Abstract
5-Hydroxytryptamine (5-HT)1-receptor-induced contraction is enhanced, or uncovered, by elevated vascular tone in many arteries including pulmonary arteries. In hypoxia-induced pulmonary hypertension, the endogenous tone of pulmonary arteries is elevated and this may contribute to increased 5-HT1-receptor-induced contraction. Here we investigate the influence of vascular tone induced by endothelin-1 (ET-1), neuropeptide Y (NPY), KCl, 4-aminopyridine (inactivator of Kv channels, 4-AP) or the calcium ionophore A23187 on contractile responses to the 5-HT1-receptor agonist 5-carboxamidotryptamine (5-CT) in small muscular pulmonary arteries from control rats and rats exposed to chronic hypoxia. The influence of the nitric oxide synthase inhibitor Nω-nitro-L-arginine methyl ester (L-NAME, 100 μM) was also studied. These conditions were chosen to mimic those that influence pulmonary vascular tone in hypoxia-induced pulmonary hypertension.
In control rat small pulmonary arteries, only high concentrations of 5-CT (>1 μM) induced vasoconstriction. Tone induced by NPY, 4-AP and A23187 had no effect on responses to 5-CT whilst responses to 5-CT were increased by ET-1- and KCl-induced tone. In the presence of L-NAME these responses to 5-CT were enhanced further.
Responses to 5-CT were enhanced 3 – 4 fold in small pulmonary arteries from hypoxia-exposed, pulmonary hypertensive rats and neither L-NAME nor increasing tone with NPY, 4-AP, A23187, ET-1 or KCl had any further effect on responses to 5-CT.
The results suggest that inhibition of nitric oxide synthase combined with KCl- or ET-1-induced vascular tone potentiates responses to 5-HT1-receptor-induced contraction in pulmonary arteries in a synergistic fashion and this mimics the effects of chronic hypoxic exposure.
Keywords: Pulmonary artery, pulmonary hypertension, pharmacological synergy, 5-HT1B receptors, vascular tone, vasoconstriction, chronic hypoxia
Introduction
5-Hydroxytryptamine (5-HT) is believed to play a role in the pathobiology of pulmonary arterial hypertension, exerting both mitogenic effects on pulmonary vascular smooth muscle and also inducing pulmonary vascular contraction (MacLean et al., 2000). There is substantial evidence that 5-HT1B receptors play an important role in mediating 5-HT-induced vasoconstriction in human large pulmonary arteries (MacLean et al., 1996a; Cortijo et al., 1997). More recently, we have demonstrated this in human small muscular pulmonary arteries (Morecroft et al., 1999). 5-HT1-receptor-mediated vasoconstriction is markedly enhanced in pulmonary arteries from the chronic hypoxic, pulmonary hypertensive rat (MacLean et al., 1996b). In control rat pulmonary arteries, 5-HT-induced vasoconstriction is mediated entirely by the Gq-coupled 5-HT2A receptor. However, in the chronic hypoxic rat, vasoconstriction to 5-HT is mediated by both the Gq-coupled 5-HT2A and the Gi-linked 5-HT1B receptors (MacLean et al., 1996b). It is possible that the 5-HT1B receptor-mediated responses are enhanced through pharmacological synergism. This occurs when a greater than additive response (e.g. vasoconstriction) ensues as a result of two agonists acting at different receptors on the same cell. Pharmacological synergism has been observed in several systemic blood vessels including the rabbit femoral artery and rabbit ear artery (Movahedi & Purdy, 1997; MacLennan & Martin, 1992) where Gi-protein coupled 5-HT1-receptor induced vasoconstriction is either ‘uncovered' or greatly potentiated by increasing vascular tone through a Gq-protein coupled receptor pathway. Hence, in the chronic hypoxic rat, increased 5-HT1B receptor-mediated responses could be due to increased activity of Gq-protein coupled receptor pathways, through an elevation of vasoconstrictors such as endothelin-1 ([ET-1], MacLean, 1999a). However, in the chronic hypoxic rat, pulmonary vascular tone can also be influenced by depolarization via inactivation of K+ channels (Osipenko et al., 1998) or decreased levels of cyclic nucleotides (MacLean et al., 1996b). Decreased levels of guanosine 3:5′-cyclic monophosphate (cyclic GMP) may be caused by increased activity of phosphodiesterase 5 (PDE5) activity (MacLean et al., 1996b, 1997). Increased Gq-protein-induced vascular tone and decreased cyclic GMP are both conditions known to synergize with, and enhance, 5-HT1-receptor-mediated vasoconstriction in pulmonary arteries (MacLean, 1999b).
