Abstract
We tested the hypothesis that sensory nerves innervating blood vessels play a role in the local and systemic regulation of the cardiovascular and respiratory (CVR) systems. We measured CVR reflexes evoked by administration of anandamide (86 – 863 nmoles) and capsaicin (0.3 – 10 nmoles) into the hindlimb vasculature of anaesthetized rats.
Anandamide and capsaicin each caused a rapid dose-dependent reflex fall in blood pressure and an increase in ventilation when injected intra-arterially into the hindlimb.
Action of both agonists at the vanilloid receptor (VR1) on perivascular sensory nerves was investigated using capsazepine (1 mg kg−1 i.a.) a competitive VR1 antagonist, ruthenium red (1 mg kg−1 i.a.), a non-competitive antagonist at VR1, or a desensitizing dose of capsaicin (200 nmoles i.a.). The cannabinoid receptor antagonist SR141716 (1 mg kg−1 i.a.) was used to determine agonist activity at the CB1 receptor.
Capsazepine, ruthenium red, or acute VR1 desensitization by capsaicin-pretreatment, markedly attenuated the reflex CVR responses evoked by anandamide and capsaicin (P<0.05; paired Student's t-test). Blockade of CB1 had no significant effect on the responses to anandamide.
Local sectioning of the femoral and sciatic nerves attenuated CVR responses to anandamide and capsaicin (P<0.05). Vagotomy or carotid sinus sectioning had no significant effect on anandamide- or capsaicin-induced responses.
These data demonstrate that both the endogenous cannabinoid, anandamide, and the vanilloid, capsaicin, evoke CVR reflexes when injected intra-arterially into the rat hindlimb. These responses appear to be mediated reflexly via VR1 located on sensory nerve endings within the hindlimb vasculature.
Keywords: Anandamide, capsaicin, sensory nerves, cardiovascular-respiratory reflexes, vanilloid receptor
Introduction
Arteries and veins receive a rich sensory innervation (Woollard, 1927; Coleridge & Coleridge, 1980), mainly comprised of fine peptidergic nerves (Lundberg et al., 1991), but their precise function has yet to be identified. Evidence suggests these nerves are nociceptive. Pain is commonly experienced during the puncture of blood vessels (Bazett & McGlone, 1928), insertion of catheters (Langham & Harrison, 1992) or on intravascular injection of anaesthetic drugs such as propofol (Tan & Onsiong, 1998). Sensory nerves innervating blood vessels have been implicated in the pain associated with angina, embolism, myocardial infarction, and migraine (Lewis, 1935; Malliani et al., 1981; Moskowitz, 1990). Vasosensory nerves might be silent nociceptors that may never be activated during a lifetime, but we hypothesize that they also play a physiological role in the local and reflex regulation of the cardiovascular and respiratory (CVR) systems. Whilst the sensory function of specialized arterial baro- and chemo-sensory afferents in cardiovascular regulation has been established (Heymans & Neil, 1958; Shepherd, 1981; Marshall, 1994; Hainsworth, 1995), that of other afferents remains uncertain, particularly sensors associated with peripheral blood vessels.
Some reports have suggested that vasosensory nerves may be involved in reflex regulation of the cardiovascular system. For example, Donnerer & Lembeck (1982; 1983) demonstrated that administration of capsaicin into the jugular vein or femoral artery evokes a reflex depressor response in the Sprague-Dawley rat. However, strain differences in the reflex evoked have been reported: Makara et al. (1967) observed a decrease in heart rate and apnoea in Wistar rats with intravenously administered capsaicin. The role of vascular sensory nerves in these studies is not clear, because the heart rate and respiratory effects were abolished after bilateral vagotomy (Makara et al., 1967), suggesting involvement of cardiopulmonary receptors. Previous studies in our laboratory have demonstrated similar vagally-mediated cardiorespiratory reflexes to intravenously administered αβ-MeATP (McQueen et al., 1998). However, in these studies a component of the response – hyperventilation and a pressor effect – remained after vagotomy, suggesting additional sensory nerves are responsible for some of the reflex responses to αβ-MeATP (McQueen et al., 1998). In humans, intravascular injection of the anaesthetic agent propofol is associated with significant falls in blood pressure (Tan & Onsiong, 1998) but it is not known whether this is a primary reflex mediated via vasosensory nerves, a secondary effect to the pain invoked, or to direct actions on blood vessels.
To test the CVR regulation hypothesis, we performed functional experiments on anaesthetized Wistar rats. General anaesthesia eliminates pain sensation without blocking reflexes, thereby avoiding problems associated with interpreting studies on conscious animals. We measured reflex cardiovascular and respiratory changes evoked by local stimulation of vascular sensory nerves in the hindlimb, using pharmacological stimuli known to activate vasosensors, namely capsaicin and anandamide. Capsaicin, the pungent ingredient in chilli peppers, is a selective activator of thinly or unmyelinated nociceptive afferents (Bevan & Szolcsanyi, 1990) and its effects are mediated through the vanilloid receptor (VR1) (Caterina et al., 1997). Anandamide is one of several endogenous cannabinoid (CB) receptor ligands that have been isolated (Devane et al., 1992). In addition to its agonist action at CB receptors, anandamide induces vasodilatation by activating VR1 on perivascular sensory nerves and causing release of calcitonin-gene-related peptide (CGRP) (Zygmunt et al., 1999). We characterized the type of receptor mediating anandamide-induced CVR reflexes by examining the effects of antagonists selective for CB1 and VR1 receptors.
