Abstract
We have studied the effect of cannabinoid agonists (CP 55,940 and cannabinol) on intestinal motility in a model of intestinal inflammation (induced by oral croton oil in mice) and measured cannabinoid receptor expression, endocannabinoids (anandamide and 2-arachidonylglycerol) and anandamide amidohydrolase activity both in physiological and pathophysiological states.
CP 55,940 (0.03 – 10 nmol mouse−1) and cannabinol (10 – 3000 nmol mouse−1) were more active in delaying intestinal motility in croton oil-treated mice than in control mice. These inhibitory effects were counteracted by the selective cannabinoid CB1 receptor antagonist SR141716A (16 nmol mouse−1). SR141716A (1 – 300 nmol mouse−1), administered alone, increased intestinal motility to the same extent in both control and croton oil-treated mice
Croton oil-induced intestinal inflammation was associated with an increased expression of CB1 receptor, an unprecedented example of up-regulation of cannabinoid receptors during inflammation.
High levels of anandamide and 2-arachidonylglycerol were detected in the small intestine, although no differences were observed between control and croton oil-treated mice; by contrast anandamide amidohydrolase activity increased 2 fold in the inflamed small intestine.
It is concluded that inflammation of the gut increases the potency of cannabinoid agonists possibly by ‘up-regulating' CB1 receptor expression; in addition, endocannabinoids, whose turnover is increased in inflamed gut, might tonically inhibit intestinal motility.
Keywords: Anandamide, 2-arachidonylglycerol, endocannabinoids, cannabinoid receptors, intestine, intestinal motility, inflammatory bowel disease
Introduction
Δ9-tetrahydrocannabinol, the major psychoactive principle of marijuana and hashish, produces a wide variety of biological effects, including inhibition of intestinal motility (Shook & Burks, 1989; Dewey, 1986). In recent years, it has been shown that Δ9-tetrahydrocannabinol binds to specific cannabinoid receptors, CB1 receptors (expressed by central and peripheral neurones) and CB2 receptors (that occur mainly in immune cells), both coupled to G-proteins (Matsuda et al., 1990; Munro et al., 1990; di marzo et al., 2000; Izzo et al., 2000a; Pertwee, 1999a, 1999b). The discovery of cannabinoid receptors has led to the demonstration that there are endogenous agonists for these receptors, namely the ‘endocannabinoids', anandamide and 2-arachidonylglycerol (2-AG) (Devane et al., 1992; Mechoulam et al., 1995; Sugiura et al., 1995). Cannabinoid receptors and their endogenous ligands together are referred to as the ‘endogenous cannabinoid system' (di marzo et al., 2000).
Cannabinoid CB1 receptors have been located in myenteric neurones and their activation can mediate the inhibition of excitatory transmission in the isolated guinea-pig (Izzo et al., 1998; Pertwee et al., 1996) and human ileum (Croci et al., 1998), inhibition of electrically-evoked acetylcholine release from guinea-pig myenteric nerves (Coutts & Pertwee, 1997) and reduction of peristalsis efficiency in the isolated guinea-pig ileum (Heinemann et al., 1999; Izzo et al., 2000c). In vivo studies have shown that cannabinoid receptor agonists decreased gastric and intestinal motility through activation of CB1 receptor (Krowichi et al., 1999; Izzo et al., 1999a, 1999b, 1999c; Calignano et al., 1997; Colombo et al., 1998; Izzo et al., 2000d), while SR141716A, a cannabinoid CB1 receptor antagonist, increased intestinal motility in rodents (Calignano et al., 1997; Colombo et al., 1998; Izzo et al., 1999a, 1999c; Izzo et al., 2000d). Both central and peripheral cannabinoid CB1 receptors could modulate upper gastrointestinal transit, but the effect of systemic (intraperitoneally injected) cannabinoid drugs was mediated by peripheral receptors (Izzo et al., 2000d).
In the present paper we have evaluated the role of the endogenous cannabinoid system in a model of intestinal inflammation induced by croton oil in mice; croton oil produces inflammation in the small intestine characterized by infiltration of lymphocytes in the submucosa (Puig & Pol, 1998). We have used the plant-derived cannabinoid agonist cannabinol (Petitet et al., 1998), the synthetic cannabinoid receptor agonist CP 55,940 (D'ambra et al., 1992), the cannabinoid CB1 receptor antagonist SR141716A (Rinaldi-Carmona et al., 1995) and the cannabinoid CB2 receptor antagonist SR144528 (Rinaldi-Carmona et al., 1998). In addition, the expression of cannabinoid receptors, the amounts of endocannabinoids and the activity of anandamide amidohydrolase (the enzyme responsible for the hydrolysis of anandamide and 2-AG) were assessed in both control and inflamed intestinal tissues.
Methods
Animals
Male ICR mice (Harlan Italy, Corezzana, MI, U.S.A.) (24 – 26 g) were used after 1 week of acclimation (temperature 23±2°C; humidity 60%). Food was withheld 6 h before transit measurement and 18 h before the induction of chronic intestinal inflammation.
Intestinal inflammation
Inflammation was induced as previously described (Puig & Pol, 1998). Mice received orally two doses of croton oil (20 μl mouse−1) on 2 consecutive days. Motility was measured 4 days after the first administration of croton oil. This time was selected on the basis of a previous work (Puig & Pol, 1998), which reported that maximal inflammatory response occurred 4 days after the first treatment.
