Abstract
Sigma receptors were first described in 1976 as opiate receptors but were later determined to be a distinct class of receptors with two subtypes, sigma1 and sigma2. Although the endogenous ligand is yet to be elucidated, the sigma1 receptor has recently been cloned.
Behavioural models used to test potential antidepressants have shown sigma ligands to produce antidepressant effects but their mechanism of action is unknown.
The goal of the present study was to assess the effects of various sigma1 ligands on the firing activity of serotonin (5-HT) neurons of the dorsal raphe nucleus (DRN) using extracellular in vivo recordings in anaesthetized rats.
The sigma1 ligands (+)-pentazocine and 4-(N-benzylpiperidin-4-yl)-4-iodobenzamide (4-IBP) (2 mg kg−1 day−1) increased markedly 5-HT firing activity after 2 days of treatment and maintained the same increased firing rate after long-term (21 days) treatments. Furthermore, the increased firing rate produced by 2 and 21 day treatments with (+)-pentazocine was prevented by the co-administration of N,N-dipropyl-2-(4-methoxy-3-(2-phenylethoxy)phenyl)-thylamine (NE-100) (10 mg kg−1 day−1) a selective sigma1 antagonist, confirming the sigma1 receptor's modulation of these effects. In contrast, the sigma1 ligands (+)-N-cyclopropylmethyl-N-methyl-1,4-diphenyl-1-1-ethyl-but-3-en-1-ylamine hydrochloride (JO-1784) and 2-(4-morpholinoethyl 1-phenyl-cyclohexane-1-carboxylate hydrochloride (PRE-084) had no effect.
Following a 21-day treatment with (+)-pentazocine there was a marked reduction in the number of neurons found per track. This decrease was not seen after chronic treatment with 4-IBP and may represent a depolarization block.
These results suggest a modulation of serotonergic neurotransmission by some sigma receptors and provide a potential mechanism for the ‘antidepressant effects' reported and provide evidence toward sigma1 ligands as potential antidepressants with a rapid onset of action.
Keywords: Antidepressant, electrophysiology, dorsal raphe nucleus, (+)-pentazocine, 4-IBP, PRE-084, JO-1784
Introduction
Sigma receptors were first described by Martin et al. (1976) as a subtype of opiate receptors. They were later distinguished from opiate receptors by the development of selective sigma ligands and classified into sigma1 and sigma2 subtypes (Quirion et al., 1987; 1992). Their endogenous ligand is not known but the endogenous steroid progesterone has high affinity for sigma1 receptors (Su et al., 1988). Many selective sigma1 ligands have been synthesized including (+)-pentazocine, 4-IBP, (+)-N-cyclopropylmethyl-N-methyl-1,4-diphenyl-1-1-ethyl-but-3-en-1-ylamine hydrochloride (JO-1784) and 2-(4-morpholinoethyl 1-phenyl-cyclohexane-1-carboxylate hydrochloride (PRE-084). Recently the sigma1 receptor was cloned and found to be different from all known mammalian receptors (Hanner et al., 1996).
Our laboratory previously used an electrophysiological model to differentiate between sigma agonists and antagonists. Specifically, it was demonstrated that sigma ligands modulate NMDA (N-methyl-D-aspartate) receptors such that low doses (0.5 – 50 μg) of sigma ligands have no effect on the spontaneous firing activity of hippocampal CA3 neurons but dose-dependently and selectively modulate the response to NMDA (Monnet et al., 1990). In this model, sigma agonists (1,3-di(2-tolyl)guanidine (DTG), (+)-pentazocine, JO-1784) potentiate the NMDA response and sigma1 antagonists (N,N-dipropyl-2-(4-methoxy-3-(2-phenylethoxy)phenyl)-thylamine (NE-100), progesterone, haloperidol) have no effect on their own but block the effects of sigma agonists (Monnet et al., 1990; 1992; Bergeron et al., 1996).
Sigma1 ligands have many potential functions one of which could be a role in the pathophysiology of depression or as antidepressants. Several sigma ligands have been shown to have antidepressant effects in behavioural models of depression such as the tail suspension and forced swimming tests (Matsuno et al., 1996; Tottori et al., 1997; Kinsora et al., 1998; Ukai et al., 1998). In addition, representatives from all classes of antidepressants have been shown to interact with sigma receptors (Bergeron et al., 1993; Narita et al., 1996; Shirayama et al., 1993). An enormous corpus of evidence suggests the involvement of serotonin in the pathophysiology of depression (Delgado, 2000). Just as an example, electrophysiological studies have demonstrated that all long-term treatments with antidepressants, through various mechanisms, increase 5-HT neurotransmission (Chaput et al., 1991; Blier & de Montigny, 1994).
The purpose of this study was to assess the effect of short and long-term treatments with several sigma1 ligands on serotonergic neurotransmission using an electrophysiological model of extracellular recordings of the firing rate of serotonin (5-HT) neurons from the dorsal raphe nucleus (DRN). Previous results using this model, demonstrated that acute and short-term treatments with SSRI's lead to a decreased firing activity of 5-HT DRN neurons, while long-term treatments lead to the restoration of 5-HT firing activity (Chaput et al., 1986; Blier et al., 1984; Blier & de Montigny, 1985). Therefore, we investigated the effects of short-term (2 days) and long-term (21 days) treatments with sigma1 ligands to assess their effects on basal 5-HT firing rate.
Methods
Animals
Experiments were performed in male Sprague-Dawley rats (Charles River, St. Constant, Québec) weighing 250 – 300 g. They were housed under standard laboratory conditions including a 12 – 12 h light-dark cycle with free access to food and water.
Treatments
For short-term treatments, rats 250 – 275 g were anaesthetized with halothane and osmotic minipumps (ALZA Corporation, Palo Alto, CA, U.S.A.) were implanted subcutaneously. Minipumps contained either JO-1784, PRE-084, (+)-pentazocine, 4-IBP (4-(N-benzylpiperidin-4-yl)-4-iodobenzamide) (all 2 mg kg−1 day−1) or saline for controls (50% saline, 50% dimethylsulphoxide (DMSO) for 4-IBP controls). Separate series of rats were implanted with two osmotic minipumps simultaneously, one containing (+)-pentazocine or 4-1BP (2 mg kg−1 day−1) and the other containing NE-100 (10 mg kg−1 day−1) for 2 days.
For long-term treatments, rats 125 – 150 g were anaesthetized and implanted in a similar fashion to that done for 2-day treatments. Pumps contained either (+)-pentazocine, JO-1784 or 4-IBP (all 2 mg kg−1 day−1) or saline for controls (50% saline 50% DMSO for 4-IBP controls). In addition, a separate series of rats were implanted with two osmotic minipumps simultaneously, one containing (+)-pentazocine or 4-1BP (2 mg kg−1 day−1) and the other containing NE-100 (10 mg kg−1 day−1) for 21 days. The electrophysiology experiments were performed with the minipumps on board.
Electrophysiology
The experiments were performed on rats anaesthetized with chloral hydrate (400 mg kg−1 intraperitoneal (i.p.)) and mounted in a stereotaxic apparatus. Supplemental doses of chloral hydrate (100 mg kg−1 i.p.) were administered as necessary to prevent any nociceptive reaction to pinching of the hind paw. The rat's body temperature was maintained at approximately 37°C by a thermistor-controlled heating pad.