Methods
Surgical procedures
Experiments were licensed under U.K. Home Office regulations (Project Licence No. PPL 60/2379). Male Wistar rats (body weight range 280 – 480 g; mean±s.e.mean 334±8 g, n=43) were anaesthetized with either urethane (25% w v−1 i.p.) or, usually, sodium pentobarbitone (60 mg kg−1 i.p., maintained with a 6 mg h−1 i.v. bolus via a cannula inserted into the right femoral vein). The trachea was cannulated and connected to a pneumotachograph head linked to an electrospirometer (AD Instruments) and a computerized recording system (MacLab 4/S and Macintosh Performa 6200) for measuring and recording tracheal airflow, and respiratory minute volume (RMV). Animals breathed room air spontaneously.
The right carotid and femoral artery were cannulated for measurement of mean arterial pressure (MAP), via a blood pressure transducer (AD Instruments) linked to the MacLab, and drug administration, respectively. The femoral artery catheter was advanced into the abdominal aorta to allow close i.a. administration of drugs into the left hindlimb. Body temperature was maintained at 38±1°C by a heating blanket (Harvard) connected to a thermistor probe inserted into the rectum. Blood samples (0.2 ml) were taken approximately every hour from the carotid artery to monitor blood gas tensions and pH (Ciba Corning 238 analyser) (Mean pH was 7.38±0.01, mean PaCO2 was 35.8±1.1 mmHg and mean PaO2 was 88.8±3.2 mmHg). Rats were killed at the end of each experiment by an overdose of anaesthetic.
Drug administration
Agonists (anandamide, capsaicin, and α,β Me-ATP) were injected intra-arterially (i.a.) in a volume of 0.1 ml, washed in with 0.2 ml saline (0.9% w v−1 sodium chloride), within a 2 s period. At the start of every experiment 0.3 ml saline was injected i.a. as a control. The minimum interval between successive doses of agonist was 20 min to prevent receptor desensitization. We have found this interval is adequate to allow reproducible responses to be obtained with repeat administration of anandamide and capsaicin. Antagonists (at VR1: ruthenium red, capsazepine, capsaicin (desensitizing dose); and at CB1: SR141716) were slowly injected i.a. over 10 s (0.1 ml 100 g−1 body weight). Exposure time to the antagonists was 3 min, except for capsazepine, which was 5 – 10 s, owing to its short duration of action.
Nerve sectioning
To demonstrate the activation of primary vasosensory afferents, the left femoral and sciatic nerves were denervated in 10 experiments. Bilateral vagotomy and carotid sinus denervation were performed in order to exclude the potential involvement of vagal (cardiopulmonary receptors) and carotid sinus (chemoreceptor) afferents in the reflex responses. The vagi were sectioned at the mid-cervical level in six experiments. The carotid sinus nerves (left and right side) were identified at their junction with the glossopharyngeal (IX cranial) trunk and both the left and right nerves were sectioned in four experiments. Denervation was confirmed by the abolition of the reflex hyperventilation evoked prior to sectioning by the peripheral chemoreceptor stimulant sodium cyanide (2 μmol i.v.).
Electrophysiology
The femoral nerve innervating the left hindlimb was sectioned and extracellular recordings of afferent activity were made from the distal end using a suction electrode (tip I.D. ∼200 μm) during administration of anandamide (575 nmoles, i.a.) and capsaicin (3 nmoles, i.a.). Signals were amplified and filtered (Neurolog, NL104, NL125) and recorded digitally on videotape before analysing off-line using a pulse-height voltage discriminator (Digitimer D130) linked to a personal computer operating Spike2 software (Cambridge Electronic Design).
Drugs
α,βMe-ATP, capsaicin, capsazepine, and ruthenium red (ammoniated ruthenium oxychloride) were purchased from Sigma (U.K.) and anandamide was purchased from Tocris. SR141716 (N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide) was a gift from Sanofi Recherche (Montpellier, France). Drugs were dissolved in saline, except for: anandamide, which was dissolved in a soya oil/water (1 : 4) emulsion; capsaicin, dissolved in Tween 80 (10% v v−1), ethanol (10% v v−1) and saline to make a stock solution of 1 mg ml−1; capsazepine, dissolved in 20% cremaphore el (Sigma, U.K.) in distilled water to make a stock solution of 10 mg ml−1; and SR141716, dissolved in Tween 80 (10% v v−1), DMSO (10% v v−1) and distilled water to make a stock solution of 10 mg ml−1. All drugs were diluted in 0.9% w v−1 sodium chloride solution (saline) for injection.
Data analysis
The effect of a drug or vehicle injection was determined by comparing blood pressure and respiration in the 1 – 10 s period immediately following drug injection with basal values in the 10 s pre-injection period (mean delay from injection to onset of reflex response evoked by 3 nmoles capsaicin was 2.8±0.2 s, n=30). Data are expressed as the mean change in MAP and RMV±s.e.mean, and n is the number of animals studied. Receptor desensitization to capsaicin and VR1 agonists occurs, so the number and maximum dose of drug injected was minimized. As full log dose-response curves could not be constructed for capsaicin, the maximum changes in the parameters measured in response to a submaximal dose of 3 nmoles (Figure 2) were measured, before and after administration of an antagonist. Maximal responses to anandamide were not obtained in every experiment. The CVR changes induced by a submaximal dose of 575 nmoles (Figure 2) of anandamide before and after antagonist are presented. A paired Student's t-test was used to compare values before and after antagonist treatments or nerve sectioning. The Null hypothesis was rejected at P<0.05.