The hypothesis tested here is that pharmacological synergism contributes to the enhanced 5-HT1-receptor mediated vasoconstriction observed in the small pulmonary arteries from the chronic hypoxic rat. We have mimicked conditions prevalent in chronic hypoxia which, as discussed above, may synergize with the 5-HT1-receptor mediated vasoconstriction. Hence, we have artificially elevated vascular tone with ET-1 (Gq-protein linked), we have depolarized the vessels with KCl and inactivated the Kv channels with 4-aminopyridine (4-AP). In addition, we increased intracellular calcium levels with the calcium ionophore A23187 and have examined the interaction of Gi-protein activation on 5-HT1-receptor mediated vasoconstriction by elevating vascular tone with neuropeptide Y (NPY), plasma levels of which are known to increase in paediatric pulmonary hypertension (Allen et al., 1989). We have studied the influence of these manipulations on 5-HT1-receptor mediated vasoconstriction in small pulmonary arteries from control and chronic hypoxic rats.
Methods
Chronic hypoxic rats
Male Wistar rats aged 28 days were placed in a specially designed perspex hypobaric chamber (Royal Hallamshire Hospital Sheffield, U.K.). This was depressurized, over 2 days, to 550 mbar (oxygen concentration equivalent to 10%). The temperature of the chamber was maintained at 21 – 22°C, and the chamber was ventilated with air at approximately 45 l min−1. Rats were maintained in these hypobaric/hypoxic conditions for 2 weeks. Age-matched normoxic animals were held in room air. The right ventricle was carefully dissected free from the septum and left ventricle, and both were blotted lightly and weighed. Pulmonary hypertension was assessed by measuring the ratio of right ventricular to total ventricular weight. This ratio is an established and reliable index for pulmonary hypertension in this model (Herget et al., 1978; Sheedy et al., 1996).
In vitro studies
Rats were killed by an overdose of sodium pentobarbitone (60 mg kg−1) and the lungs removed and placed in cold Krebs-buffer solution. Small pulmonary arteries (150 – 200 μm i.d.) were dissected out, cleared of adherent tissue and mounted on Mulvany-Halpern wire myographs (JP Trading, Aarhus, Denmark) in Krebs-buffer solution (pH 7.4) (composition in mM: NaCl 118.4, NaHCO3 25, KCl 4.7, KH2PO4 1.2, MgSO4 0.6, CaCl2 2.5, glucose 11.0, and EDTA 0.023) at 37°C and bubbled with 16% O2/6% CO2 balance N2. Small pulmonary arteries from control rats were tensioned ∼15 mmHg in order to mimic in vivo pulmonary arterial transmural pressure in the rat; vessels from the chronic hypoxic rats were tensioned to a transmural pressure of ∼34 mmHg to mimic pulmonary artery and arteriolar pressure of rats exposed to chronic hypoxic in vivo (e.g. Herget et al., 1978). No attempt was made to remove the endothelium in any vessel as this damages the fragile underlying layer of smooth muscle cells. The small pulmonary arteries were pre-constricted with either ET-1 (0.1 – 1.0 nM), KCl (10 mM), NPY (1 – 10 μM), A23187 (1 – 10 μM) or 4-AP (1 – 3 mM) to give a preconstriction 10 – 13% of that achieved by 50 mM KCl (in the absence of the pre-contractile agent) in each preparation. Once a stable plateau had been reached, cumulative concentration response curves to 5-CT (1 nM – 0.1 mM) were obtained either in the presence or absence of the nitric oxide synthase inhibitor, Nω-nitro-L-arginine methyl ester (L-NAME, 100 μM).
Data analysis
Contractile responses are expressed as a percentage of the contraction to 50 mM KCl determined at the start of the experiment in each preparation. In the presence of the pre-constricting agents, responses to 5-CT are calculated from the new baseline such that the zero response is at the level of pre-constriction. The results are shown as the mean±s.e.mean. pEC50 values were calculated where maximum responses were achieved (i.e. in the chronic hypoxic rat vessels), by BBC microcomputer graphical interpolation from individual cumulative concentration response curves. Statistical analysis was by either unpaired Student's t-test or analysis of variance (ANOVA) with Tukeys post hoc test where appropriate. P<0.05 were considered to be statistically significant.
Drugs
Calcimycin (A23187), 4-AP, neuropeptide-Y (human), Nω-nitro-L-arginine methylester (L-NAME) were purchased from Sigma-Aldrich (Poole, Dorset, U.K.); 5-carboxamidotryptamine (5-CT) maleate was purchased from Tocris Cookson (U.K.); endothelin-1 was purchased from Thistle Peptides Ltd., U.K. All drugs were dissolved in distilled water except A23187 and 4-AP, where stock solutions were made in DMSO. Subsequent dilutions of all stock solutions were made in distilled water.
Results
Assessment of pulmonary hypertension
The right ventricular to total ventricular weight ratio of the control rats was 0.251±0.009 and of the chronic hypoxic was 0.387±0.008 (P>0.001; unpaired Student's t-test, n=12); this is indicative of severe pulmonary hypertension.
