Abstract
To elucidate possible mechanisms underlying the effects of carbamazepine (CBZ), valproate (VPA) and zonisamide (ZNS) on neurotransmitter exocytosis, the interaction between these three antiepileptic drugs (AEDs) and botulinum toxins (BoNTs) on basal, Ca2+- and K+-evoked release of dopamine (DA) and serotonin (5-HT) were determined by microdialysis in the hippocampus of freely moving rats.
Basal release of monoamine was decreased by pre-microinjection of the syntaxin inhibitor, BoNT/C, but only weakly affected by the synaptobrevin inhibitor, BoNT/B. Ca2+-evoked release was inhibited by BoNT/C selectively. K+-evoked release was reduced by BoNT/B predominantly and BoNT/C weakly.
Perfusion with low and high concentrations of CBZ and ZNS increased and decreased basal monoamine release, respectively. Perfusion with VPA increased basal 5-HT release concentration-dependently, whereas basal DA release was affected by VPA biphasic concentration-dependently, similar to CBZ and ZNS. This stimulatory action of AEDs on basal release was inhibited by BoNT/C predominantly.
Ca2+-evoked monoamine release was increased by low concentrations of CBZ, ZNS and VPA, but decreased by high concentrations. These effects of the AEDs on Ca2+-evoked release were inhibited by BoNT/C, but not by BoNT/B.
K+-evoked monoamine release was reduced by AEDs concentration-dependently. The inhibitory effect of these three AEDs on K+-evoked release was inhibited by BoNT/B, but not by BoNT/C,
These findings suggest that the therapeutic-relevant concentration of CBZ, VPA and ZNS affects exocytosis of DA and 5-HT, the enhancement of syntaxin-mediated monoamine release during resting stage, and the inhibition of synaptobrevin-mediated release during depolarizing stage.
Keywords: Carbamezepine, valproate, zonisamide, botulinum toxin, serotonin, dopamine, exocytosis
Introduction
The antiepileptic drugs (AEDS), carbamazepine (CBZ), zonisamide (ZNS) and valproate (VPA) have a wide clinical spectrum of use in epileptic (Scheffer et al., 1994; Bourgeois, 1995; Loiseau & Duche, 1995; Seino & Ito, 1997) and mood (Okuma et al., 1990; Kanba et al., 1994; Norton & Quarles, 2000) disorders. Especially, CBZ has been established as a highly effective first line AED against both simple and complex partial seizures (Scheffer et al., 1994; Loiseau & Duche, 1995), whereas VPA has been recognized as a highly-effective first line AED against idiopathic and primary generalized seizures (Bourgeois, 1995). Several clinical trials have demonstrated ZNS to be a promising AED for treating a wide variety of seizures, including partial and generalised seizures (Seino & Ito, 1997).
The major mechanisms of antiepileptic actions of CBZ and ZNS were considered to be its inhibitory effects on voltage-gated Na+ channel (VGSC) activity (McLean & Macdonald, 1986; Rock et al., 1989; Mattson, 1997). The effect of VPA on VGSC has been less extensively studied, and it is uncertain whether VPA has the same mechanism of action as CBZ and ZNS. Although VPA blocked sustained high-frequency repetitive firing (SRF) (Despland, 1994), detailed voltage-clamp investigations regarding the effects of VPA on Na+ current have not been reported. It cannot be concluded that VPA has a mechanism of action similar to those of ZNS and CBZ until these studies have been performed (Bourgeois, 1995). Recently, ZNS was considered as one of the most effective AEDs against absence and myoclonic epilepsies similar to VPA (Seino & Ito, 1997). It has been considered that the putative mechanisms of action of VPA and ZNS may be modulated by their inhibition of T-type voltage-sensitive Ca2+ channel (VSCC) activity (Suzuki et al., 1992; Kito et al., 1994; 1996); however, their inhibitory effects were attained by doses higher than the plasma-concentration associated with their antiepileptic action (higher than therapeutic-relevant concentration) (Kito et al., 1994; 1996; Bourgeois, 1995).
Numerous investigators have already studied the effects of these three AEDs on monoaminergic transmission (Pratt et al., 1985; Elphick et al., 1990; Okada et al., 1992; 1995; 1997a, 1997b, 1997d; 1998a, 1998b; 1999a; Takebayashi et al., 1995; Ichikawa & Meltzer, 1999; Kawata et al., 1999; 2001; Mizuno et al., 1994; 2000), because monoaminergic function has been related to the aetiology of several psychiatric and neurological disorders. It has been established that inhibition of VGSC activity reduces basal neurotransmitter release (Westerink et al., 1988; 1989). However, we have already demonstrated concentration-dependent biphasic effects of CBZ and ZNS on various neurotransmitter releases (Okada et al., 1992; 1995; 1997a, 1997d; 1998a, 1998b; 1999a; Mizuno et al., 1994; Kawata et al., 1999; 2001). The plasma-concentration of CBZ and ZNS associated with their antiepileptic action (therapeutic-relevant concentration), enhanced basal release of monoamines and acetylcholine without affecting basal glutamate release, and inhibited the depolarization-induced release of monoamine and glutamate (Okada et al., 1992; 1995; 1997a, 1997d; 1998a, 1998b; 1999a; Mizuno et al., 1994; Kawata et al., 1999; 2001), whereas higher than therapeutic-relevant concentration of CBZ and ZNS reduced them (Okada et al., 1992; 1995; 1997a, 1997d; 1998a; 1998b; 1999a; Kawata et al., 1999; 2001; Mizuno et al., 2000). Contrary to CBZ or ZNS, several initial studies could not demonstrate the concentration-dependent biphasic action of VPA monoaminergic system (Takebayashi et al., 1995).
It has been considered that a mechanistic model for neurotransmitter release, known as the soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein (SNAP) receptor (SNARE) hypothesis, proposes that vesicle membrane SNAREs, synaptotagmin and synaptobrevin, binds to cognate proteins on the target membrane SNAREs, syntaxin and SNAP-25, in order to form complexes that are then recognized and dissociated by the general membrane trafficking factors α-SNAP and NSF (Sollner et al., 1993; Sudhof, 1995). Recently, we have demonstrated that hippocampal serotonin (5-HT) release was regulated by two major functional complexes, ‘N-type VSCC/calcium-phospholipid-dependent protein kinase (PKC)/syntaxin' and ‘P-type VSCC/cyclic AMP-dependent protein kinase (PKA)/synaptobrevin', using in vivo microdialysis (Okada et al., 2001).
Hence, using the in vivo microdialysis technique, the present study was designed to determine the interaction between AEDs (CBZ, VPA and ZNS) and SNAREs inhibitors, botulinum toxins (BoNTs) on hippocampal release of dopamine (DA) and 5-HT using in vivo microdialysis, in order to: (1) clarify the mechanisms of concentration-dependent biphasic action of CBZ and ZNS on hippocampal neurotransmitter release, including basal, Ca2+- and K+-evoked, (2) explore whether there is a concentration-dependent biphasic action of VPA on neurotransmitter release, and (3) determine the effects of these three AEDs on exocytosis.
Methods
All of the experiments described in this report were performed in accordance with the specifications of the Ethical Committee of Hirosaki University and met the guidelines of the responsible governmental agency. Male Wistar rats (Clea, Tokyo, Japan), weighing 250 – 300 g, were housed under conditions of constant temperature 22±2°C with a 12 h light-dark cycle.
Microdialysis system preparation
Each rat was placed in a stereotaxic frame and kept under halothane anaesthesia (1.5% mixture of halothane and O2 with N2O). Before inserting the microdialysis probe, all rats used in this study were pre-treated with a microinjection of 0.3 μl modified Ringer's solution (MRS) with or without 0.3 ng botulinum toxins (BoNTs) (Capogna et al., 1997; Pierce and Kalivas, 1997; Okada et al., 2001). A concentric I-type dialysis probe (0.22 mm diameter; 3 mm exposed membrane; Eicom, Kyoto, Japan) was implanted in the hippocampus at anterior=−5.8 mm, lateral=4.8 mm and vertical=−4.0 mm (relative to bregma) (Paxinos & Watson, 1986) and the perfusion experiments were started 18 h after the rats had recovered from anaesthesia (Okada et al., 1997c; 1999b). The perfusion rate was always 1 μl min−1, using MRS composed of (in mM): Na+ 145, K+ 2.7, Ca2+ 1.2, Mg2+ 1.0, Cl−1 154.4, and buffered with 2 mM phosphate buffer and 1.1 mM Tris buffer adjusted to pH 7.40 (Okada et al., 1998b, 1998c). To study the effects of an increase in extracellular levels of Ca2+ (Ca2+-evoked stimulation) and K+ (K+-evoked stimulation) on the hippocampal extracellular monoamine level, MRS containing 3.4 mM Ca2+ (HCMRS) and 50 mM K+ (HKMRS) was perfused for 20 min (Okada et al., 1998c; 2001). The ionic composition was modified and isotonicity was maintained by an equimolar decrease of Na+ (Okada et al., 1998b). Each hippocampal dialysate was injected every 20 min into a high performance liquid-chromatograph (HPLC).
