Abstract
The effects of agents that inactivate free radical nitric oxide (carboxy-PTIO, hydroxocobalamin and pyrogallol) were tested on relaxations produced by the nitroxyl anion (NO−) donor Angeli's salt in rat aortic rings and anococcygeus muscles. The amount of NO• generated from Angeli's salt in the presence of these agents was measured using a NO•-selective electrode sensor.
Carboxy-PTIO (100, 300 μM), hydroxocobalamin (30, 100 μM) and pyrogallol (10, 30 μM) significantly reduced relaxations produced by Angeli's salt (0.3 μM) in aortic rings but not in anococcygeus muscles.
NO• generated from Angeli's salt (0.1 – 10 μM), as detected by the sensor electrode, was less than 0.5% of the amount of Angeli's salt added. Carboxy-PTIO (100 μM) and hydroxocobalamin (30 μM), but not pyrogallol significantly increased the amount of NO• detected.
In the presence of an oxidizing agent copper [II] (as CuSO4 100 μM), the amount of NO• detected from 0.3 μM of Angeli's salt increased from an undetectable level of 142.7±15.7 nM (equivalent to 47.6% of Angeli's salt added). Under these conditions, carboxy-PTIO, hydroxocobalamin and pyrogallol significantly reduced the amount of NO• detected from Angeli's salt as well as the signal generated by an equivalent amount of authentic NO (0.33 μM).
The difference in effects of these agents on relaxations to Angeli's salt in the aorta and the anococcygeus muscle may be explained by the ready conversion of NO− to NO• in the aorta through an unidentified mechanism, which makes NO− susceptible to inactivation by these agents. Furthermore, in addition to inactivating NO•, carboxy-PTIO and hydroxocobalamin may themselves oxidize NO− to NO•, albeit slightly.
Keywords: Angeli's salt, anococcygeus muscle (rat), aorta (rat), carboxy-PTIO, hydroxocobalamin, nitric oxide, nitroxyl anions, NO sensor electrode, pyrogallol
Introduction
The product formed by nitric oxide synthases (NOSs) in endothelial cells and nitrergic nerves signals the relaxation of smooth muscle. The free radical species of nitric oxide (NO•) is the best-known redox form of nitrogen monoxide (NO). Most studies on the physiological and biochemical aspects of NO have been carried out with the free radical form; thus, many assumptions made about endogenously – produced NO are based on what is known about NO•. In addition to the free radical species, NO can exist in the oxidized form as the nitrosonium cation (NO+), and in the reduced form as the nitroxyl anion (NO−). Of these redox forms, it appears that the nitroxyl form is the more physiologically relevant as there is abundant evidence to suggest that NO− may be formed endogenously (Murphy & Sies, 1991; Fukuto et al., 1992; Arnelle & Stamler, 1995; Schmidt et al., 1996; Gow & Stamler, 1998; Wong et al., 1998; Hughes, 1999; Komarov et al., 2000; Adak et al., 2000).
NO− can be generated from Angeli's salt (sodium trioxodinitrate, Na2N2O3), which dissociates to yield nitroxyl and nitrite anions. Only nitroxyl anions account for the relaxant activity of Angeli's salt because concentrations of at least 100 fold higher of sodium nitrite are required to produce relaxations of a similar magnitude to those produced by Angeli's salt (Pino & Feelisch, 1994; Ellis et al., 2000). Angeli's salt relaxes smooth muscle with reported EC50 values of 0.59 μM in the rabbit aorta (Maragos et al., 1991), 0.32 μM in the rat aorta (Pino & Feelisch, 1994) and 0.3 μM in the anococcygeus muscle (Li et al., 1999).
Like authentic NO, smooth muscle relaxations to NO− arise from an increase in cyclic GMP levels since relaxations to Angeli's salt are abolished by the soluble guanylate cyclase inhibitor ODQ (1H [1,2,4] oxadiazolo [4,3,-a]quimoxalin-1-one) in the rat aorta and anococcygeus muscle (Ellis et al., 2000; Li et al., 1999). However, according to Dierks & Burstyn (1996), NO• is the only redox form of nitrogen monoxide that activates soluble guanylate cyclase. If this is so, it follows that the relaxant activity of NO− is due to its oxidation to NO•.
Many studies have been undertaken to compare the activity of NO• with that of the nitrergic transmitter in the presence of substances that are known to inhibit NO• (Hobbs et al., 1991; Barbier & Lefebvre, 1992; Rand & Li, 1993; 1995; Paisley & Martin, 1996; Jiang et al., 1997; Ellis et al., 1998; La & Rand, 1998). These substances include the superoxide generators xanthine oxidase/xanthine and pyrogallol, the selective free radical NO scavenger carboxy-PTIO and other NO scavenging agents such as oxyhaemoglobin. The outcome was that these substances have differential effects, reducing the activity of NO• to a much greater extent than that of the nitrergic transmitter; we therefore describe them as discriminating agents. However, the effect of these discriminating agents on the activity of NO− has been examined in only a few studies. Li et al. (1999) showed that concentrations of pyrogallol and carboxy-PTIO that greatly reduced relaxations produced by NO• had little effect on relaxations induced by NO− in the rat anococcygeus muscle. However, in the rat aorta, the selective NO• scavenger carboxy-PTIO (300 μM) abolished relaxations to both NO− and NO• (Ellis et al., 2000). The reason for this difference between the anococcygeus muscle and the aorta is not clear, and indicates that the selectivity of these agents as inactivators of NO• may need to be reviewed. Therefore, the aim of the present study was to investigate the effects of the superoxide generator, pyrogallol and the NO• scavengers carboxy-PTIO and hydroxocobalamin on relaxations to NO− (donated from Angeli's salt) in rat aorta and anococcygeus muscle. In addition, the amount of NO• generated by Angeli's salt was detected by a NO•-selective electrode in the presence of the discriminating agents under various conditions, including in the presence of an oxidizing agent. We chose copper (Cu[II]) as an oxidizing agent since it has been shown that it is readily coupled in a redox reaction with NO− (Murphy & Sies, 1991; Nelli et al., 2000).