Control rat small pulmonary arteries
The internal diameter of the small pulmonary arteries was 169.7±9.8 μm and the effective transmural pressure was 14.6±0.3 mmHg (n=10). 5-CT evoked a small, concentration-dependent vasoconstriction only at concentrations >1 μM (Figure 1, Table 1). All contractile agents produced an equivalent degree of pre-constriction (Table 2) and L-NAME itself did not induce any contraction. Pre-constriction with NPY, A23187 and 4-AP did not enhance 5-CT-induced vasoconstriction (Figure 1, Table 1). Prior exposure to ET-1 or KCl, however, significantly augmented the 5-CT-induced contraction in control rat vessels, causing significant contractions to concentrations as low as 10 – 30 nM, whereas no significant contraction to 5-CT was observed in control vessels below 30 μM (Figure 2). For example, a contractile response to 1 μM 5-CT was uncovered in the presence of ET-1- and KCl-induced tone (Table 1). L-NAME had no effect on the response to 1 μM 5-CT (Figure 1, Table 1) but, in the presence of ET-1, L-NAME greatly enhanced 5-CT-induced contraction. For example, the magnitude of the response to 1 μM 5-CT was increased 2 fold (Figure 2, Table 1). In the presence of KCl, L-NAME also enhanced 5-CT-induced contraction, increasing the response achieved to 1 μM 5-CT 2 fold (Figure 2, Table 1).
Figure 1.
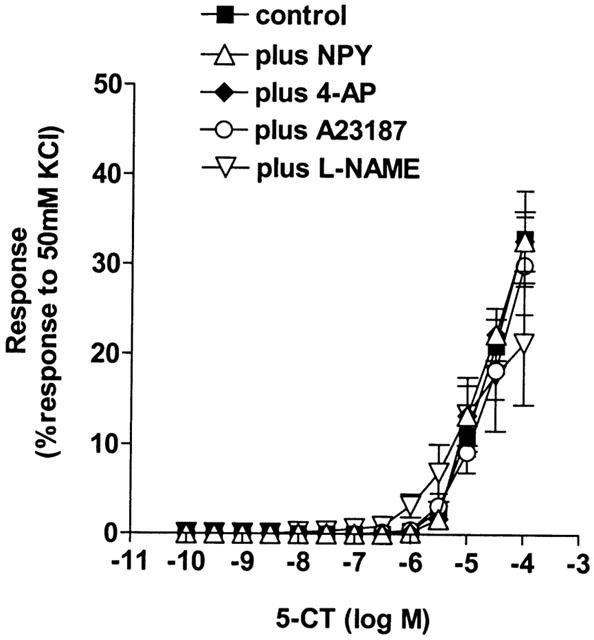
Effect of increasing vascular tone with neuropeptide Y (NPY, n=6), 4-amidopyridine (4-AP, n=6), the calcium ionophore A23187 (n=6) and the nitric oxide synthase inhibitor Nω-nitro-L-arginine methyl ester (L-NAME, 100 μM, n=7) on contractile responses to 5-carboxamidotryptamine (5-CT, control: n=12) in control rat isolated small muscular pulmonary arteries. Responses are shown as the percentage response to a contraction to 50 mM KCl. Data is shown as means±s.e.mean. n=number of rats.
Table 1.
Effect of pharmacologically-induced vascular tone on vasoconstrictor responses to 5-CT in control and chronic hypoxic rat small muscular pulmonary arteries
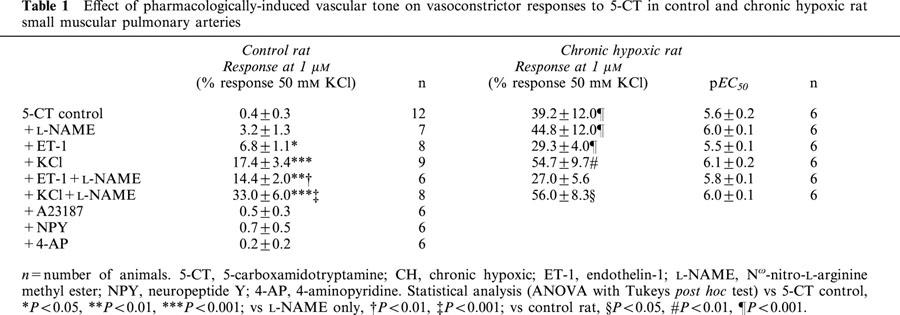
Table 2.
Pulmonary vascular tone (expressed as a percentage of the response to 50 mM KCl in each vessel) induced by contractile agents in control and chronic hypoxic rats
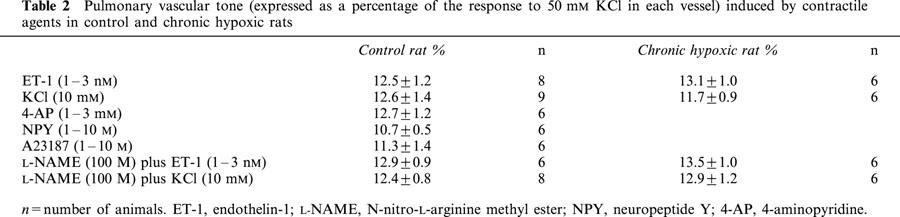
Figure 2.
