Abstract
The nitric oxide synthase (NOS) inhibitor, Nω-nitro-L-arginine methyl ester (L-NAME), inhibits both rat and human eosinophil chemotaxis in vitro. Here, the role of nitric oxide (NO) in human eosinophil cell surface integrin expression and function was investigated.
Human peripheral blood eosinophils were treated with L-NAME (0.01 – 1.0 mM) and their adhesion to human fibronectin and serum observed. Adhesion of cells to fibronectin and serum increased by 24.0±4.6 and 43.8±4.7%, respectively, when eosinophils were treated with 1.0 mM L-NAME. Increased adhesion by L-NAME could be abolished when cells were co-incubated with VLA-4- and Mac-1-specific monoclonal antibodies (mAbs).
The NO donor, sodium nitroprusside (2.5 mM), significantly inhibited eosinophil adhesion to fibronectin and serum by 34.3±4.5 and 45.2±5.6%, respectively. This inhibition was accompanied by a 4 fold increase in the levels of intracellular cyclic GMP.
Flow cytometrical analysis demonstrated that L-NAME induced an increased expression of CD11b (Mac-1) on the eosinophil cell surface of 36.3±7.4%. L-NAME had no effect upon CD49d (VLA-4) expression.
Treatment of human eosinophils, in vitro, with H-[1,2,4] oxadiazolo quinoxalin-1-one (ODQ) (0.1 mM), an inhibitor of soluble guanylate cyclase, also significantly increased eosinophil adhesion to fibronectin and serum by 73.5±17.9 and 91.7±12.9%, respectively. This increase in adhesion could also be inhibited by co-incubation with the Mac-1 and VLA-4-specific mAbs.
In conclusion, results indicate that NO, via a cyclic GMP-dependent mechanism, inhibits the adhesion of human eosinophils to the extracellular matrix (ECM). This inhibition is accompanied by a decrease in the expression and function of the eosinophil's adhesion molecules, in particular, the expression of the Mac-1 integrin and the function of the VLA-4 integrin.
Keywords: Eosinophil, nitric oxide, cyclic GMP, VLA-4, Mac-1, adhesion molecule, leukocyte migration, allergic inflammation
Introduction
Leukocyte emigration from the blood vessel into surrounding tissue is a major feature of inflammatory diseases. Migration involves a number of steps involving adhesion molecule interactions. Tethering and rolling of the leukocyte on the endothelium is followed by its firm adhesion to the endothelial cell layer. The adhesion is mediated largely by the β2 integrins on the leukocyte surface before final transendothelial migration of the cells into tissue (Issekutz & Issekutz, 1992; Alon et al., 1995). Whilst the mechanism involved in leukocyte adhesion to the endothelium has been well documented, it is becoming increasingly clear that a ‘de-adhesion' step follows adhesion allowing cell locomotion from one point on the endothelium to another (Sendo et al., 1998). This locomotion is likely to be facilitated by changes in integrin-ligand binding properties. Changes in integrin function may be brought about in a number of ways; activation of cells may induce a conformational change in the integrin structure and thus a change in integrin affinity for ligand (inside-out signalling) (Diamond & Springer, 1994) or divalent cations close to integrin-ligand complexes may affect ligand-binding avidity. In addition, recent research has suggested the involvement of integrin-associated glycosyl phosphatidyl inositol (GPI)-anchored proteins (such as urokinase-type plasminogen activator receptor, uPAR) in integrin function (Sendo et al., 1998). The mechanism of this adhesion/de-adhesion stage has still to be elucidated, but it is clear that the integrins and regulation of their function play a central role in it.
Human neutrophil chemotaxis in vitro can be inhibited by nitric oxide (NO) synthesis blockade and there is evidence that NO stimulates neutrophil chemotaxis via a cyclic GMP-dependent pathway (Belenky et al., 1993; Moilanen et al., 1993; Wanikiat et al., 1997; Kaplan et al., 1989). Chronic treatment of rats with the NO synthase (NOS) inhibitor Nω-nitro-L-arginine methyl ester (L-NAME) (Moore et al., 1991) inhibits stimulated eosinophil migration both in vivo and ex-vivo (Ferreira et al., 1996; Zanardo et al., 1997) and this effect is coupled to the cyclic GMP transduction pathway in both rat (Zanardo et al., 1997) and human (Conran et al., 1998) eosinophils. The NOS inhibitor NGMonomethyl-L-arginine (L-NMMA) increased the expression of CD11/CD18 on cat neutrophils (Kubes et al., 1991) and NO has a role in enhancing VCAM-1/ICAM-1 expression on endothelial cells (de caterina et al., 1995; Niu et al., 1994; Khan et al., 1996).
Both Mac-1 and VLA-4 are known to be important mediators of eosinophil function, Mac-1 has a role in degranulation and superoxide anion production in GM-CSF-(granulocyte macrophage-colony stimulating factor) and PAF-(platelet activating factor) induced eosinophils (Horie & Kita, 1994). Focal adhesion kinase (FAK) is found on focal adhesion contacts and can bind to integrins transferring signals via src (Clark & Brugge, 1995), crosslinking of Mac-1 on eosinophils leads to intracellular signalling events such as activation of protein tyrosine kinase leading to eosinophil degranulation (Kato et al., 1998). Binding of eosinophil VLA-4 with VCAM-1 promotes eosinophil cell superoxide production (Nagata et al., 1995) and adhesion of eosinophils to fibronectin via VLA-4 prolongs eosinophil survival possibly by Fas antigen signalling (Higashimoto et al., 1996) indicating a role for integrin adhesion and signalling in regulation of eosinophil function and death.
Since inhibition of eosinophil migration by L-NAME may be mediated by changes in eosinophil-integrin function and expression, we have investigated the effect of blocking eosinophil NO synthesis upon human eosinophil cell surface integrin expression and cell adhesion interactions with extracellular matrix components.