Upper gastrointestinal transit
Gastrointestinal transit was measured in control and in croton oil-treated mice. At this time, a black marker (10% charcoal suspension in 5% gum arabic, 0.1 ml per 10 g body weight) was administered orally to assess upper gastrointestinal transit as previously described (Puig & Pol, 1998). After 20 min the mice were killed by asphyxiation with CO2 and the gastrointestinal tract removed. The distance travelled by the marker was measured and expressed as a percentage of the total length of the small intestine from pylorus to caecum. In some experiments, the inhibitory effect of cannabinoid agonists on upper gastrointestinal transit was expressed as a percentage of inhibition as follows:
Drugs administration
The cannabinoid agonists CP 55,940 (0.03 – 10 nmol mouse−1), cannabinol (10 – 3000 nmol mouse−1), the CB1 receptor antagonist SR141716A (1 – 300 nmol mouse−1), the CB2 receptor antagonist SR144528 (52 nmol mouse−1) or vehicle (DMSO, 4 – 8 μl mouse−1) were given intraperitoneally (i.p.) or intracerebroventricularly (i.c.v.) 20 min before charcoal administration. In some experiments SR141716A (16 nmol mouse−1=0.3 mg kg−1), SR144528 (52 nmol mouse−1=1 mg kg−1) or hexamethonium (69 nmol mouse−1=1 mg kg−1) were given (i.p.) 10 min before the cannabinoid agonists. These doses were selected on the basis of previous published work (Izzo et al., 2000d).
Intracerebroventricular injections
Intracerebroventricular injections were performed as described by Haley & McCormich, (1957). Mice were briefly anaesthetized with enflurane and the drugs were delivered in a volume of 4 μl, using a Hamilton microlitre syringe fitted with 26-gauge needle. In preliminary experiments, the correct location of the intracerebroventricular injection has been verified by injecting 5 μl Evans blue dye (0.5%). The brains were then removed and examined to verify dye distribution.
Western blot analysis
Jejunum was homogenized in a buffer containing (mM): 100 HEPES, NaCl 1 M, EDTA 200, EGTA 100, dithiothreitol 1 M, phenylmethylsulphonyl fluoride 100, trypsin inhibitor 15 mg/ml−1, pepstatin A 750 μ−1/ml, benzamidine 10, glycerol 20% v v−1, MgCl2 100, Nonidet P40 1%, H2O, using a glass homogenizer and a teflon pestle. Homogenates were centrifuged at 4°C at 13,000 r.p.m. for 15 min and the supernatants were separated from pellet and aliquoted and stored at −80°C. Protein concentration was determined before use by Bio-Rad protein assay kit. Immunoblotting analysis of CB1 receptors was performed as previously described (Melek et al., 2000). Equivalent amounts (100 μg) of proteins from intestinal homogenates were separated by SDS – PAGE polyacrilamide minigel and transferred onto a nitro-cellulose membrane. The blots were blocked with 10% non fat dry milk in phosphate buffered saline (PBS) and incubated overnight at 4°C with a polyclonal rabbit antiserum raised against human CB1 receptor (dil. 1 : 800; Cayman Chemicals). After repeated washing with 1% Triton X-100 in PBS a peroxidase-conjugated goat anti-rabbit antibody (dil. 1 : 2000; Amersham) was added for 1 h at room temperature. Finally the immunoreactive bands were visualized by enhanced chemiluminescence (ECL; Amersham).
The relative expression of CB1 receptors was quantified by densitometric scanning of the X-ray films with a GS 700 Imaging Densitometer (Bio-Rad) and a computer program (Molecular Analyst IBM).
Anandamide and 2-arachidonylglycerol assay
The small intestines of control and croton oil-treated mice were removed and tissue specimens were immediately weighed, immersed into liquid nitrogen, then stored at −70° until analysis. Tissue was then extracted with chloroform/methanol (2 : 1, by volume) containing 1 nmol each of d8-anandamide and 2-AG, synthesized as described from the corresponding deuterated fatty acids and either ethanolamine or glycerol (Bisogno et al., 1997). The lipid extracts were purified by silica column chromatography and normal phase high pressure liquid chromatography (NP-HPLC), carried out as described previously (Bisogno et al., 1997), and the fractions corresponding to either anandamide (retention time 26 – 27 min) or 2-AG (retention time 18 – 22 min) were derivatized and analysed by isotope dilution gas chromatography-mass spectrometry (GC – MS) carried out in the selected monitoring mode as described in detail elsewhere (Bisogno et al., 1999). Results were expressed as pmol mg−1 tissue. As during tissue extraction/purification both d8- and native 2-AG are partly transformed into the 1(3)-isomers (which are eluted from the GC column 0.5 min later), and only little arachidonic acid is present on the sn-1(3) position of (phospho)glycerides, the amounts of 2-AG reported here represent the combined mono-arachidonoyl-glycerol peaks.
Anandamide amidohydrolase activity
To measure anandamide amidohydrolase activity [14C]-anandamide (5 mCi nmol−1), synthesized as described previously from [14C]-ethanolamine and arachidonic acid (Bisogno et al., 1997), was used as the radioligand at a 10 μM concentration. Membrane fractions prepared from small intestine of either control or croton oil-treated mice were assayed (Bisogno et al., 1999). The assay was carried out in 50 mM Tris-HCl, pH=9, at 37°C for 30 min. [14C]-ethanolamine produced from the reaction was quantified as described previously (Bisogno et al., 1997), and the activity was expressed as pmol of [14C]-ethanolamine produced min mg protein−1.