Methods
Functional experiments
Male Sprague-Dawley rats (250 – 350 g) were anaesthetized with CO2 and then decapitated. The thoracic aorta was removed and cleaned of surrounding fat before being cut into rings of 5 mm width. The endothelium was removed by gently rubbing the luminal surface of the aorta with forceps. The rings were mounted on hooks in 8 ml organ baths in PSS at a resting tension of 2 g and allowed to equilibrate for 90 min. The two anococcygeus muscles were removed and set up as previously described (Li & Rand, 1989).
Tone was raised in the aortic rings with phenylephrine (1 μM), and in the anococcygeus muscles with guanethidine (20 μM) plus clonidine (0.1 μM). Once the tone reached a steady level, relaxations to Angeli's salt were produced and compared in the absence and presence of carboxy-PTIO (100, 300 μM), hydroxocobalamin (30, 100 μM) or pyrogallol (10, 30 μM).
NO sensor experiments
The measurement of NO• release was performed with the inNO nitric oxide measurement system using the amiNO-700 sensor electrode (Innovative Instruments Inc., U.S.A.). The output current was monitored and recorded on a Windows 95/98 compatible system using analysis software provided with the inNO system.
The sensor was calibrated by adding known volumes of standard nitrite solution to an acidified solution in the presence of iodide ion according to the manufacturer's specifications. The sensor was then immersed in an 8 ml organ bath containing PSS that was constantly gassed with 95% O2 and 5% CO2 and maintained at 37°C, so that the conditions were the same as in the functional experiments. Disodium EDTA 0.067 mM was included in the PSS to chelate any metal ions that may have remained in the Millipore-filtered distilled water or the salts used to make the PSS. When the effect of copper was tested on NO• detection from Angeli's salt or NO gas in aqueous solution, the EDTA was omitted.
NO• levels were determined after addition to the PSS of either Angeli's salt (0.1 – 10 μM) or a saturated aqueous solution of NO gas (0.33 μM) in the absence or in the presence of carboxy-PTIO (100 μM), hydroxocobalamin (30 μM) and pyrogallol (30 μM). These experiments were repeated in the presence of Cu[II] (as CuSO4, 100 μM). In between each test the sensor was washed with PSS containing EDTA at least three times to remove any contaminants from the previous observation and left to recover for at least 10 min before commencing with the next test.
Drugs and materials used
The composition of the physiological salt solution (PSS) was as follows (mM, pH 7.4): NaCl 118, KCl 4.7; NaHCO3 25; MgSO4 0.45; KH2PO4 1.03; CaCl2 2.5; D-(+)-glucose 11.1. The PSS also contained disodium EDTA (ethylene diaminetetraacetic acid disodium salt, 0.067 mM) except in experiments in which Cu[II] was added to the organ bath.
The following chemicals and drugs were used: sodium nitrite standard solution (0.01 M; Innovative Instruments Inc. U.S.A.); acetylcholine hydrochloride, guanethidine sulphate, phenylephrine hydrochloride, potassium iodide, hydroxocobalamin hydrochloride, pyrogallol, cupric sulphate, sulphuric acid and sodium hydroxide (Sigma-Aldrich, St. Louis, MO, U.S.A.); clonidine hydrochloride (Boehringer Ingelheim GmbH, Germany); carboxy-PTIO (2-[4[carboxyphenyl]-4,4,5,5,-tetramethylimidazoline-1-oxyl 3-oxide); Angeli's salt (disodium trioxodinitrate) (Cayman Chemical Company, Ann Arbor, MI, U.S.A.). All drugs used were dissolved in distilled water except Angeli's salt, which was dissolved in NaOH (0.01 M).
Saturated solutions of nitric oxide gas (2 mM) were prepared freshly on the day of each experiment by bubbling compressed nitric oxide gas (Linde Gas Pty. Ltd., Melbourne, Australia) through deoxygenated distilled water, as described by Rajanayagam et al. (1993).
Data and statistical analysis of results
Tone induced in the isolated tissues was expressed in grams tension. Relaxations were expressed as percentages of induced tone. In experiments with the NO sensor, results are expressed as nM of NO• detected. Numerical data are given as means and standard error of the means for the number (n) of experiments indicated.
Evaluation of statistical significance was established using analysis of variance (ANOVA) or Student t-test. Probability values less than 0.05 were deemed significant.
Ethnics
The experiments were approved by the Animal Experimentation Ethics Committee of RMIT University and conformed to guidelines laid down by the National Health & Medical Research Council of Australia.
Results
Effect of carboxy-PTIO, hydroxocobalamin and pyrogallol on nitroxyl-mediated relaxations in aortic rings
The tension produced by 1 μM phenylephrine was 2.08±0.1 g (n=14). Successful removal of the endothelium was confined when 10 μM acetylcholine failed to induce a relaxation. Angeli's salt (0.3 μM) produced relaxations that were on average 68.3±5.0% of the phenylephrine-induced tone.
Carboxy-PTIO (100, 300 μM), hydroxocobalamin (30, 100 μM), and pyrogallol (10, 30 μM) significantly reduced relaxations produced by Angeli's salt (Figure 1).
Figure 1.