HPLC system preparation
The HPLC system used for determination of the extracellular levels of DA and 5-HT was equipped with an electrochemical detector (ECD-300, Eicom) with a EP-300 Eicom pump and a graphite carbon electrode set at +450 mV (versus an Ag/AgCl reference electrode). The analytical column (100 mm×1.5 mm internal diameter) was packed with mightysil RP-18 (3 μm particle size; a gift from Kanto Chemicals, Tokyo, Japan), by Masis INC. (Hirosaki, Japan). The mobile phase comprised 0.1 M phosphate buffer, containing 20% (v v−1) methanol, 900 mg l−1 octansulfonic sodium and 50 mg l−1 EDTANa2. The final pH was 5.9 and the column temperature was maintained at 25°C with the flow rate set at 200 μl min−1 (Okada et al., 2001).
Chemical agents
The chemical agents used in this study were carbamazepine (Tokyo Chemical Industry, Tokyo, Japan), sodium valproate (Wako Chemicals, Osaka, Japan), zonisamide (Dainippon Pharmaceutical Co., Osaka, Japan), the synaptobrevin inhibitor, BoNT/B (Wako Chemicals) and the syntaxin inhibitor, BoNT/C (Wako Chemicals).
Drug administration
The perfusions were initiated with MRS, and hippocampal extracellular levels of DA and 5-HT were measured only at 6 h after starting the perfusion. When the coefficients of variation of hippocampal extracellular levels of DA and 5-HT reached less than 5% over 60 min (stabilization) (Okada et al., 1998c), control data were obtained over a further 60 min, then the perfusion medium (MRS) was switched to MRS containing the target AED (pre-treatment period), in order to study the effects of CBZ, VPA and ZNS on basal monoamine release.
To study the effects of target agents on hippocampal Ca2+- and K+-evoked monoamine release, after confirming stabilization, the pre-treatment perfusion medium was switched to HCMRS or HKMRs containing the same agents for 20 min. After Ca2+- or K+-evoked stimulation, the perfusion medium was switched to the pre-treatment perfusion medium for another 100 min.
Diffusion rates of AEDs and recovery rate of monoamine
In order to accurately measure the concentration of CBZ, ZNS and VPA in the extracellular fluid perfused from hippocampus, in vivo probe diffusion was determined according to the ‘reverse dialysis' procedure (Le Quellec et al., 1995). Because solute diffusion occurs in both directions across the dialysis membrane, loss of solute from the perfusate occurs at the same rate as recovery of solute into the perfusate. During analysis the temperature was maintained at 37°C with a perfusion warmer. The concentration of CBZ and ZNS was measured by HPLC according to the methods of Juergens (1987). The concentration of VPA was measured by HPLC according to the methods of Liu et al. (1992). The rate at which CBZ, VPA and ZNS diffused from the dialysis probe (internal to external probes) was 19±2, 23±4 and 21±4% (mean±s.d., n=12), respectively.
In in vitro experiments, the recovery rates of probes for DA and 5-HT (external to internal probes) were of 16.7±2.4 and 13.6±1.9% (mean±s.d., n=36), respectively (Okada et al., 1997c; 1998c; 2001).
Statistics
The concentration-dependent effects of CBZ, VPA and ZNS on basal, Ca2+ and K+-evoked releases of hippocampal DA and 5-HT were compared using two-way analysis of variance (ANOVA) and Tukey's multiple comparison. The interaction between AEDs and BoNTs on basal, Ca2+ and K+-evoked releases of DA and 5-HT were compared using one-way ANOVA and Tukey's multiple comparison.
Results
The basal hippocampal extracellular levels of DA and 5-HT were 3.1±0.3 and 7.5±0.6 fmol sample−1 (20 μl) (mean±s.d., n=36) in 20 min, respectively (Figures 1, 2 and 3). In the pilot study, MRS containing tetrodotoxin (TTX) and Ca2+-free decreased (P<0.01) extracellular levels of DA and 5-HT to lower than 0.2 and 0.5 fmol sample−1, respectively (data not shown). An increase in the extracellular K+ from 2.7 to 50 mM (K+-evoked stimulation for 20 min) increased (P<0.01) from 3.1 to 21.3 and from 7.5 to 42.5 fmol sample−1 the extracellular DA and 5-HT levels, respectively (K+-evoked release; DA 18.2±2.6 fmol sample−1, 5-HT 35.0±5.6 fmol sample−1, mean±s.d., n=36) (Figures 1, 6 and 7). Therefore, these experiments show that under the microdialysis conditions employed, DA and 5-HT levels (basal release) were of neuronal origin, since there were TTX-sensitive, Ca2+-dependent and K+-sensitive (Westerink et al., 1988; 1989). In addition, an increase in the extracellular Ca2+ level from 1.2 to 3.4 mM (Ca2+-evoked stimulation) for 20 min increased (P<0.01) from 3.1 to 6.0 and from 7.5 to 12.4 fmol sample−1 the extracellular DA and 5-HT levels, respectively (Ca2+-evoked release; DA 2.9±0.3 fmol sample−1, 5-HT 4.9±0.6 fmol sample−1, mean±s.d., n=36) (Figures 1, 4 and 5).
Figure 1.
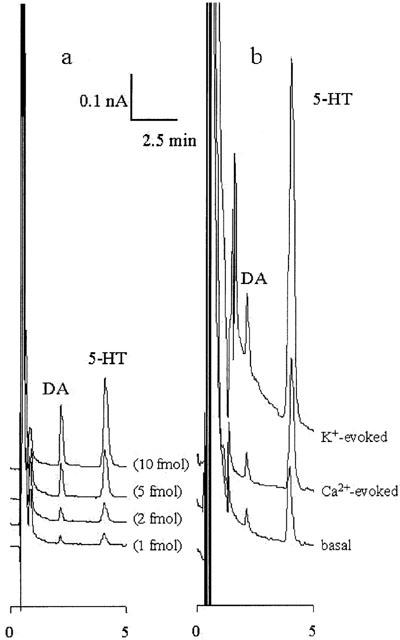
Typical chromatograms of ECD-HPLC. (a,b) Typical chromatograms obtained from 20 μl of a standard solution containing DA and 5-HT, and hippocampal perfusate, respectively. (a) The four lines express chromatograms of different concentrations of DA and 5-HT (1, 2, 5 and 10 fmol). The quantification limits for DA and 5-HT were both 100 amol 20 μl−1. (b) The three lines express chromatograms of basal, Ca2+- and K+-evoked releases.
Figure 2.
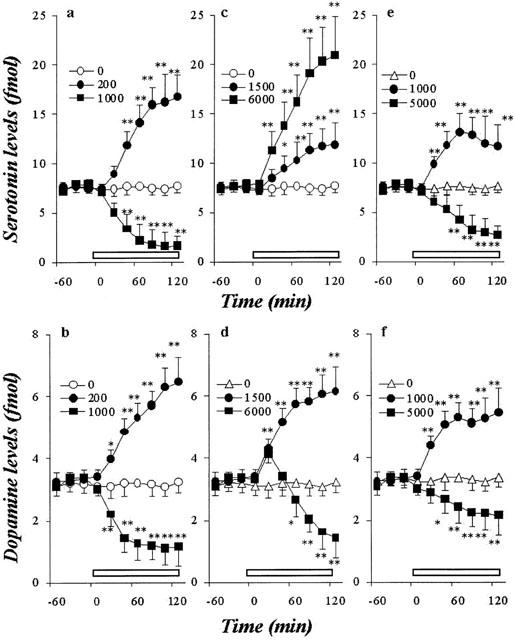
Effects of AEDs on hippocampal basal releases of DA and 5-HT. The effects of CBZ, VPA and ZNS on the hippocampal basal 5-HT release are shown in a, c and e, respectively. The effects of CBZ, VPA and ZNS on the hippocampal basal DA release are shown in b, d and f, respectively. The ordinates indicate the mean±s.d. (n=6) of extracellular levels of 5-HT or DA (fmol sample−1), and abscissas show the time in minutes (min). The open bars indicate perfusion with AEDs. The effects of CBZ, VPA and ZNS on hippocampal basal releases of DA and 5-HT were compared using one-way ANOVA and Tukey's multiple comparison (*P<0.05; **P<0.01).