Extracellular unitary recording of DRN 5-HT neurons was obtained with single-barrelled glass micropipettes pulled in a conventional manner (Haigler & Aghajanian, 1974) with the tips broken back 1 – 3 μm and filled with 3% fast green solution. Electrode impedance ranged between 2 and 4 MΩ. A burr hole 4 mm in diameter was drilled 1 mm anterior to lambda on the midline. The electrode was then lowered along descents covering the DRN from 300 μm to approximately 1500 μm anterior of lambda. Spontaneously firing DRN 5-HT neurons were identified by their characteristic slow and regular rhythmical firing (Aghajanian & Vandermaelen, 1982). Following the experiments each rat was sacrificed with an intravenous injection of air (1 ml).
Data collection
For each treatment group, the mean DRN 5-HT basal firing rate was determined by averaging the firing rate of all the neurons measured in the population (treatment). Each neuron was recorded for 90 s, and five descents were performed per rat in the DRN of 3 – 6 rats with the total number of neurons averaged being greater than 40. Student's paired t-tests were done comparing treatments to controls using the program Sigmaplot 4.0. A value was considered significant if P<0.05.
Drugs
The following substances were used: JO-1784 (a gift from F. Roman, Institut de Recherche Jouveinal, Fresnes, France), (+)-pentazocine, 8-hydroxy-2-(di-n-propylamino)tetralin(8-OH-DPAT), (−)bicuculline methiodide (RBI Pharmaceuticals, Natick, MA, U.S.A.), PRE-084 (a gift from Dr T.-P. Su, NIDA/NIH, Baltimore, MD, U.S.A.). NE-100 (a gift from Taisho Pharmaceutical Co. Ltd. Tokyo, Japan), 4-IBP and R(+)baclofen (Tocris Cookson Inc. Ballwin, MO, U.S.A.).
Results
The doses used in the present series of experiments were chosen according to data obtained previously. We have shown that doses of (+)-pentazocine and JO-1784 between 500 – 3000 μg kg−1 induced the maximal agonistic effect on the potentiation of the NMDA response (Monnet et al., 1990; 1992). The same doses of PRE-084 and 4-IBP were used since these molecules possess very high affinity for sigma1 receptors, similar to that of (+)-pentazocine and JO-1784 (Su et al., 1991; John et al., 1994; Steinfels et al., 1988; Roman et al., 1990).
In control animals, 5-HT neurons were encountered, starting at a depth of 5033 μm with an average of 2.8 neurons per track and a firing activity of 1.0 Hz. Figure 1 depicts a representative tracing of serotonergic neurons recorded in the DRN along a descent. In this example, the tracing is from a control rat or one treated for 2 days with (+)-pentazocine (2 mg kg−1 day−1) showing an increased firing rate.
Figure 1.
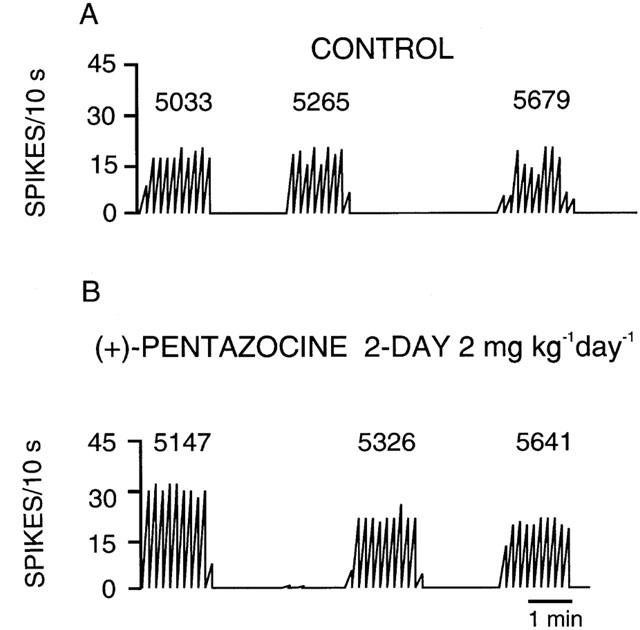
Integrated firing rate histograms of dorsal raphe 5-HT neurons obtained in anaesthetized rats following 2-day treatment with saline (control) (A) or (+)-pentazocine (2 mg kg−1 day−1) (B). The numbers above the histogram represent the depth at which the neuron was found.
Treatment with JO-1784
The short-term (2 days) administration of JO-1784 (2 mg kg−1 day−1) produced no significant change in the basal firing rate of DRN 5-HT neurons (Figure 2). In addition, a 21-day administration of the same dose of JO-1784 also did not affect the average firing rate of the 5-HT neurons of the DRN (Figure 2).
Figure 2.
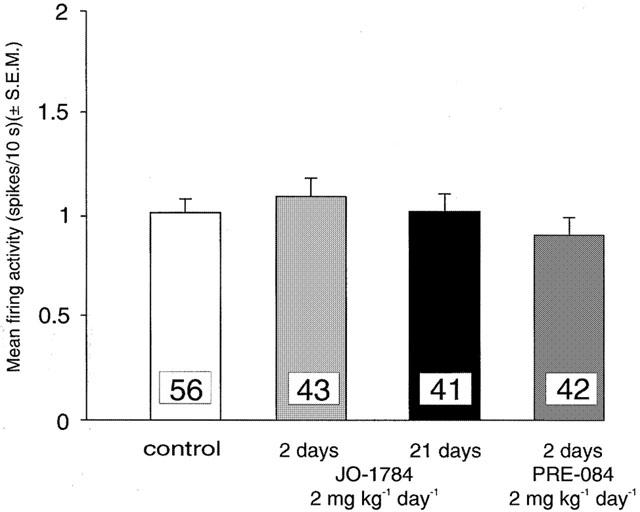
Mean firing activity expressed as spikes/10 s (mean±s.e.m.) of dorsal raphe nucleus serotonergic neurons measured in anaesthetized rats. Rats were treated with saline (control), JO-1784 (2 mg kg−1 day−1 for 2 days), PRE-084 (2 mg kg−1 day−1 for 2 days) or JO-1784 (2 mg kg−1 day−1 for 21 days). In this and the following figures, numbers in columns indicated the number of neurons tested.
Treatment with PRE-084
The 2-day administration of PRE-084 (2 mg kg−1 day−1) produced no significant change in the basal firing rate of the 5-HT neurons of the DRN (Figure 2).
Treatment with 4-IBP
A 2-day treatment with 4-IBP (2 mg kg−1 day−1) produced a 35% increase in the basal firing rate of 5-HT neurons compared to saline-treated animals (P=0.002) (Figure 3). Furthermore, a 21-day treatment with 4-IBP maintained a 36% increase in firing rate as seen after 2 days (Figure 3). The co-administration of NE-100 (10 mg kg−1 day−1) with 4-IBP (2 mg kg−1 day−1) for 2 days did not modify the effect of 4-IBP and a significant increase in the average firing activity of 5-HT neurons was observed (Figure 3).
Figure 3.