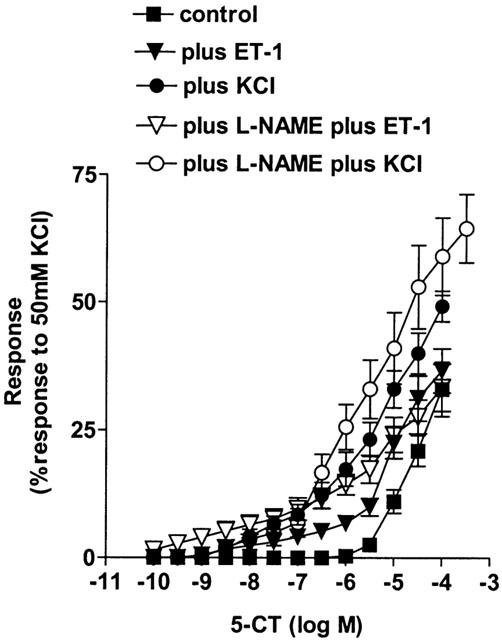
Effect of increasing vascular tone with endothelin-1 (ET-1, n=8) and KCl (n=9), ET-1 plus the nitric oxide synthase inhibitor Nω-nitro-L-arginine methyl ester (L-NAME, 100 μM, n=6) and KCl plus L-NAME (n=8) on contractile responses to 5-carboxamidotryptamine (5-CT, control: n=12) in control rat isolated small muscular pulmonary arteries. Responses are shown as the percentage response of a contraction to 50 mM KCl. Data is shown as means±s.e.mean. n=number of rats. Control data shown is as for Figure 1.
Chronic hypoxic rat small pulmonary arteries
The internal diameter of the small pulmonary arteries was 172±11.1 μm and the effective transmural pressure was 34.5±0.5 mmHg (n=10). Chronic hypoxia substantially increased the response to 5-CT (Figure 3, Table 1). All contractile agents produced an equivalent degree of pre-constriction (Table 2) and L-NAME itself did not induce any contraction. Pre-contracting the small pulmonary arteries from chronic hypoxic rats with ET-1 or KCl either in the presence or absence of L-NAME did not significantly affect responses to 5-CT in small pulmonary arteries (Figure 4, Table 1).
Figure 3.
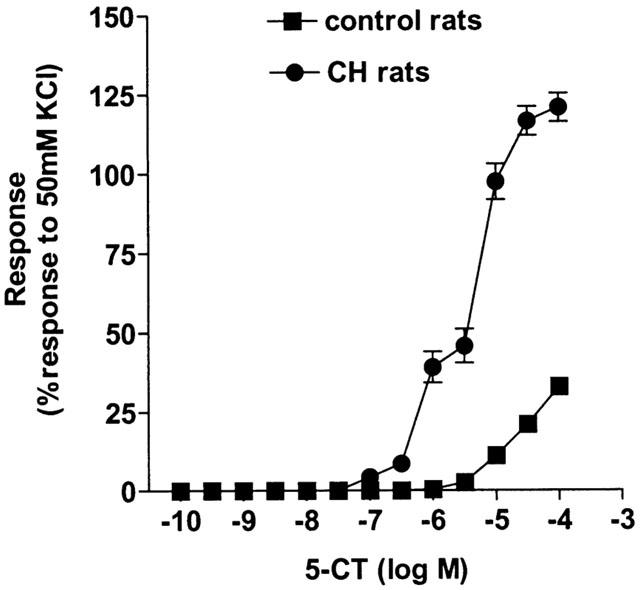
Responses to 5-carboxamidotryptamine (5-CT) in control (n=12) and chronic hypoxic (n=6) rat isolated small muscular pulmonary arteries. Responses are shown as the percentage response of a contraction to 50 mM KCl. Data is shown as means±s.e.mean. n=number of rats.
Figure 4.
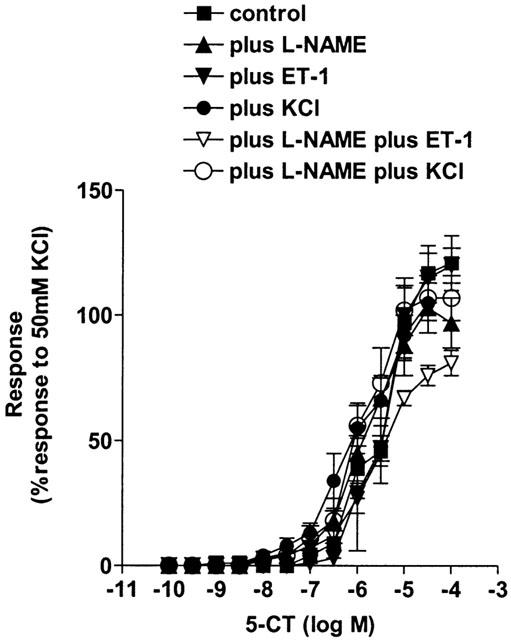
Effect of increasing vascular tone with the nitric oxide synthase inhibitor Nω-nitro-L-arginine methyl ester (L-NAME, 100 μM, n=6), endothelin-1 (ET-1, in the presence (n=6) and absence (n=6) of L-NAME), KCl (in the presence (n=6) and absence (n=6) of L-NAME) on contractile responses to 5-carboxamidotryptamine (5-CT, control: n=6) in chronic hypoxic rat isolated small muscular pulmonary arteries. Responses are shown as the percentage response of a contraction to 50 mM KCl. Data is shown as means±s.e.mean. n=6 rats. Control data shown is as for Figure 3.
