitory effects of ODQ has previously been put forward (Homer & Wanstall, 1999; Waldron et al., 1999). The NO donor that most resembled NO gas solution, based on the ODQ data, was spermine NONOate; this may be because spermine NONOate, like NO gas solution, is an extracellular (or ‘global') source of NO. Despite this similarity, spermine NONOate was not pharmacologically identical to NO gas solution since it appeared to produce NO− as well as NO·. It remains to be established whether other spontaneous generators of NO, including other NONOates, have pharmacological profiles that are even closer to that of ‘authentic' NO. Our data suggest that NONOates, despite being ready sources of NO (Keefer et al., 1996), may not necessarily be good imitators of endogenous, endothelium-derived NO. The findings of this study emphasise that one should give careful consideration to the choice of NO donor depending on whether the purpose is to mimic endogenous NO, released from the endothelium, or exogenous authentic NO gas.
Acknowledgments
The financial support of the National Health and Medical Research Council of Australia NHMRC (J.C. Wanstall, A. Gambino and T.K. Jeffery) and the Heart and Stroke Foundation of Canada (C.R. Triggle) is gratefully acknowledged. We are grateful to Dr Chun Guang Li for the provision of solutions of NO gas.
Abbreviations
- ACh
acetylcholine
- ANOVA
analysis of variance
- carboxy-PTIO
2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- GTN
glyceryl trinitrate
- L-NAME
Nω-nitro-L-arginine methyl ester
- NO
nitric oxide
- NO·
nitric oxide free radical
- NO+
nitrosonium ion
- NO−
nitroxyl ion
- ODQ
1H-(1,2,4-)oxadiazolo(4,3-a)-quinoxalin-1-one
- PSS
physiological salt solution
- SNP
sodium nitroprusside
- SperNO
spermine NONOate
References
- ARVOLA P., PORSTI I., VUORINEN P., HUHTAJA H., METSAKETELA T., VAPAATALO H. L-Cysteine augments the vasorelaxation induced by sodium nitrite and SIN-1 but not that due to acetylcholine. Eur. J. Pharmacol. 1992;214:289–292. doi: 10.1016/0014-2999(92)90133-o. [DOI] [PubMed] [Google Scholar]
- BOLOTINA V.M., NAJIBI S., PALACINO J.J., PAGANO P.J., COHEN R.A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- BONNER F.T., STEDMAN G.The chemistry of nitric oxide and redox-related species Methods in nitric oxide research 1996Chichester: Wiley and Sons; 3–18.ed. Feelisch, M. & Stamler, J.S. pp [Google Scholar]
- DIERKS E.A., BURSTYN J.N. Nitric Oxide (NO·), the only nitrogen monoxide redox form capable of activating soluble guanylate cyclase. Biochem. Pharmacol. 1996;51:1593–1600. doi: 10.1016/0006-2952(96)00078-0. [DOI] [PubMed] [Google Scholar]
- DOYLE M.P., MAHAPATRO S.N., BROENE R.D. , GUY J.K. Oxidation and reductions of hemoproteins by trioxodinitrate(II). The role of nitrosyl hydride and nitrite. J. Am. Chem. Soc. 1988;110:593–599. [Google Scholar]
- ELLIS A., LI C.G., RAND M.J. Differential actions of L-cysteine on responses to nitric oxide, nitroxyl anions and EDRF in the rat aorta. Br. J. Pharmacol. 2000;129:315–322. doi: 10.1038/sj.bjp.0703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEELISCH M., KOTSONIS P., SIEBE J., CLEMENT B., SCHMIDT H.H.H.W. The soluble guanylyl cyclase inhibitor 1H-(1,2,4)oxadiazolo-(4,3-a)quinoxalin-1-one is a nonselective heme protein inhibitor of nitric oxide synthase and cytochrome P-450 enzymes involved in nitric oxide donor bioactivation. Mol. Pharmacol. 1999;56:243–253. doi: 10.1124/mol.56.2.243. [DOI] [PubMed] [Google Scholar]
- FEELISCH M., STAMLER J.S.Donors of nitrogen oxides Methods in nitric oxide research 1996Chichester: Wiley and Sons; 71–115.ed. Feelisch, M. & Stamler, J.S. pp [Google Scholar]
- FEELISCH M., TE POEL M., ZAMORA R., DEUSSEN A., MONCADA S. Understanding the controversy over the identity of EDRF. Nature. 1994;368:62–65. doi: 10.1038/368062a0. [DOI] [PubMed] [Google Scholar]