Figure 2.
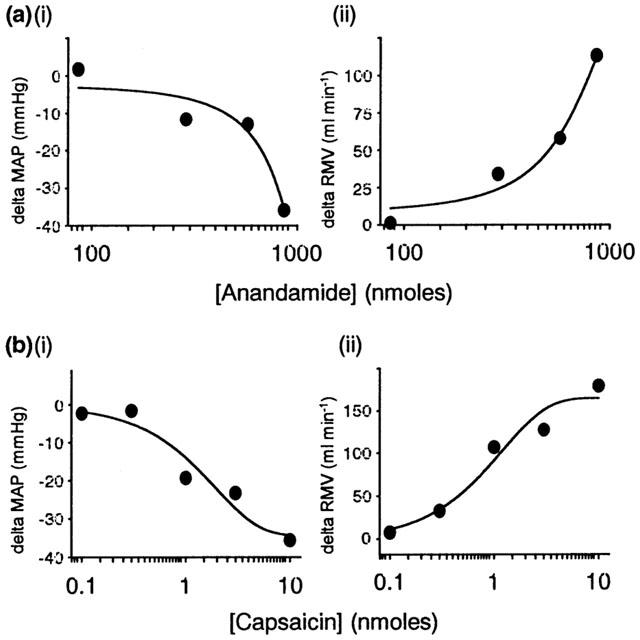
Individual log dose-response curves to (a) anandamide (86 – 863 nmoles i.a.) and (b) capsaicin (0.03 – 10 nmoles i.a.) in the anaesthetized rat, expressed as (i) change in mean arterial pressure (MAP, mmHg) and (ii) respiratory minute volume (RMV, ml min−1).
Results
Anandamide and capsaicin each caused a reflex fall in MAP and an increase in ventilation (RMV) when injected intra-arterially into the hindlimb (Figure 1), although anandamide only induced the depressor component in ∼70% of animals studied. The responses evoked by each agonist were dose-dependent (Figure 2). Similar results were obtained under both pentobarbitone and urethane anaesthesia and so data were pooled. Injection of saline vehicle had no effect on blood pressure or ventilation (MAP=113±3 mmHg versus 113±3 mmHg, before and after saline respectively; P=0.80, and RMV=145.9±10.8 ml min−1 versus 146.3±10.9 ml min−1, before and after saline respectively; P=0.69, n=43). Basal cardiovascular and respiratory parameters did not change significantly following antagonist treatment or denervations.
Figure 1.
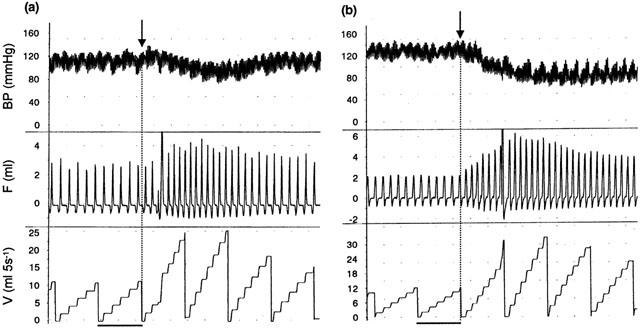
Representative traces showing the effects of (a) anandamide (575 nmoles i.a.) and (b) capsaicin (3 nmoles i.a.) on blood pressure (mmHg, top trace), respiratory volume (ml, middle trace) and respiratory frequency-volume (number of breaths (ml) per 5 s ramp, bottom trace) in rats under pentobarbitone anaesthesia. Arrows indicate time of injection, i.e. at the start of a new ramp. Scale bar=5 s.
Effects of VR1 blockade
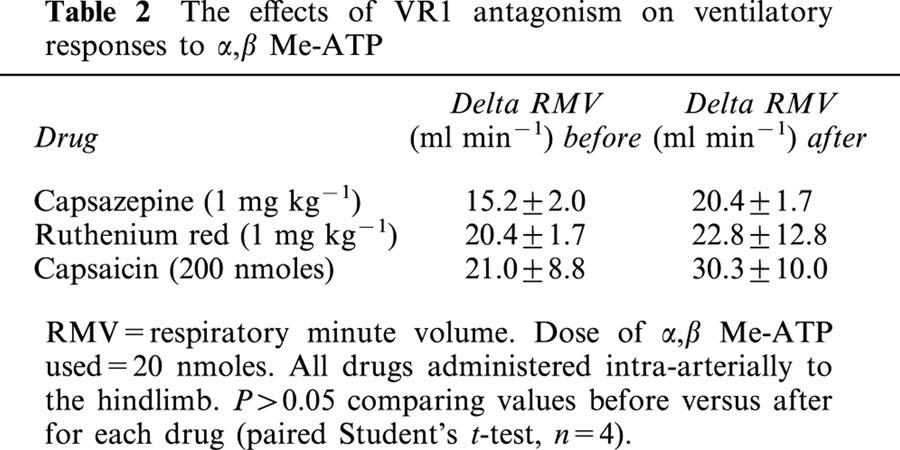
The non-competitive VR1 antagonist ruthenium red (1 mg kg−1) significantly attenuated the CVR reflexes evoked by anandamide and capsaicin (Figures 3 and 4). Similar results were obtained using capsazepine (1 mg kg−1), a competitive antagonist at VR1 receptors (Figure 3), although its inhibitory effects on the ventilatory responses to either anandamide or capsaicin failed to reach statistical significance (Figure 4). Pretreatment with a desensitizing dose of capsaicin (200 nmoles) substantially reduced the reflex depressor response and hyperventilation evoked by anandamide and capsaicin (Figures 3 and 4). The effects of ruthenium red, capsazepine and capsaicin-pretreatment were specific for agonists at the VR1 receptor; pressor responses and hyperventilation to α,β Me-ATP were not significantly changed following antagonist administration (Tables 1 and 2).