Methods
Eosinophil isolation
Eosinophils were isolated from peripheral blood as described by Hansel et al. (1991), with minor modifications. Briefly, 60 ml of blood collected in 3.13% (w v−1) sodium citrate from a healthy subject was diluted 1 : 1 with phosphate buffered saline (PBS) and 35 ml of diluted blood overlaid onto a 15 ml Percoll gradient (1.088 g ml−1, pH 7.4, 340 mosmol Kg−1 H2O). Gradients were centrifuged at 700×g for 20 min, 4°C (Hermle model Z360k centrifuge, Germany) and the red cell pellet collected. Red cells in the granulocyte pellet were lysed with lysing buffer (155 mM NH4Cl, 10 mM KHCO3 0.1 M EDTA). Washed granulocytes were incubated with anti-CD16 immunomagnetic microbeads (Miltenyi Biotec Inc., U.S.A.) before passing on a steel-matrix column in a magnetic field (Miltenyi Biotec Inc., U.S.A.) and collecting CD16-negative eosinophils. Eosinophils were resuspended in Eagle's minimum essential medium, pH 7.2 (MEM) (>92% eosinophils, contaminating cells were mononuclear cells).
Treatment of isolated eosinophils before performance of assays
Cells were treated with various drugs or antibodies before performance of adhesion assays or flow cytometry. Cells suspended in MEM/0.1% ovalbumin were incubated with the drug and/or antibody of choice for 25 min at 37°C, 5% CO2. Cells to be used in flow cytometry in conjunction with the 44H6 monoclonal antibody were also pre-incubated with 20 μg ml−1 fibronectin before monoclonal antibody incubation. Treated cells were then used immediately in the assay desired.
Eosinophil cell adhesion assays
96-well plates were prepared by coating individual wells with 60 μl of desired ligand (20 μg ml−1 fibronectin or 10% (v v−1) human serum in PBS) overnight at 4°C. Wells were then washed twice with PBS before blocking non-coated sites with 0.1% (w v−1) BSA for 60 min at 37°C. Wells were washed twice again with PBS before allowing plates to dry. Eosinophils were added in a volume of 50 μl of MEM/ovalbumin (7×105 cells ml−1) to the coated wells of a 96-well plate. Cells were allowed to adhere to wells for 15 min at 37°C, 5% CO2. After incubation non-adhered cells were removed and the remaining cells were washed twice with PBS. Fifty μl of MEM were added to each well and varying concentrations of the original cell suspension (in MEM) were added to empty wells to form a standard curve. Eosinophil adhesion was calculated by measuring residual eosinophil peroxidase (EPO) activity of adherent cells (Nagata et al., 1995). Fifty μl of EPO substrate (1 mM H2O2, 1 mM o-phenylenediamine and 0.1% Triton X-100 in Tris buffer, pH 8.0) were added to each well. After a 30 min incubation at room temperature, 25 μl of 4 M H2SO4 were added to each well to stop the reaction and absorbance measured at 490 nM in a microplate reader (Multiscan MS, Labsystems, U.S.A.). Adherence was calculated by comparing absorbance of unknowns to that of the standard curve.
Extraction of cyclic GMP from eosinophil cell suspensions
Eosinophils were isolated and resuspended to a concentration of 1×107 cells ml−1 in PBS. Cells were incubated with the phosphodiesterase inhibitor, 3-isobutyl-l-methylxanthine (2 mM, IBMX) for 30 min at room temperature before stimulating cells, or not, with the NO donor, sodium nitroprusside (0.01 – 3.0 mM) for 12 min in the presence of IBMX at 37°C in a humidified atmosphere. The reaction was interrupted by the addition of cold acidified absolute ethanol to a final concentration of 67% (v v−1) and samples were vigorously agitated by hand for 30 s. Cell samples were then incubated on ice for 30 min before centrifuging at 4000×g for 30 min at 4°C. Supernatants were collected and retained and the precipitates washed with 0.5 ml 67% (v v−1) acidified ethanol before centrifuging again at 14,000×g for 5 min at room temperature. Supernatants from these washed samples were collected and added to the first supernatants collected and dried at 55 – 60°C under a stream of nitrogen in a water bath and stored at −20°C until measurement of cyclic GMP.
Measurement of cyclic GMP
Cyclic GMP in 3×106 cells was measured using a Cayman kit (Cayman Chemical Co., Ann Arbor, MI, U.S.A.) employing the method described by Pradelles & Grassi (1989).
Flow cytometry
Expression of adhesion molecules on the surface of eosinophils was detected using flow cytometry. Isolated eosinophils (50 μl, 5×106 cells ml−1) were incubated with a saturating concentration of adhesion molecule monoclonal antibody or a suitable isotype control (30 min, 4°C). After centrifugation of cells (300×g, 10 min) and removal of supernatant, cells were incubated with FITC-conjugated secondary antibody (30 min, 4°C in dark). The cells were then fixed in 0.5 ml 1% paraformaldehyde (10 min 4°C) before washing twice with buffer (PBS/0.1% BSA). Cells (50,000) were analysed at 488 nm on a Becton-Dickinson FACScalibur. SSC/FSC (side scatter/forward scatter) dot plots were used to gate the eosinophil population ensuring that only cell populations of interest were analysed. Fluorescence intensity of each cell was compared to that of isotype-control reacted cells.
Statistical analysis
Results are expressed as means±s.e.mean and the statistical significance between groups was determined using the Tukey test for analysis of variance. Where appropriate Student's t-test (unpaired) was used to compare specific groups. Significance was established at P<0.05.