Drugs
Drugs used were: CP 55,940 mesylate (Tocris Cookson, Bristol, U.K.), hexamethonium bromide and cannabinol (SIGMA, Milan, Italy). SR141716A [(N-piperidin-1-yl)-5-(4-chlorophenyl)-1-2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride and SR144528 (N-[-1S-endo-1,3,3-trimethyl bicyclo [2.2.1] heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide-3-carboxamide) were a gift from Dr Madaleine Mossè and Dr Francis Barth (SANOFI-Recherche, Montpellier, France). Cannabinoid drugs were dissolved in dimethyl sulphoxide (DMSO), while hexamethonium was dissolved in saline.
Statistics
Data are mean±s.e.mean. To determine statistical significance, Student's t-test for unpaired data or one-way analysis of variance followed by Tukey – Kramer multiple comparisons test was used. A P-value less than 0.05 was considered significant. ED50 (dose which produced a 50% variation of gastrointestinal transit) values were calculated using the computer program of Tallarida & Murray, 1986.
Results
Effect of cannabinoid agonists
Administration of croton oil produced a significant increase of upper gastrointestinal transit (per cent transit: control 46±4; croton oil 66±4, n=12, P<0.01). Both CP 55,940 and cannabinol administration (i.p.) produced a dose-related inhibition of transit (Figure 1) and both agonists had a lower ED50 value compared to the corresponding treatment (i.p.) in control mice (Table 1). CP 55,940 and cannabinol were approximately equipotent in slowing gastrointestinal transit after both i.p. and i.c.v. routes of administration in croton oil-treated mice (Figure 2). The EC50 values of i.c.v.-administered CP 55,940 and cannabinol in croton oil-treated mice were 2.35±0.31 nmol mouse−1 and 1360±154 nmol mouse−1 respectively and were not statistically different from the corresponding i.p. treatment.
Figure 1.
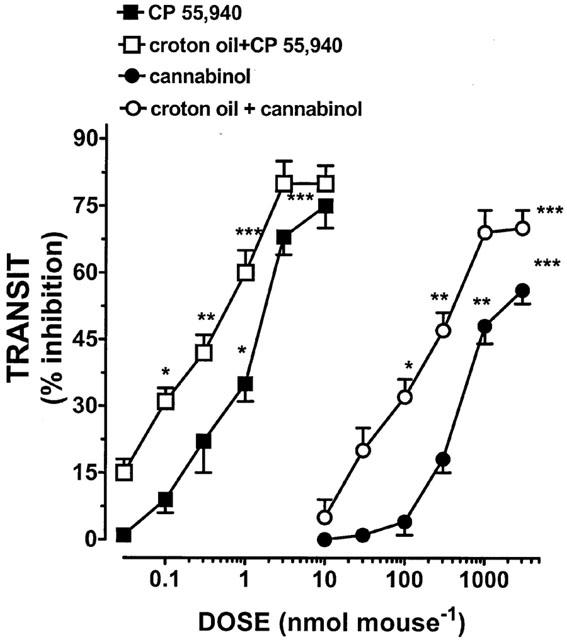
Dose related inhibition of upper gastrointestinal transit by CP 55,940 and cannabinol (both given i.p.) in control mice or mice receiving croton oil. Each point represents the mean±s.e.mean of 11 – 13 animals for each experimental group. *P<0.05, **P<0.01 and ***P<0.001 vs corresponding control.
Table 1.
ED50 (nmol mouse−1±s.e.mean) of cannabinoid drugs after i.p. administration in control mice and in mice with chronic inflammation (croton oil-treated mice)
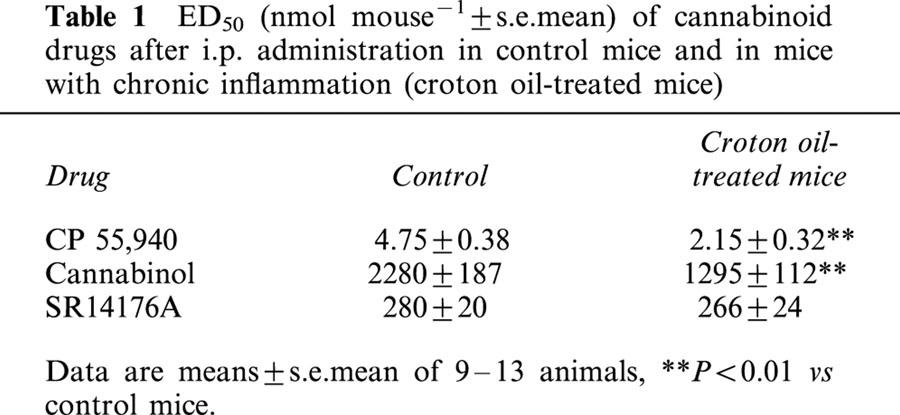
Figure 2.

Effect of CP 55,940 and cannabinol on upper gastrointestinal transit after i.p. or i.c.v. administration in mice treated with croton oil. Results are mean±s.e.mean of 8 – 11 animals for each experimental group. *P<0.05, **P<0.01 and ***P<0.001 vs corresponding control.
The cannabinoid CB1 receptor antagonist SR141716A (16 nmol mouse−1, i.p.), but not the cannabinoid CB2 receptor antagonist SR144528 (52 nmol mouse−1, i.p.) counteracted the inhibitory effect of CP 55,940 (0.3 nmol mouse−1, i.p.) and cannabinol (300 nmol mouse−1, i.p.) in croton oil-treated mice (Figure 3). Hexamethonium (69 nmol mouse−1, i.p.) did not modify significantly the effect of either (i.p.-injected) CP 55,940 (0.3 nmol mouse−1, i.p.) or cannabinol (300 nmol mouse−1, i.p.) in croton oil-treated mice (Figure 3). Hexamethonium (69 nmol mouse−1, i.p.), per se, did not significantly modify gastrointestinal transit in croton oil-treated mice (12±6% increase, n=10). DMSO (4 μl mouse−1, i.c.v or 4 – 8 μl mouse−1, i.p.) had no effect on the response under study either in control or in croton oil-treated mice (data not shown).