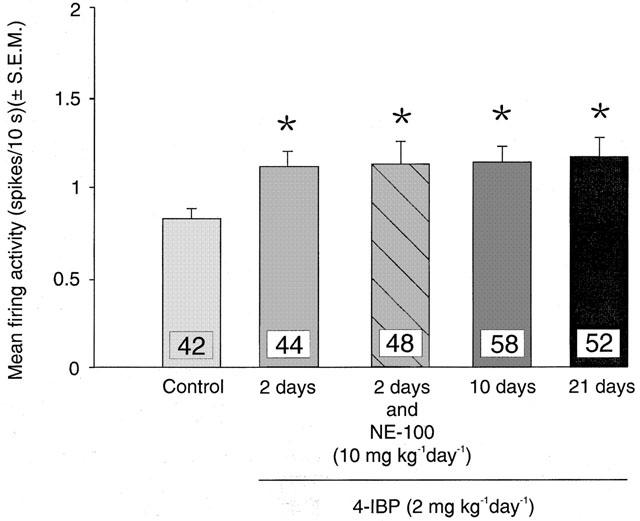
Mean firing activity expressed as spikes/10 s (mean±s.e.m.) of dorsal raphe nucleus serotonergic neurons measured in anaesthetized rats. Rats were treated with saline (control) for 2 days or 4-IBP (2 mg kg−1 day−1) for 2, 10 or 21 days or co-administered 4-IBP (2 mg kg−1 day−1) and NE-100 (10 mg kg−1 day−1) for 2 days. *P<0.05.
Treatment with (+)-pentazocine
As illustrated in Figure 1, a 2-day treatment with (+)-pentazocine (2 mg kg−1 day−1) produced a 33% increase in the basal firing rate compared to saline-treated rats (P=0.001) (Figure 4). Co-administration of NE-100 (10 mg kg−1 day−1) with (+)-pentazocine (2 mg kg−1 day−1) for 2 days completely prevented the increase of 5-HT firing activity caused by a 2-day treatment with (+)-pentazocine (Figure 4). (+)-Pentazocine treatment (2 mg kg−1 day−1) for 21 days maintained a 43% increase in basal firing rate compared to saline-treated rats (Figure 5). Similarly, co-administration of NE-100 (10 mg kg−1 day−1) with (+)-pentazocine (2 mg kg−1 day−1) for 21 days produced no significant change in average firing rate nor neurons found per track compared to controls (Figures 5 and 6).
Figure 4.
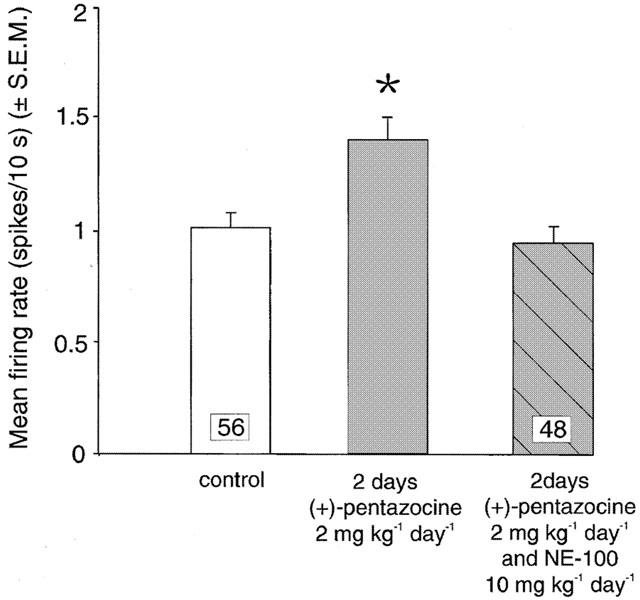
Mean firing activity expressed as spikes/10 s (mean±s.e.m.) of dorsal raphe nucleus serotonergic neurons measured in rats treated with saline (control), (+)-pentazocine (2 mg kg−1 day−1) or co-administered (+)-pentazocine (2 mg kg−1 day−1) and NE-100 (10 mg kg−1 day−1) for 2 days. *P<0.05.
Figure 5.
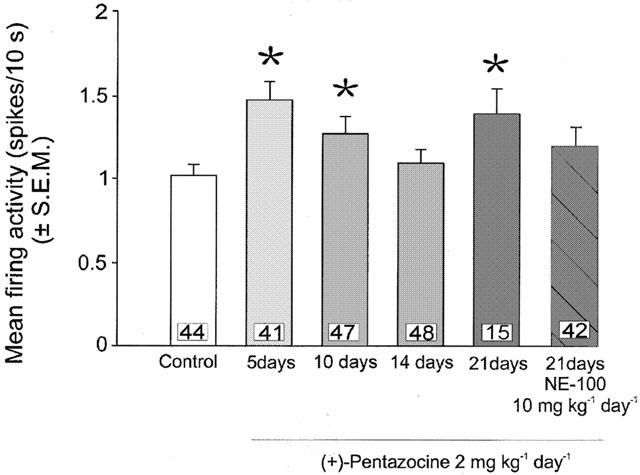
Mean firing activity expressed as spikes/10 s (mean±s.e.m.) of dorsal raphe nucleus serotonergic neurons in rats treated with saline (control) or with (+)-pentazocine (2 mg kg−1 day−1) for 5, 10, 14 or 21 days or co-administered (+)-pentazocine (2 mg kg−1 day−1) with NE-100 (10 mg kg−1 day−1) for 21 days. *P<0.05.
Figure 6.
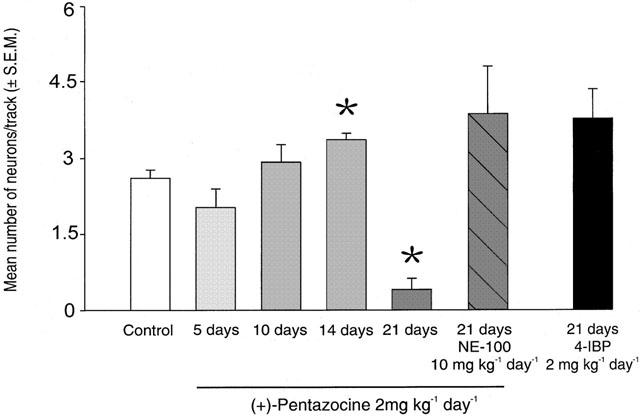
Mean number of neurons per track (±s.e.m.) encountered in rats treated with saline (control) or with (+)-pentazocine (2 mg kg−1 day−1) for 5, 10, 14 or 21 days or co-administered (+)-pentazocine (2 mg kg−1 day−1) with NE-100 (10 mg kg−1 day−1) for 21 days or treated with 4-IBP (2 mg kg−1 day−1) for 21 days. *P<0.05.
Neurons found per track following long-term treatments
As shown in Figure 6, in rats treated for 21 days with (+)-pentazocine (2 mg kg−1 day−1), 94% less neurons were encountered per track. In rats treated for 21 days with 4-IBP there was no significant difference in the amount of neurons found per track compared to saline-treated rats. Various durations of treatment with (+)-pentazocine (2 mg kg−1 day−1) did not significantly change the amount of neurons found per track versus controls until day 21. Specifically, following 5- and 10-day treatments, increased firing rates were maintained (40% and 27% respectively) compared to controls, without decreasing the number of neurons found per track (Figures 5 and 6). In addition, when treated with a lesser dose of (+)-pentazocine (0.5 mg kg−1 day−1) no change was seen for neurons yielded per track nor average firing rate compared to controls (Figure 7). To investigate the nature of the finding of decreased number of neurons per track, we injected animals treated with (+)-pentazocine for 21 days (2 mg kg−1 day−1) with 8-OH-DPAT (4 μg kg−1 i.v.), bicuculline (375 μg kg−1 i.v.) or (+)baclofen (5 – 15 mg kg−1 i.v.). These different approaches did not restore the amount of neurons found per track to that of saline-treated rats (8-OH-DPAT 0.0±0, bicuculline 0.2±0.2 and baclofen 0.63±0.24 versus (+)-pentazocine 0.41±0.16, not significant).