Figure 3.
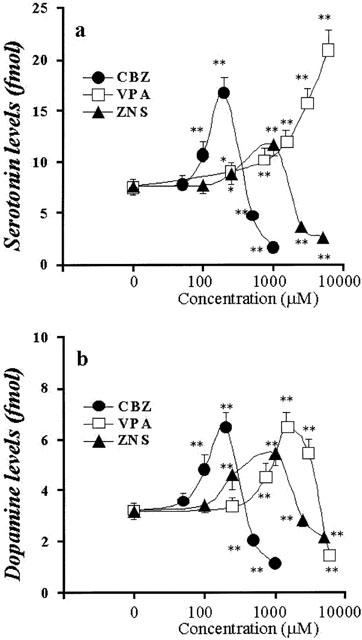
Concentration-dependent effects of AEDs on basal releases of hippocampal DA and 5-HT. The concentration-dependent effects of CBZ, VPA and ZNS on basal releases of hippocampal 5-HT and DA are shown in a and b, respectively. The ordinates indicate the mean±s.d. (n=6) of levels of basal releases of DA and 5-HT (fmol sample−1), and abscissas indicate the concentration of AEDs (μM). The concentration-dependent effects of CBZ, VPA and ZNS on basal releases of hippocampal DA and 5-HT were compared using one-way ANOVA and Tukey's multiple comparison (*P<0.05; **P<0.01).
Figure 6.
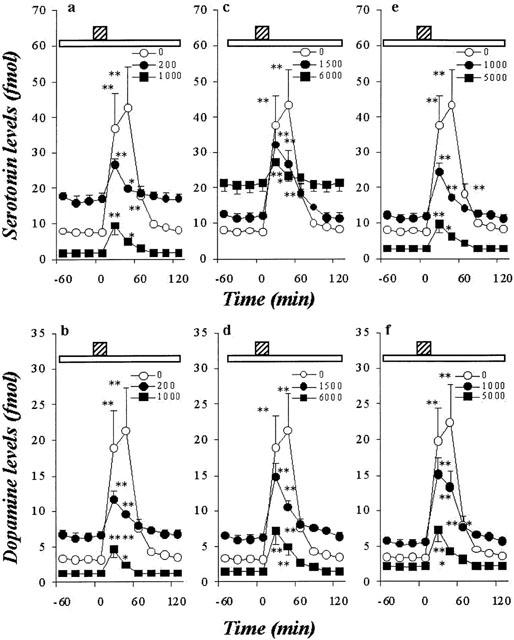
Effects of AEDs on hippocampal K+-evoked releases of DA and 5-HT. The effects of CBZ, VPA and ZNS on hippocampal K+-evoked 5-HT release are shown in a, c and e, respectively. The effects of CBZ, VPA and ZNS on the hippocampal K+-evoked DA release are shown in b, d and f, respectively. The ordinates indicate the mean±s.d. (n=6) of extracellular levels of 5-HT or DA (fmol sample−1), and abscissas show the time in minutes (min). The open bars indicate perfusion with AEDs, and stripped bars indicate the K+-evoked stimulation for 20 min. The concentration-dependent effects of CBZ, VPA and ZNS on hippocampal K+-evoked releases of DA and 5-HT were compared using one-way ANOVA and Tukey's multiple comparison (*P<0.05; **P<0.01).
Figure 7.
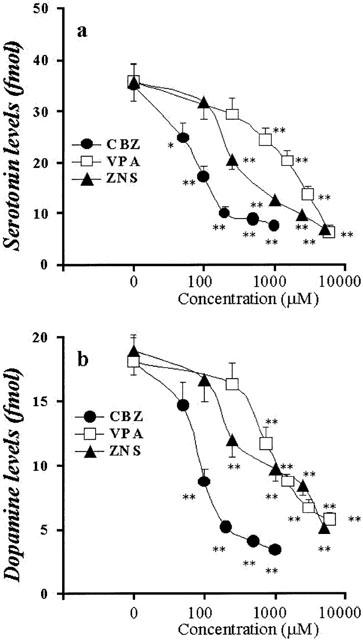
Concentration-dependent effects of AEDs on K+-evoked releases of hippocampal DA and 5-HT. The concentration-dependent effects of CBZ, VPA and ZNS on K+-evoked releases of hippocampal 5-HT and DA are shown in a and b, respectively. The ordinates indicate the mean±s.d. (n=6) of levels of K+-evoked releases of DA and 5-HT (fmol sample−1), and the abscissas indicate the concentration of AEDs (μM). The concentration-dependent effects of CBZ, VPA and ZNS on hippocampal K+-evoked releases of DA and 5-HT were compared using one-way ANOVA and Tukey's multiple comparison (*P<0.05; **P<0.01).
Figure 4.
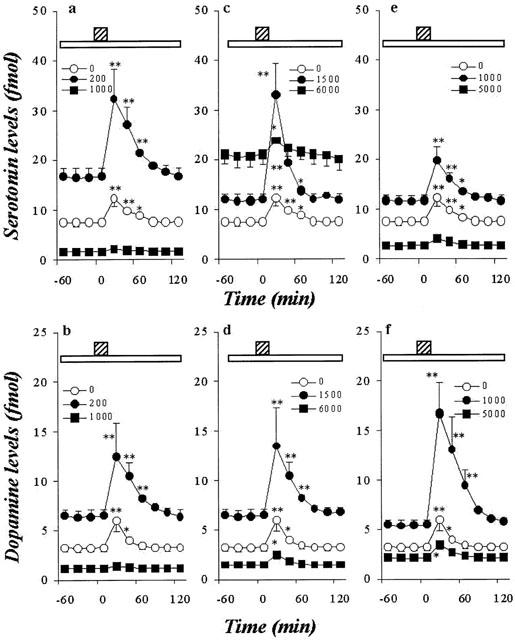
Effects of AEDs on hippocampal Ca2+-evoked releases of DA and 5-HT. The effects of CBZ, VPA and ZNS on the hippocampal Ca2+-evoked 5-HT release are shown in a, c and e, respectively. The effects of CBZ, VPA and ZNS on the hippocampal Ca2+-evoked DA release are shown in b, d and f, respectively. The ordinates indicate the mean±s.d. (n=6) of extracellular levels of 5-HT or DA (fmol sample−1), and abscissas show the time in minutes (min). The open bars indicate perfusion with AEDs, and stripped bars indicate the Ca2+-evoked stimulation for 20 min. The effects of CBZ, VPA and ZNS on hippocampal Ca2+-evoked releases of DA and 5-HT were compared using one-way ANOVA and Tukey's multiple comparison (*P<0.05; **P<0.01).
Figure 5.
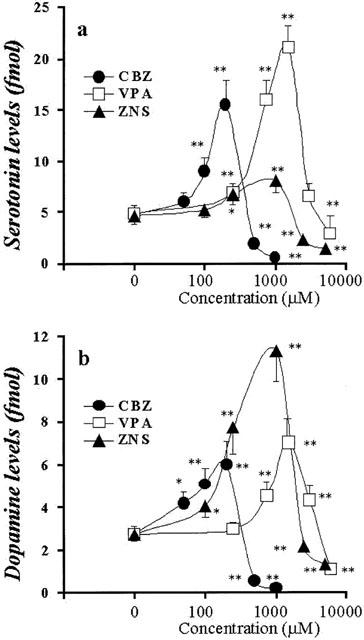
Concentration-dependent effects of AEDs on Ca2+-evoked releases of hippocampal DA and 5-HT. The concentration-dependent effects of CBZ, VPA and ZNS on Ca2+-evoked releases of hippocampal 5-HT and DA are shown in a and b, respectively. The ordinates indicate the mean±s.d. (n=6) of levels of Ca2+-evoked releases of DA and 5-HT (fmol sample−1), and the abscissas indicate the AEDs concentration (μM). The concentration-dependent effects of CBZ, VPA and ZNS on Ca2+-evoked releases of hippocampal DA and 5-HT were compared using one-way ANOVA and Tukey's multiple comparison (*P<0.05; **P<0.01).