Materials
The VarioMACS system complete with columns and microbeads was purchased from Miltenyi Biotec Inc., CA, U.S.A. Antibodies were purchased from Serotec U.S.A. (HP2/1, 44H6, ICRF 44 and FITC-secondary control) and Rockland, Gilbertsville, U.S.A. (Isotype control monoclonal antibodies). All other products were bought from Sigma Co. U.S.A. unless otherwise stated.
Results
Time courses of eosinophil adhesion to fibronectin and serum
The time courses of eosinophil adhesion (37°C, 5% CO2) to fibronectin (20 μg ml−1)-coated plates and to serum (10% v v−1)-coated plates are illustrated in Figure 1. Fifteen minutes was chosen as the optimal time point for examination of eosinophil adhesion.
Figure 1.
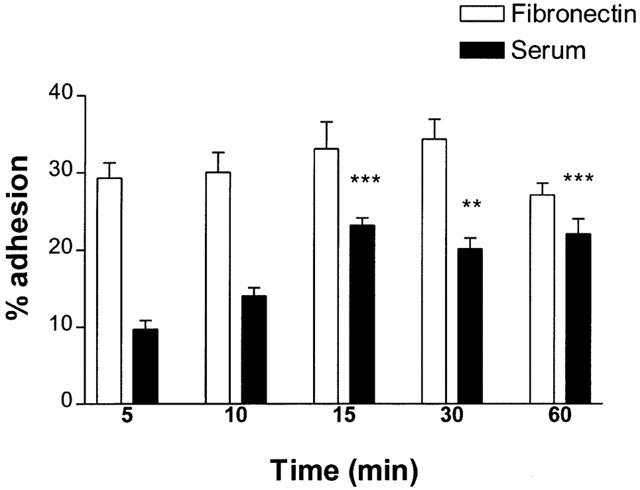
Time course of eosinophil adhesion to fibronectin (20 μg ml−1) and serum (10%, v v-1)-coated 96 well plates. 3.5×104 cells were added to wells and incubated at 37°C, 5% CO2. Following incubation, non-adhered cells were removed and percentage cell adhesion was calculated by comparing eosinophil peroxidase activity of adherent cells to that of a standard curve. Results are expressed as mean adhered cell percentages of total cell number±s.e. of three independent experiments with three replicates in each. **P<0.01, ***P<0.001 compared to adhesion at 5 min.
Effect of L-NAME upon human eosinophil adhesion to fibronectin and serum
Incubation of eosinophils with L-NAME (0.01, 0.1 and 1.0 mM) (25 min, 37°C, 5% CO2) before allowing adhesion to fibronectin-coated plates (15 min, 37°C, 5% CO2) increased eosinophil cell adhesion from 33.5±1.4% (untreated) to 39.13±0.92% (n=4, P>0.05), 42.2±1.2% (n=4, P<0.05) and 44.0±1.6% (n=4, P<0.01), respectively. Eosinophil cell adhesion to serum was also increased significantly from 23.3±0.8 to 29.5±1.5% (n=4, P<0.01), 33.8±0.8% (n=4, P<0.01) and 33.5±1.1% (n=4, P<0.01) after treatment with 0.01, 0.1 and 1.0 mM L-NAME, respectively. Increased adhesion following treatment with L-NAME to both fibronectin and serum was observed after 15 min of incubation (results not shown). D-NAME (1.0 mM) did not significantly affect adhesion of human eosinophils to human fibronectin (36.2±1.0%, n=4) or serum (23.6±1.1%, n=4).
Effect of SNP upon eosinophil adhesion to fibronectin and serum
Pre-incubation of eosinophils with the NO donor, sodium nitroprusside (SNP, 0.025 – 2.5 mM, 25 min, 37°C, 5% CO2), significantly inhibited cell adhesion to fibronectin and serum (15 min, 37°C, 5% CO2) in a concentration-dependent manner. Adhesion to fibronectin was reduced from 35.6±2.3 to 28.5±2.1% (n=4, P<0.05), 23.5±1.1% (n=4, P<0.001) and 23.4±1.6% (n=4, P<0.001) after treatment with 0.025, 0.25 and 2.5 mM SNP, respectively. Cell adhesion to serum after treatment with SNP (0.025, 0.25 and 2.5 mM) fell from 25.0±1.0 to 24.0±1.7% (n=4, P>0.05), 16.4±1.1% (n=4, P<0.01) and 13.7±1.4% (n=4, P<0.001), respectively.
Effect of SNP upon intracellular levels of cyclic GMP in eosinophils
Treatment of eosinophils with SNP (0.01 – 3.0 mM) significantly increased the concentration of intracellular cyclic GMP in a dose-dependent manner (Figure 2). SNP increased cyclic GMP levels after an incubation of 12 min (results not shown).
Figure 2.
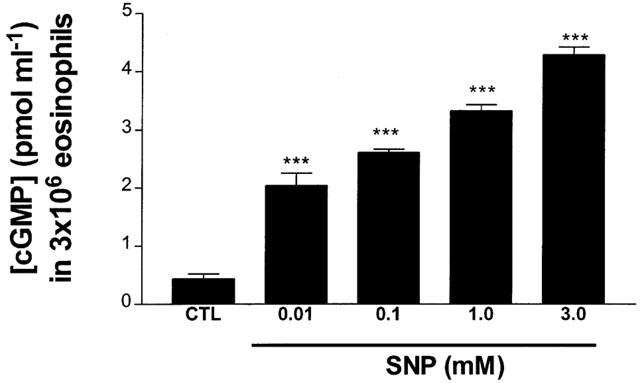
Effect of SNP upon intracellular cyclic GMP levels in eosinophils. Eosinophils (1×107 cells ml−1) were incubated with IBMX (2 mM) for 30 min before treating with SNP for 12 min (37°C) and measuring the concentration of cyclic GMP in 3×106 eosinophils. Results are expressed as mean concentration of cyclic GMP (pmol ml−1) in 3×106 eosinophils±s.e. of three independent experiments with three replicates in each. ***P<0.001 compared to control sample.