Figure 7.
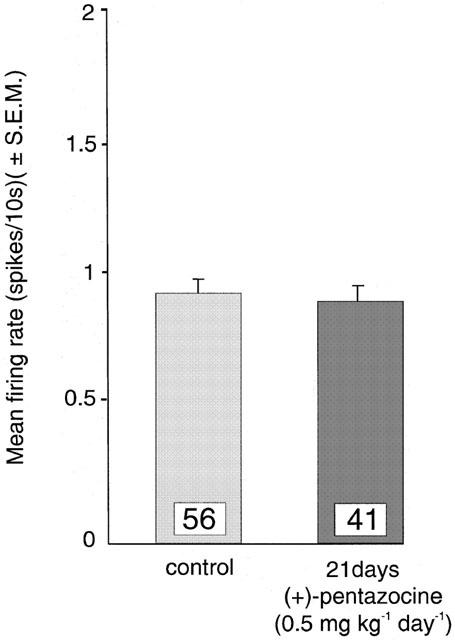
Mean firing activity expressed as spikes/10 s (mean±s.e.m.) of dorsal raphe nucleus serotonergic neurons in rats treated with saline (control) or for 21 days with (+)-pentazocine (0.5 mg kg−1 day−1).
Discussion
4-IBP is a selective sigma ligand with a high affinity for the sigma1 receptor (Ki=1.7 nM) and moderate affinity for the sigma2 receptor (Ki=25.2 nM) (John et al., 1994). Short-term treatments with 4-IBP (2 mg kg−1 day−1) for 2 days, produced a significant 35% increase in the basal firing rate of DRN 5-HT neurons (Figure 3). Similarly, the selective sigma1 ligand (+)-pentazocine produced a 33% increase in the firing activity of 5-HT neurons of the DRN (Figures 1 and 4). This increase was not seen after treatment with the selective sigma1 ligands PRE-084 and JO-1784 as their firing rates did not differ significantly from controls (Figure 2).
The increased firing rates observed after both short- and long-term treatments with (+)-pentazocine were completely prevented by co-administration with NE-100 (10 mg kg−1 day−1), a selective sigma1 antagonist (Figures 4 and 5). This confirms that the modulation of serotonergic firing activity demonstrated here is indeed mediated by sigma1 receptors. However, as shown in Figure 3, when NE-100 (10 mg kg−1 day−1) was co-administered with 4-IBP for 2 days, the increase in the firing activity of the 5-HT neurons which was induced by 2 day treatments with 4-IBP was not prevented. Thus, the average firing activity remained significantly increased versus controls.
Various preclinical results for a variety of sigma ligands have already suggested that these compounds could produce antidepressant effects. Specifically, the sigma ligands 1-(3,4-dimethoxyphenethyl)-4-(3-phenylpropyl) piperazine dihydrochloride (SA-4503), (+)-pentazocine, DTG, JO-1784 and 1-[3-[4-(3-chlorophenyl)-1-piperazinyl]propyl-5-methoxy-3,4-dihydro-2-quinolinone monomethanesulphonate (OPC-14523) dose-dependently decreased immobility in mice in the forced swimming test and this decrease was antagonized by pretreatment with the sigma antagonist NE-100 (Matsuno et al., 1996; Tottori et al., 1997; Kinsora et al., 1998). In keeping with these data, the acute administration of SA-4503 and (+)-pentazocine decreased immobility in mice exposed to the tail suspension test, at doses that failed to influence motor activity and these effects were antagonized by NE-100 (Ukai et al., 1998).
A second line of evidence suggesting sigma receptor's potential involvement in the pathophysiology and/or the treatment of depression comes from many antidepressant's interaction with and/or high affinity for sigma receptors. For example, serotonin (5-HT) reuptake inhibitors (SSRI's) and monoamine oxidase inhibitors (MAOI's) prevent [3H](+)3-PPP binding to sigma receptors in rat and mouse brains (Schmidt et al., 1989; Itzhak & Kassim, 1990). Furthermore, sertraline, an SSRI, and clorgyline, an MAOI, potentiate the NMDA response with a bell-shaped dose response curve, potentiations, which are reversed by haloperidol (a sigma1 antagonist). Paroxetine and tranylcypromine, with monoaminergic profiles similar to sertraline and clorgyline except that they are devoid of sigma affinity, did not affect the NMDA response, therefore, indicating that the effects of sertraline and clorgyline were not due to monoaminergic effects (Bergeron et al., 1993). Thirdly, in rats, chronic treatments with imipramine or fluoxetine result in a down regulation of sigma receptors in the striatum, hippocampus and cerebral cortex, brain regions implicated in regulation of emotions. This down regulation involves a decrease in Bmax and depends on cerebral serotonergic transmission as it was reversed by p-chlorophenylalanine (Shirayama et al., 1993).
The significant increase in the firing activity of 5-HT neurons observed after only 2 days of treatment contrasts what has been seen up to now in electrophysiological studies assessing the effects of antidepressant medications. More specifically, electrophysiological data demonstrate that all antidepressants after chronic treatments, through various mechanisms, increase 5-HT neurotransmission (Chaput et al., 1991; Blier & de Montigny, 1994). For example, acute treatments with MAOI's or SSRI's lead to decreased firing activity of 5-HT neurons in the DRN but as treatment continues the 5-HT neurons regain normal firing activity due to desensitization of the 5-HT1A somatodendritic autoreceptors. This desensitization may be an adaptive change that explains the delayed enhancement of 5-HT mediated neurotransmission, which is consistent with the clinical onset of action of SSRI's (Chaput et al., 1986; Blier et al., 1984; Blier & de Montigny, 1985; 1994). In agreement with electrophysiologial results, microdialysis experiments show that following a 14-day administration of an SSRI, there is a 6 fold increase in extracellular 5-HT concentration in the frontal cortex (Bel & Artigas, 1993). Indeed up to now only one antidepressant (mirtazapine) has been shown to induce an increase in the firing activity of DRN neurons following acute and long-term treatments (Haddjeri et al., 1998; Besson et al., 2000). Interestingly, mirtazapine was recently reported a showing a more rapid onset of action of its antidepressant properties (Benkert et al., 2000). Thus, the present data could suggest that not only sigma agonists might have antidepressant properties, but also that their onset of action might be more rapid than those of classical antidepressants.
Following 21 days of treatment with (+)-pentazocine or 4-IBP (2 mg kg−1 day−1) the increase in firing activity seen after 2 days persists, suggesting this is not a transient effect. Interestingly, after 21 days of treatment with (+)-pentazocine but not with 4-IBP there was a drastic decrease in neurons found per track (Figure 6). This did not occur after shorter treatments of 10 or 14 days (Figure 6) nor after 21-day treatment with a lower dose of 0.5 mg kg−1 day−1 of (+)-pentazocine (Figure 7). Furthermore, the co-administration of NE-100 prevented the decreased neurons per track seen after 21 days of treatment with (+)-pentazocine (Figure 6). Therefore, this phenomenon appears to be selective for (+)-pentazocine and specific to long-term treatments over a certain dosage.