Table 1 demonstrates that, comparing the data for the control vs chronic hypoxic rats, in the presence of L-NAME plus ET-1-induced tone, responses were enhanced to such an extent that there was no significant difference, between the control and chronic hypoxic rat data, in the response to 1 μM 5-CT in the presence of both ET-1 and L-NAME.
Discussion
We have previously demonstrated that the 5-HT1-receptor mediates vasoconstriction in human large pulmonary arteries (MacLean et al., 1996a). Further, we recently confirmed this in human small pulmonary arteries and identified the 5-HT1B-receptor as that mediating vasoconstriction at physiological concentrations of 5-HT (Morecroft et al., 1999). We have previously demonstrated that there is a marked increase in the vasoconstrictor response to 5-HT in pulmonary arteries from the chronic hypoxic rat and this is partly mediated by increased 5-HT1-receptor mediated contraction (MacLean et al., 1996b). Hence, the 5-HT1B-receptor may play a critical role in normal pulmonary vascular tone and perhaps in the enhanced pulmonary vasoconstrictor responses to 5-HT observed in pulmonary hypertension, both in animal models and man (Brink et al., 1988). The current study was designed to test the hypothesis that pharmacological synergy contributes to increased pulmonary vascular 5-HT1B-receptor activity in the chronic hypoxic rat (MacLean, 1999b). We have artificially mimicked conditions prevalent in the chronic hypoxic rat pulmonary artery such as decreased cyclic GMP accumulation, increased intracellular calcium, depolarization, inactivation of K+ channels and increased Gq-receptor stimulation (MacLean, 1999b).
Here we confirm that there is a marked augmentation of vasoconstrictor responses to the 5-HT1 receptor agonist 5-CT in the chronic hypoxic rat small pulmonary arteries, in agreement with our previous observations (MacLean et al., 1996b). ET-1 mediates vasoconstriction through Gq-protein coupled receptors and subsequent activation of phospholipase C (Resink et al., 1988; Ohlstein et al., 1995). The augmented response to 5-CT in normoxic rat small pulmonary arteries observed in this study, in the presence of ET-1-induced tone is consistent with several reports showing that pre-contraction with agonists acting via Gq-coupled receptors causes an augmentation or uncovering of Gi-coupled receptor responses such as 5-HT1-receptor mediated vasoconstriction (Selbie & Hill, 1998). This synergistic interaction has mainly been demonstrated in large systemic arteries (De le Lande, 1992; MacLennan & Martin, 1992) or large pulmonary arteries (MacLean et al., 1994). This is the first study to demonstrate this phenomenon in small pulmonary arteries, which are the major contributors to increased pulmonary vascular resistance in the pulmonary hypertension (Singhal et al., 1973). Systemic arteries in vivo are under a degree of tonic sympathetic tone and therefore, the synergistic interactions between receptors in these vessels may be normally operative in vivo. In contrast, pulmonary arteries in the normal lung are full dilated and therefore synergistic effects may only operate when the pulmonary arteries are under the influence of increased vascular tone as in pulmonary hypertension. Importantly, in pulmonary hypertension, there is evidence for an increased production of ET-1 (MacLean, 1999a) as well as thromboxane (Christman, 1998) and angiotensin-II (Cargill & Lipworth, 1995). These all are agonists acting via Gq-coupled receptors and collectively, supports the possibility that increased Gq-coupled-receptor activation may synergize with Gi-coupled responses in pulmonary hypertension.
KCl-induced vascular tone also potentiated 5-CT-induced vasoconstriction in the normoxic small pulmonary arteries. In fact the amplification effect was greater than in the presence of ET-1. In addition to membrane depolarization, KCl has been shown to increase the formation of inositol (1,4,5) triphosphate (IP3) in rat pulmonary arteries (Jin et al., 1993). It is possible that different mechanisms of amplification by ET-1 and KCl are operating, however. For example, in dog artery and vein, precontraction with KCl and ET-1 potentiates Gi-receptor linked contraction to the α2-adrenoceptor agonist UK14304. Protein kinase C predominantly mediates the ET-1-induced amplification but not KCl-induced amplification in these dog vessels (Shimamoto et al., 1995).