Figure 3.
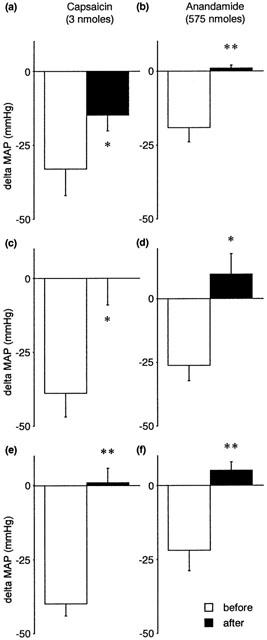
The effects of VR1 antagonism on capsaicin-induced (left panels) and anandamide-induced (right panels) changes in mean arterial pressure (MAP, mmHg) in the anaesthetized rat. Values are shown as mean±s.e.mean before and after (a, b) ruthenium red (1 mg kg−1 i.a.) (n=4), (c, d) capsazepine (1 mg kg−1 i.a.) (n=4), and (e, f) capsaicin pre-treatment (200 nmoles i.a.) (n=5 – 6). *P<0.05, **P<0.01 vs before antagonist; paired Student's t-test.
Figure 4.
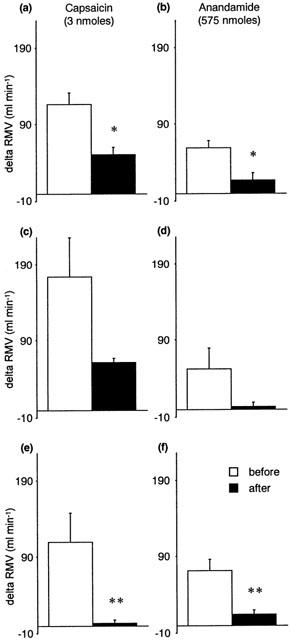
The effects of VR1 antagonism on capsaicin-induced (left panels) and anandamide-induced (right panels) changes in respiratory minute volume (RMV, ml min−1) in the anaesthetized rat. Values are shown as mean±s.e.mean before and after (a, b) ruthenium red (1 mg kg−1 i.a.) (n=4), (c, d) capsazepine (1 mg kg−1 i.a.) (n=4), and (e, f) capsaicin pre-treatment (200 nmoles i.a.) (n=5 – 6). *P<0.05, **P<0.01 vs before antagonist; paired Student's t-test.
Table 1.
The effects of VR1 antagonism on blood pressure responses to α,β Me-ATP
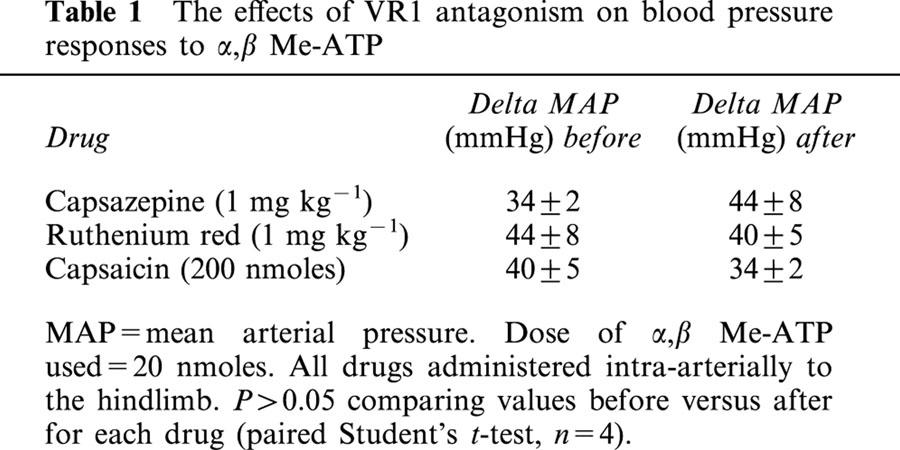
Table 2.
The effects of VR1 antagonism on ventilatory responses to α,β Me-ATP
Effects of CB1 receptor antagonist
CVR reflexes to anandamide were not significantly affected by the CB1 receptor antagonist, SR141716 (1 mg kg−1 i.a.) (delta MAP: −28±12 mmHg versus −28±13 mmHg, before and after SR141716 respectively; P=0.87, and delta RMV: 88±20 ml min−1 versus 73±6 ml min−1, before and after SR141716 respectively; P=0.31, for a 575 nmole dose of anandamide; n=3).