One possible explanation for the decrease in the number of neurons found per track after chronic (+)-pentazocine treatment is a decrease of spontaneously active 5-HT neurons, due to a depolarization blockade, as seen in the dopaminergic neurons of the midbrain following chronic haloperidol administration (Grace & Bunney, 1986; Hollerman et al., 1992). Thus far, we have first investigated the reality of this potential depolarization blockade by testing if it could be reversed by a 5-HT1A agonist. Following the intravenous administration of 8-OH-DPAT, a 5-HT1A agonist at somatodendritic autoreceptors (Peroutka, 1985), the amount of neurons found per track was not changed. One would expect that the activation of the somatodendritic 5-HT1A autoreceptor by 8-OH-DPAT would reverse a depolarization blockade since it repolarizes the neuron, as the depolarization blockade seen in dopaminergic neurons was reversed by apomorphine, a dopamine autoreceptor agonist (Grace & Bunney, 1986; Hollerman et al., 1992). Thus, the lack of effect of 8-OH-DPAT suggests, either that the decreased number of neurons per track was not due to a depolarization blockade, or that higher doses of the 8-OH-DPAT were required. However, the latter appears unlikely as we used the dose previously shown to completely suppress 5-HT firing activity in the DRN (Blier et al., 1998). In a second attempt to repolarize the neurons, rats were injected with (+)baclofen (5 – 15 mg kg−1 i.v.), a γ-aminobutyric acidB (GABA) agonist, which also, did not restore the number of spontaneously firing neurons suggesting that the silent neurons were not depolarized. However, a lack of repolarizing effect of the GABAB agonist could not be totally excluded based on recent findings suggesting that, under some circumstances, (+)baclofen might disinhibit DRN 5-HT neurons by preferentially activating GABAB autoreceptors (Abellan et al., 2000). Therefore, at present the possibility of a depolarization blockade cannot be completely ruled out and we will be further investigating this phenomenon as this would be the first report of such a phenomenon occurring in 5-HT neurons.
A second possible explanation for the decreased number of neurons found per track is an increased endogenous tonic GABA inhibition of the 5-HT neurons of the DRN (Hajos et al., 1999; Abellan et al., 2000). It has been suggested that the inhibitory effect of 8-OH-DPAT on firing activity of DRN neurons involves, in part, the activation of a 5-HT1A receptor-mediated postsynaptic long feedback loop centred on the medial prefrontal cortex (Ceci et al., 1994; Hajos et al., 1999; Casanovas et al., 1999). This inhibition by the prefrontal cortex is thought to involve activation of GABA interneurons by glutaminergic cortical input (Hajos et al., 1999; Haddjeri et al., 2000; Abellan et al., 2000). To test this possibility we injected (−)bicuculline (375 μg kg−1 i.v.), a GABAA antagonist, but this did not restore the number of neurons found per track, suggesting overactive GABA tonic inhibition is not responsible.
It has also been shown that in addition to GABAergic modulation of neurons in the long feedback loop cholinergic and glutamatergic systems play key roles. This was demonstrated by the finding that the muscarinic antagonist atropine, the M2 antagonist 11-[[2-[(diethylamino)methyl]-1-piperidinyl]acetyl]-5-11-dihydro-6H-pyrido[2,3-6][1,4]benzodiazepine-6-one (AF-DX116), the NMDA antagonist (+)-5-methyl-10,11-dihydro-5H-dibenzo(a,d)cyclohepten-5-10-imine maleate (MK-801) and GABAB antagonist (25)(+)-5,5-dimethyl-2-morpholineacetic acid (SCH-50911) all dampened the suppressant effect of 8-OH-DPAT on the firing activity of DRN 5-HT neurons while (−)bicuculline did not (Haddjeri et al., 2000). Therefore, the possible effect(s) of these other systems on the firing activity of neurons of the DRN and thus the neurons found per track should be assessed especially in the light of the known interaction of sigma ligands with NMDA receptors discussed previously. Nevertheless, this dramatic decrease in the number of spontaneously active 5-HT neurons could suggest that the net effect of some sigma ligands will not always be beneficial from an ‘antidepressant' perspective.
The effects of (+)-pentazocine changed over the duration of the treatments, as shown in Figures 5 and 6. The magnitude of the increase in the average firing rate of DRN 5-HT neurons progressively reduced in parallel to a progressive increase in the number of neurons found per track. We do not have any definite explanation for this phenomenon, however, if one assumes that the decreased number of spontaneously active neurons is due to a depolarization blockade, one possible explanation could be that, at the beginning of the treatment, spontaneously active neurons and silent neurons will see their firing activity progressively increase and then decrease before reaching the final stage of the depolarization blockade. In such a paradigm, day 14 could represent the time with the maximum number of neurons firing, with some being already on the descending phase of the curve before the depolarization, while the initially silent ones have not yet reached their maximal firing activity. We are currently investigating the potential mechanisms responsible for this phenomenon.
The discrepancy between (+)-pentazocine and 4-IBP producing an increase in firing activity while PRE-084 and JO-1784 did not, is surprising. Firstly, like (+)-pentazocine, JO-1784 was shown to be a sigma agonist in our model of modulation of the NMDA response (Monnet et al., 1992). Secondly, although PRE-084 has not been tested in our model of NMDA modulation, it was found to act as an agonist in several behavioural models of learning and memory deficits. Specifically, PRE-084 attenuated MK-801-induce learning impairments in mice similar to sigma1 agonists (+)-pentazocine and (+)-N-allyl-normetazocine ((+)SKF-10,047) and was antagonized by the sigma1 antagonist α-(4-fluorophenyl)-4-(5-fluoro-2-pyrimidinyl)-l-piperazinebutanol (BMY-14802) (Maurice et al., 1994a, 1994b). Similarly, treatments with JO-1784 and PRE-084 (0.1 – 3 mg kg−1) improved learning impairments in a BMY-14802 sensitive manner in senescense accelerated mice (Maurice et al., 1996).
This lack of effect of JO-1784 and PRE-084 on the firing activity of serotonergic neurons of the DRN may be explained by the existence of subtypes of sigma1 receptors, which has been previously suggested by results from our laboratory. Specifically, potentiation of the NMDA response by DTG and JO-1784 is mediated by a subtype of sigma1 receptor linked to a Gi/o protein, whereas potentiation induced by (+)-pentazocine is mediated by another subtype of the sigma1 receptor not linked to a Gi/o protein, as only this response is pertussis toxic insensitive (Monnet et al., 1994). Furthermore, following colchicine pretreatment, which destroys the mossy fibre system, the neuronal response induced by DTG and JO-1784 was abolished while (+)-pentazocine's effect persisted, indicating the sigma1 receptor subtype mediating (+)-pentazocine's effect is located postsynaptically on pyramidal neurons while the sigma1 receptor subtype mediating DTG and JO-1784's effects is located presynaptically (Debonnel et al., 1996). Further evidence in support of the existence of subtypes of sigma1 receptors was demonstrated recently as the potentiation of the NMDA response by (+)-pentazocine is reversed by naloxone, an opiate antagonist, while the potentiating effects of JO-1784, (+)-cis-N-[2,(3,4-dichlorophenyl)ethyl]-2-(1-pyrrolidinyl-cyclohexlamine (BD 737) and 1-benzylspiro[1,2,3,4-tetrahydronaphthalene-1,4-piperidine (L 687-384) were not (Couture & Debonnel, 2001). Thus, the modulation of serotonergic firing activity seen after a 2 day treatment with (+)-pentazocine and 4-IBP may be mediated by a specific subtype of sigma1 receptor to which (+)-pentazocine and 4-IBP possess high affinity, while JO-1784 and PRE-084 may not.