Figure 3.

Croton oil-treated mice: effect of CP 55,940 (0.3 nmol mouse−1, i.p.) and cannabinol (300 nmol mouse−1, i.p.) on upper gastrointestinal transit alone or in mice treated with SR141716A (16 nmol mouse−1, i.p.) or SR144528 (52 nmol mouse−1, i.p.) or hexamethonium (69 nmol mouse−1, i.p.). Results are mean±s.e.mean of 10 – 11 animals for each experimental group @P<0.01 vs control **P<0.01 vs croton oil and #P<0.01 vs CP 55,940 (or cannabinol).
Effect of the cannabinoid CB1 receptor antagonist SR141716A alone
In absence of any drugs, the cannabinoid CB1 receptor antagonist SR141716A (i.p.) increased gastrointestinal transit both in control and in croton oil-treated mice with the same potency (Figure 4 and Table 1). SR141716A (i.c.v.) also increased gastrointestinal transit, but only at doses which were also active when injected i.p. (data not shown). Hexamethonium (69 nmol mouse−1, i.p.) did not modify significantly the inhibitory effect of SR141716A (100 nmol mouse−1 i.p., data not shown) in croton oil-treated mice. SR144528 (52 nmol mouse−1, i.p.) did not modify significantly intestinal motility either in control (per cent transit: control 46±6%; SR144528 48±5, n=6 for each experimental group) or croton oil-treated mice (per cent transit: control 65±4, SR144528 66±5, n=6 for each experimental group).
Figure 4.

Dose-related increase of upper gastrointestinal transit by SR141716A (i.p.) in control mice or mice treated with croton oil. Results are mean±s.e.mean of 8 – 10 animals for each experimental group. **P<0.01 vs corresponding control.
Western blot analysis
Densitometric analysis of immunoreactive bands showed a significant (P<0.01) increase of CB1 expression in the inflamed jejunum compared to control tissues (Figure 5).
Figure 5.

Western blot analysis, carried out with an antibody against CB1, of proteins of the jejunum of control and croton oil-treated mice. The Figure is representative of three separate experiments.
Endocannabinoid and amidohydrolase activity
Table 2 shows that anandamide and 2-AG were detected in control tissues and that this production was not significantly modified in the small intestine of croton oil-treated mice. By contrast, a significant increase in anandamide amidohydrolase activity was observed in croton oil-treated mice.
Table 2.
Levels of anadamide, 2-arachidonylglycerol and anandamide amidohydrolase activity in the mouse small intestine during control and croton oil-induced gut inflammation conditions

Discussion
We have shown that the synthetic cannabinoid agonist CP 55,940 and the natural cannabinoid receptor agonist cannabinol reduce upper gastrointestinal motility both in control and in croton oil-treated mice. However, the anti-transit effects of both agonists were augmented during intestinal inflammation. The relative potencies of CP 55,940 and cannabinol reflected their affinity for CB1 receptors (Pertwee, 1999b). More importantly, the inhibitory effects of the two cannabinoid agonists were counteracted by SR141716A, a cannabinoid CB1 receptor antagonist, but not by SR144528, a cannabinoid CB2 receptor antagonist, thus indicating an involvement of cannabinoid CB1, but not cannabinoid CB2 receptors. The present results are consistent with previous studies carried out in control animals, in which it has been shown that cannabinoid agonists reduce intestinal motility by activating cannabinoid CB1 receptors, either in vitro (Izzo et al., 1998; 2000c; Pertwee et al., 1996; Croci et al., 1998; Coutts & Pertwee, 1997; Heinemann et al., 1999) or in vivo (Krowichi et al., 1999; Izzo et al., 1999a, 1999c; 2000d; Calignano et al., 1997; Colombo et al., 1998). In addition, acute diarrhoea enhances the potency of cannabinoid agonists in reducing intestinal motility (Izzo et al., 2000d).
The central nervous system has a significant role in the initiation and co-ordination of gastrointestinal motor activity (Krowicki & Hornby, 1995); thus, we were interested in the extent to which the inhibitory effect of cannabinoid agonists is mediated via a central or peripheral site of action. This is because the cannabinoid CB1 receptor is located within both the central (Matsuda et al., 1990; Izzo et al., 2000a; Pertwee, 1999a) and the enteric nervous system (Griffin et al., 1997; Izzo et al., 2000b). Cannabinoid agonists are lipophilic molecules which can easily cross blood brain barrier (Petitet et al., 1999). In a previous work, carried out in control mice, we have shown that cannabinoid agonists were significantly more active in reducing motility in mice when administered i.c.v. than when administered i.p. The higher central-to-peripheral potency of cannabinoid agonists suggested a central site of action (Izzo et al., 2000d). However, central CB1 receptors probably contribute little to the effects of peripherally administered cannabinoid as the effect of i.p.-injected cannabinoid agonists was not modified by the ganglionic blocker hexamethonium (Izzo et al., 2000d). In the present study, the anti-transit effect likely involves peripheral (enteric) CB1 receptors since: (i) cannabinoid agonists, injected i.c.v. inhibited transit, but only at doses which were also active when injected i.p. (i.e. cannabinoid agonists were equally active after i.p. or i.c.v. administration) and (ii) the ganglion blocker hexamethonium, at a dose previously shown to antagonize the effect of i.c.v.-injected cannabinoid drugs (Izzo et al., 2000d), did not alter the inhibitory effect of i.p.-injected cannabinoids. However, we cannot definitely rule out the possibility that also central CB1 receptors may have a role in the control of intestinal motility.