Effect of VLA-4 and Mac-1-blocking monoclonal antibodies upon eosinophil adhesion to fibronectin and serum
Incubation of eosinophils (25 min, 37°C, 5% CO2) with the anti-VLA-4 and anti-Mac-1 monoclonal antibodies, HP2/1 and ICRF 44 respectively (at saturating concentrations of 10 μg/ml and 1 : 12 supernatant dilution, respectively) did not significantly affect eosinophil basal adhesion to fibronectin (29.7±5.9, 29.2±3.1%, respectively, compared to control cell adhesion, 30.4±1.6%). Similar results were obtained when cells were incubated with both HP2/1 and ICRF 44 together (30.9±3.0%) and a non-specific control monoclonal antibody (29.5±2.1%).
Pre-incubation of eosinophils with either HP2/1 (10 μg ml−1) or ICRF 44 (1 : 12 dilution) as well as L-NAME (25 min, 37°C, 5% CO2) abolished the increase in adhesion to fibronectin seen when cells were pre-incubated with L-NAME alone (Figure 3a). A non-specific control monoclonal antibody (10 μg ml−1) had no effect on the adhesion of L-NAME treated cells to fibronectin.
Figure 3.
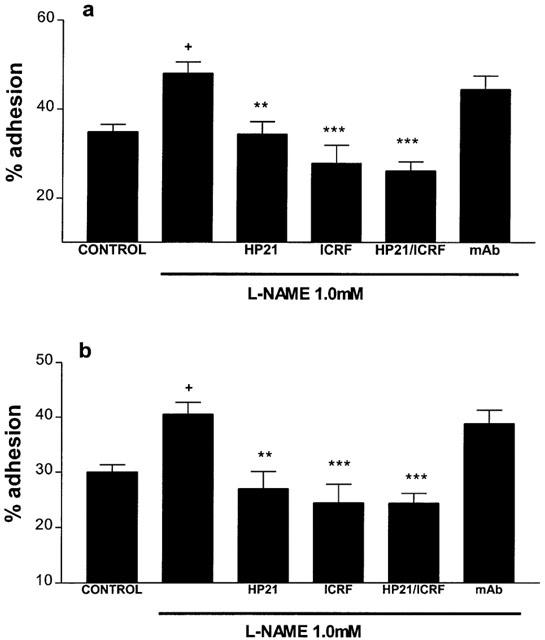
Effects of the anti-VLA-4 (HP2/1, 10 μg ml−1), anti-Mac-1 (ICRF 44, 1 : 12 diln) monoclonal antibodies and a non-specific control monoclonal antibody (mAb, 10 μg ml−1) upon human eosinophil adhesion after treatment with L-NAME, 1.0 mM, (a) to 20 μg ml−1 fibronectin, (b) to 10% (v v−1) human serum. Eosinophils (>95% pure) were incubated with monoclonal antibodies (+L-NAME) (25 min, 37°C, 5% CO2) before allowing cells to adhere to fibronectin/serum-coated plates (15 min, 37°C, 5% CO2). Results are expressed as mean adhered cell percentages of total cell number±s.e. of four independent experiments with three replicates in each. +P<0.05 compared to untreated cells, **P<0.01, ***P<0.001 compared to cells treated with L-NAME alone (1.0 mM).
Incubation of eosinophils (25 min, 37°C, 5% CO2) with the anti-VLA-4 and anti-Mac-1 monoclonal antibodies, HP2/1 and ICRF 44, respectively (at saturating concentrations of 10 μg ml−1 and 1 : 12 supernatant dilution, respectively) did not significantly affect basal eosinophil adhesion to serum (15.1±1.5, 18.4±1.7% respectively, compared to control cell adhesion, 19.0±1.0%). Similar results were obtained when cells were incubated with both HP2/1 and ICRF 44 together (14.0±1.8%) and a non-specific control monoclonal antibody (15.8±1.3%).
Co-incubation of L-NAME (1.0 mM)-treated cells with HP2/1 (10 μg ml−1) or ICRF 44 (1 : 12 dilution) (25 min, 37°C, 5% CO2) inhibited the increase in eosinophil adhesion to serum seen when eosinophils were treated with L-NAME alone (Figure 3b). A non-specific monoclonal antibody (10 μg ml−1) had no effect on the adhesion of L-NAME treated cells to serum.
Effect of ODQ upon eosinophil cell adhesion to fibronectin and serum
Incubation of eosinophils with ODQ significantly increased human eosinophil adhesion to fibronectin and serum. Adhesion to fibronectin increased from 29.5±2.2 to 40.5±4.6% (n=5, P>0.05), 51.2±5.3% (n=5, P<0.01) and 38.0±3.7% (n=5, P>0.05) following treatment with 0.01, 0.10 and 0.25 mM ODQ, respectively (25 min, 37°C, 5% CO2), DMSO (5 μg ml−1), the vehicle in which ODQ was diluted, did not significantly increase adhesion (33.9±2.1%).
Adhesion to serum increased from 23.5±2.5 to 39.9±3.0% (n=5, P<0.01), 45.1±3.0% (n=5, P<0.001) and 43.7±4.3% (n=5, P<0.001) respectively, after the same treatment. DMSO (5 μg ml−1) did not significantly increase cell adhesion (29.2±2.8%).
The augmentation in adhesion to fibronectin seen when eosinophils were treated with ODQ (0.10 mM) was abolished upon co-incubation with HP2/1 and ICRF 44 (Figure 4a). The increased adhesion to serum seen when eosinophils were treated with ODQ (0.01 mM) was inhibited by co-incubation with ICRF 44 and with HP2/1 (although the difference in results for HP2/1 did not reach significance). See Figure 4b.