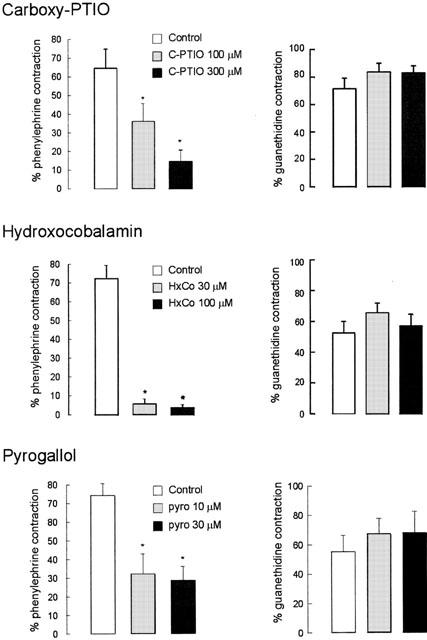
Mean data on the effects of carboxy-PTIO (100, 300 μM), hydroxocobalamin (30, 100 μM) and pyrogallol (30, 100 μM) on relaxations produced by Angeli's salt (0.3 μM) in rat aortic rings (n=4 – 5) and anococcygeus muscles (n=4 – 7). Responses are expressed as a percentage of contractile tone produced by phenylephrine (1 μM) in aortic rings and by guanethidine (20 μM) plus clonidine (0.1 μM) in anococcygeus muscles. Columns represent means and T-bars indicate s.e.means. Asterisks indicate significant differences from the corresponding control values (P<0.05, paired t-test).
Effect of carboxy-PTIO, pyrogallol and hydroxocobalamin on nitroxyl-mediated relaxations in anococcygeus muscles
Guanethidine and clonidine raised the tension of the anococcygeus muscle to 7.62±0.39 g (n=17). Relaxations induced by Angeli's salt (0.3 μM) were 63.9±7.3% of the induced tone.
In contrast to findings with aortic rings, relaxations of the anococcygeus produced by Angeli's salt (0.3 μM) were not affected by carboxy-PTIO (100, 300 μM), hydroxocobalamin (30, 100 μM) or pyrogallol (10, 30 μM) (Figure 1).
Effect of carboxy-PTIO, hydroxocobalamin and pyrogallol on the NO• signal generated from Angeli's salt in the absence and presence of CuSO4
When added to PSS containing EDTA to ensure absence of contaminant metal ions, Angeli's salt (⩽1 μM) did not produce a detectable signal. At 10 μM of Angeli's salt the signal was less than 50 nM NO• (Figure 2), which is equivalent to less than 0.5% of the amount of Angeli's salt added to the organ bath.
Figure 2.
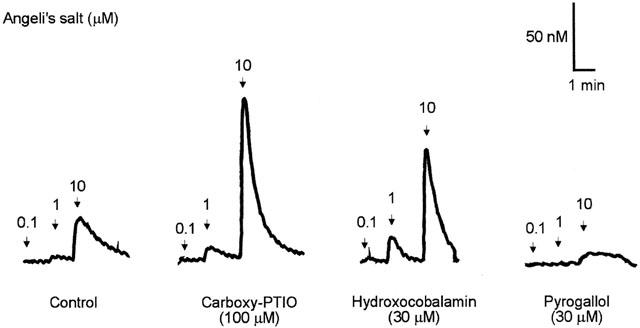
Traces illustrating the electrochemical detection of NO• from Angeli's salt (0.1, 1, 10 μM) in PSS containing edetate (0.067 mM) in the absence (control) and presence of carboxy-PTIO (100 μM), hydroxocobalamin (30 μM) and pyrogallol (30 μM) using the amiNO-700 sensor electrode.
In the presence of carboxy-PTIO (100 μM) and hydroxocobalamin (30 μM), the amounts of NO• generated by 1 and 10 μM of Angeli's salt was significantly increased (P<0.05, ANOVA) (Figure 3). On the other hand, the amount of NO• generated from 10 μM of Angeli's salt was slightly reduced by pyrogallol (30 μM), but this reduction was not statistically significant.
Figure 3.
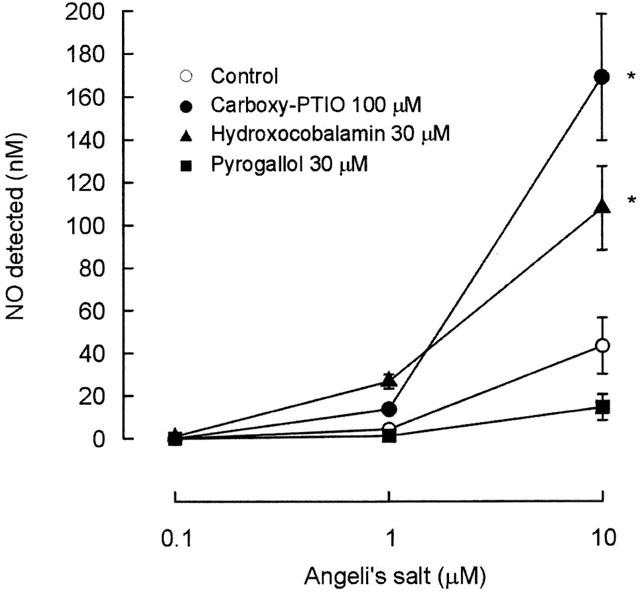
Mean data on the effects of carboxy-PTIO (100 μM), hydroxocobalamin (30 μM) and pyrogallol (30 μM) on NO• generated from Angeli's salt (0.1, 1 and 10 μM) in PSS containing edetate (0.067 mM). NO• is expressed in nanomoles. Symbols represent means and T-bars indicate s.e.means (n=4). Asterisks indicate significant differences from control (P<0.05, ANOVA).
When 0.3 μM Angeli's salt was added to PSS containing Cu[II] (as CuSO4, 100 μM), it produced a large signal (Figure 4), with a mean value equivalent to 142.7.1±15.7 nM of NO•. The signals produced by Angeli's salt in the presence of Cu[II] were significantly reduced by the presence of carboxy-PTIO, hydroxocobalamin and pyrogallol (Figures 4 and 5).
Figure 4.