Concentration-dependent effects of AEDs on basal hippocampal monoamine release
Figures 2 and 3 show the concentration-dependent effects of CBZ, VPA and ZNS on basal hippocampal releases of 5-HT and DA, respectively. The perfusion with CBZ (100 and 200 μM, estimated concentrations in hippocampal tissue were 19 and 38 μM, respectively) and ZNS (250 and 1000 μM, estimated concentrations in hippocampal tissue were 53 and 210 μM, respectively) increased hippocampal basal DA and 5-HT releases, in a concentration-dependent manner (P<0.01), whereas higher doses of CBZ (500 and 1000 μM, estimated concentrations in hippocampal tissue were 95 and 190 μM, respectively) and ZNS (2500 and 5000 μM, estimated concentrations in hippocampal tissue were 530 and 1050 μM, respectively) decreased them (P<0.01) (Figures 2 and 3). The perfusion with VPA (250 – 6000 μM, tissue concentrations 58 – 1380 μM, respectively) increased basal 5-HT release concentration-dependently (P<0.01) (Figures 2 and 3), whereas the hippocampal basal DA release was increased by VPA concentration-dependently from 250 to 1500 μM (tissue concentrations 58 – 345 μM) (P<0.01); however, a dose of VPA higher than 3000 μM decreased DA release (Figures 2 and 3).
Concentration-dependent effects of AEDs on Ca2+-evoked hippocampal monoamine release
Figures 4 and 5 show the concentration-dependent effects of CBZ, VPA and ZNS on Ca2+-evoked hippocampal release of 5-HT and DA. The perfusion with CBZ (100 and 200 μM) and ZNS (250 and 1000 μM) increased hippocampal Ca2+-evoked DA and 5-HT release (P<0.01), whereas perfusion with CBZ (500 and 1000 μM) and ZNS (2500 and 5000 μM) decreased them (P<0.01) (Figures 4 and 5). The perfusion with VPA (250 to 1500 μM) increased Ca2+-evoked DA and 5-HT release concentration-dependently (P<0.01), whereas VPA at doses greater than 3000 μM decreased them (P<0.01) (Figures 4 and 5).
Concentration-dependent effects of AEDs on K+-evoked hippocampal monoamine release
Figures 6 and 7 show the concentration-dependent effects of CBZ, VPA and ZNS on K+-evoked hippocampal releases of 5-HT and DA. The perfusion with these drugs reduced hippocampal K+-evoked releases of DA and 5-HT in a concentration-dependent manner (P<0.01) (Figures 6 and 7).
Interaction between AEDs and BoNTs on basal monoamine release
The microinjection of both 0.3 ng BoNT/B and BoNT/C decreased the basal releases of 5-HT (P<0.01) and DA (BoNT/B: P<0.05; BoNT/C: P<0.01) (Figure 8). The inhibitory effect of BoNT/C on basal releases of 5-HT and DA was more predominant than that of BoNT/B (P<0.05) (Figure 8).
Figure 8.
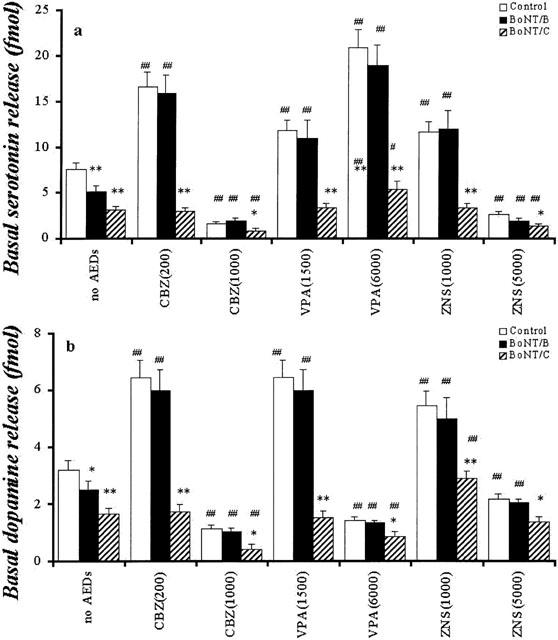
Interaction between AEDs and BoNTs on hippocampal basal releases of DA and 5-HT. The interactions between AEDs and BoNTs on hippocampal basal release of 5-HT and DA are shown in a and b, respectively. The ordinates indicate the mean±s.d. (n=6) of levels of basal releases of DA and 5-HT (fmol sample−1). The interaction between AEDs and BoNTs on hippocampal basal releases of DA and 5-HT were compared using two-way ANOVA and Tukey's multiple comparison (control vs *P<0.05; **P<0.01, no AEDs vs #P<0.05; ##P<0.01).
Inhibition of synaptobrevin by BoNT/B did not affect low concentrations of CBZ (200 μM) and ZNS (1000 μM) mediated stimulatory response of basal DA and 5-HT release, and high concentrations of CBZ (1000 μM) and ZNS (5000 μM) mediated inhibitory response of them (Figure 8). The concentration-dependent stimulatory effects of VPA on basal 5-HT release and the biphasic concentration-dependent effects of VPA (elevation by 1500 μM VPA and inhibition by 6000 μM VPA) on basal DA release also were not affected by BoNT/B (Figure 8).
After inhibition of syntaxin with BoNT/C, the stimulatory effects of low concentrations of CBZ on basal releases of 5-HT and DA were abolished, whereas the high concentrations of CBZ decreased them (P<0.01) (Figure 8). Under the same conditions, the stimulatory effect of low concentrations of VPA on basal release was abolished. High concentrations of VPA increased basal 5-HT release (P<0.05), but this stimulatory effect was reduced by BoNT/C (P<0.01). Contrary to 5-HT, high concentrations of VPA reduced basal DA release (P<0.05), under the inhibition of syntaxin (Figure 8). Under the same conditions, low concentrations of ZNS did not affect and increased (P<0.01) basal 5-HT and DA release, respectively, but the low concentrations of ZNS-mediated stimulatory response of DA release was reduced by BoNT/C (P<0.01) (Figure 8). The high concentrations of ZNS decreased (P<0.01) and did not affect basal releases of 5-HT and DA, respectively (Figure 8).
Interaction between AEDs and BoNTs on Ca2+-evoked monoamine release
The microinjection of 0.3 mg BoNT/C reduced the Ca2+-evoked releases of DA and 5-HT (P<0.01), whereas 0.3 ng BoNT/B did not affect Ca2+-evoked releases (Figure 9).
Figure 9.
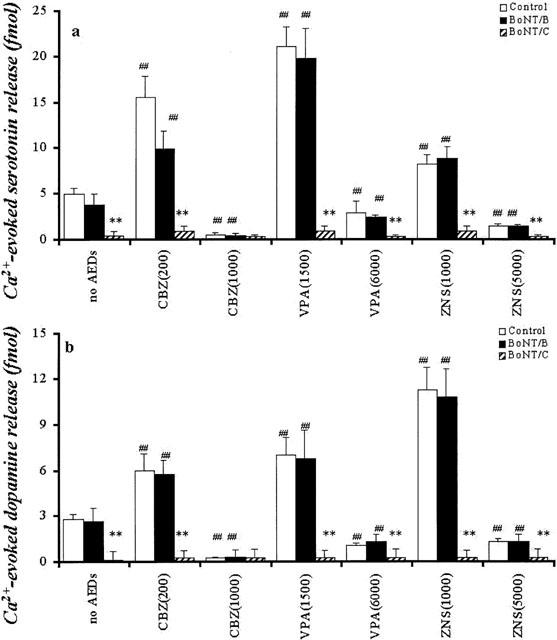
Interaction between AEDs and BoNTs on hippocampal Ca2+-evoked releases of DA and 5-HT. The interactions between AEDs and BoNTs on hippocampal Ca2+-evoked release of 5-HT and DA are shown in a and b, respectively. The ordinates indicate the mean±s.d. (n=6) of levels of Ca2+-evoked releases of DA and 5-HT (fmol sample−1). The interaction between AEDs and BoNTs on hippocampal Ca2+-evoked releases of DA and 5-HT were compared using two-way ANOVA and Tukey's multiple comparison (control vs *P<0.05; **P<0.01, no AEDs vs #P<0.05; ##P<0.01).