- GUPTA S., MCARTHUR A., GRADY C, RUDERMANN N.B. Stimulation of vascular Na+-K+-ATPase activity by nitric oxide: a cGMP-independent effect. Am. J. Physiol. 1994;266:H2146–H2151. doi: 10.1152/ajpheart.1994.266.5.H2146. [DOI] [PubMed] [Google Scholar]
- HOMER K.L., FIORE S.A., WANSTALL J.C. Inhibition by 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) of responses to nitric oxide-donors in rat pulmonary artery: influence of the mechanism of nitric oxide generation. J. Pharm. Pharmacol. 1999;51:135–139. doi: 10.1211/0022357991772240. [DOI] [PubMed] [Google Scholar]
- HOMER K.L., WANSTALL J.C. In vitro comparison of two NONOates (novel nitric oxide donors) on rat pulmonary arteries. Eur. J. Pharmacol. 1998;356:49–57. doi: 10.1016/s0014-2999(98)00511-1. [DOI] [PubMed] [Google Scholar]
- HOMER K.L., WANSTALL J.C. Cyclic GMP-independent relaxation of rat pulmonary artery by spermine NONOate, a diazeniumdiolate nitric oxide donor. Br. J. Pharmacol. 2000;131:673–682. doi: 10.1038/sj.bjp.0703613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG P.L., HUANG Z., MASHIMO H., BLOCH K.D., MOSKOWITZ M.A., BEVAN J.A., FISHMAN M.C. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- KEEFER L.K., NIMS R.W., DAVIES K.M., WINK D.A. ‘NONOates' (1-substituted diazen-1,2-diolates) as nitric oxide donors: convenient nitric oxide dosage forms. Methods Enzymol. 1996;268:281–293. doi: 10.1016/s0076-6879(96)68030-6. [DOI] [PubMed] [Google Scholar]
- KLESCHYOV A.L., SEDOV K.R., MORDINTCEV P.I., VANIN A.F. Biotransformation of sodium nitroprusside into dinitrosyl ion complexes in tissue of ascites tumors of mice. Biochem. Biophys. Res. Commun. 1994;202:168–173. doi: 10.1006/bbrc.1994.1908. [DOI] [PubMed] [Google Scholar]
- LAURENT M., LEPOIVRE M., TENU J.P. Kinetic modelling of the nitric oxide gradient generated in vitro by adherent cells expressing inducible nitric oxide synthase. Biochem. J. 1996;314:109–113. doi: 10.1042/bj3140109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C.G., RAND M.J. Comparison of the effects of hydroxocobalamin and oxyhaemoglobin on responses to NO, EDRF and the nitrergic transmitter. Br. J. Pharmacol. 1996;117:805–810. doi: 10.1111/j.1476-5381.1996.tb15264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C.H., KARAGIANNIS J., RAND M.J. Comparison of the redox forms of nitrogen monoxide with the nitrergic transmitter in the rat anococcygeus muscle. Br. J. Pharmacol. 1999;127:826–834. doi: 10.1038/sj.bjp.0702540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILLEY E., GIBSON A. Antioxidant protection of NO-induced relaxations of the mouse anococcygeus against inhibition by superoxide anions, hydroquinone and carboxy-PTIO. Br. J. Pharmacol. 1996;119:432–438. doi: 10.1111/j.1476-5381.1996.tb16004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOVREN F., TRIGGLE C.R. Nitric oxide and sodium nitroprusside-induced relaxation of the human umbilical artery. Br. J. Pharmacol. 2000;131:521–529. doi: 10.1038/sj.bjp.0703588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKS G.S., MCLAUCHLIN B.E., BROWN L.B., BEATON D.E., BOOTH B.P., NAKATSU K., BRIEN J. Interaction of glyceryl trinitrate and sodium nitroprusside with bovine pulmonary vein homogenate and 10000×g supernatant: biotransformation and nitric oxide formation. Can. J. Physiol. Pharmacol. 1991;69:889–892. doi: 10.1139/y91-135. [DOI] [PubMed] [Google Scholar]
- MCDONALD L.J., MURAD F.Nitric oxide and cGMP signalling Advances in Pharmacology 199534New York: Academic Press; 263–273.ed. Ignarro, L. & Murad, F., vol [DOI] [PubMed] [Google Scholar]