(+)-Pentazocine and 4-IBP are probably not acting via the same sigma1 receptors. Evidence for this includes the fact that (+)-pentazocine after chronic treatments induced a decrease in the number of neurons encountered per track while chronic treatment with 4-IBP did not. In addition, (+)-pentazocine's effect of increasing the 5-HT firing activity was reversed by the co-administration of NE-100 while 4-IBP's effect was not. These differences are likely due to effects mediated by different subtypes of the sigma1 receptor. There has been previous evidence of multiple binding sites for (+)-pentazocine in addition to the aforementioned results by Couture & Debonnel (2001), for example, saturation studies, in the presence of ions including Zn2+, Ca2+, Mg2+ and in Krebs-Ringer buffer have demonstrated multiple (+)-[3H]-pentazocine binding sites in vivo (Basile et al., 1992). Further evidence showed [3H]-pentazocine to label three different sites with different Kd values when various cell lines were tested (Vilner et al., 1995).
It is important to mention that JO-1784 or PRE-084's ability to modulate serotonergic neurotransmission cannot be completely ruled out. Maurice et al. (1994b), has shown that PRE-084 follows a bell-shaped dose-response curve, which has been previously described in the modulation of the NMDA response by sigma ligands, including JO-1784, in the hippocampus (Bergeron et al., 1995). Our doses were chosen based on those shown to produce an optimal response in the modulation of the NMDA response previously tested in our laboratory (Monnet et al., 1990; 1992). Thus, it is indeed possible that the dose of PRE-084 or JO-1784 tested may be too low to reach the agonist range, or conversely, it may be too high and functioning as an antagonist. After chronic treatments with sigma ligands in the NMDA model, our laboratory has shown that low doses of JO-1784 or DTG potentiate the response to NMDA, however, at high doses they function as antagonists having no effect on their own but blocking the effect of sigma agonists (Bergeron et al., 1997). Thus, the effect of these two ligands on serotonergic neurotransmission cannot be completely ruled out until a range of doses is tested.
Even if this was not the case, it is also possible that PRE-084 and JO-1784 could possess some antidepressant properties but act via another mechanism. This may involve the modulation of NMDA receptors as other compounds that antagonize NMDA receptors have been shown to produce antidepressant effects in behavioural models of depression (Trullas & Skolnick, 1990; Maj et al., 1992; Papp & Moryl, 1994). In addition, an alternative theory is that these sigma ligands could be modulating noradrenergic activity.
The precise mechanisms underlying the modulation of serotonergic neurotransmission evidenced in the present study remain to be elucidated and are the focus of current investigations in our laboratory.
In conclusion, this series of experiments provides the first evidence of sigma receptor interaction with the 5-HT system. Thus, it strengthens the argument for sigma receptor's role in depression and provides a plausible mechanism of action to explain the antidepressant-like effects observed with some sigma ligands in behavioural models of depression. Importantly, these experiments show sigma ligands to produce an increase in 5-HT firing activity in just 2 days, a more rapid and robust effect than the vast majority of known antidepressant medications, thus, indicating its potential as an antidepressant with a rapid onset of action.
Abbreviations
- 4-IBP
4-(N-benzylpiperidin-4-yl)-4-iodobenzamide
- 5-HT
serotonin
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino)tetralin
- AF-DX116
11-[[2-[(diethylamino)methyl]-1-piperidinyl]acetyl]-5-11-dihydro-6H-pyrido[2,3-6][1,4]benzodiazepine-6-one
- BD-737
(+)-cis-N-[2,(3,4-dichlorophenyl)ethyl]-2-(1-pyrrolidinyl-cyclohexlamine
- BMY-14802
α-(4-fluorophenyl)-4-(5-fluoro-2-pyrimidinyl)-1-piperazinebutanol
- DHSO
dimethylsulphoxide
- DRN
dorsal raphe nucleus
- DTG
1.3-di(2-tolyl)guanidine
- i.p.
intraperitoneal
- GABA
γ-aminobutyric acid
- JO-1784
(+)-N-cyclopropylmethyl-N-methyl-1,4-diphenyl-1-1-ethyl-but-3-en-l-ylamine hydrochloride
- L687-384
1-benzylspiro[1,2,3,4-tetrahydronaphthalene-1,4-piperidine
- MAOI
monoamine oxidase inhibitor
- MK-801 (dizocilpine)
(+)-5-methyl-10,11-dihydro-5H-dibenzo(a,d)cyclohepten-5-10-imine maleate
- NE-100
N,N-dipropyl-2-(4-methoxy-3-(2-phenylethoxy)phenyl)-thylamine
- NMDA
N-methyl-D-aspartate
- OPC-14523
1-[3-[4-(3-chlorophenyl)-1-piperazinyl]propyl]-5-methoxy-3,4-dihydro-2-quinolinone monomethanesulphonate
- PRE-084
2-(4-morpholinoethyl 1-phenyl-cyclohexane-1-carboxylate hydrochloride
- SA-4503
1-(3,4-dimethoxyphenethyl)-4-(3-phenylpropyl) piperazine dihydrochloride
- SCH-50911
(25)(+)-5,5-dimethyl-2-morpholineacetic acid
- SEM
standard error mean
- SSRI
selective serotonin reuptake inhibitor
- (+)SKF-10,047
(+)-N-allyl-normetazocine
References
- ABELLAN M., JOLAS T., AGHAJANIAN G., ARTIGAS F. Dual control of dorsal raphe serotonergic neurons by GABAB receptors. Electrophysiological and microdialysis studies. Synapse. 2000;36:21–34. doi: 10.1002/(SICI)1098-2396(200004)36:1<21::AID-SYN3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- AGHAJANIAN G., VANDERMAELEN C. Intracellular recordings from serotonergic dorsal raphe neurons: Pacemaker potentials and the effect of LSD. Brain Res. 1982;238:463–469. doi: 10.1016/0006-8993(82)90124-x. [DOI] [PubMed] [Google Scholar]
- BASILE A., PAUL I., MIRCHEVITCH A., KURJPERS G., DE COSTA B. Modulation of (+)-(3H) pentazocine binding to guinea pig cerebellum by divalent cations. Mol. Pharm. 1992;42:882–889. [PubMed] [Google Scholar]
- BEL N., ARTIGAS F. Chronic treatment with fluvoxamine increases extracellular serotonin in frontal cortex but not in raphe nuclei. Synapse. 1993;15:243–245. doi: 10.1002/syn.890150310. [DOI] [PubMed] [Google Scholar]
- BENKERT O., SZEGEDI A., KOHNEN R. Mirtazapine compared with paroxetine in major depression. J. Clin. Psychiatry. 2000;61:656–663. doi: 10.4088/jcp.v61n0911. [DOI] [PubMed] [Google Scholar]