Figure 4.
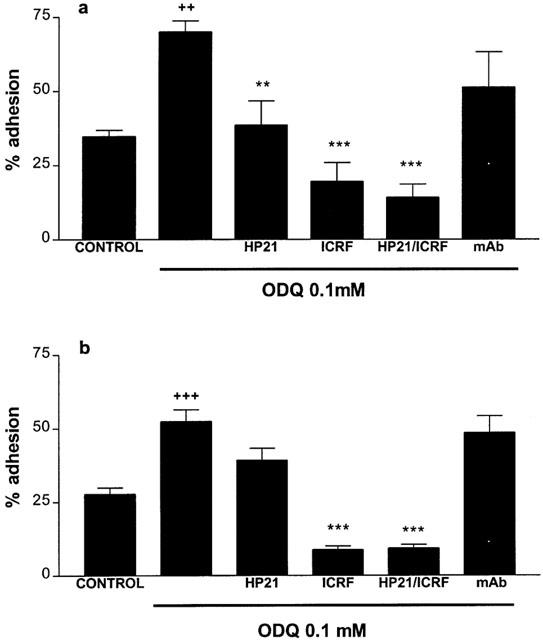
Effects of the anti-VLA-4 (HP2/1, 10 μg ml−1), anti-Mac-1 (ICRF 44, 1 : 12 diln) monoclonal antibodies and a non-specific control monoclonal antibody (mAb, 10 μg ml−1), upon ODQ-treated human eosinophil adhesion to (a) 20 μg ml−1 fibronectin, (b) 10% (v v−1) serum. Eosinophils (>95% pure) were incubated with ODQ/monoclonal antibodies (25 min, 37°C, 5% CO2) before allowing cells to adhere to ligand-coated plates (15 min, 37°C, 5% CO2). Results are expressed as mean adhered cell percentages of total cell number±s.e. of three independent experiments with three replicates in each ++P<0.01, +++P<0.001 compared to untreated cells, **P<0.01, ***P<0.001 compared to cells treated with ODQ alone.
Co-incubation with a non-specific control mAb had no effect upon the adhesion of ODQ-treated cells to either fibronectin or serum.
Effect of L-NAME upon human eosinophil VLA-4 and Mac-1 expression
Expression of VLA-4 on the eosinophil cell surface was determined by fluorescent-immunolabelling cells and detection by flow cytometry.
Untreated and treated cells were incubated with the anti-α4 monoclonal antibody, 44H6 (1 : 80 dilution), before reaction with a FITC-conjugated secondary antibody. Average mean fluorescence bound to each cell was detected by flow cytometry.
Weak expression of VLA4 (α4β1) on the surface of 12.7±2.3% of human eosinophils could be detected using either the HP2/1 or 44H6 α4-specific monoclonal antibodies showing a mean binding of 9.0±1.3 fluorescent units per cell. Treatment of eosinophils with either fMLP (0.05 – 2.00 μM) or L-NAME (0.1 – 2.5 mM) (25 min, 37°C, 5% CO2) did not significantly affect the number of cells expressing VLA-4 or the mean expression of the integrin on each cell. See Table 1.
Table 1.
Effect of L-NAME/fMLP upon α4 and αm expression on human eosinophils (50,000) as determined by flow cytometry
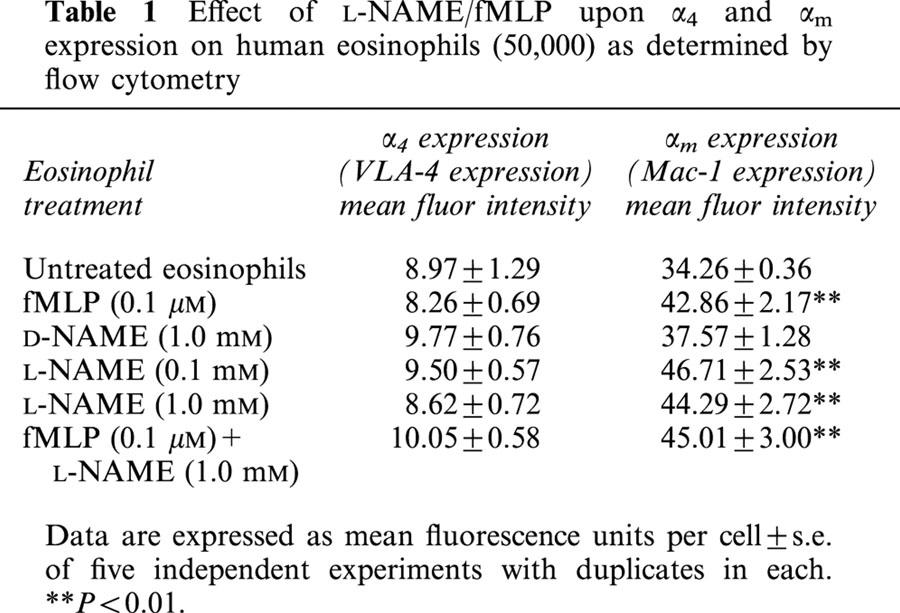
The Mac-1 (αMβ2) integrin was detected on the cell surface of 97.0±0.3% of human eosinophils using the monoclonal anti-αM antibody ICRF 44 in conjunction with flow cytometry.
Treated and untreated cells were incubated with the anti-αM monoclonal antibody, ICRF 44 (1 : 50 dilution), before reaction with a FITC-conjugated secondary antibody and detection of bound fluorescence by flow cytometry. Mean expression of αMβ2 on each eosinophil was significantly increased after treatment of cells with either fMLP (0.1 μM) or L-NAME (0.1 mM, 1.0 mM) (25 min, 37°C, 5% CO2). See Table 1.