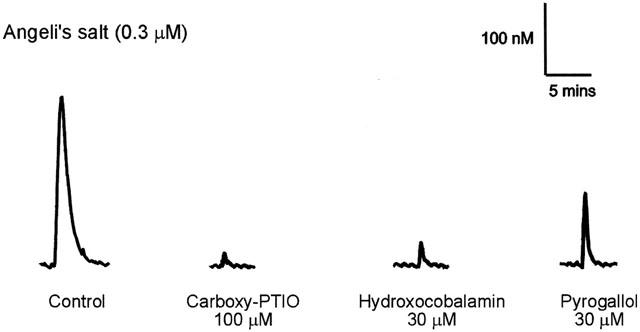
Traces illustrating the detection of NO• from Angeli's salt (0.3 μM) in PSS containing CuSO4 (100 μM) in the absence (control) and presence of carboxy-PTIO (100 μM), hydroxocobalamin (30 μM) and pyrogallol (30 μM) using the amiNO-700 sensor electrode.
Figure 5.
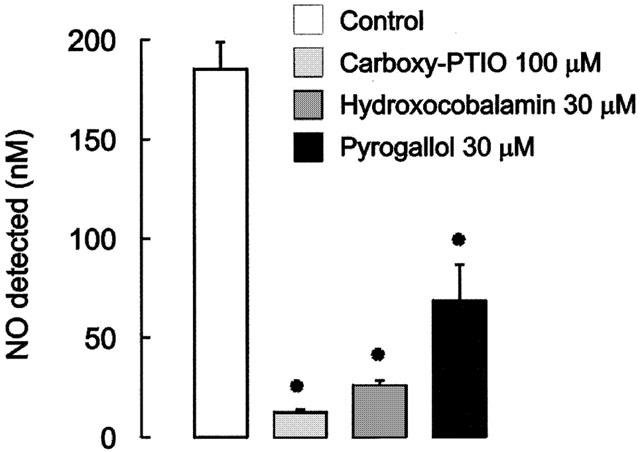
Mean data of the effects of carboxy-PTIO (100 μM), hydroxocobalamin (30 μM) and pyrogallol (30 μM) on NO• generated from Angeli's salt (0.3 μM) in PSS containing CuSO4 (100 μM). NO• is expressed in nanomoles. Column heights represents mean and T-bars indicate s.e.means (n=4). Asterisks indicate significant differences from control values (P<0.05, paired t-test).
Effect of carboxy-PTIO, hydroxocobalamin and pyrogallol on aqueous solution of NO in the absence and presence of CuSO4
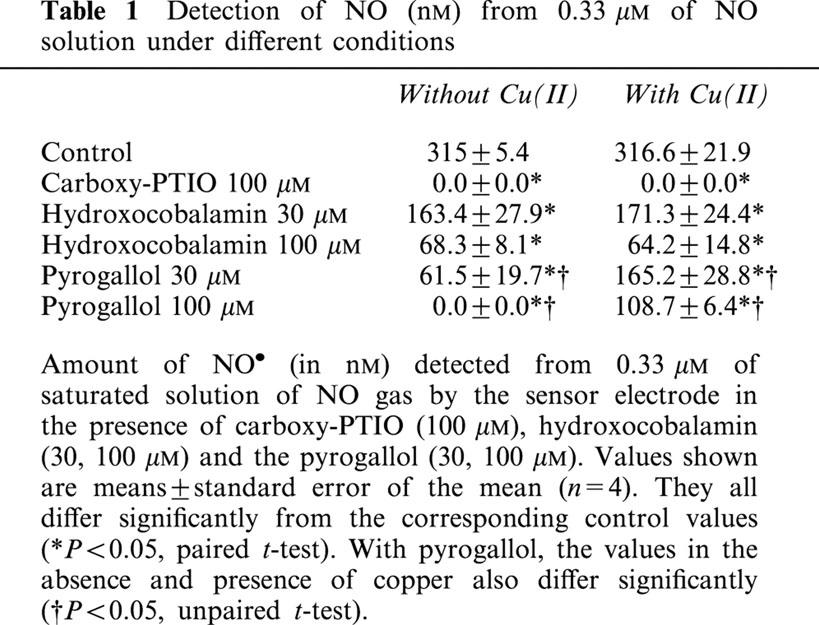
Since the detection of a NO• signal by Angeli's salt is dependent on how much NO− is converted to NO•, it was necessary to compare the extents to which carboxy-PTIO, hydroxocobalamin and pyrogallol affected the NO• signal when NO• (as an aqueous solution of the gas) was added. Carboxy-PTIO (100 μM) abolished the NO• signal while hydroxocobalamin (30, 100 μM) and pyrogallol (30, 100 μM) produced significant reductions of the signal. The effect of carboxy-PTIO and hydroxocobalamin did not differ appreciably when tested in the presence or absence of Cu[II], but pyrogallol was less effective in reducing the NO• signal when Cu[II] was present (Table 1).
Table 1.
Detection of NO (nM) from 0.33 μM of NO solution under different conditions
Discussion
There is evidence to suggest that the pharmacological activity of endogenous NO (i.e. NO formed by NOS) can be attributed to the combination of different redox forms of nitrogen monoxide. For example, we recently reported that both NO• and NO− may contribute to the activity of EDRF (Ellis et al., 2000). Therefore the interconversion between the redox forms of nitrogen monoxide needs to be studied in greater detail. In the present study we investigated interactions between the nitroxyl anion derived from Angeli's salt and agents that inactivate the free radical form, NO•, using both pharmacodynamic experiments and an electrochemical system with a NO electrode sensor that selectively detects the free radical.
Oxidation of nitroxyl by Cu[II]
When copper (as Cu[II] in CuSO4, 100 μM) was present in the PSS, the amount of NO• detected from 0.3 μM Angeli's salt was increased from an undetectable level to 142.7 nM, indicating that NO− was being oxidized to NO• coupled to the reduction of copper from Cu[II] to Cu[I], an effect reported recently by Nelli et al. (2000). However, the conversion ratio, that is the proportion of Angeli's salt added to the bath detected by the sensor as NO•, was only 47.6%, yet when a saturated solution of NO gas was used, almost 100% of the added NO• was detected by the electrode. It appears, therefore, that the oxidation of NO− from Angeli's salt to NO• is incomplete.