- BERGERON R., DE MONTIGNY C., DEBONNEL G. Biphasic effects of sigma ligands on the neuronal response to N-methyl-D-aspartate. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995;351:252–260. doi: 10.1007/BF00233244. [DOI] [PubMed] [Google Scholar]
- BERGERON R., DEBONNEL G., DE MONTIGNY C. Modification of the N-methyl-D-aspartate response by antidepressant σ receptor ligands. Eur. J. Pharmacol. 1993;240:319–323. doi: 10.1016/0014-2999(93)90918-8. [DOI] [PubMed] [Google Scholar]
- BERGERON R., DE MONTIGNY C., DEBONNEL G. Potentiation of neuronal response induced by dehydroepiandrosterone and its suppression by progesterone: Effects mediated by sigma receptors. J. Neurosci. 1996;16:1193–1202. doi: 10.1523/JNEUROSCI.16-03-01193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGERON R., DE MONTIGNY C., DEBONNEL G. Effect of short-term and long-term treatments with sigma ligands on the N-methyl-D-aspartate response in the CA3 region of the rat dorsal hippocampus. Br. J. Pharmacol. 1997;120:1351–1359. doi: 10.1038/sj.bjp.0701042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BESSON A., HADDJERI N., BLIER P., DE MONTIGNY C. Effects of the co-administration of mirtazapine and paroxetine on serotonergic neurotransmission in the rat brain. Eur. Neuropsychopharmacol. 2000;10:177–188. doi: 10.1016/s0924-977x(00)00069-9. [DOI] [PubMed] [Google Scholar]
- BLIER P., DE MONTIGNY C. Short-term lithium administration enhances serotonergic neurotransmission: Electrophysiological evidence in the rat CNS. Eur. J. Pharmacol. 1985;113:69–77. doi: 10.1016/0014-2999(85)90344-9. [DOI] [PubMed] [Google Scholar]
- BLIER P., DE MONTIGNY C. Current advances and trends in the treatment of depression. Trends Pharmacol. Sci. 1994;15:220–225. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- BLIER P., DE MONTIGNY C., TARDIF D. Effects of the two antidepressant drugs mianserin and indalpine on the serotonergic system: Single-cell studies in the rat. Psychopharmacology. 1984;84:242–249. doi: 10.1007/BF00427453. [DOI] [PubMed] [Google Scholar]
- BLIER P., PINEYRO G., EL MANSARI M., BERGERON R., DE MONTIGNY C. Role of somatodendritic 5-HT autoreceptors in modulating 5-HT neurotransmission. Ann. N.Y. Acad. Sci. 1998;861:204–216. doi: 10.1111/j.1749-6632.1998.tb10192.x. [DOI] [PubMed] [Google Scholar]
- CASANOVAS M., HERVÁS I., ARTIGAS F. Postsynaptic 5-HT1A receptors control 5-HT release in the rat medial prefrontal cortex. NeuroReport. 1999;10:1441–1445. doi: 10.1097/00001756-199905140-00010. [DOI] [PubMed] [Google Scholar]
- CECI A., BASCHIROTTO A., BORSINI F. The inhibitory effect of 8-OH-DPAT on the firing activity of dorsal raphe serotonergic neurons in rats is attenuated by lesion of the frontal cortex. Neuropharmacology. 1994;33:709–713. doi: 10.1016/0028-3908(94)90177-5. [DOI] [PubMed] [Google Scholar]
- CHAPUT Y., DE MONTIGNY C., BLIER P. Effects of a selective 5-HT reuptake blocker, citalopram, on the sensitivity of 5-HT autoreceptors: Electrophysiological studies in the rat brain. Naunyn-Schmied. Arch. Pharmacol. 1986;333:342–348. doi: 10.1007/BF00500007. [DOI] [PubMed] [Google Scholar]
- CHAPUT Y., DE MONTIGNY C., BLIER P. Presynaptic and postsynaptic modifications of the serotonin system by long-term administration of antidepressant treatments. Neuropsychopharmacology. 1991;5:219–229. [PubMed] [Google Scholar]
- COUTURE S., DEBONNEL G. Some of the effects of the selective sigma ligand (+)pentazocine are mediated via a naloxone-sensitive receptor. Synapse. 2001;39:323–331. doi: 10.1002/1098-2396(20010315)39:4<323::AID-SYN1016>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- DEBONNEL G., BERGERON R., MONNET F.P., DE MONTIGNY C. Differential effects of sigma ligands on the N-methyl-D-aspartate response in the CA1 and CA3 regions of the dorsal hippocampus: effect of mossy fiber lesioning. Neuroscience. 1996;71:977–987. doi: 10.1016/0306-4522(96)80001-7. [DOI] [PubMed] [Google Scholar]
- DELGADO P.L. Depression: the case for a monoamine deficiency. J. Clin. Psychiat. 2000;61 Suppl 6:7–11. [PubMed] [Google Scholar]
- GRACE A., BUNNEY B. Induction of depolarization block in midbrain dopamine neurons by repeated administration of haloperidol: Analysis using in vivo intracellular recording. J. Pharmacol. Exp. Ther. 1986;238:1092–1100. [PubMed] [Google Scholar]
- HADDJERI N., BLIER P., DE MONTIGNY C. Acute and long-term actions of the antidepressant drug mirtazapine on central 5-HT neurotransmission. J. Aff. Dis. 1998;51:255–266. doi: 10.1016/s0165-0327(98)00223-7. [DOI] [PubMed] [Google Scholar]
- HADDJERI N., LUCAS G., BLIER P. Role of cholinergic and GABAergic system in the feedback inhibition of dorsal raphe 5-HT neurons. NeuroReport. 2000;11:3397–3401. doi: 10.1097/00001756-200010200-00026. [DOI] [PubMed] [Google Scholar]
- HAIGLER H., AGHAJANIAN G. Lysergic acid diethylamide and serotonin: A comparison of effects of serotonergic neurons receiving a serotonergic input. J. Pharmacol. Exp. Ther. 1974;168:688–699. [PubMed] [Google Scholar]
- HAJOS M., HAJOS-KORCSOK E., SHARP T. Role of the medial prefrontal cortex in 5-HT1A-induced inhibition of 5-HT neuronal activity in the rat. Br. J. Pharmacol. 1999;126:1741–1750. doi: 10.1038/sj.bjp.0702510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANNER M., MOEBIUS F., FLANDORFER A., KNAUS H.-G., STRIESSNIG J., KEMPNER E., GLOSSMAN H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. PNAS. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLERMAN J., ABERCROMBIE E., GRACE A. Electrophysiological, biochemical, and behavioral studies of acute haloperidol-induced depolarization block of nigral dopamine neurons. Neuroscience. 1992;47:589–601. doi: 10.1016/0306-4522(92)90168-2. [DOI] [PubMed] [Google Scholar]
- ITZHAK Y., KASSIM C. Clorgyline displays high affinity for σ binding sites in C57BL/6 mouse brain. Eur. J. Pharmacol. 1990;176:107–108. doi: 10.1016/0014-2999(90)90139-w. [DOI] [PubMed] [Google Scholar]
- JOHN C., VILNER B., BOWEN W. Synthesis and characterization of [125I]-N-(N-Benzylpiperidin-4-yl)-4-iodobenzamide, a new σ receptor radiopharmaceutical: High-affinity binding to MCF-7 breast tumour cells. J. Med. Chem. 1994;37:1737–1739. doi: 10.1021/jm00038a002. [DOI] [PubMed] [Google Scholar]