Effects of nerve sectioning
Figure 5 shows that sectioning of the ipsilateral femoral and sciatic nerves attenuated the CVR responses evoked by anandamide and capsaicin injected locally into the hindlimb. Denervation of carotid sinuses or cardiopulmonary receptors by bilateral cervical vagotomy did not prevent the reflex responses to anandamide (Figures 6 and 7). Sectioning the carotid sinus nerves tended to reduce the fall in blood pressure induced by capsaicin (P=0.05, paired Student's t-test) (Figure 6), suggesting that part of this response is mediated by chemosensory afferents. Vagotomy had no significant effect on the CVR reflexes induced by capsaicin (Figure 7).
Figure 5.
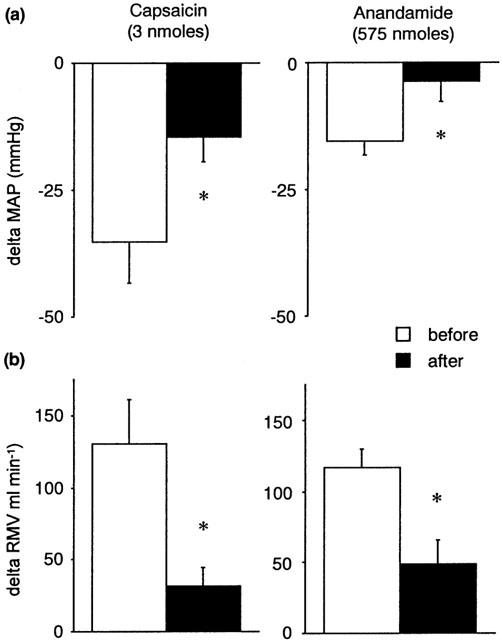
The effects of ipsilateral sectioning of the femoral and sciatic nerves on capsaicin-induced (left panels) and anandamide-induced (right panels) changes in (a) mean arterial pressure (MAP, mmHg) and (b) respiratory minute volume (RMV, ml min−1) in the anaesthetized rat. Values shown as mean±s.e.mean (n=5). *P<0.05 vs before denervation; paired Student's t-test.
Figure 6.
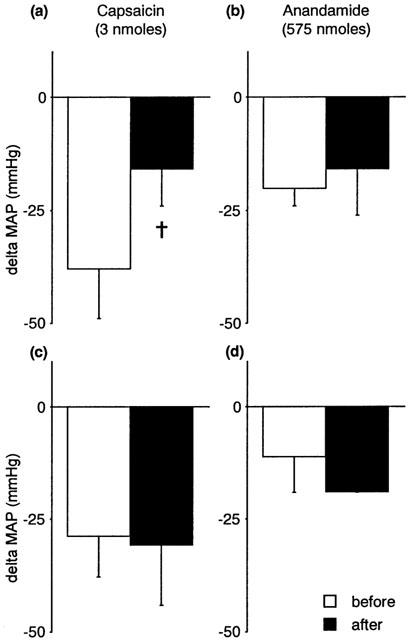
The effects of sectioning the carotid sinus nerves (a, b; n=4) or the vagus nerves (c, d; n=4) on capsaicin-induced (left panels) and anandamide-induced (right panels) changes in mean arterial pressure (MAP, mmHg) in the anaesthetized rat. Values are shown as mean±s.e.mean. †P=0.05 vs before denervation; paired Student's t-test.
Figure 7.
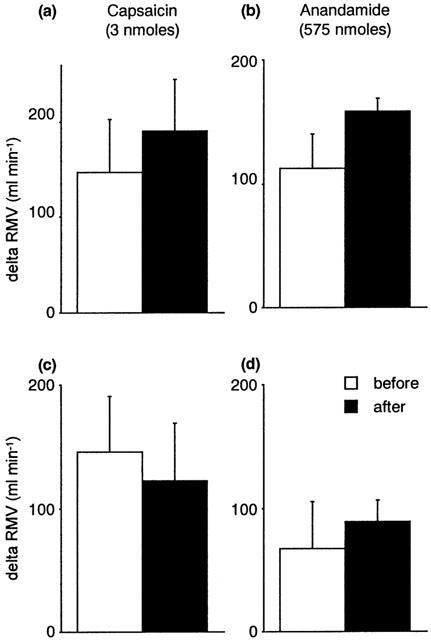
The effects of sectioning the carotid sinus nerves (a, b; n=4) or the vagus nerves (c, d; n=4) on capsaicin-induced (left panels) and anandamide-induced (right panels) changes in respiratory minute volume (RMV, ml min−1) in the anaesthetized rat. Values are shown as mean±s.e.mean.
Neural recordings
The recordings of femoral afferent activity during administration of anandamide and capsaicin reveals that changes in discharge correlate with the reflexes evoked by sensory stimuli (Figure 8). Because the femoral nerve was sectioned in order to record afferent activity from the distal end, there is an attenuated depressor and ventilatory response usually observed with administration of the agonists.
Figure 8.