After inhibition of synaptobrevin with BoNT/B, the low concentrations of CBZ, VPA and ZNS increased Ca2+-evoked releases of 5-HT and DA (P<0.01), whereas the high concentrations of CBZ, VPA and ZNS decreased them (P<0.01) (Figure 9). After inhibition of syntaxin with BoNT/C, neither low nor high concentrations of CBZ, VPA and ZNS affected Ca2+-evoked releases of 5-HT and DA (Figure 9).
Interaction between AEDs and BoNTs on K+-evoked monoamine release
The microinjection of 0.3 ng BoNT/B and BoNT/C reduced the K+-evoked releases of DA and 5-HT (Figure 10). The inhibitory effect of BoNT/B on K+-evoked releases of 5-HT and DA was more predominant than that of BoNT/C (P<0.01) (Figure 10).
Figure 10.
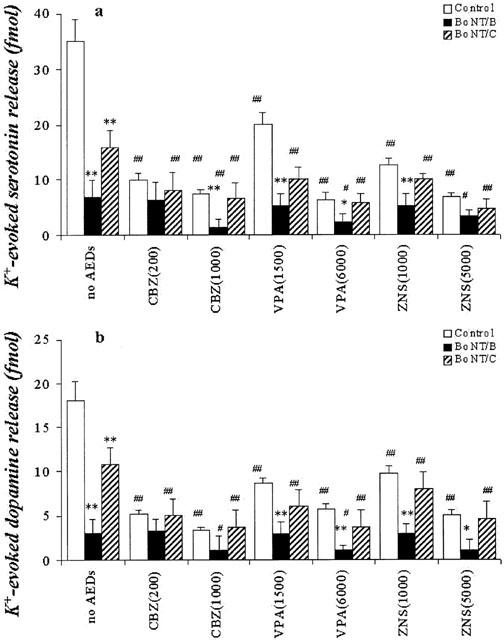
Interaction between AEDs and BoNTs on hippocampal K+-evoked releases of DA and 5-HT. The interactions between AEDs and BoNTs on hippocampal K+-evoked release of 5-HT and DA are shown in a and b, respectively. The ordinates indicate the mean±s.d. (n=6) of levels of K+-evoked releases of DA and 5-HT (fmol sample−1). The interaction between AEDs and BoNTs on hippocampal K+ (control vs *P<0.05; **P<0.01, no AEDs vs #P<0.05; ##P<0.01).
Inhibition of syntaxin with BoNT/C did not affect the concentration-dependent inhibitory effects of CBZ, VPA and ZNS on K+-evoked releases of 5-HT and DA (Figure 10). Inhibition of synaptobrevin with BoNT/B abolished the inhibitory effects of low concentrations of CBZ, VPA and ZNS on K+-evoked releases of 5-HT and DA (P<0.01) (Figure 10). The high concentrations of CBZ, VPA and ZNS decreased on K+-evoked releases of 5-HT and DA (P<0.01), but these inhibitory effects were reduced by BoNT/B (P<0.05) (Figure 10).
Discussion
Exocytosis mechanisms of hippocampal DA and 5-HT
Different exocytosis mechanisms are implicated in both spontaneous and depolarization-induced neurotransmitter release (Yoshihara et al., 1999; Kawata et al., 2001; Okada et al., 2001). The mechanism for depolarization-induced neurotransmitter release has been considered to occur as follows: after arrival of an action potential at the presynaptic terminal leading to Ca2+ entry through specific channels and thus increasing abruptly its levels to submillimolar concentration (Roberts et al., 1990). On the other hand, spontaneous fusion of the synaptic vesicle at the resting state generates miniature synaptic potentials. The frequency of these events changes when the cytosolic Ca2+ level is altered in the micromolar range (Delaney & Tank, 1994; Xu et al., 1998). Recent studies have indicated that hippocampal 5-HT release was regulated by the two functional complexes, N-type VSCC/PKC/syntaxin and P-type VSCC/PKA/synaptobrevin (Kawata et al., 2001; Okada et al., 2001). This study demonstrated that hippocampal basal and K+-evoked DA release is regulated by the similar mechanisms of 5-HT exocytosis, since the basal and K+-evoked releases of hippocampal DA were predominantly inhibited by inhibitors of syntaxin (BoNT/C) and synaptobrevin (BoNT/B), respectively. Furthermore, we have already demonstrated that the Ca2+-evoked releases of striatal DA and hippocampal 5-HT were inhibited by N-type VSCC inhibitor, ω-conotoxin GVIA selectively but was unaffected by P-type VSCC inhibitor, ω-agatoxin IVA (Okada et al., 1998c; Kawata et al., 2001). Taken together with this evidence, the present results, the Ca2+-evoked releases of hippcampal DA and 5-HT were inhibited by BoNT/C but not by BoNT/B, suggests that the Ca2+-evoked releases of DA and 5-HT may require specific exocytosis mechanisms, only N-type VSCC/PKC/syntaxin. To clarify this hypothesis, we are determining the exocytosis mechanisms of Ca2+-evoked hippocampal monoamine release.
Effects of CBZ and ZNS on monoamine release
It has been well established that therapeutic-relevant doses of CBZ and ZNS inhibit VGSC activity (McLean & MacDonald, 1986; Mattson, 1997) and this could decrease basal monoamine release (Westerink et al., 1988; 1989). Both our previous and present studies have demonstrated that the therapeutic-relevant concentration of CBZ (from 17 to 42 μM) and ZNS (47 to 330 μM) that inhibit VGSC activity, increased basal and Ca2+-evoked release of DA and 5-HT (Okada et al., 1992; Kawata et al., 1999; 2001). In contrast, the therapeutic-relevant concentration of CBZ and ZNS decreased K+-evoked release of DA, 5-HT and glutamate without affecting basal glutamate release (Okada et al., 1998b; Kawata et al., 1999; 2001). On the other hand, higher than plasma-concentration of CBZ and ZNS associated with their antiepileptic action decreased basal, Ca2+- and K+-evoked releases of DA, 5-HT and K+-evoked glutamate (Okada et al., 1992; 1995; 1997a, 1997d; 1998a, 1998b; Kawata et al., 1999; 2001) without affecting basal glutamate release (Okada et al., 1998b). This contradiction between the effects of CBZ and ZNS on basal, Ca2+- and K+-evoked monoamine release may be caused by the sensitivities of two functional complex pathways for monoamine release, N-type VSCC/PKC/syntaxin and P-type VSCC/PKA/synaptobrevin.
In this study, the stimulatory effects of therapeutic-relevant concentrations of CBZ and ZNS on Ca2+-evoked monoamine release, which was sensitive to BoNT/C but insensitive to BoNT/B, were inhibited by BoNT/C, whereas under the condition of microinjection of BoNT/C, higher than therapeutic-relevant concentrations of CBZ and ZNS did not affect Ca2+-evoked monoamine release. These results suggest that therapeutic-relevant concentrations of CBZ and ZNS enhance syntaxin-related monoamine exocytosis mechanism; however, higher than therapeutic-relevant concentrations of CBZ and ZNS inhibit it.
The concentration-dependent biphasic effects of CBZ and ZNS on basal monoamine release were not affected by inhibition of synaptobrevin with BoNT/B. The inhibition of syntaxin with BoNT/C abolished the stimulatory effects of therapeutic-relevant concentrations of CBZ on monoamine basal release. Under the same conditions, higher than therapeutic-relevant concentrations of CBZ remained able to reduce basal monoamine release. These results suggest that the major mechanisms of stimulatory effects of therapeutic-relevant concentrations of CBZ on basal monoamine release were stimulation of syntaxin-related monoamine exocytosis. Contrary to therapeutic-relevant concentration, the major mechanisms of inhibitory effects of higher than therapeutic-relevant concentrations of CBZ on basal monoamine release may be inhibition of syntaxin-related monoamine exocytosis; however, the present study could not deny the possibility that the higher than therapeutic-relevant concentrations of CBZ inhibit other basal monoamine exocytosis mechanisms, i.e. adenosine receptor, N-type VSCC and VGSC (Okada et al., 1997d; Kawata et al., 2001). The inhibition of syntaxin with BoNT/C abolished the stimulatory effects of therapeutic-relevant concentrations of ZNS on 5-HT basal release, but higher than therapeutic-relevant concentrations of ZNS reduced it, similar to CBZ. However, under the same conditions, the basal DA release was increased and unaffected by within and higher than therapeutic-relevant concentrations of ZNS, respectively. These different results between ZNS and CBZ on basal DA release, under the conditions of inhibition of syntaxin activity, might be modulated by inhibition of monoamine oxidase activity induced by ZNS (Okada et al., 1992; 1995).