- MURPHY M.E. Influence of redox compounds on nitrovasodilator-induced relaxations of rat coronary arteries. Br. J. Pharmacol. 1999;128:435–443. doi: 10.1038/sj.bjp.0702777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYERS P.R., MINOR R.L., JR, GUERRA R., JR, BATES J.N., HARRISON D.G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990;345:161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- NELLI S., HILLEN M., BUYUKAFSAR K., MARTIN W. Oxidation of nitroxyl anion to nitric oxide by copper ions. Br. J. Pharmacol. 2000;131:356–362. doi: 10.1038/sj.bjp.0703550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINO R.Z., FEELISCH M. Bioassay discrimination between nitric oxide (NO·) and nitroxyl (NO−) using L-cysteine. Biochem. Biophys. Res. Commun. 1994;201:54–62. doi: 10.1006/bbrc.1994.1668. [DOI] [PubMed] [Google Scholar]
- RAJANAYAGAM M.A.S., LI C.G., RAND M.J. Differential effects of hydroxocobalamin on NO-mediated relaxations in rat aorta and anococcygeus muscle. Br. J. Pharmacol. 1993;108:3–5. doi: 10.1111/j.1476-5381.1993.tb13429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAND M.J., LI C.G. Differential effects of hydroxocobalamin on relaxations induced by nitrosthiols in rat aorta and anococcygeus muscle. Eur. J. Pharmacol. 1993;241:249–254. doi: 10.1016/0014-2999(93)90210-9. [DOI] [PubMed] [Google Scholar]
- SCHRAMMEL A., BEHRENDS S., SCHMIDT K., KOESLING D., MAYER B. Characterization of 1H (1,2,4-)oxadiazolo(4,3-a)quinoxalin-1-one as a heme-site inhibitor of nitric oxide-sensitive guanylyl cyclase. Mol. Pharmacol. 1996;50:1–5. [PubMed] [Google Scholar]
- TREPAKOVA E.S., COHEN R.A., BOLOTINA V.M. Nitric oxide inhibits capacitative cation influx in human platelets by promoting sarco/endoplasmic reticulum Ca2+-ATPase-dependent refilling of Ca2+ stores. Circ. Res. 1999;84:201–209. doi: 10.1161/01.res.84.2.201. [DOI] [PubMed] [Google Scholar]
- TROTTIER G., TRIGGLE C.R., O'NEILL S.K., LOUTZENHISER R. Cyclic GMP-dependent and cyclic GMP-independent actions of nitric oxide on the renal afferent arteriole. Br. J. Pharmacol. 1998;125:563–569. doi: 10.1038/sj.bjp.0702090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSENG C-M.L., TABRIZI-FARD M.A., FUNG H-L. Differential sensitivity among nitric oxide donors toward ODQ-mediated inhibition of vascular relaxation. J. Pharmacol. Exp. Therap. 2000;292:737–742. [PubMed] [Google Scholar]
- TSUNODA R., OKUMURA K., ISHIZAKA H., MATSUNAGA T., TABUCHI T., YASUE H., AKAIKE T., SATO K., MAEDA H. Vasodilator effect of carboxy-2-phenyl-4,4,5,5,-tetramethylomidazoline-I-oxyl in the coronary circulation: in vivo and in vitro studies. Eur. J. Pharmacol. 1994;262:55–63. doi: 10.1016/0014-2999(94)90028-0. [DOI] [PubMed] [Google Scholar]
- VAN DER ZYPP A., MAJEWSKI H. Effect of cGMP inhibitors on the actions of nitrodilators in rat aorta. Clin. Exp. Pharmacol. Physiol. 1998;25:38–43. doi: 10.1111/j.1440-1681.1998.tb02141.x. [DOI] [PubMed] [Google Scholar]
- WALDMAN S.A., MURAD F. Biochemical mechanisms underlying vascular smooth muscle relaxation: the guanylate cyclase-cyclic GMP system. J. Cardiovasc. Pharmacol. 1988;12:S115–S118. [PubMed] [Google Scholar]
- WALDRON G.J., DING H., LOVREN F., KUBES P., TRIGGLE C.R. Acetylcholine-induced relaxation of arteries isolated from mice lacking endothelial nitric oxide synthase. Br. J. Pharmacol. 1999;128:653–658. doi: 10.1038/sj.bjp.0702858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANSTALL J.C., GAMBINO A., JEFFERY T.K., LOVREN F., TRIGGLE C.R. Vascular smooth muscle relaxation mediated by nitric oxide donors: a comparison with acetylcholine, nitric oxide gas and nitroxyl ion. Proc. Aust. Soc. Clin. Exp. Pharmacol. Toxicol. 2000;8:130. doi: 10.1038/sj.bjp.0704269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANSTALL J.C., KAYE J.A., GAMBINO A. The in vitro pulmonary vascular effects of FK409 (nitric oxide donor): a study in normotensive and pulmonary hypertensive rats. Br. J. Pharmacol. 1997;121:280–286. doi: 10.1038/sj.bjp.0701105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAMORA R., FEELISCH M. Bioassay discrimination between nitric oxide and nitroxyl using L-cysteine. Biochem. Biophys. Res. Commun. 1994;201:54–62. doi: 10.1006/bbrc.1994.1668. [DOI] [PubMed] [Google Scholar]