Decreased levels of cyclic GMP have previously been shown to potentiate α2-adrenoceptor- (Gi-coupled receptor) induced vasoconstriction in rabbit large pulmonary arteries (MacLean et al., 1993). In bovine pulmonary arteries, responses to the 5-HT1B/1D-Gi-linked receptor agonist sumatriptan are potentiated by decreased cyclic GMP (MacLean et al., 1994). In this study, we observed that inhibition of nitric oxide synthase with L-NAME further potentiates the augmented responses to 5-CT in the presence of ET-1- and KCl-induced tone. This pharmacological manipulation potentiated the response to 5-CT to an extent that mimicked the effect of chronic hypoxia. This suggests that levels of cyclic GMP may also play an important synergistic role in the response of pulmonary resistance arteries to Gi-coupled receptor-induced vasoconstriction. In pulmonary arteries removed from chronic hypoxic rats, decreased levels of cyclic GMP, as well as decreased cyclic AMP, along with increased vascular tone have been observed (MacLean et al., 1996b). This is likely to be due to increased PDE activity (MacLean et al., 1997). The observation here that Gq- and Gi-coupled interactions can be further modulated by cyclic nucleotide levels may therefore be of pathophysiological importance in pulmonary hypertension.
We have demonstrated that there are enhanced pulmonary contractile responses to 5-HT in the chronic hypoxic rat and that responses to the 5-HT1 receptor agonists 5-CT and sumatriptan were enhanced in the small pulmonary arteries of the chronic hypoxic rat. In addition, the 5-HT2A antagonist ketanserin was less potent against 5-HT in vessels from chronic hypoxic rats whilst the 5-HT1 receptor antagonist GR555612 was more potent (MacLean et al., 1996b). There is also increased expression of 5-HT1B receptor mRNA in pulmonary arteries from the chronic hypoxic (Heeley et al., 1998). Hence, there is increased 5-HT1 receptor-mediated contraction in the pulmonary arteries of chronic hypoxic rats and this may contribute to the enhanced contractile responses to 5-HT. In the current study, whilst we have examined responses to 5-CT, the results suggest a mechanism for the increased response to 5-HT1 receptor agonists and hence 5-HT, observed in the chronic hypoxic rat.
Membrane depolarization is known to occur in isolated pulmonary artery smooth muscle cells from our hypobaric, chronic hypoxic rats and this is possibly due to a down regulation of K+ channels in these cells (Osipenko et al., 1998). Based on this, we expected that the observed augmented response to 5-HT receptor-induced vasoconstriction in pulmonary hypertension would be mimicked by blocking Kv channels with 4-AP. Surprisingly, our findings here did not support this hypothesis, suggesting that down regulation of Kv channels may not be involved in the observed augmentation of 5-HT1 receptor-mediated vasoconstriction in small pulmonary arteries from the chronic hypoxic rat. Similarly, Doggrell et al. (1999) observed that 4-AP did not augment responses to 5-HT in the rat intra-PA, but did in the main-PA, suggesting membrane depolarization plays a more important role in 5-HT-induced vasoconstriction in large, conduit PAs.
Neuropeptide Y (NPY) mediates its vasoconstrictor effects via the same intracellular pathway as 5-CT, namely activation of a Gi-coupled receptor, inhibition of adenylate cyclase and subsequent decrease in the intra-cellular levels of adenosine 3′:5′-cyclic monophosphate (cyclic AMP) (Lundberg et al., 1988). Not surprisingly, prior exposure to threshold concentrations of NPY did not significantly alter the response to 5-CT in the rat small pulmonary arteries. A23187 mediates its vasoconstrictor effects as a calcium ionophore, however it was also unable to significantly alter the response to 5-CT. This suggests that simply elevating i.c. calcium does not produce synergistic amplification of vasoconstriction in these vessels.
Increasing vascular tone and/or decreasing levels of cyclic GMP in the small pulmonary arteries from chronic hypoxic rats did not significantly change the sensitivity or maximum contraction to 5-CT. This suggests that the amplification factors necessary for pharmacological synergism already exist in these vessels and collectively provide an ‘optimum' environment for 5-HT1, Gi-protein-coupled receptor mediated vasoconstriction. This synergism may play an important role in pulmonary vasoconstriction associated with pulmonary hypertension.
In conclusion, these results show that in rat small pulmonary arteries, increased vascular tone via either Gq-coupled receptor activation or elevated KCl can potentiate Gi-coupled 5-HT1 receptor-induced vasoconstriction. This synergism may therefore become pathophysiologically important in pulmonary hypertension which is characterized by elevated pulmonary vascular tone, increased Gq-coupled receptor activation and membrane deplolarization. The observation that nitric oxide synthase inhibition can further augment the Gq-/Gi-coupled receptor interactions in these small pulmonary arteries strengthens the suggestion that pharmacological synergism is pathophysiologically important in pulmonary hypertension.