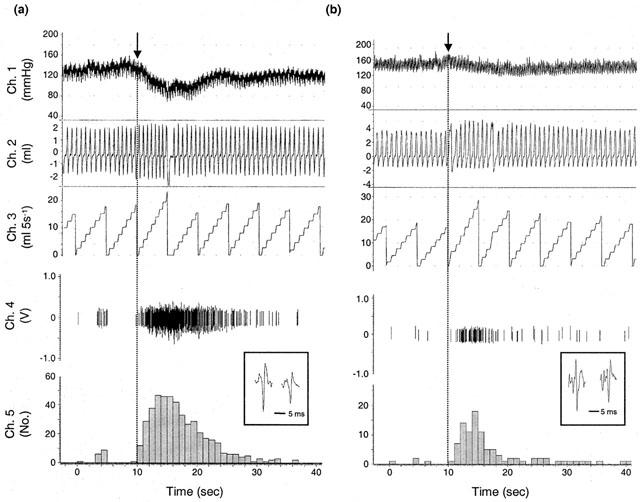
Evoked discharge recorded from the distal end of a sectioned femoral nerve, together with cardiorespiratory measurements, following injection of (a) capsaicin (3 nmoles, i.a.) and (b) anandamide (575 nmoles, i.a.). Delay to increased firing <1.0 s. Channel 4 (volts) shows the action potentials recorded from the afferent fibres (inset=individual units), and channel 5 (impulses) represents pooled discharge of the individual units recorded. Upper traces show mean arterial pressure (mmHg, channel 1), respiratory volume (ml, channel 2) and respiratory frequency-volume (no. of breaths (ml) per 5 s ramp, channel 3).
Discussion
Our findings support the hypothesis being tested, namely that vasosensory nerves have a physiological regulatory role in the CVR systems. A rapid, dose dependent fall in blood pressure and an increase in ventilation were observed after intra-arterial administration of either anandamide or capsaicin into the hindlimb of anaesthetized rats. We propose that primary afferents from the hindlimb vasculature mediate the reflex depressor response and hyperventilation, and suggest that the sensory nerve terminals are located in the adventitia/peri-arterial space, with a significant proportion of their fibres running in the femoral and sciatic nerves. Consistent with this, sectioning of the femoral and sciatic nerves markedly attenuated the CVR reflexes to anandamide and capsaicin. Furthermore, the responses were found to persist following denervation of the carotid sinus and vagus. We have also provided evidence from neural recordings, which showed that increase in afferent discharge correlates with the CVR responses evoked by anandamide and capsaicin when administered to the femoral artery. This further supports a reflex generated via vasosensory nerves. We cannot rule out the possibility that a proportion of the response is mediated via extra-vascular afferents e.g. those found in surrounding skeletal muscle (Tallarida et al., 1979). Recording neural activity directly from the surface of blood vessels would help to ascertain the location of sensory nerves and such studies are ongoing in our laboratory.
Kaczyñska & Szereda-Przestaszewska (2000) observed that apnoea preceded the hyperventilatory response when capsaicin, at a dose similar to that used in the present study, was administered via the femoral vein in anaesthetized Wistar rats. The difference from our findings reflects the different routes of drug administration. When we injected capsaicin intravenously via the femoral or jugular vein, we occasionally observed an initial apnoea (lasting between 5 – 10 s) before the hyperventilation that is typical of intra-arterial injection (Smith & McQueen, unpublished observations).
Several theories have been advanced to explain the depressor action of intravascularly administered capsaicin. In a series of experiments on rats, Donnerer & Lembeck (1983) demonstrated that the reflex depressor response to capsaicin is relayed within the brainstem and involves substance P-containing primary afferent fibres. This could result from a direct effect of capsaicin on either the brainstem reflex centre (capsaicin can easily cross the blood-brain barrier – Saria et al., 1982), or to an action on carotid sinus chemo- and/or baro-receptors. In our experiments sectioning of the carotid sinus nerves partly reduced the fall in blood pressure induced by capsaicin, suggesting the depressor response to this vanilloid partially involves carotid sensors, as has been reported by others in dogs (Brender & Webb-Peploe, 1969). From the rapid onset of observed responses (<2 s), we propose that capsaicin can also mediate its depressor response through activation of local primary vascular afferents, which in turn activate the brainstem to cause a reflex fall in blood pressure.