The concentration-dependent inhibitory effects of CBZ and ZNS on K+-evoked monoamine release were not affected by BoNT/C. In contrast to BoNT/C, under the inhibition of synaptobrevin using BoNT/B, they were inhibited, but the higher than therapeutic-relevant concentrations of CBZ and ZNS could reduce it. Therefore, these results suggest that both CBZ and ZNS inhibit the synaptobrevin-related exocytosis mechanism concentration-dependently. Furthermore, higher than therapeutic-relevant concentrations of CBZ and ZNS may inhibit other K+-evoked monoamine release mechanisms, i.e. P-type VSCC or VGSC (Kawata et al., 1999; 2001).
CBZ reduces Ca2+ influx (Kito et al., 1994; 1996; Yoshimura et al., 1995), but the effects of CBZ on VSCC subtypes have not yet been clarified. Kito et al. (1994; 1996) demonstrated, using a patch-clamp technique, the lack of effect of CBZ on various subtypes of Ca2+ channels in the human neuroblastoma NB1 cell; however, Yoshimura et al. (1995) demonstrated that CBZ inhibited N-type VSCC function in cultured bovine adrenal medullary cells. Therefore, taken together with these previous demonstrations, present results suggest that CBZ affects, at least partially, the N-type VSCC/PKC/syntaxin pathway biphasic concentration-dependently without Ca2+ inflow through N-type VSCC. On the other hand, the effect of ZNS on VSCC has been studied less extensively. It remains uncertain whether ZNS has the same mechanism of action as CBZ. Although ZNS inhibited T-type VSCC activity (Suzuki et al., 1992), which does not affect monoamine release (Kato et al., 1992), detailed voltage-clamp investigations regarding the effects of ZNS on other subtypes of VSCC has not been demonstrated.
Nevertheless, the present study demonstrated that therapeutic-relevant concentrations of CBZ and ZNS enhanced syntaxin-related exocytosis mechanism, whereas higher than therapeutic-relevant concentrations of CBZ and ZNS reduced both syntaxin- and synaptobrevin-related exocytosis mechanisms.
Effects of VPA on monoamine release
VPA affected basal DA release biphasic concentration-dependently, similar to CBZ and ZNS, whereas VPA increased basal 5-HT release concentration-dependently. Contrary to basal release, both Ca2+-evoked release of DA and 5-HT were enhanced and reduced by therapeutic- and higher than therapeutic-relevant concentrations of VPA, respectively (biphasic concentration-dependently). The stimulatory effects of VPA on basal and Ca2+-evoked releases of DA and 5-HT were inhibited by BoNT/C but unaffected by BoNT/B, whereas the inhibitory effects of higher than therapeutic-relevant concentrations of VPA were reduced by BoNT/C but were not affected by BoNT/B. Therefore, therapeutic- and higher than therapeutic-relevant concentrations of VPA enhance and inhibit syntaxin-related exocytosis mechanism, similar to CBZ and ZNS. It remains uncertain why higher than therapeutic-relevant concentrations of VPA increase and decrease basal release of DA and 5-HT, respectively. In our previous study, VPA increased 5-HT levels in hippocampal tissue, whereas DA levels increased and decreased by therapeutic- and higher than therapeutic-relevant doses of VPA, respectively (Mizuno et al., 1994). Although VPA acts on the syntaxin-related exocytosis mechanism biphasic concentration-dependently similar to CBZ and ZNS, VPA may have another effect on serotonergic transmission, i.e. enhancement of 5-HT synthesis or turnover (Mizuno et al., 1994). On the other hand, the concentration-dependent inhibitory effect of VPA on K+-evoked monoamine release, which was low-sensitive to BoNT/C and high-sensitive to BoNT/B, was not affected by BoNT/B. Therefore, these results suggest that VPA inhibits synaptobrevin-mediated exocytosis concentration-dependently, similar to CBZ and ZNS.
Mechanisms of antiepileptic action of CBZ, VPA and ZNS
The occurrence of an epileptic seizure may be generated by the relative imbalance between excitatory and inhibitory neurotransmission, resulting in neurones showing increased excitability and abnormally frequent patterns of discharge (Hirose et al., 2000). Glutamate is the principal excitatory neurotransmitter, and excessive release of glutamate may produce seizures in epileptic patients (During et al., 1995), and in animal models of epilepsy (Ueda & Tsuru, 1994). We have already demonstrated that therapeutic-relevant concentrations of CBZ and ZNS reduce both hippocampal K+-evoked glutamate release and spreading depression induced glutamate release, without affecting basal release (Okada et al., 1998b). In addition, enhancement of monoaminergic transmission reduced seizure threshold (Wahnschaffe & Loscher, 1991; Wada et al., 1993). Taken together with our previous results, the present study indicates that the mechanisms of antiepileptic action of therapeutic-relevant concentrations of CBZ, VPA and ZNS may be produced by the combination of effects resulting from enhancement of basal release of inhibitory neurotransmitter without affecting basal glutamate release, and reduction of neurotransmitter release at the event of neuronal excitation (i.e. during seizures).
In this study we determined the concentration-dependent different action of CBZ, VPA and ZNS on the basal, Ca2+- and K+-evoked hippocampal release of DA and 5-HT, in this study. In conclusion, higher than therapeutic-relevant concentrations of CBZ, VPA and ZNS inhibited the activities of both synaptobrevin- and syntaxin-related monoamine release mechanisms, which resulted in inhibition of monoamine exocytosis. In contrast, the low concentrations of CBZ, VPA and ZNS enhanced the syntaxin-related exocytosis mechanism at the resting stage, and synaptobrevin-related exocytosis mechanism at the neuronal depolarizing stage, i.e. during K+-evoked stimulation. CBZ, VPA and ZNS possibly exert their antiepileptic action, at least partially, by combined effect on the enhancement of syntaxin-related monoamine exocytosis mechanism (enhancement of basal release) during neuronal resting stage, which elevates seizure threshold, and inhibition of synaptobrevin-related monoamine exocytosis mechanism during neuronal depolarization. It can now be proposed that at least partially, therapeutic-relevant concentrations of CBZ, VPA and ZNS exert their antiepileptic action by combined effects on modulation of two functional pathways for exocytosis, namely the N-type VSCC/PKC/syntaxin and the P-type VSCC/PKA/synaptobrevin, during both resting and depolarizing stages, respectively.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Science and Culture (05454309 and 11770532), a Grant from Hirosaki Research Institute for Neurosciences, a Grant from Pharmacopsycyhiatry Research Foundation and a Grant from Japan Epilepsy Research Foundation.