- KINSORA J., JR, CORBIN A., SNYDER B., WILEY J., MELTZER L., HEFFNER T. Effects of igmesine in preclinical antidepressant tests. Soc. Neurosci. Abstr. 1998;24:744. [Google Scholar]
- MAJ J., ROGOZ Z., SKUZA G., SOWINSKA H. Effects of MK-801 and antidepressant drugs in the forced swimming test in rats. Eur. Neuropsychopharmacol. 1992;2:37–41. doi: 10.1016/0924-977x(92)90034-6. [DOI] [PubMed] [Google Scholar]
- MARTIN W., EADES C., THOMPSON J., HUPPLER R., GILBERT P. The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- MATSUNO K., KOBAYASHI T., TANAKA M., MITA S. σ1 receptor subtype is involved in the relief of behavioral despair in the mouse forced swimming test. Eur. J. Pharmacol. 1996;312:267–271. doi: 10.1016/0014-2999(96)00497-9. [DOI] [PubMed] [Google Scholar]
- MAURICE T., HIRAMATSU M., ITOH J., KAMEYAMA T., HASEGAWA T., NABESHIMA T. Behavioral evidence for a modulating role of sigma ligands in memory processes: I. Attenuation of dizocilpine (MK-801)-induced amnesia. Brain Res. 1994a;647:44–56. doi: 10.1016/0006-8993(94)91397-8. [DOI] [PubMed] [Google Scholar]
- MAURICE T., ROMAN F.J., SU T.-S., PRIVAT A. Beneficial effects of sigma agonists on the age-related learning impairment in the senescence-accelerated mouse (SAM) Brain Res. 1996;733:219–230. doi: 10.1016/0006-8993(96)00565-3. [DOI] [PubMed] [Google Scholar]
- MAURICE T., SU T.-S., PARISH D.W., NABESHIMA T., PRIVAT W. PRE-084, a σ selective PCP derivative, attenuates MK-801-induced impairment of learning in mice. Pharmacol. Biochem. Behav. 1994b;49:859–869. doi: 10.1016/0091-3057(94)90235-6. [DOI] [PubMed] [Google Scholar]
- MONNET F.P., DEBONNEL G., DE MONTIGNY C. In vivo electrophysiological evidence for a selective modulation of N-methyl-D-aspartate-induced neuronal activation in rat CA3 dorsal hippocampus by sigma ligands. J. Pharmacol. Exp. Ther. 1992;261:123–130. [PubMed] [Google Scholar]
- MONNET F.P., DEBONNEL G., BERGERON R., GRONIER B., DE MONTIGNEY C. The effects of sigma ligands and of neuropeptide Y or N-methyl-D-aspartate-induced neuronal activation of CA3 hippocampus neurones are differentially affected by pertussin toxin. Br. J. Pharmacol. 1994;112:709–715. doi: 10.1111/j.1476-5381.1994.tb13134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONNET F.P., DEBONNEL G., JUNIEN J.-L., DE MONTIGNY C. N-methyl-D-aspartate-induced neuronal activation is selectively modulated by σ receptors. Eur. J. Pharmacol. 1990;179:441–445. doi: 10.1016/0014-2999(90)90186-a. [DOI] [PubMed] [Google Scholar]
- NARITA N., HASHIMOTO K., TOMITAKA S.-I., MINABE Y. Interaction of selective serotonin reuptake inhibitors with subtypes of σ receptors in the rat brain. Eur. J. Pharmacol. 1996;307:117–119. doi: 10.1016/0014-2999(96)00254-3. [DOI] [PubMed] [Google Scholar]
- PAPP M., MORYL E. Antidepressant activity of non-competitive and competitive NMDA receptor antagonists in a chronic mild stress model of depression. Eur. J. Pharmacol. 1994;263:1–7. doi: 10.1016/0014-2999(94)90516-9. [DOI] [PubMed] [Google Scholar]
- PEROUTKA S.J. Selective labeling of 5-HT1A and 5-HT1B binding sites in bovine brain. Brain Res. 1985;344:167–171. doi: 10.1016/0006-8993(85)91204-1. [DOI] [PubMed] [Google Scholar]
- QUIRION R., BOWEN W., ITZHAK Y, , JUNIEN J.-L., MUSACCHIO J., ROTHMAN R., SU T.-P., TAM S., TAYLOR D. A proposal for the classification of sigma binding sites. Trends Pharmacol. Sci. 1992;13:85–86. doi: 10.1016/0165-6147(92)90030-a. [DOI] [PubMed] [Google Scholar]
- QUIRION R., CHICHEPORTICHE R., CONTRERAS P., JOHNSON K., LODGE D., TAM S., WOODS J., ZUKIN S. Classification and nomenclature of phencyclidine and sigma receptor sites. Trends Pharmacol. Sci. 1987;10:444–446. [Google Scholar]
- ROMAN F.J., PASCAUD X., MARTIN B., VAUCHE D., JUNIEN J.L. JO-1784, a potent and selective ligand for rat and mouse brain sigma sites. J. Pharm. Pharmacol. 1990;42:439–440. doi: 10.1111/j.2042-7158.1990.tb06588.x. [DOI] [PubMed] [Google Scholar]
- SCHMIDT A., LEBEL L., KOE B., SEEGER T., HEYM J. Sertraline potently displaces (+)-[3H]3-PPP binding to σ sites in rat brain. Eur. J. Pharmacol. 1989;165:335–336. doi: 10.1016/0014-2999(89)90734-6. [DOI] [PubMed] [Google Scholar]
- SHIRAYAMA Y., NISHIKAWA T., UMINO A., TAKAHASHI K. p-Chlorophenylalanine-reversible reduction of σ binding sites by chronic imipramine treatment in rat brain. Eur. J. Pharmacol. 1993;237:117–126. doi: 10.1016/0014-2999(93)90100-v. [DOI] [PubMed] [Google Scholar]
- STEINFELS G.F., ALBERICI G.P., TAM S.W., COOK L. Biochemical, behavioral and electrophysiologic actions of the selective sigma receptor ligand (+)-pentazocine. Neuropsychopharmacology. 1988;1:321–327. [PubMed] [Google Scholar]
- SU T.-P., LONDON E., JAFFE J. Steroid binding at σ receptors suggests a link between endocrine, nervous and immune systems. Science. 1988;240:219–221. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- SU T.-P., WU X., CONE E., SHUKLA K., GUND T., DODGE A., PARISH D. Sigma compounds derived from phencyclidine: identification of PRE-084, a new, selective sigma ligand. J. Pharmacol. Exp. Ther. 1991;249:543–548. [PubMed] [Google Scholar]
- TOTTORI K., KIKUCHI T., UWAHODO Y., YAMADA S., OSHIRO Y., KOGA N. Antidepressant effect of OPC-14523 in the forced swimming test in mice. Japan J. Pharmacol. 1997;73:59P. [Google Scholar]
- TRULLAS R., SKOLNICK P. Functional antagonists at the NMDA receptor exhibit antidepressant actions. Eur. J. Pharmacol. 1990;185:1–10. doi: 10.1016/0014-2999(90)90204-j. [DOI] [PubMed] [Google Scholar]
- UKAI M., MAEDA H., NANYA Y., KAMEYAMA T., MATSUNO K. Beneficial effects of acute and repeated administrations of σ receptor agonists on behavioral despair in mice exposed to tail suspension. Pharmacol. Biochem. Behav. 1998;61:247–252. doi: 10.1016/s0091-3057(98)00093-8. [DOI] [PubMed] [Google Scholar]
- VILNER B.J., JOHN C.S., BOWEN W.D. Sigma-1 and sigma-2 receptors are expressed in a wide variety of human and rodent tumor cell lines. Cancer Res. 1995;55:408–413. [PubMed] [Google Scholar]