Anandamide is a non-selective, endogenous agonist for cannabinoid receptors (CB1 and CB2). CB1 receptors are located mainly on central and peripheral neurones (Matsuda et al., 1990), and CB2 receptors are expressed predominantly by immune cells (Munro et al., 1993). Anandamide has also been shown to act through VR1 receptors in various preparations, including HEK293 cells transfected with human vanilloid receptors (Smart et al., 2000). Zygmunt et al. (1999) reported that anandamide causes vasodilation in isolated arteries by activating VR1 on perivascular sensory nerves which trigger the release of calcitonin-gene-related peptide (CGRP). They confirmed the presence of nerve fibres within the adventitia of arteries using immunohistochemical techniques, which is in accord with our hypothesis. We showed that anandamide evokes depressor and hyperventilatory responses by acting on VR1 on sensory nerves, as evidenced by blockade of reflexes following administration of capsazepine – a competitive VR1 antagonist, or ruthenium red – a non-competitive antagonist at VR1, or acute desensitization with capsaicin. Anandamide was significantly less potent than capsaicin in activating VR1, consistent with the findings of others who report differences of one to two orders of magnitude between these agonists (Zygmunt et al., 1999; Smart et al., 2000; Tucker et al., 2001). CB1 receptors do not appear to play a role in mediating the observed responses because the antagonist SR141716 had no significant effect on anandamide-induced reflexes. Previous studies have demonstrated CB1-mediated hypotensive effects of anandamide when administered intra-venously to anaesthetized rats (Lake et al., 1997), resulting from a pre-synaptic inhibition of noradrenaline release from sympathetic nerve terminals (Varga et al., 1996). These results perhaps differ from ours due to the route of administration of anandamide. Whilst CB1 and VR1 receptors have been shown to be co-expressed in nociceptive primary sensory neurons (Ahluwalia et al., 2000), it is possible that regional differences in receptor expression exist between arteries and veins. Perhaps arterial vasosensory nerves express a greater proportion of VR1 relative to CB1, compared to the venous neurons. In support of this notion, it has been demonstrated that anandamide-induced vasodilatation of the mesenteric arterial bed is mediated by VR1, not CB1 (Zygmunt et al., 1999; Ralevic et al., 2000).
Like capsaicin, the anandamide site of action on VR1 is intracellular (de petrocellis et al., 2001). Anandamide is rapidly hydrolysed in vivo to arachidonic acid and ethanolamine by the intracellular enzyme fatty acid amide hydrolase (FAAH) (see Pertwee, 2000), which perhaps explains why we needed to use high doses in our study. de petrocellis et al. (2001) have shown that inhibitors of FAAH greatly enhance the activity of anandamide on VR1 in HEK293 cells expressing human vanilloid receptors. Similar findings have been reported in CHO cells transfected with rat VR1 (Ross et al., 2001). If we were to repeat the present studies in rats pre-treated with a FAAH inhibitor, or use the stable analogue of anandamide, methanandamide, we might expect to find greater potency at VR1. Alternatively, the route of administration could be another explanation why high doses of anandamide had to be used in the present study. Ralevic et al. (2000) have demonstrated that VR1-mediated vasorelaxation to methanandamide is greater in arterial segments, where there is ready access to the adventitial surface, compared to luminal application in the perfused mesenteric arterial bed. It is likely that local production of anandamide in vivo provides sufficiently high concentrations to activate vascular afferents: leukocytes (Bisogno et al., 1997; Wagner et al., 1997; di marzo et al., 1999), endothelial cells (Deutsch et al., 1997), and vascular sensory neurons (Ishioka & Bukoski, 1999) have been suggested as the source of endocannabinoids in the vasculature. Furthermore, accompanying the release of anandamide are an entourage of additional fatty acid derivatives, such as palmitoylethanolamide (PEA), which lack cannabinoid activity but inhibit the hydrolysis of anandamide (Bisogno et al., 1997; Mechoulam et al., 1998), thereby potentiating its effects. Thus, anandamide or similar compounds may have a physiological role in the control of CVR systems via vasosensory nerves.
In conclusion, our results demonstrate that anandamide can evoke CVR reflexes with a rapid onset following intra-arterial injection into the rat hindlimb. These responses appear to be mediated reflexly via VR1 receptors located on sensory nerve endings within the hindlimb vasculature because (a) low doses of capsaicin gave similar responses and (b) antagonism of VR1 blocked responses to both capsaicin and anandamide. Vasosensory nerves probably provide information to the central nervous system regarding the metabolic status of a particular vascular bed, which can lead to either local or systemic effects, depending on the magnitude and duration of the stimulation. Local or neurogenic regulation of microvessels would occur when low concentrations of anandamide cause VR1-mediated release of CGRP from sensory nerves, without any major action on blood pressure. Higher concentrations would evoke systemic reflexes by an action in the brainstem to cause changes in blood pressure and breathing patterns. We believe sensory nerve endings are likely to exist in the adventitia/peri-arterial space, although their exact location remains to be established by further investigations.
Acknowledgments
This work is supported by the Wellcome Trust (Grant No. 058011). We thank Sanofi Recherche (Montpellier, France) for the gift of SR141716.
Abbreviations
- α,β Me-ATP
α,β Methylene-adenosine 5′triphosphate
- anandamide
arachidonylethanolamide
- CGRP
calcitonin-gene-related peptide
- CB
cannabinoid, capsaicin, 8-methyl-N-vanillyl-6-nonenamide
- CVR
cardiovascular and respiratory
- FAAH
fatty acid amide hydrolase
- i.a.