Treatment of cells with both fMLP (0.1 μM) and L-NAME (1.0 mM) did not further increase Mac-1 expression. Pre-incubation of cells with D-NAME (1.0 mM) had no significant effect upon αM-integrin expression on eosinophils.
Discussion
Whilst nitric oxide has a well documented role in leukocyte migration, the mechanism by which it exerts its effect is not well understood. fMLP-induced chemotaxis in human neutrophils is thought to result from a rise in cyclic GMP levels after production of NO (Kaplan et al., 1989; Belenky et al., 1993).
Nitric oxide indirectly decreases neutrophil migration by blocking attachment to endothelium possibly by a superoxide-dependent effect involving the ADP-ribosylation of actin (Clancy et al., 1995). Other theories mention a role for interleukin 8 (Corriveau et al., 1998) and NO inhibits neutrophil β2-integrin function (Banick et al., 1997; Thom et al., 1994) and VLA-4 expression (Conran et al., unpublished data). Our findings demonstrate a role for NO in regulating adhesion molecule function and expression on human eosinophils.
Data presented herein demonstrate that the in vitro inhibition of NO expression in human eosinophils significantly increases the capacity of the cell to adhere to fibronectin and serum components. This increase in adhesion, whilst small, can be seen to correlate with results previously demonstrated in rat eosinophils (Ferreira et al., 1996; Zanardo et al., 1997), where L-NAME was observed to inhibit rat eosinophil migration both in vivo and in vitro.
Thus, NO can be seen to have an attenuating role in the expression of Mac-1 on eosinophils and an inhibiting effect on the function of eosinophil VLA-4. Miller et al. (1987) showed that fMLP stimulation of monocytes increases the surface expression of Mac-1 by rapid mobilization of intracellular stores, whilst Molad et al. (1994) demonstrated that when neutrophils are stimulated by such chemoattractants as C5a, IL-8 or fMLP the cell surface expression of Mac-1 is increased via exocytosis. Although we found that fMLP (0.1 μM) increased Mac-1 expression on the eosinophil cell surface, no significant increase in eosinophil cell adhesion was observed following incubation of cells with fMLP (0.01 – 1.0 μM, results not shown). The present study demonstrates that L-NAME induces a rapid increase in eosinophil adhesion and Mac-1 expression after an incubation of just 25 min in a manner similar to that of fMLP, indicating that Mac-1 expression is indeed increased by a rapid mobilization of internal stores of the adhesion molecule. Mac-1 expression may also be affected at gene-transcriptional levels or by post-transcriptional mechanisms. Both the αM (CD11b) and β2 (CD18)-subunits have promoter regions allowing regulation of gene transcription (Hickstein et al., 1992; Agura et al., 1992).
VLA-4-integrin function may be modulated by a change in integrin avidity or affinity. Integrin avidity (i.e. clustering or multimerization of molecules on the plasma membrane) may be modulated by chelate effects, whilst the affinity of an integrin can be affected by structural changes in the integrin protein conformation. Different affinity states for the β1-subunit have been characterized (Faull et al., 1993; Sung et al., 1997), unmasking ligand-binding sites. Since we have not observed changes in VLA-4 expression, the inhibitory effect of NO should occur via modification of VLA-4 conformation. Studies have shown that when integrin affinity is high, migration may be inhibited, thus inappropriate activation of integrins might inhibit leukocyte migration, explaining the suppressive role that NO may have upon integrin activity.
Basal adhesion to fibronectin and serum was not significantly reduced by co-incubation with the anti-VLA 4 and Mac-1 blocking antibodies. These antibodies, though, were able to reduce L-NAME- and ODQ-induced adhesion to levels below those of the untreated cells, in particular the ICRF 44 monoclonal antibody was able to significantly reduce ODQ-induced cell adhesion to serum below levels of basal adhesion (P<0.01, Figure 4). These results may imply that basal adhesion is partially mediated by the VLA-4 and Mac-1 integrins in conjunction with adhesion mediated by other adhesion molecules, such as LFA-1 and non-specific binding mechanisms. It may also be postulated that once the expression or function of these integrins is up-regulated on the cells, their adhesion mechanisms dominate those which might have been utilized before activation and that the subsequent inhibition of these mechanisms by specific mAbs decreases cell adhesion to below basal adhesion levels.
The mechanism by which NO induces these changes in integrin activity and expression is as yet unknown. Exogenous NO, donated by SNP, inhibits eosinophil adhesion to fibronectin and serum and this inhibition is accompanied by an increase in intracellular cyclic GMP levels. ODQ, an inhibitor of soluble guanylate cyclase, also increases eosinophil-cell adhesion to serum and fibronectin via activation of VLA-4 and Mac-1 activity. These results imply that a cyclic GMP-dependent kinase pathway is involved in the mechanism by which NO affects eosinophil adhesion. Cyclic GMP in turn may facilitate a number of reactions; cyclic GMP-dependent protein kinase I, in addition to its ability to mediate cyclic GMP inhibition of IP3-evoked Ca2+ release from intracellular stores, is known to phosphorylate vasodilator stimulated phosphoprotein (VASP) which seems, in turn, to have a modulating effect upon the activity of adhesion molecules such as GPIIb/IIIa found on human platelets (Lohmann et al., 1997; Horstrup et al., 1994).
In conclusion, we demonstrate here that the role of NO as a regulator of eosinophil migration may be mediated through its attenuating effect (via a cyclic GMP-dependent mechanism) upon the ability of the human eosinophil to adhere to the extracellular matrix by altering the expression and function of the adhesion molecules on the eosinophil surface, in particular, those of the Mac-1 and VLA-4 adhesion molecules.