The NO• signal produced by Angeli's salt in the presence of copper was almost abolished by carboxy-PTIO (to about 8% of control), markedly attenuated by hydroxocobalamin (to about 18% of control), and reduced to a lesser extent by pyrogallol (to about 48% of control), indicating that NO• was rapidly inactivated. Likewise, the presence of these agents abolished or reduced the NO• signal when NO• from a saturated solution of NO gas was added in the presence or absence of copper. However, pyrogallol reduced the NO• signal to a greater extent in the absence of copper than in its presence. A likely explanation for this is that the copper participates in a dismutation reaction with superoxide with the net reaction products being hydrogen peroxide and molecular oxygen (Halliwell & Gutteridge, 1999), as in the reaction catalysed by Cu/Zn superoxide dismutase.
Difference between aorta and anococcygeus
As with previous findings (Li et al., 1999), relaxations of anococcygeus muscles induced by Angeli's salt were not affected by carboxy-PTIO, hydroxocobalamin or pyrogallol, agents which are widely reported to reduce NO•-induced relaxations (Gillespie & Sheng, 1990; La & Rand, 1998; Li et al., 1999). These agents, however, reduced relaxations to Angeli's salt in endothelium-denuded rat aortic rings.
Many agents that reduce relaxations in vascular tissue to endogenously generated NO (EDRF) or exogenously applied NO• (as an aqueous solution of the gas) are ineffective in blocking nitrergic transmission in nitrergically-innervated tissues (Rajanayagam et al., 1993; Rand & Li, 1993; 1995; La et al., 1996; Jiang et al., 1997; La & Rand, 1998). A possible reason for the difference in susceptibility between exogenous NO• and the nitrergic transmitter is that the latter may be the nitroxyl redox form of nitrogen monoxide, as recently suggested (Li et al., 1999). However, this does not explain the difference between anococcygeus muscles and aortic rings in the susceptibility to blockade of relaxant responses to Angeli's salt. A possible explanation for this is that the oxidative environment may differ between the two tissues.
Such a difference between the aorta and a non-vascular tissue (the gastric fundus of the rat) was invoked by Guilmard et al. (1998). They suggested that differences in reactivities to NO-mediated relaxants and of inhibition of relaxations by guanylate cyclase inhibitors were attributable to greater inactivation by superoxide anions of NO• released into the extracellular space in vascular tissue than in the gastric fundus. It is unlikely that in the present study the different sensitivities to the agents could be accounted for by the different contractile agents used to raise tone in the two issues. When the aorta was incubated with 20 μM guanethidine to replicate conditions used in the anococcygeus muscle, relaxations to Angeli's salt were still reduced by carboxy-PTIO, hydroxocobalamin and pyrogallol (data not shown).
One possible reason for the difference between the aorta and the anococcygeus muscle is that the NO− released from Angeli's salt can be oxidized to NO• by a cellular component of the aorta which is not present in the anococcygeus muscle. A recent study indicates that cytochrome P450 may play a role in the oxidation of NO− into NO• in vascular tissue (Nelli et al., 2001), therefore this could represent a potential mechanism to explain our present findings. An attempt to demonstrate this possibility by adding Angeli's salt to PSS containing a crude homogenate of aorta failed to show an increase in the amount of NO• detected by the sensor electrode (data not shown). However, the fact that no change was observed in the NO• signal does not necessarily indicate the absence of a potential oxidant. In the intact tissue, it would be expected that the oxidation of NO− would occur in close proximity to the endogenous ‘detector' of NO, guanylate cyclase, and may even be coupled to the ‘detector'. Experiments with the electrode sensor and an aortic homogenate dispersed throughout the 8 ml bath may not faithfully emulate the conditions that operate in the intact organized tissue.
Another possible explanation for the difference between the aorta and the anococcygeus muscle is that the anococcygeus muscle may contain an antioxidant that may prevent the oxidation of NO− to NO•, the form which is more susceptible to inactivation. Lilley & Gibson (1997) reported that the antioxidants, ascorbate and urate were released from the mouse anococcygeus muscle, and suggested that their presence explained the resistance of the nitrergic transmitter to inactivation by superoxide generators. We found that homogenates of anococcygeus muscles slightly decreased the NO• signal from Angeli's salt in the presence of copper. However, this effect was also produced by aortic homogenates, indicating that this decrease was not due specifically to an antioxidant factor found only in the anococcygeus muscle.
It has been stated that only the free radical form of nitrogen monoxide can activate guanylate cyclase (Dierks & Burstyn, 1996). If this is so, then the NO− from Angeli's salt must be oxidized to NO• before it can produce a relaxation. However, our findings with the anococcygeus muscle in the presence of NO•-inactivating agents indicate that NO− is not oxidized to NO•. It was also shown that Angeli's salt can stimulate human neutrophil migration and elevate cyclic GMP levels in either aerobic or anaerobic conditions, and this was taken as evidence that NO− does not need to be oxidized extracellularly to mediate a guanylate cyclase-dependent effect (Vanuffelen et al., 1998). It should be noted that Dierks & Burstyn (1996) assayed the activity of soluble guanylate cyclase in the presence of a high concentration of the thiol, dithiothreitol (10 mM). Since, thiols are known to trap NO− (Pino & Feelisch, 1994; Hughes, 1999) it is likely that this may have interfered with the activity of the NO− derived from Angeli's salt, therefore the possibility that the NO− anion can directly activate guanylate cyclase cannot be completely ruled out yet. However, since NO− is charged and presumably not lipid-soluble, the mechanism by which it traverses the cell membrane is not clear and requires investigation.
In the aorta on the other hand, our present study indicates that NO− may be oxidized to NO• at the smooth muscle cell surface, where the NO• would be exposed to the inactivating agents. Furthermore, the uncharged, lipid-soluble NO• could diffuse readily across the cell membrane.