Abbreviations
- AEDs
antiepileptic drugs
- ANOVA
analysis of variance
- BoNTs
botulinum toxins
- BoNT/B
botolinum toxin type B
- BoNT/C
botulinum toxin type C
- CBZ
carbamazepine
- DA
dopamine
- HCMRS
high Ca2+ (3.4 mM) containing modified Ringer's solution
- HKMRS
high K+ (50 mM) containing modified Ringer's solution
- HPLC
high performance liquid chromatograph
- 5-HT
serotonin
- MRS
modified Ringer's solution
- NSF
N-ethylmaleimide-sensitive factor
- PKA
cyclic AMP-dependent protein kinase
- PKC
calcium-phospholipid-dependent protein kinase
- SNAP
soluble N-ethylmaleimide-sensitive factor attachment protein
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- SRF
sustained high-frequency repetitive firing
- TTX
tetrodotoxin
- VGSC
voltage-gated Na+ channel
- VPA
valproate
- VSCC
voltage-sensitive Ca2+ channel
- ZNS
zonisamide
References
- BOURGEOIS B.F.D.Valproic acid Clinical Use Antiepileptic drugs, Fourth edition 1995New York: Raven Press; 633–639.ed. Levy, R.H., Mattson, R.H. & Meldrum, B.S [Google Scholar]
- CAPOGNA M., MCKINNEY R.A., O'CONNOR V., GAHWILER B.H., THOMPSON S.M. Ca2+ or Sr2+ partially rescues synaptic transmission in hippocampal cultures treated with botulinum toxin A and C, but not tetanus toxin. J. Neurosci. 1997;17:7190–7202. doi: 10.1523/JNEUROSCI.17-19-07190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELANEY K.R., TANK D.W. A quantitative measurement of the dependence of short-term synaptic enhancement on synaptic residual calcium. J. Neurosci. 1994;14:5885–5902. doi: 10.1523/JNEUROSCI.14-10-05885.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESPLAND P.A. A retrospective study of 113 epileptic patients treated with sustained-release valproate. Epilepsia. 1994;35 Suppl 5:S99–S100. doi: 10.1111/j.1528-1157.1994.tb05981.x. [DOI] [PubMed] [Google Scholar]
- DURING M.J., RYDER K.M., SPENCER D.D. Hippocampal GABA transporter function in temporal-lobe epilepsy. Nature. 1995;376:174–177. doi: 10.1038/376174a0. [DOI] [PubMed] [Google Scholar]
- ELPHICK M., ANDERSON S.M., HALLIS K.F., GRAHAME-SMITH D.G. Effects of carbamazepine on 5-hydroxytryptamine function in rodents. Psychopharmacology. 1990;100:49–53. doi: 10.1007/BF02245789. [DOI] [PubMed] [Google Scholar]
- HIROSE S., OKADA M., KANEKO S., MITUDOME A. Are some idiopathic epilepsies disorders of the ion channels?: A working hypothesis. Epilepsy Res. 2000;41:191–204. doi: 10.1016/s0920-1211(00)00141-8. [DOI] [PubMed] [Google Scholar]
- ICHIKAWA J., MELTZER H.Y. Valproate and carbamazpine increase prefrontal dopamine release by 5-HT1A receptor activation. Eur. J. Pharmacol. 1999;380:R1–R3. doi: 10.1016/s0014-2999(99)00517-8. [DOI] [PubMed] [Google Scholar]
- JUERGENS U. Simultaneous determination of zonisamide and nine other anti-epileptic drugs and metabolites in serum. J. Chromatogr. 1987;385:233–240. doi: 10.1016/s0021-9673(01)94635-7. [DOI] [PubMed] [Google Scholar]
- KANBA S., YAGI G., KAMIJIMA K., SUZUKI T., TAJIMA O., OTAKI J., ARATA E., KOSHIKAWA H., NIBUYA M., KINOSHITA N., ASAI M. The first open study of zonisamide, a novel anticonvulsant, shows efficacy in mania. Prog. Neuropsychopharmacol. Biol. Psychiat. 1994;18:707–715. doi: 10.1016/0278-5846(94)90078-7. [DOI] [PubMed] [Google Scholar]
- KATO T., OTSU Y., FURUNE Y., YAMAMOTO T. Different effects of L-, N- and T-type calcium channel blockers on striatal dopamine release measured by microdialysis in freely moving rats. Neurochem. Int. 1992;21:99–107. doi: 10.1016/0197-0186(92)90072-y. [DOI] [PubMed] [Google Scholar]
- KAWATA Y., OKADA M., MURAKAMI T., KAMATA A., ZHU G., KANEKO S. Pharmacological discrimination between effects of carbamazepine on hippocampal basal, Ca2+- and K+-evoked serotonin releases. Br. J. Pharamcol. 2001;133:557–567. doi: 10.1038/sj.bjp.0704104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWATA Y., OKADA M., MURAKAMI T., MIZUNO K., WADA K., KONDO T., KANEKO S. Effects of zonisamide on K+ and Ca2+ evoked release of monoamine as well as K+ evoked intracellular Ca2+ mobilization in rat hippocampus. Epilepsy Res. 1999;35:173–182. doi: 10.1016/s0920-1211(99)00010-8. [DOI] [PubMed] [Google Scholar]
- KITO M., MAEHARA M., WATANABE K. Antiepileptic drugs-calcium current interaction in cultured human neuroblastoma cells. Seizure. 1994;3:141–149. doi: 10.1016/s1059-1311(05)80205-5. [DOI] [PubMed] [Google Scholar]
- KITO M., MAEHARA M., WATANABE K. Mechanisms of T-type calcium channel blockade by zonisamide. Seizure. 1996;5:115–119. doi: 10.1016/s1059-1311(96)80104-x. [DOI] [PubMed] [Google Scholar]
- LE QUELLEC A., DUPIN S., GENISSEL P., SAIVIN S., MARCHAND B., HOUIN G. Microdialysis probes calibration: gradient and tissue dependent changes in no net flux and reverse dialysis methods. J. Pharmacol. Toxicol. Methods. 1995;33:11–16. doi: 10.1016/1056-8719(94)00049-a. [DOI] [PubMed] [Google Scholar]
- LIU H., FORMAN L.J., MONTOYA J., EGGERS C., BARHAM C., DELGADO M. Determination of valproic acid by high-performance liquid chromatography with photodiode-array and fluorescence detection. J. Chromatogr. 1992;576:163–169. doi: 10.1016/0378-4347(92)80189-w. [DOI] [PubMed] [Google Scholar]
- LOISEAU P., DUCHE B.Carbamazepine Clinical Use Antiepileptic drugs, Fourth edition 1995New York: Raven Press; 555–566.ed. Levy, R.H., Mattson, R.H. & Meldrum, B.S [Google Scholar]
- MATTSON R.H.Carbamazepine Epilepsy: a comprehensive textbook 1997Philadelphia: Lippincott-Raven Publishers; 1491–1502.ed. Engel, Jr, J. & Pedley, T.A [Google Scholar]
- MCCLEAN M.J., MACDONALD R.L. Carbamazepine and 10,11-epoxycarbamazepine produce use- and voltage-dependent limitation of rapidly firing action potentials of mouse central neurons in cell culture. J. Pharmacol. Exp. Ther. 1986;238:727–738. [PubMed] [Google Scholar]
- MIZUNO K., OKADA M., KANEKO S., HIRANO T., TOKINAGA N., CHIBA T., OTANI K., FUKUSHIMA Y. Effects of carbamazepine on acetylcholine release and metabolism. Epilepsy Res. 2000;40:187–195. doi: 10.1016/s0920-1211(00)00129-7. [DOI] [PubMed] [Google Scholar]
- MIZUNO K., OKADA M., MURAKAMI T., KAMATA A., ZHU G., KAWATA Y., WATA K., KANEKO S. Effects of zonisamide, carbamazepine and valproate on monoamine metabolism in rat striatum and hippocampus. Jpn. J. Psychiatry Neurol. 1994;48:406–408. [Google Scholar]
- NORTON J.W., QUARLES E. Intravenous valproate in neuropsychiatry. Pharmacotherapy. 2000;20:88–92. doi: 10.1592/phco.20.1.88.34657. [DOI] [PubMed] [Google Scholar]
- OKADA M., HIRANO T., KAWATA Y., MURAKAMI T., WADA K., MIZUNO K., KONDO T., KANEKO S. Biphasic effects of zonisamide on serotonergic system in rat hippocampus. Epilepsy Res. 1999a;34:187–197. doi: 10.1016/s0920-1211(98)00109-0. [DOI] [PubMed] [Google Scholar]
- OKADA M., HIRANO T., MIZUNO K., CHIBA T., KAWATA Y., KIRYU K., WADA K., TASAKI H., KANEKO S. Biphasic effects of carbamazepine on the dopaminergic system in rat striatum and hippocampus. Epilepsy Res. 1997a;28:143–153. doi: 10.1016/s0920-1211(97)00042-9. [DOI] [PubMed] [Google Scholar]
- OKADA M., HIRANO T., MIZUNO K., KAWATA Y., WADA K., MURAKAMI T., TASAKI H., KANEKO S. Effects of carbamazepine on hippocampal serotonergic system. Epilepsy Res. 1998a;31:187–198. doi: 10.1016/s0920-1211(98)00025-4. [DOI] [PubMed] [Google Scholar]
- OKADA M., KANEKO S., HIRANO T., ISHIDA M., KONDO T., OTANI K., FUKUSHIMA Y. Effects of zonisamide on extracellular levels of monoamine and its metabolite, and on Ca2+ dependent dopamine release. Epilepsy Res. 1992;13:113–119. doi: 10.1016/0920-1211(92)90066-3. [DOI] [PubMed] [Google Scholar]