Acknowledgments
We thank Fernanda Pereira, Elisângela Ribeiro and Mônia Lodo for their technical help. This work was supported by FAPESP, Brazil.
Abbreviations
- BSA
bovine serum albumin
- cyclic GMP
cyclic guanosine 3′5′-monophosphate
- ECM
extracellular matrix
- fMLP
N-formyl-methionyl-leucyl-phenylalanine
- GTP
guanosine 5′ triphosphate
- IBMX
3-isobutyl-l-methylxanthine
- L-NAME
Nω-nitro-L-arginine methyl ester
- mAb
monoclonal antibody
- ODQ
H-[1,2,4]-oxadiazolo quinoxalin-1-one
- SNP
sodium nitroprusside
References
- AGURA E.D., HOWARD M., COLLINS S.J. Identification and sequence analysis of the promoter for the integrin β-subunit (CD18): A retonoic acid-inducible gene. Blood. 1992;79:602–609. [PubMed] [Google Scholar]
- ALON R., KASSNER P.P., CARR M.W., FINGER E.B., HEMLER M.E., SPRINGER T.A. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J. Cell. Biol. 1995;128:1243–1253. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANICK P.D., CHEN Q., ANNE Xu Y., THOM S.R. Nitric oxide inhibits neutrophil β2 integrin function by inhibiting membrane-associated cyclic GMP synthesis. J. Cell. Physiol. 1997;172:12–24. doi: 10.1002/(SICI)1097-4652(199707)172:1<12::AID-JCP2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- BELENKY S.N., ROBBINS R.A., RENNARD S.I., GOSSMAN G.L., NELSON K.J., RUBINSTEIN I. Inhibitors of nitric oxide attenuate neutrophil chemotaxis in vitro. J. Lab. Clin. Med. 1993;122:388–394. [PubMed] [Google Scholar]
- CLANCY R., LESZCZYNSKA J., AMIN A., LEVARTOVSKY D., ABRAMSON S.B. Nitric oxide stimulates ADP ribosylation of actin in association with the inhibition of actin polymerization in human neutrophils. J. Leukoc. Biol. 1995;58:196–202. doi: 10.1002/jlb.58.2.196. [DOI] [PubMed] [Google Scholar]
- CLARK E.A., BRUGGE J.S. Integrins and signal transduction pathways. The road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- CONRAN N., FERREIRA H.H.A., ANTUNES E., DE NUCCI G. Inhibition of fMLP-stimulate eosinophil in vitro chemotaxis by Nω-nitro-L-arginine methyl. ester. Br. J. Pharmacol. 1998;125:30P. [Google Scholar]
- CORRIVEAU C.C., MADARA P.J., VAN DERVORT A.L., TROPEA M.M., WESLEY R.A., DANNER R.L. Effects of nitric oxide on chemotaxis and endotoxin-induced interleukin-8 production in human neutrophils. J. Infect. Dis. 1998;177:116–126. doi: 10.1086/513829. [DOI] [PubMed] [Google Scholar]
- DE CATERINA R., LIBBY P., PENG H.-B., THANNICKAL V.J., RAJAVASHISTH T.B., GIMBRONE M.A., JR, SHIN W.S., LIAO J.K. Nitric oxide decreases cytokine-induced endothelial activation. J. Clin. Invest. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND M.S., SPRINGER T.A. The dynamic regulation of integrin adhesiveness. Curr. Biol. 1994;4:506–517. doi: 10.1016/s0960-9822(00)00111-1. [DOI] [PubMed] [Google Scholar]
- FAULL R.J., KOVACH N.L., HARLAN J.M., GINSBERG M.H. Affinity modulation of integrin α5β1 regulation of the functional response by soluble fibronectin. J. Cell Biol. 1993;121:155–162. doi: 10.1083/jcb.121.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERREIRA H.H.A., MEDEIROS M.V., LIMA C.S.P., FLORES C.A., SANNOMIYA P., ANTUNES E., DE NUCCI G. Inhibition of eosinophil chemotaxis by chronic blockade of nitric oxide biosynthesis. Eu. J. Pharmacol. 1996;310:201–207. doi: 10.1016/0014-2999(96)00379-2. [DOI] [PubMed] [Google Scholar]
- HANSEL T.T., DE VRIES J.M., IFF T., RIHS S., WANDZILAK M., BETZ S., BLASER K., WALKER C. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J. Immun. Meth. 1991;145:105–110. doi: 10.1016/0022-1759(91)90315-7. [DOI] [PubMed] [Google Scholar]
- HICKSTEIN D.D., BAKER D.M., GOLLAHON K.A., BACK A.L. Identification of the promoter of the myelomonocytic leukocyte integrin CD11b. Proc. Natl. Acad. Sci. U.S.A. 1992;89:2105–2109. doi: 10.1073/pnas.89.6.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGASHIMOTO I., CHIHARA J., KAKAZU T., KAWABATA M., NAKAJIMA S., OSAME M. Regulation of eosinophil cell death by adhesion to fibronectin. Int. Arch. Allergy Immunol. 1996;111 Suppl 1:66–69. doi: 10.1159/000237420. [DOI] [PubMed] [Google Scholar]
- HORIE S., KITA H. CD11b/CD18 (Mac-1) is required for degranulation of human eosinophils induced by human recombinant granulocyte-macrophage colony-stimulating factor and platelet activating factor. J. Immunol. 1994;152:5457–5467. [PubMed] [Google Scholar]
- HORSTRUP K., JABLONKA B., HONIG-LIEDL P., JUST M., HOCHSIEK K., WALTER U. Phosphorylation of focal adhesion vasodilator-stimulated phosphoprotein at Ser157 in intact human platelets correlates with fibrinogen receptor inhibition. Eur. J. Biochem. 1994;225:21–27. doi: 10.1111/j.1432-1033.1994.00021.x. [DOI] [PubMed] [Google Scholar]