Oxidation of NO− by inactivating agents
When Angeli's salt was added to PSS that contained edetate to chelate heavy metal ions, the level of NO• detected by the sensor was equivalent to less than 0.5% of the amount of Angeli's salt added. This indicates that the sensor is highly selective for the free radical form of NO, and the residual signal may have been the result of slight oxidation of NO− to NO• in the oxygenated PSS. The presence of carboxy-PTIO or hydroxocobalamin in the PSS markedly increased the amounts of NO• detected, but pyrogallol did not have this effect.
Carboxy-PTIO
This is an imidazole-derived free radical compound that inactivates NO• by forming an imidazoleoxyl compound and a NO2 radical that subsequently reacts with water to form nitrite and nitrate (Akaike et al., 1993). Since carboxy-PTIO has been reported to be highly selective for the free radical form of NO (Akaike et al., 1993; Rand & Li, 1995; Li et al., 1999), the finding that it reduced relaxations produced by NO− in the aorta was unexpected. As discussed above, a possible explanation is that NO− is rapidly oxidized to NO• extracellularly in the presence of the aorta. However, this does not fit with our previous observation that L-cysteine reduced relaxations produced by Angeli's salt in rat aorta (Ellis et al., 2000). If NO− is converted to NO•, the L-cysteine would be expected to enhance the relaxations of Angeli's salt through S-nitrosothiol formation (Stamler, 1994), but this did not occur. Another possible explanation is that there may be a direct reaction between NO− and carboxy-PTIO, such as a coupled redox reaction, and this is more evident in the aorta than in anococcygeus muscles. Carboxy-PTIO is understood to be coupled to a redox reaction with ascorbate where it is reduced into N-hydroxy-carboxy-PTIO (Tsunoda et al., 1994), therefore it is possible that Angeli's salt may modify carboxy-PTIO in a similar manner. The results with the sensor indicated that carboxy-PTIO does indeed oxidize NO−; however, the amount of NO• detected was less than 2% of the total amount of Angeli's salt added, therefore this property of carboxy-PTIO may not be critical to explaining the differences between the aorta and the anococcygeus muscle. In addition, the concentration of carboxy-PTIO administered was much higher than that of Angeli's salt, hence it was expected that the residual carboxy-PTIO that did not participate in the oxidation of NO− would reduce the NO• signal through a direct scavenging action, but this did not occur. The findings with the sensor would indicate that the redox reaction between NO− and carboxy-PTIO occurs at a much faster rate than the reaction between residual carboxy-PTIO and NO•.
Hydroxocobalamin
(vitamin B12a) has a corrin core containing cobalt (as CoIII) that can combine with NO• to form nitrosocobalamin (Kaczka et al., 1951), and it inactivates NO• by binding with it (Rajanayagam et al., 1993). However, there are reports of other mechanisms of action (Rochelle et al., 1995; Kruszyna et al., 1998; Brouwer et al., 1996). With respect to our findings, it would appear that the most likely explanation would be that NO− might form a complex with hydroxocobalamin in an analogous manner to its reaction with haem. However, cobalamins in their oxidized state may bind with either redox form of NO if they follow a similar reaction pathway to that of haemoglobin, since NO− can be oxidized by oxyhaemoglobin to NO• (Doyle et al., 1988). This could explain why the generation of NO• from Angeli's salt was increased in the presence of hydroxocobalamin.
Pyrogallol
This is a generator of superoxide anions (Marklund & Marklund, 1974). It is well known that the reaction between superoxide anions and NO• is rapid and results in the formation of peroxynitrite (Beckman et al., 1990), which generally does not possess potent relaxant activity. However, it is unlikely that such a reaction would occur between NO− and superoxide, since both are negatively charged and would thus be mutually repulsive. Angeli's salt did not yield a NO• signal in the presence of pyrogallol, as was also recently reported by Buyukafsar et al. (2001). The mechanism by which pyrogallol reduced relaxations to Angeli's salt in the aorta is probably as in the case with carboxy-PTIO; that is, NO− from Angeli's salt undergoes oxidation to NO•, which reacts with superoxide.
Conclusions
Nitroxyl anions generated by Angeli's salt appear to mediate relaxations differently in the aorta than in non-vascular smooth muscle since NO-inactivating agents inhibited these responses in the aorta but not in the anococcygeus muscle. Such differences can be explained by the possibility that the aorta may have a more oxidative environment, thus allowing the oxidation of NO− to NO•, while in the anococcygeus muscle, little or no conversion occurs. Because of mounting evidence for the participation of the NO− anion in the activity of endogenously-generated NO, the findings from this study could provide an insight into how oxidant agents, whether occurring endogenously or used in an experimental setting, alter its reactivity.
Acknowledgments
This work was supported by a grant from the National Health and Medical Research Council of Australia.
Abbreviations
- carboxy-PTIO
2-[4[carboxyphenyl]-4,4,5,5,-tetramethylimidazoline-1-oxyl 3-oxide
- EC50
concentration producing 50% of maximal effect
- EDRF
endothelium-derived relaxing factor
- NO
nitric oxide without reference to redox state (nitrogen monoxide)
- NO•
nitric oxide free radical
- NO−
nitroxyl anion
- NO+
nitrosonium cation
- NOS
nitric oxide synthase
- PSS
physiological salt solution.