Cannabinoid CB1 receptors are located on peripheral neurones, including the myenteric plexus (Griffin et al., 1997; Izzo et al., 2000b; Kulkarni-Narla & Brown, 2000). The myenteric plexus of the guinea-pig small intestine contains CB1-, but not CB2, cannabinoid receptor mRNA (Griffin et al., 1997). In the porcine gut, cannabinoid CB1 receptors have been immunohistochemically localized on enteric cholinergic neurones (Kulkarni-Narla & Brown, 2000). Using Western blot analysis, we have detected the presence of CB1 receptors in the mouse jejunum and its increased expression in the inflamed gut. This is, to the best of our knowledge, the first example of up-regulation of CB1 cannabinoid receptors during an inflammatory state. This up-regulation explains the increased potency of exogenous cannabinoid agonists during inflammation. Others have shown that croton oil-induced inflammation increases the effect of μ-opioid receptor agonists, suggesting a sensitization of peripheral μ-opioid receptors (Puig & Pol, 1998; Valle et al., 2000).
There is evidence in the literature that intestinal motility can be tonically inhibited by the endogenous cannabinoid system (Izzo et al., 2000b). Indeed SR141716A increased electrically-induced contractions in the isolated guinea-pig ileum (Izzo et al., 1998; Pertwee et al., 1996) as well as intestinal motility and defecation in rodents (Colombo et al., 1998; Izzo et al., 1999a, 1999c Izzo et al., 2000d). The observation that SR141716A, per se, increased intestinal motility does not necessarily imply that endogenous cannabinoid agonists are involved in the control of intestinal motility as SR141716A behaves as inverse agonist at the human CB1 and CB2 cannabinoid receptors (Landsman et al., 1997; MacLennan et al., 1998). In view of the increased CB1 expression in the inflamed small intestine observed in the present study, one may have expected that, if SR141716A behaved as an inverse agonist in this context, this compound would have exerted a higher enhancement of intestinal transit in croton oil-treated than in vehicle-treated mice. However, this hypothesis was not confirmed in the present study, in which we have shown that SR141716A increased motility in control and inflamed mice with exactly the same potency. This finding would suggest, on the contrary, that SR141716A exerts an antagonistic action on a possible tonic anti-transit effect of endocannabinoids, and that, during inflammation, increased expression of CB1 receptors does not result in an increased tonic activity of these receptors, possibly due to the lack of increased concentrations of endogenous ligands.
In order to investigate the mechanism of action of SR141716A we measured the levels and the metabolism of endogenous cannabinoids in normal and inflamed small intestine under the same conditions used to study the effect of the cannabinoid receptor agonists and the expression of CB1 receptors. Anandamide amidohydrolase is responsible for the hydrolysis of anandamide amide bond, and was shown to recognize as substrates also palmitoylethanolamide (a saturated acylethanolamide co-released with anandamide) and 2-AG (Mechoulam et al., 1998). The presence of this enzyme in the rat small intestine has been previously demonstrated and it was shown that, due to the presence of endogenous inhibitors, low activity, similar to those found here in mice, is found in the intestine under normal conditions (Katayama et al., 1997). Nevertheless, this activity would still ensure the hydrolysis of 40 pmol of anandamide per min and per g of tissue, which reflects the amounts of anandamide that we found in the tissue (Table 2). In fact, while Mechoulam et al. (1995) detected 2-AG, but not anandamide, in the canine small intestine, in the present study we have revealed, in mice, amounts of anandamide and, particularly, 2-AG compatible with a tonic activation of CB1 receptors. In fact, these amounts are about three (for anandamide) or 10 (for 2-AG) times higher than those measured in the rat brain (Sugiura et al., 1995), and are very likely to yield tissue concentrations similar to or higher than the Ki values for the activation of CB1 cannabinoid receptors (Devane et al., 1992; Facci et al., 1995; Calignano et al., 1998). Anandamide is known to reduce intestinal motility in mice via activation of CB1 receptors (Fride, 1995; Calignano et al., 1997; Izzo et al., 2001a) and thus, the displacement from these receptors of the endocannabinoid by SR141716A could explain, at least in part, the prokinetic action of the antagonist in the small intestine (see above). A relatively low basal rate of endocannabinoid inactivation in normal intestine might explain these high levels. In fact, as mentioned above, anandamide amidohydrolase activity in the intestine of rodents is relatively low under normal conditions (Katayama et al., 1997) [and our present data], whereas Calignano et al. (1997) previously suggested the lack, in this tissue, of a functional transport mechanism limiting anandamide action.
In the present study 2-AG levels in the mouse small intestine were more than 1000 fold higher than those of anandamide. There are no data in the literature concerning the pharmacological effects of 2-AG on intestinal motility. However, in the vas deferens assay, which, like the model used here, involves peripheral CB1 receptors, 2-AG was about 100 times less potent than anandamide as a depressant of electrically-induced contractions (Mechoulam et al., 1995). Thus, collectively, these results tend to suggest a physiological role for both anandamide and 2-AG as physiological modulators of intestinal motility, and indicate that counteraction of their action at CB1 receptors underlies at least in part the effect of SR141716A on intestinal motility in normal small intestine.