- ISSEKUTZ A.C., ISSEKUTZ T.B. The contribution of LFA-1 (CD11a/CD18) and MAC-1 (CD11b/CD18) to be in vivo migration of PMN leukocytes to inflammatory reactions in the rat. Immunology. 1992;76:655–661. [PMC free article] [PubMed] [Google Scholar]
- KAPLAN S.S., BILLIAR T., CURRAN R.D., ZDZIARSKI U.E., SIMMONS R.L., BASFORD R.E. Inhibition of chemotaxis with NG-monomethyl-L-arginine: A role for cyclic GMP. Blood. 1989;74:1885–1887. [PubMed] [Google Scholar]
- KATO M., KITA H., TOKUYAMA K., MORIKAWA A. Cross-linking of the β2-integrin, CD11b/CD18 on human eosinophils induces protein tyrosine phosphorylation and cellular granulation. Int. Arch. Allergy Immunol. 1998;117 Supp 1:68–71. doi: 10.1159/000053576. [DOI] [PubMed] [Google Scholar]
- KHAN B.V., HARRISON D.G., OLBRYCH M.T., ALEXANDER R.W. Nitric oxide regulates VCAM-1 gene expression and redox-sensitive transcriptional events in human vascular endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9114–9119. doi: 10.1073/pnas.93.17.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUBES P., SUZUKI M., GRANGER D.N. Nitric oxide: An endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. U.S.A. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOHMANN S.M., VAANDRAGER A.B., SMOLENSKI A., WALTER U., DE JONGE H.R. Distinct and specific functions of cGMP-dependent protein kinases. Trends Biochem. Sci. 1997;22:307–312. doi: 10.1016/s0968-0004(97)01086-4. [DOI] [PubMed] [Google Scholar]
- MILLER L.J., BAINTON D.J., BORREGARD N., SPRINGER T.A. Stimulated mobilization of monocyte Mac-1 and p150,95 adhesion proteins from an intracellular vesicular compartment to the cell surface. J. Clin. Invest. 1987;80:535–544. doi: 10.1172/JCI113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOILANEN E., VUORINEN H., KANKAANRANTA H., METSA-KETELA T., VAPAATALO H. Inhibition by nitric oxide-donors of human PMN leucocyte functions. Br. J. Pharmacol. 1993;109:852–858. doi: 10.1111/j.1476-5381.1993.tb13653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLAD Y., HAINES K.A., ANDERSON D.C., BUYON J.P., CRONSTIEN B.N. Immunocomplexes stimulate different signalling events to chemoattractants in the neutrophil and regulate L-selectin and β2-integrin expression differently. Biochem. J. 1994;299:881–887. doi: 10.1042/bj2990881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE P.K., OLUYOMI A.O., BABBEDGE R.C., WALLACE P., HART S.L. L-NG-nitro arginine methyl ester inhibits antinociceptive activity in the mouse. Br. J. Pharmacol. 1991;102:198–202. doi: 10.1111/j.1476-5381.1991.tb12153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGATA M., SEDGWICK J.B., BATES M.E., KITA H., BUSSE W.W. Eosinophil adhesion to vascular cell adhesion molecule-1 activates superoxide anion generation. J. Immunol. 1995;155:2194–2202. [PubMed] [Google Scholar]
- NIU X.-F., SMITH W., KUBES P. Intracellular oxidative stress induced by nitric oxide synthesis inhibition increases endothelial cell adhesion to neutrophils. Circ. Res. 1994;74:1133–1140. doi: 10.1161/01.res.74.6.1133. [DOI] [PubMed] [Google Scholar]
- PRADELLES P., GRASSI J. Enzyme immnoassays of adenosine cyclic 3′, 5′-monophosphate and guanosine cyclic 3′, 5′-monophosphate using acetylcholinesterase. Anal. Chem. 1989;61:447–453. doi: 10.1021/ac00180a014. [DOI] [PubMed] [Google Scholar]
- SENDO F., SUZUKI K., WATANABE T., TAKEDA Y., ARAKI Y. Modulation of leukocyte transendothelial migration by integrin-associated glycosyl phosphatidyl inositol (GPI)-anchored proteins. Inflamm. Res. 1998;47:S133–S136. doi: 10.1007/s000110050302. [DOI] [PubMed] [Google Scholar]
- SUNG P.K.-L., YANG L., ELICES M., JIN G., SRIRAMARAO P., BROIDE D.H. Granulocyte-macrophage colony-stimulating factor regulates the functional adhesive state of VLA-4 expressed by eosinophils. J. Immunol. 1997;158:919–927. [PubMed] [Google Scholar]
- THOM S.R., OHNISHI S.T., ISCHIROPOULOS H. Nitric oxide released by platelets inhibits neutrophil β2 integrin function following acute carbon monoxide poisoning. Toxicol. Appl. Pharmacol. 1994;128:105–110. doi: 10.1006/taap.1994.1186. [DOI] [PubMed] [Google Scholar]
- WANIKIAT P., WOODWARD D.F., ARMSTRONG R.A. Investigation into the role of nitric oxide and cGMP in both the activation and inhibition of human neutrophils. Br. J. Pharmacol. 1997;122:1135–1145. doi: 10.1038/sj.bjp.0701477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZANARDO R.C.O., COSTA E., FERREIRA H.H.A., ANTUNES E., MARTINS A.R., MURAD F., DE NUCCI G. Pharmacological and immunological histochemical evidence for a functional nitric oxide synthase system in rat peritoneal eosinophils. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14111–14114. doi: 10.1073/pnas.94.25.14111. [DOI] [PMC free article] [PubMed] [Google Scholar]