Acknowledgments
This work was funded by the Wellcome Trust.
Abbreviations
- 4-AP
4-aminopyridine
- cyclic GMP
guanosine 3:5′-cyclic monophosphate
- 5-CT
5-carboxamidotryptamine
- 5-HT
5-hydroxytryptamine
- ET-1
endothelin-1
- L-NAME
Nω-nitro-L-arginine methyl ester
- NPY
neuropeptide Y
- PDE
phosphodiesterase
References
- ALLEN K.M., WHARTON J., POLAK J.M., HAWORTH S.G. A study of nerves containing peptides in the pulmonary vasculature of healthy infants and children and of those with pulmonary hypertension. Br. Heart J. 1989;62:353–360. doi: 10.1136/hrt.62.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRINK C., CERRINA C., LABAT C., VERLEY J., BENVENISTE J. The effect of contractile agonists on isolated pulmonary arterial and venous muscle preparations derived from patients with primary pulmonary hypertension. Am. Rev. Resp. Dis. 1988;137:A106. [Google Scholar]
- CARGILL R.I., LIPWORTH B.J. The role of the renin-angiotensin and neuropeptide systems in the pulmonary vasculature. Br. J. Clin. Pharmacol. 1995;40:11–18. doi: 10.1111/j.1365-2125.1995.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTMAN B.W. Lipid mediator dysregulation in primary pulmonary hypertension. Chest. 1998;114:205S–207S. doi: 10.1378/chest.114.3_supplement.205s. [DOI] [PubMed] [Google Scholar]
- CORTIJO J., MARTI-CABRERA M., BERNABEU E., DOMENECH T., BOU J., FERNANDEZ A.G., BELETA J., PALACIOS J.M., MORCILLO E.J. Characterisation of 5-HT receptors on human pulmonary artery and vein: functional and binding studies. Br. J. Pharmacol. 1997;122:1455–1463. doi: 10.1038/sj.bjp.0701509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE LA LANDE I.S. Evidence for the 5-HT1-like receptor mediating the amplifying action of 5-HT in the rabbit ear artery. Br. J. Pharmacol. 1992;106:550–555. [PMC free article] [PubMed] [Google Scholar]
- DOGGRELL S.A., WANSTALL J.C., GAMBINO A. Functional effects of 4-aminopyridine (4-AP) on pulmonary and systemic vessel from normoxic control and hypoxic pulmonary hypertensive rats. Naunyn-Schmiedebergs Arch. Pharmacol. 1999;360:317–323. doi: 10.1007/s002109900064. [DOI] [PubMed] [Google Scholar]
- HEELEY R.P., PRENTICE H., MORECROFT I., MACLEAN M.R. Evidence for increased expression of the r5-HT1B receptor in small pulmonary arteries from rats with chronic hypoxic pulmonary hypertension. Am. J. Resp. Crit. Care Med. 1998;157:A590. [Google Scholar]
- HERGET J., SUGGET A.J., LEACH E., BARER G.R. Resolution of pulmonary hypertension and other features induced by chronic hypoxia in rats during complete and intermittent normoxia. Thorax. 1978;33:468–473. doi: 10.1136/thx.33.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIN N., PACKER C.S., ENGLISH D., RHOADES R.A. Inositol trisphosphate is involved in norepinephrine- but not hypoxia-induced pulmonary arterial contraction. Am. J. Physiol. 1993;264:L160–L164. doi: 10.1152/ajplung.1993.264.2.L160. [DOI] [PubMed] [Google Scholar]
- LUNDBERG J.M., HEMSON A, , LARSSON O, , RUDEHILL A., SARIA A., FREDHOLM B.B. Neuropeptide Y receptors in pig spleen: binding characteristics, reduction of cyclic AMP formation and calcium antagonist inhibition of vasoconstriction. Eur. J. Pharmacol. 1988;145:21–29. doi: 10.1016/0014-2999(88)90344-5. [DOI] [PubMed] [Google Scholar]
- MACLEAN M.R. Endothelin-1 and serotonin: mediators of primary and secondary pulmonary hypertension. J. Lab. Clin. Med. 1999a;134:105–114. doi: 10.1016/s0022-2143(99)90114-2. [DOI] [PubMed] [Google Scholar]
- MACLEAN M.R. Pulmonary hypertension, anorexigens and 5-hydroxytryptamine: Pharmacological synergism in action. Trends Pharmacol. Sci. 1999b;20:490–495. doi: 10.1016/s0165-6147(99)01389-9. [DOI] [PubMed] [Google Scholar]
- MACLEAN M.R., CLAYTON R.A., MCINTYRE P.D., HILLIS S.W., PEACOCK A.J., TEMPLETON A.G.B. 5-HT1-receptor-mediated vasoconstriction in bovine isolated pulmonary arteries: influence of vascular endothelium. Pulm. Pharmacol. 1994;7:65–72. doi: 10.1006/pulp.1994.1007. [DOI] [PubMed] [Google Scholar]