References
- ADAK S., WANG Q., STUEHR D.J. Arginine conversion to nitroxide by tetrahydrobiopterin-free neuronal nitric oxide synthase. Implications for mechanism. J. Biol. Chem. 2000;275:33554–33561. doi: 10.1074/jbc.M004337200. [DOI] [PubMed] [Google Scholar]
- AKAIKE T., YOSHIDA M., MIYAMOTO Y., SATO K., KOHNO M., SASAMOTO K., MIYAZAKI K., UEDA S., MAEDA H. Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factors/nitric oxide through a radical reaction. Biochemistry. 1993;32:827–832. doi: 10.1021/bi00054a013. [DOI] [PubMed] [Google Scholar]
- ARNELLE D.R., STAMLER J.S. NO+, NO, NO− donation by S-nitrosothiols: implications for regulation of physiological functions by S-nitrosylation and acceleration of disulfide formation. Arch. Biochem. Biophys. 1995;318:279–285. doi: 10.1006/abbi.1995.1231. [DOI] [PubMed] [Google Scholar]
- BARBIER A.J.M., LEFEBVRE R.A. Effect of LY 83583 on relaxation induced by non-adrenergic non-cholinergic nerve stimulation and exogenous nitric oxide in the rat gastric fundus. Eur. J. Pharmacol. 1992;219:331–334. doi: 10.1016/0014-2999(92)90315-u. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S., BECKMAN T.W., CHEN J., MARSHALL P.A., FREEMAN B.A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROUWER M., CHAMULITRAT W., FERRUZZT G., SAULS D.L., WEINBERG J.B. Nitric oxide interactions with cobalamins: biochemical and functional consequences. Blood. 1996;88:1857–1864. [PubMed] [Google Scholar]
- BUYUKAFSAR K., NELLI S., MARTIN W. Formation of nitric oxide from nitroxyl anion: role of quinones and ferricytochrome c. Br. J. Pharmacol. 2001;132:165–172. doi: 10.1038/sj.bjp.0703812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIERKS E.A., BURSTYN J.N. Nitric oxide (NO) the only nitrogen monoxide redox form capable of activating soluble guanylate cyclase. Biochem. Pharmacol. 1996;51:1593–1600. doi: 10.1016/0006-2952(96)00078-0. [DOI] [PubMed] [Google Scholar]
- DOYLE M.P., MAHAPATRO S.N., BROENE R.D., GUY J.K. Oxidation and reduction of hemoproteins by trioxodinitrate(II). The role of nitroxyl hydride and nitrite. J. Am. Chem. Soc. 1988;110:593–599. [Google Scholar]
- ELLIS A., LI C.G., RAND M.J. Effect of xanthine oxidase inhibition on endothelium dependent and nitrergic relaxations. Eur. J. Pharmacol. 1998;356:41–47. doi: 10.1016/s0014-2999(98)00510-x. [DOI] [PubMed] [Google Scholar]
- ELLIS A., LI C.G., RAND M.J. Differential actions of L-cysteine on responses to nitric oxide, nitroxyl anions and EDRF in the rat aorta. Br. J. Pharmacol. 2000;129:315–322. doi: 10.1038/sj.bjp.0703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKUTO J.M., WALLACE G.C., HSZEIH R., CHAUDHURI G. Chemical oxidation of N-hydroxyguanidine compounds. Release of nitric oxide, nitroxyl and possible relationship to the mechanism of biological nitric oxide generation. Biochem. Pharmacol. 1992;43:607–613. doi: 10.1016/0006-2952(92)90584-6. [DOI] [PubMed] [Google Scholar]
- GILLESPIE J.S., SHENG H. The effects of pyrogallol and hydroquinone on the response to NANC nerve stimulation in the rat anococcygeus and the bovine retractor penis muscle. Br. J. Pharmacol. 1990;99:194–196. doi: 10.1111/j.1476-5381.1990.tb14677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOW A.J., STAMLER J.S. Reaction between nitric oxide and haemoglobin under physiological conditions. Nature. 1998;391:169–173. doi: 10.1038/34402. [DOI] [PubMed] [Google Scholar]
- GUILMARD C., AUGUET M., CHABRIER P.E. Comparison between endothelial and neuronal nitric oxide pathways in rat aorta and gastric fundus. Nitric Oxide. 1998;2:147–154. doi: 10.1006/niox.1998.0170. [DOI] [PubMed] [Google Scholar]
- HALLIWELL B., GUTTERIDGE J.M.C.The chemistry of free radicals and related ‘reactive specie's Free Radicals in Biology and Medicine 1999Oxford University Press; 3rd Edition [Google Scholar]
- HOBBS A.J., TUCKER J.F., GIBSON A. Differentiation by hydroquinone of relaxations induced by exogenous and endogenous nitrates in non-vascular smooth muscle: role of superoxide anions. Br. J. Pharmacol. 1991;104:645–650. doi: 10.1111/j.1476-5381.1991.tb12483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES M. Relationship between nitric oxide, nitroxyl ion, nitrosonium cation and peroxynitrite. Biochem. Biophys. Acta. 1999;1411:263–272. doi: 10.1016/s0005-2728(99)00019-5. [DOI] [PubMed] [Google Scholar]
- JIANG F., LI C.G., RAND M.J. Effect of hydroxocobalamin on vasodilatations to nitrergic transmitter, nitric oxide and endothelium-derived relaxing factor in guinea-pig basilar artery. Eur. J. Pharmacol. 1997;340:181–186. doi: 10.1016/s0014-2999(97)01381-2. [DOI] [PubMed] [Google Scholar]
- KACZKA E.E., WOLF D.E., KUEHL F.A., FOLKERS K. Vitamin B12. Modifications of cyano-cobalamin. J. Am. Chem. Soc. 1951;73:3569–3572. [Google Scholar]
- KOMAROV A.M., WINK D.A., FEELISCH M., SCHMIDT H.H. Electron-paramagnetic resonance spectroscopy using N-methyl-D-glucamine dithiocarbamate iron cannot discriminate between nitric oxide and nitroxyl: implications for the detection of reaction products for nitric oxide synthase. Free Radic. Biol. Med. 2000;28:739–742. doi: 10.1016/s0891-5849(00)00156-8. [DOI] [PubMed] [Google Scholar]
- KRUSZYNA H., MAGYAR J.S., ROCHELLE L.G., RUSSELL M.A., SMITH R.P., WILCOX D.E. Spectroscopic studies of nitric oxide (NO) interactions with cobalamins: reactions of NO with superoxocabalamin(III) likely accounts for cobalamin reversal of the biological effects of NO. J. Pharmacol. Exp. Ther. 1998;285:665–671. [PubMed] [Google Scholar]