Beaulieu et al. (2000) have recently observed that the rat paw skin concentration of anandamide and 2-AG did not differ between control and inflamed tissues. Similarly, we did not find significant difference between inflamed and non-inflamed intestinal tissues. However, the fact that we could not observe an increase in endogenous cannabinoid levels could be due to up-regulation of anandamide amidohydrolase, since we have showed here that the activity of this enzyme markedly increased in the inflamed intestine, whereas no change in the activity of this enzyme was observed in rat skin after formalin injection (Beaulieu et al., 2000). At any rate, the finding of no change in endocannabinoid levels following croton oil treatment may suggest that, despite the over-expression of CB1 receptors under these conditions, the overall anti-transit cannabinoid tone in the small intestine does not increase during inflammation, in agreement with the observation that SR141716A exerts the same effects in normal as well as inflamed small intestine and via counteraction of endocannabinoid actions rather than as an inverse agonist.
In conclusion, some major issues emerged from this study: we have shown that inflammation enhances the potency of cannabinoid receptor agonists probably by up-regulating CB1 expression in the small intestine, thus raising the possibility of a clinical use of selective, non-psychotropic cannabinoid CB1 receptor agonists for the treatment of the motility disorders associated with intestinal inflammation. The presence of high amounts of endogenous cannabinoids in the small intestine strongly suggests a physiological role in the regulation of intestinal motility. The increased activity of the enzyme anandamide amidohydrolase, in consideration of the lack of decrease of endocannabinoids in the inflamed small intestine of croton oil-treated mice, suggests a more rapid turnover (biosynthesis plus degradation) of endogenous cannabinoids in the inflamed gut. These substances might be produced in order to better counteract the effects of inflammation in the gut by activating the up-regulated CB1 receptors in this tissue, but would be immediately inactivated by an increased amidohydrolase activity. We also found that the CB1 receptor antagonist SR141716A stimulates intestinal transit to the same extent in control and inflamed small intestine. This finding: (i) argues against a direct, inverse agonist effect of this compound on CB1 receptors (which being over-expressed in inflamed tissue should have led to a higher effect of the antagonist under these conditions), and (ii) supports the existence of an endocannabinoid tone that counteracts intestinal motility during both physiological and pathological states.
Acknowledgments
This work was supported by Cofinanziamento Murst and Enrico and Enrica Sovena Foundation (Roma). SR141716A and SR144528 were a kind gift from SANOFI (Montpellier, France).
Abbreviations
- 2-AG
2-arachidonylglycerol
- CB
cannabinoids
- DMSO
dimethyl sulphoxide
References
- BEAULIEU P., BISOGNO T., PUNWAR S., FARQUHAR-SMITH P., AMBROSINO G., DI MARZO V., RICE A.S.C. Role of the endogenous cannabinoid system in the formalin test of persistent pain in the rat. Eur. J. Pharmacol. 2000;396:85–92. doi: 10.1016/s0014-2999(00)00226-0. [DOI] [PubMed] [Google Scholar]
- BISOGNO T., BERRENDERO F., AMBROSINO G., CEBEIRA M., RAMOS J.A., FERNANDEZ-RUIZ J.J., DI MARZO V. Brain regional distribution of endocannabinoids: implications for their biosynthesis and biological function. Biochem. Biophys. Res. Commun. 1999;256:377–380. doi: 10.1006/bbrc.1999.0254. [DOI] [PubMed] [Google Scholar]
- BISOGNO T., MAURELLI S., MELCK D., DE PETROCELLI L., DI MARZO V. Biosynthesis, uptake, and degradation of anandamide and palmitoylethanolamide in leukocytes. J. Biol. Chem. 1997;272:3315–3323. doi: 10.1074/jbc.272.6.3315. [DOI] [PubMed] [Google Scholar]
- CALIGNANO A., LA RANA G., GIUFFRIDA A., PIOMELLI D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- CALIGNANO A., LA RANA G., MAKRYANNIS A., LIN S.Y., BELTRAMO M., PIOMELLI D. Inhibition of intestinal motility by anandamide, an endogenous cannabinoid. Eur. J. Pharmacol. 1997;340:R7–R8. [PubMed] [Google Scholar]
- COLOMBO G.R., AGABIO C., LOBINA R., REALI R., GESSA G.L. Cannabinoid modulation of intestinal propulsion in mice. Eur. J. Pharmacol. 1998;344:67–69. doi: 10.1016/s0014-2999(97)01555-0. [DOI] [PubMed] [Google Scholar]
- COUTTS A.A., PERTWEE R.G. Inhibition by cannabinoid receptor agonists of acetylcholine release from the guinea-pig myenteric plexus. Br. J. Pharmacol. 1997;121:1557–1566. doi: 10.1038/sj.bjp.0701301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROCI T., MANARA L., AUREGGI G., GUAGNIN F., RINALDI-CARMONA M., MAFFRAND J.P., LE FUR G., MUKENGE S., FERLA G. In vitro functional evidence of neuronal cannabinoid CB1 receptors in human ileum. Br. J. Pharmacol. 1998;125:1393–1396. doi: 10.1038/sj.bjp.0702190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'AMBRA T.E., ESTEP K.G., BELL M.R., EISSENSTAT M.A., JOSEF K.A., WARD S.J., HAYCOCK D.A., BAIZMAN E.R., CASIANO F.M., BEGLIN N.C. Conformationally restrained analogues of pravadoline: nanomolar potent, enantioselective, (aminoalkyl)indole agonists of the cannabinoid receptor. J. Med. Chem. 1992;35:124–135. doi: 10.1021/jm00079a016. [DOI] [PubMed] [Google Scholar]