- OKADA M., KANEKO S., HIRANO T., MIZUNO K., KONDO T., OTANI K., FUKUSHIMA Y. Effects of zonisamide on dopaminergic system. Epilepsy Res. 1995;22:193–205. doi: 10.1016/0920-1211(95)00078-x. [DOI] [PubMed] [Google Scholar]
- OKADA M., KAWATA Y., KIRYU K., MIZUNO K., WADA K., INOMATA H., TASAKI H., KANEKO S. Effects of non-toxic and toxic concentrations of phenytoin on monoamines levels in rat brain. Epilepsy Res. 1997b;28:155–163. doi: 10.1016/s0920-1211(97)00043-0. [DOI] [PubMed] [Google Scholar]
- OKADA M., KAWATA Y., KIRYU K., MIZUNO K., WADA K., TASAKI H., KENEKO S. Effects of adenosine receptor subtypes on hippocampal extracellular serotonin level and serotonin reuptake activity. J. Neurochem. 1997c;69:2581–2588. doi: 10.1046/j.1471-4159.1997.69062581.x. [DOI] [PubMed] [Google Scholar]
- OKADA M., KAWATA Y., MIZUNO K., WADA K., KONDO T., KENEKO S. Interaction between Ca2+, K+, carbamazepine and zonisamide on hippocampal extracellular glutamate monitored with a microdialysis electrode. Br. J. Pharmacol. 1998b;124:1277–1285. doi: 10.1038/sj.bjp.0701941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKADA M., KAWATA Y., MURAKAMI T., WADA K., MIZUNO K., KONDO T., KANEKO S. Differential effects of adenosine receptor subtypes on release and reuptake of hippocampal serotonin. Eur. J. Neurosci. 1999b;11:1–9. doi: 10.1046/j.1460-9568.1999.00415.x. [DOI] [PubMed] [Google Scholar]
- OKADA M., KIRYU K., KAWATA Y., MIZUNO K., WADA K., TASAKI H., KANEKO S. Determination of the effects of caffeine and carbamazepine on striatal dopamine release by in vivo microdialysis. Eur. J. Pharmacol. 1997d;32:181–188. doi: 10.1016/s0014-2999(96)00938-7. [DOI] [PubMed] [Google Scholar]
- OKADA M., NUTT D.J., MURAKAMI T., ZHU G., KAMATA A., KAWATA Y., KANEKO S. Adenosine receptor subtypes modulate two major functional pathways for hippocampal serotonin release. J. Neurosci. 2001;21:628–640. doi: 10.1523/JNEUROSCI.21-02-00628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKADA M., WADA K., KIRYU K., KAWATA Y., MIZUNO K., KONDO T., TASAKI H., KANEKO S. Effects of Ca2+ channel antagonists on striatal dopamine and DOPA release, studied by in vivo microdialysis. Br. J. Pharmacol. 1998c;123:805–814. doi: 10.1038/sj.bjp.0701675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKUMA T., YAMASHITA I., TAKAHASHI R., ITOH H., OTUKI S., WATANABE S., HAZAMA H., INAGAWA K. Comparison of the antimanic efficacy of carbamazepine and lithium carbonate by double-blind controlled study. Pharmacopsychiatry. 1990;23:143–150. doi: 10.1055/s-2007-1014497. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The rat brain in stereotaxic coordinates: second edition. San Diego: Academic Press; 1986. [Google Scholar]
- PIERCE R.C., KALIVAS P.W. Repeated cocaine modifies the mechanism by which amphetamine releases dopamine. J. Neurosci. 1997;17:3254–3261. doi: 10.1523/JNEUROSCI.17-09-03254.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRATT J.A., JENNER P., MARSDEN C.D. Comparison of the effects of benzodiazepines and other anticonvulsant drugs on synthesis and utilization of 5-HT in mouse brain. Neuropharmacology. 1985;24:59–68. doi: 10.1016/0028-3908(85)90096-6. [DOI] [PubMed] [Google Scholar]
- ROBERTS W.M., JACOBS R.A., HUDSPETH A.J. Colocalization of ion channels involved in frequency selective and synaptic transmission at presynaptic active zone of hair cells. J. Neurosci. 1990;10:3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROCK D.M., MACDONALD R.L., TAYLOR C.P. Blockade of sustained repetitive action potentials in culture spinal cord neurons by zonisamide (AD 810, CI 912), a novel anticonvulsant. Epilepsy Res. 1989;3:138–143. doi: 10.1016/0920-1211(89)90041-7. [DOI] [PubMed] [Google Scholar]
- SCHEFFER I.E., BHATIA K.P., LOPES-CENDES I., FISH D.R., MARSDEN C.D., ANDERMANN F., ANDERMANN E., DESBIENS R., CENDES F., MANSON J.I. Autosomal dominant frontal epilepsy misdiagnosed as sleep disorder. Lancet. 1994;343:515–517. doi: 10.1016/s0140-6736(94)91463-x. [DOI] [PubMed] [Google Scholar]
- SEINO M., ITO T. Epilepsy: A Comprehensive Textbook: Zonisamide 1997Philadelphia: Lippincott-Raven Publishers; 1619–1626.ed. Engel J., Pedley, T [Google Scholar]
- SOLLNER T., WHITEHEART S.W., BRUNNER M., ERDJUMENT-BROMAGE H., GEROMANOS S., TEMPST P., ROTHMAN J.E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- SUDHOF T.C. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- SUZUKI S., KAWAKAMI K., NISHIMURA S., WATANABE Y., YAGI K., SEINO M., MIYAMOTO K. Zonisamide blocks T-type calcium channel in culture neurons of rat cerebral cortex. Epilspsy Res. 1992;12:21–27. doi: 10.1016/0920-1211(92)90087-a. [DOI] [PubMed] [Google Scholar]
- TAKEBAYASHI M., MOTOHASHI N., SAITO H., KAGAYA A., YAMAWAKI S. Effect of acute treatment with sodium valproate on catecholamine and serotonin synthesis in mouse cerebral cortex. Neuropsychobiology. 1995;32:124–127. doi: 10.1159/000119224. [DOI] [PubMed] [Google Scholar]
- UEDA Y., TSURU N. Bilateral seizure-related changes of extracellular glutamate concentration in hippocampi during development of amygdaloid kindling. Epilepsy. Res. 1994;18:85–88. doi: 10.1016/0920-1211(94)90036-1. [DOI] [PubMed] [Google Scholar]
- WADA Y., NAKAMURA M., HASEGAWA H., YAMAGUCHI N. Intra-hippocampal injection of 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) inhibits partial and generalized seizures induced by kindling stimulation in cats. Neurosci. Lett. 1993;159:179–182. doi: 10.1016/0304-3940(93)90828-9. [DOI] [PubMed] [Google Scholar]
- WAHNSCHAFFE U., LOSCHER W. Anticonvulsant effects of ipsilateral but not contralateral microinjections of the dopamine D2 agonost LY 171555 into the nucleus accumbens of amygdala-kindled rats. Brain Res. 1991;553:181–187. doi: 10.1016/0006-8993(91)90822-d. [DOI] [PubMed] [Google Scholar]
- WESTERINK B.H.C., HOFSTEEDE H.M., DAMSMA G., DEVRIES J.B. The significance of extracellular calcium for the release of dopamine, acetylcholine and amino acids in conscious rats, evaluated by brain microdialysis. Naunyn-Schmiedeberg's Arch. Pharmacol. 1988;337:373–378. doi: 10.1007/BF00169526. [DOI] [PubMed] [Google Scholar]
- WESTERINK B.H.C., HOFSTEEDE R.M., TUNTLER J., DE VRIES J.B. Use of calcium antagonism for the characterization of drug-evoked dopamine release from the brain of conscious rats determined by microdialysis. J. Neurochem. 1989;52:722–729. doi: 10.1111/j.1471-4159.1989.tb02514.x. [DOI] [PubMed] [Google Scholar]
- XU, BINZ T., NIEMANN H., NEHER E. Multiple kinetic components of exocytosis distinguished by neurotoxin sensitivity. Nat. Neurosci. 1998;1:192–200. doi: 10.1038/642. [DOI] [PubMed] [Google Scholar]
- YOSHIHARA M., UEDA A., ZHANG D., DEITCHER D.L., SCHWARZ T.L., KIDOKORO Y. Selective effects of neuronal-synaptobrevin mutations on transmitter release evoked by sustained versus transient Ca2+ increases and by cAMP. J. Neurosci. 1999;19:2432–2441. doi: 10.1523/JNEUROSCI.19-07-02432.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIMURA R., YANAGIHARA N., TERAO T., MINAMI K., ABE K., IZUMI F. Inhibition by carbamazepine of various ion channels-mediated catecholamine secretion in cultured bovine adrenal medullary cells. Naunyn Schmiedebergs Arch. Pharmacol. 1995;352:297–303. doi: 10.1007/BF00168560. [DOI] [PubMed] [Google Scholar]