- LA M., LI C.G., RAND M.J. Comparison of the effects of hydroxocobalamin and oxyhaemoglobin on responses to NO, EDRF and nitrergic transmitter. Br. J. Pharmacol. 1996;117:805–810. doi: 10.1111/j.1476-5381.1996.tb15264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LA M., RAND M.J. Effect of pyrogallol, hydroquinone and duroquinone on responses to nitrergic nerve stimulation and NO in the rat anococcygeus muscle. Br. J. Pharmacol. 1998;126:342–348. doi: 10.1038/sj.bjp.0702277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C.G., KARAGIANNIS J., RAND M.J. Comparison of the redox forms of nitrogen monoxide with the nitrergic transmitter in the rat anococcygeus muscle. Br. J. Pharmacol. 1999;127:826–834. doi: 10.1038/sj.bjp.0702540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C.J., RAND M.J. Evidence for a role of nitric oxide in the neurotransmitter system mediating relaxation of the rat anococcygeus muscle. Clin. Exp. Pharmacol. Physiol. 1989;16:933–938. doi: 10.1111/j.1440-1681.1989.tb02404.x. [DOI] [PubMed] [Google Scholar]
- LILLEY E., GIBSON A. Release of the antioxidants ascorbate and urate from a nitrergically-innervated smooth muscle. Br. J. Pharmacol. 1997;122:1746–1752. doi: 10.1038/sj.bjp.0701571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARAGOS C.M., MORLEY D., WINK D.A., DUNAMS T.M., SAAVEDRA J.E., HOFFMAN A., BOVE A.A., ISSACS L., HRABIE J.A., KEEFER L.K. Complexes of NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. J. Med. Chem. 1991;34:3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- MARKLUND S., MARKLUND G. Involvement of the superoxide anion radical in the autooxidation of pyrogallol and convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- MURPHY M.E., SIES H. Reversible conversion of nitroxyl anion to nitric oxide by superoxide dismutase. Proc. Natl. Acad. Sci. U.S.A. 1991;88:10860–10864. doi: 10.1073/pnas.88.23.10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELLI S., HILLEN M., BUYUKAFSAR K., MARTIN W. Oxidation of nitroxyl anion to nitric oxide by copper ions. Br. J. Pharmacol. 2000;131:356–362. doi: 10.1038/sj.bjp.0703550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELLI S., MCINTOSH L., MARTIN W. Role of copper ions and cytochrome P450 in the vasodilator actions of the nitroxyl anion generator, Angeli's salt, on rat aorta. Eur. J. Pharmacol. 2001;412:281–289. doi: 10.1016/s0014-2999(00)00845-1. [DOI] [PubMed] [Google Scholar]
- PAISLEY K., MARTIN W. Blockade of nitrergic transmission by hydroquinone, hydroxocobalamin and carboxy-PTIO in bovine retractor penis: role of superoxide anion. Br. J. Pharmacol. 1996;117:1633–1638. doi: 10.1111/j.1476-5381.1996.tb15333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINO R., FEELISCH M. Bioassay discrimination between nitric oxide and nitroxyl using L-cysteine. Biochem. Biophys. Res. Comm. 1994;201:54–62. doi: 10.1006/bbrc.1994.1668. [DOI] [PubMed] [Google Scholar]
- RAJANAYAGAM M.A., LI C.G., RAND M.J. Differential effects of hydroxocobalamin on NO-mediated relaxations in rat aorta and anococcygeus muscle. Br. J. Pharmacol. 1993;108:3–5. doi: 10.1111/j.1476-5381.1993.tb13429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAND M.J., LI C.G. Differential effects of hydroxocobalamin on relaxations induced by nitrosothiols in rat aorta and anococcygeus muscle. Eur. J. Pharmacol. 1993;241:249–254. doi: 10.1016/0014-2999(93)90210-9. [DOI] [PubMed] [Google Scholar]
- RAND M.J., LI C.G. Discrimination by the NO-trapping agent, carboxy-PTIO, between NO and EDRF. Br. J. Pharmacol. 1995;116:1906–1910. doi: 10.1111/j.1476-5381.1995.tb16681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROCHELLE L.G., MORNAN S.J., KRUSZYNA H., RUSSELL M.A., WILCOX D.E., SMITH R.P. Interactions between hydroxocobalamin and nitric oxide (NO): evidence for a redox reaction between NO and reduced cobalamin and reversible NO binding to oxidised cobalamin. J. Pharmacol. Exp. Ther. 1995;275:48–52. [PubMed] [Google Scholar]
- SCHMIDT H.H., HOFMANN H., SCHINDLER U., SHUTENKO Z.S., CUNNINGHAM D.D., FEELISCH H. No .NO from NO synthase. Proc. Natl. Acad. Sci. U.S.A. 1996;93:14492–14497. doi: 10.1073/pnas.93.25.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAMLER J.S. Redox signalling: nitrosylation and related target interactions of nitric oxide. Cell. 1994;78:931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- TSUNODA T., OKUMURA K., ISHIZAKA H., MATSUNAGA T., TABUCHI T., YASUE H., AKAIKE T., SATO K., MAEDA H. Vasodilator effect of carboxy-PTIO in the coronary circulation. Eur. J. Pharmacol. 1994;262:55–63. doi: 10.1016/0014-2999(94)90028-0. [DOI] [PubMed] [Google Scholar]
- VANUFFELEN B.E., VAN DER ZEE J., DE KOSTER B.M., VANSTEVENINCK J., ELEFERINK J.G. Intracellular but not extracellular conversion of nitroxyl anion into nitric oxide leads to stimulation of human neutrophil migration. Biochem. J. 1998;330:719–722. doi: 10.1042/bj3300719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WONG P.S., HYUN J., FUKUTO J.M., SHIROTA F.N., DEMASTER E.G., SHOEMAN D.W., NAGASAWA H.T. Reaction between S-nitrosothiols and thiols: generation of nitroxyl (HNO) and subsequent chemistry. Biochemistry. 1998;37:5362–5371. doi: 10.1021/bi973153g. [DOI] [PubMed] [Google Scholar]