- DEVANE W.A., HANUS L., BREUER A., PERTWEE R.G., STEVENSON L.A., GRIFFIN G., GIBSON D., MANDELBAUM A., ETINGER A., MECHOULAM R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- DEWEY W.L. Cannabinoid Pharmacology. Pharmacol. Rev. 1986;38:151–178. [PubMed] [Google Scholar]
- DI MARZO V., BISOGNO T., DE PETROCELLIS L. Endocannabinoids: new targets for drug development. Curr. Pharm. Design. 2000;6:1361–1380. doi: 10.2174/1381612003399365. [DOI] [PubMed] [Google Scholar]
- FACCI L., DAL TOSO R., ROMANELLO S., BURIANI A., SKAPER S.D., LEON A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc. Natl. Acad. Sci. U.S.A. 1995;92:3376–3380. doi: 10.1073/pnas.92.8.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIDE E. Anandamides: tolerance and cross-tolerance to Δ9-tetrahydrocannabinol. Brain. Res. 1995;697:83–90. doi: 10.1016/0006-8993(95)00790-w. [DOI] [PubMed] [Google Scholar]
- GRIFFIN G., FERNANDO S.R., ROSS R.A., MCKAY N.G., ASHFORD M.L.J., SHIRE D., HUFFMAN J.W., YU S., LAINTON J.A.H., PERTWEE R.G. Evidence for the presence of CB2-like cannabinoid receptors on peripheral nerve terminals. Eur. J. Pharmacol. 1997;339:53–61. doi: 10.1016/s0014-2999(97)01336-8. [DOI] [PubMed] [Google Scholar]
- HALEY T.J., MCCROMICK W.G. Pharmacological effects produced by intracerebral injection of drugs in the conscous mouse. Br. J. Pharmacol. 1957;12:12–15. doi: 10.1111/j.1476-5381.1957.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEINEMANN A., SHAHBAZIAN A., HOLZER P. Cannabinoid inhibition of guineapig intestinal peristalsis via inhibition of excitatory and activation of inhibitory neural pathways. Neuropharmacology. 1999;38:1289–1297. doi: 10.1016/s0028-3908(99)00056-8. [DOI] [PubMed] [Google Scholar]
- IZZO A.A., CAPASSO R., PINTO L., DI CARLO G., MASCOLO N., CAPASSO F.Effect of vanilloid drugs on gastroitnestinal transit in mice Br. J. Pharmacol. 2001a13344P(Abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- IZZO A.A., CAPASSO R., PINTO L., IUVONE T., ESPOSITO G., MASCOLO N., CAPASSO F.Effect of cannabinoid agonists on intestinal motility in a chronic model of intestinal inflammation Br. J. Pharmacol. 2001b13344P(Abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- IZZO A.A., MASCOLO N., BORRELLI F., CAPASSO F. Excitatory transmission to the circular muscle of the guinea-pig ileum: evidence for the involvement of cannabinoid CB1 receptor. Br. J. Pharmacol. 1998;124:1363–1368. doi: 10.1038/sj.bjp.0701964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IZZO A.A., MASCOLO N., BORRELLI F., CAPASSO F. Defaecation, intestinal fluid accumulation and motility in rodents: implications of cannabinoid CB1 receptors. Naunyn-Schmiedeberg's Arch Pharmacol. 1999a;359:65–70. doi: 10.1007/pl00005325. [DOI] [PubMed] [Google Scholar]
- IZZO A.A., MASCOLO N., CAPASSO F. Forgotten target for Marijuana: the endocannabinoid system in the gut. Trends. Pharmacol. Sci. 2000b;21:372. doi: 10.1016/s0165-6147(00)01543-1. [DOI] [PubMed] [Google Scholar]
- IZZO A.A., MASCOLO N., CAPASSO F. Marijuana in the new millennium: perspectives for cannabinoid research. Trends. Pharmacol. Sci. 2000a;21:81–82. doi: 10.1016/s0165-6147(00)01515-7. [DOI] [PubMed] [Google Scholar]
- IZZO A.A., MASCOLO N., CAPASSO R., GERMANÒ M.P., DE PASQUALE R., CAPASSO F. Inhibitory effect of cannabinoid agonists on gastric emptying in the rat. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999b;360:221–223. doi: 10.1007/s002109900054. [DOI] [PubMed] [Google Scholar]
- IZZO A.A., MASCOLO N., PINTO L., CAPASSO R., CAPASSO F. The role of cannabinoid receptors in intestinal motility, defaecation and diarrhoea in rats. Eur. J. Pharmacol. 1999c;384:37–42. doi: 10.1016/s0014-2999(99)00673-1. [DOI] [PubMed] [Google Scholar]
- IZZO A.A., MASCOLO N., TONINI M., CAPASSO F. Modulation of peristalsis by cannabinoid CB1 ligands in the isolated guinea pig ileum. Br. J. Pharmacol. 2000c;129:984–990. doi: 10.1038/sj.bjp.0703116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IZZO A.A., PINTO L., BORRELLI F., CAPASSO R., MASCOLO N., CAPASSO F. Central and peripheral cannabinoid modulation of gastrointestinal transit in physiological states or during the diarrhoea induced by croton oil. Br. J. Pharmacol. 2000d;129:1627–1632. doi: 10.1038/sj.bjp.0703265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATAYAMA K., UEDA N., KURAHASHI Y., SUZUKU H., YAMAMOTO S., KATO I. Distribution of anandamide amidohydrolase in rat tissues with special reference to small intestine. Biochim. Biophys. Acta. 1997;1347:212–218. doi: 10.1016/s0005-2760(97)00078-7. [DOI] [PubMed] [Google Scholar]