- MACLEAN M.R., CLAYTON R.A., TEMPLETON A.G.B., MORECROFT I. Evidence for 5-HT1-like receptor mediated vasoconstriction in human pulmonary artery. Br. J. Pharmacol. 1996a;119:277–282. doi: 10.1111/j.1476-5381.1996.tb15982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACLEAN M.R., HERVE P., EDDAHIBI S., ADNOT S. 5-hydroxytryptamine and the pulmonary circulation: receptors, transporters and relevance to pulmonary arterial hypertension. Br. J. Pharmacol. 2000;131:161–168. doi: 10.1038/sj.bjp.0703570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACLEAN M.R., JOHNSTON E.D., MCCULLOCH K.M., POOLEY L., HOUSLAY M., SWEENEY G. Phosphodiesterase isoforms in the pulmonary arterial circulation of the rat: changes in pulmonary hypertension. J. Pharmacol. Exp. Ther. 1997;283:619–624. [PubMed] [Google Scholar]
- MACLEAN M.R., MCCULLOCH K.M., MCGRATH J.C. Influences of endothelium and hypoxia on α1- and α2-adrenoceptor-mediated responses in the rabbit isolated pulmonary artery. Br. J. Pharmacol. 1993;108:155–161. doi: 10.1111/j.1476-5381.1993.tb13456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACLEAN M.R., SWEENEY G., BAIRD M., MCCULLOCH K.M., HOUSLAY M., MORECROFT I. 5-hydroxytryptamine receptors mediating vasoconstriction in pulmonary arteries from control and pulmonary hypertensive rats. Br. J. Pharmacol. 1996b;119:917–930. doi: 10.1111/j.1476-5381.1996.tb15760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACLENNAN S.J., MARTIN G.R. Effect of thromboxane A2-mimetic U46619 on 5-HT1-like and 5-HT2 receptor mediated contraction of the rabbit isolated femoral artery. Br. J. Pharmacol. 1992;107:418–421. doi: 10.1111/j.1476-5381.1992.tb12761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORECROFT I., HEELEY R.P., PRENTICE H.M., KIRK A., MACLEAN M.R. 5-hydroxytryptamine receptors mediating contraction in human small muscular pulmonary arteries: importance of the 5-HT1B receptor. Br J Pharmacol. 1999;128:730–734. doi: 10.1038/sj.bjp.0702841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOVAHEDI H., PURDY RE. Pharmacological characterisation of the silent 5-hydroxytryptamine1B-like receptors of the rabbit ear artery. J Pharmacol. Exp. Ther. 1997;283:653–660. [PubMed] [Google Scholar]
- OHLSTEIN E.H., DOUGLAS S.A., BROOKS D.P., HAY D.W.P., FEURSTEIN G.Z., RUFFOLO R.R.Functions mediate by peripheral endothelin receptors Endothelin receptors – from the gene to the human 1995CRC Press: Boca, Raton; 109–185.In: Ruffolo, R.R. (ed) [Google Scholar]
- OSIPENKO O.N., ALEXANDER D., MACLEAN M.R., GURNEY A.M. Influence of chronic hypoxia on the contributions of non-activating and delayed rectifier K+ currents to the resting potential and tone of rat pulmonary artery smooth muscle. Br. J. Pharmacol. 1998;124:1335–1337. doi: 10.1038/sj.bjp.0702006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RESINK T.J., SCOTT-BURDEN T., BUHLER F.R. Endothelin stimulates phospholipase C in cultured vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 1988;157:1360–1368. doi: 10.1016/s0006-291x(88)81025-8. [DOI] [PubMed] [Google Scholar]
- SELBIE L.A., HILL S.J. G protein-coupled-receptor cross-talk: the fine-tuning of multiple receptor-signalling pathways. Trends Pharmacol. Sci. 1998;19:87–93. doi: 10.1016/s0165-6147(97)01166-8. [DOI] [PubMed] [Google Scholar]
- SHEEDY W., THOMPSON J.S., MORICE A.H. A comparison of pathophysiological changes during hypobaric and normobaric hypoxia in rats. Resp. 1996;63:217–222. doi: 10.1159/000196548. [DOI] [PubMed] [Google Scholar]
- SHIMAMOTO H., SHIMAMOTO Y., KWAN C-Y., DANIEL E.E. Activation of protein kinase C as a modulator of potentiated UK-14304- induced contractions in dog mesenteric artery and vein. J. Cardiovasc. Pharmacol. 1995;26:923–931. doi: 10.1097/00005344-199512000-00011. [DOI] [PubMed] [Google Scholar]
- SINGHAL S., HENDERSON R., HORSFIELD K., CUMMING G. Morphometry of the human pulmonary arterial tree. Circ. Res. 1973;33:190–197. doi: 10.1161/01.res.33.2.190. [DOI] [PubMed] [Google Scholar]