- KROWICKI Z.K., HORNBY P.J.Hindbrain neuroactive substances controlling gastrointestinal functions Regulatory mechanisms in gastrointestinal function 1995New York: CRC Press; 277–319.ed. Gaginella T.S. pp [Google Scholar]
- KROWICHI Z.K., MOERSCHBAECHER J.M., WINSAUER P.J., DIVAGALLI S.V., HORNBY P.J. Δ9-tetrahydrocannabinol inhibits gastric motility in the rat through cannabinoid CB1 receptors. Eur. J. Pharmacol. 1999;371:187–196. doi: 10.1016/s0014-2999(99)00165-x. [DOI] [PubMed] [Google Scholar]
- KULKARNI-NARLA A., BROWN D.R. Localization of CB1-cannabinoid receptor immunoreactivity in the porcine enteric nervous system. Cell. Tissue Res. 2000;302:73–80. doi: 10.1007/s004410000261. [DOI] [PubMed] [Google Scholar]
- LANDSMAN R.S., BURKEY T.H., CONSROE P., ROESKE W.R. SR14171A is an inverse agonist at the human cannabinoid CB1 receptors. Eur. J. Pharmacol. 1997;334:R1–R2. doi: 10.1016/s0014-2999(97)01160-6. [DOI] [PubMed] [Google Scholar]
- MACLENNAN S.J., REYNEN P.H., BONHAUS D.W. Evidence for inverse agonism of SR141716A at human recombinant cannabinoid CB1 and CB2 receptors. Br. J. Pharmacol. 1998;24:619–622. doi: 10.1038/sj.bjp.0701915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUDA L.A., LOLAIT S.J., BROWNSTEIN B.J., YOUG A.C., BONNER T.L. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- MECHOULAM R., BEN-SHABAT S., HANUS L., LIGUMSKY M., KAMINSKI N.E., SCHATZ A.R., GOPHER A., ALMOG S., MARTIN B.R., COMPTON D.R., PERTWEE R.G., GRIFFIN G., BAYEWITCH M., BARG J., VOGEL Z. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to a cannabinoid receptor. Biochem. Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- MECHOULAM R., FRIDE E., DI MARZO V. Endocannabinoids. Eur. J. Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- MELEK D., DE PETROCELLIS L., ORLANDO P., BISOGNO T., LAEZZA C., BIFULCO M., DI MARZO V. Suppression of nerve growth factor Trk receptors and prolactin receptors by endocannabinoids leads to inhibition of human breast and prostate cancer cell proliferation. Endocrinology. 2000;141:118–126. doi: 10.1210/endo.141.1.7239. [DOI] [PubMed] [Google Scholar]
- MUNRO S., THOMAS K.L., SHAAR M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1990;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Evidence for the presence of CB1 cannabinoid receptors on peripheral neurones and for the existence of neuronal non-CB1 cannabinoid receptors. Life Sci. 1999a;65:597–605. doi: 10.1016/s0024-3205(99)00282-9. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Pharmacology of cannabinoid receptor ligands. Curr. Med. Chem. 1999b;6:635–664. [PubMed] [Google Scholar]
- PERTWEE R.G., FERNANDO S.R., NASH J.E., COUTTS A.A. Further evidence for the presence of cannabinoid CB1 receptors in guinea-pig small intestine. Br. J. Pharmacol. 1996;118:2199–2205. doi: 10.1111/j.1476-5381.1996.tb15663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETITET F., JEANTAUD B., BERTRAND P., IMPERATO A. Cannabinoid penetration into mouse brain as determined by ex vivo binding. Eur. J. Pharmacol. 1999;374:417–721. doi: 10.1016/s0014-2999(99)00189-2. [DOI] [PubMed] [Google Scholar]
- PETITET F., JEANTAUD B., REIBAUD M., IMPERATO A., DUBROEUCQ M.C. Complex pharmacology of natural cannabinoids: evidence for partial agonist activity of Δ9-tetrahydrocannabinol and antagonist activity of cannabidiol on rat brain cannabinoid receptors. Life Sci. 1998;63:PL1–PL6. doi: 10.1016/s0024-3205(98)00238-0. [DOI] [PubMed] [Google Scholar]
- PUIG M.M., POL O. Peripheral effects of opioids in a model of chronic intestinal inflammation in mice. J. Pharmacol. Exp. Ther. 1998;287:1068–1075. [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., HEAULME M., ALFONSO R., SHIRE D., CONGY C., SOBRIÈ P., BRELIERE J.C., LE FUR G. Biochemical and pharmacological characterization of SR141716A, the first potent and selective brain cannabinoid receptor antagonist. Life Sci. 1995;56:1941–1947. doi: 10.1016/0024-3205(95)00174-5. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., MILLAN J., DEROCQ J.M., CASELLAS P., CONGY C., SOBRIÈ P., BRELIER J.C., LE FUR G. SR144528, the first potent and selective antagonist of the CB2 cannabinoid receptors. J. Pharmacol. Exp. Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- SHOOK J.E., BURKS T.F. Psychoactive cannabinoids reduce gastrointestinal propulsion and motility in rodents. J. Pharmacol. Exp. Ther. 1989;249:444–449. [PubMed] [Google Scholar]
- SUGIURA T., KONDO S., SUKAGAWA A., NAKANE S., SHINODA A., ITOH K., YAMASHITA A., WAKU K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- TALLARIDA R.J., MURRAY R.B. Manual of Pharmacological Calculations with computer programs. New York: Springer-Verlag; 1986. [Google Scholar]
- VALLE L., PUIG M.M., POL O. Effects of mu-opioid receptor agonists on intestinal secretion and permeability during acute intestinal inflammation in mice. Eur. J. Pharmacol. 2000;389:235–242. doi: 10.1016/s0014-2999(99)00871-7. [DOI] [PubMed] [Google Scholar]