intra-arterially
- MAP
mean arterial pressure
- PEA
palmitoylethanolamide
- RMV
respiratory minute volume
- VR1
vanilloid receptor
References
- AHLUWALIA J., URBAN L., CAPOGNA M., BEVAN S., NAGY I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100:685–688. doi: 10.1016/s0306-4522(00)00389-4. [DOI] [PubMed] [Google Scholar]
- BAZETT H.C., MCGLONE B. Note on the pain sensations which accompany deep punctures. Brain. 1928;51:18–23. [Google Scholar]
- BEVAN S., SZOLCSANYI J. Sensory neuron-specific actions of capsaicin: mechanisms and applications. Trends Pharmacol Sci. 1990;11:330–333. doi: 10.1016/0165-6147(90)90237-3. [DOI] [PubMed] [Google Scholar]
- BISOGNO T., MAURELLI S., MELCK D., DE PETROCELLIS L., DI MARZO V. Biosynthesis, uptake, and degradation of anandamide and palmitoylethanolamide in leukocytes. J. Biol. Chem. 1997;272:3315–3323. doi: 10.1074/jbc.272.6.3315. [DOI] [PubMed] [Google Scholar]
- BRENDER D., WEBB-PEPLOE M.M. Vascular responses to stimulation of pulmonary and carotid baroreceptors by capsaicin. Am. J. Physiol. 1969;217:1837–1845. doi: 10.1152/ajplegacy.1969.217.6.1837. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- COLERIDGE H.M., COLERIDGE J.C. Cardiovascular afferents involved in regulation of peripheral vessels. Ann. Rev. Physiol. 1980;42:413–427. doi: 10.1146/annurev.ph.42.030180.002213. [DOI] [PubMed] [Google Scholar]
- DE PETROCELLIS L., BISOGNO T., MACCARRONE M., DAVIS J.B., FINAZZI-AGRO A., DI MARZO V. The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism. J. Biol. Chem. 2001;276:12856–12863. doi: 10.1074/jbc.M008555200. [DOI] [PubMed] [Google Scholar]
- DEVANE W.A., HANUS L., BREUER A., PERTWEE R.G., STEVENSON L.A., GRIFFIN G., GIBSON D., MANDELBAUM A., ETINGER A., MECHOULAM R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- DEUTSCH D.G., GOLIGORSKY M.S., SCHMID P.C., KREBSBACH R.J., SCHMID H.H.O., DAS S.K., ARREAZA G., THORUP C., STEFANO G., MOORE L. Production and physiological actions of anandamide in the vasculature of the rat kidney. J. Clin. Invest. 1997;100:1538–1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DI MARZO V., BISOGNO T., DE PETROCELLIS L., MELCK D., ORLANDO P., WAGNER J.A., KUNOS G. Biosynthesis and inactivation of the endocannabinoid 2-arachidonoylglycerol in circulating and tumoral macrophages. Eur. J. Biochem. 1999;264:258–267. doi: 10.1046/j.1432-1327.1999.00631.x. [DOI] [PubMed] [Google Scholar]
- DONNERER J., LEMBECK F. Analysis of the effects of intravenously injected capsaicin in the rat. Naunyn-Schmiedeberg's Arch. Pharmacol. 1982;320:54–57. doi: 10.1007/BF00499072. [DOI] [PubMed] [Google Scholar]
- DONNERER J., LEMBECK F. Capsaicin-induced reflex fall in rat blood pressure is mediated by afferent substance P-containing neurones via a reflex centre in the brain stem. Naunyn-Schmiedeberg's Arch. Pharmacol. 1983;324:293–295. doi: 10.1007/BF00502626. [DOI] [PubMed] [Google Scholar]
- HAINSWORTH R. Cardiovascular reflexes from ventricular and coronary receptors. Adv. Exp. Med. Biol. 1995;381:157–174. doi: 10.1007/978-1-4615-1895-2_15. [DOI] [PubMed] [Google Scholar]
- HEYMANS C., NEIL E. J. & A. Churchill, London; 1958. Reflexogenic areas of the cardiovascular system. [DOI] [PubMed] [Google Scholar]
- ISHIOKA N., BUKOSKI R.D. A role for N-arachidonylethanolamine (anandamide) as the mediator of sensory nerve-dependent Ca2+-induced relaxation. J. Pharmacol. Exp. Ther. 1999;289:245–250. [PubMed] [Google Scholar]
- KACZYÑSKA K., SZEREDA-PRZESTASZEWSKA M. Respiratory effects of capsaicin occur beyond the lung vagi in anaesthetized rats. Acta Neurobiol. Exp. 2000;60:159–165. doi: 10.55782/ane-2000-1333. [DOI] [PubMed] [Google Scholar]
- LAKE K.D., COMPTON D.R., VARGA K., MARTIN B.R., KUNOS G. Cannabinoid-induced hypotension and bradycardia in rats is mediated by CB1-like cannabinoid receptors. J. Pharmacol. Exp. Ther. 1997;281:1030–1037. [PubMed] [Google Scholar]
- LANGHAM B.T., HARRISON D.A. Local anaesthetic: does it really reduce the pain of insertion of all sizes of venous cannula. Anaesthesia. 1992;47:890–891. doi: 10.1111/j.1365-2044.1992.tb03157.x. [DOI] [PubMed] [Google Scholar]
- LEWIS T. Pain as an early symptom of arterial embolism and its causation. Clin. Sci. 1935;2:237–251. [Google Scholar]
- LUNDBERG J.M., FRANCO-CERECEDA A., LACROIX J.S., PERNOW J. Release of vasoactive peptides from autonomic and sensory nerves. Blood Vessels. 1991;28:27–34. doi: 10.1159/000158840. [DOI] [PubMed] [Google Scholar]
- MAKARA G.B., GYORGY L., MOLNAR J. Circulatory and respiratory responses to capsaicin, 5-hydroxytryptamine and histamine in rats pretreated with capsaicin. Arch. Int. Pharmacodyn. 1967;170:39–45. [PubMed] [Google Scholar]
- MALLIANI A., LOMBARDI F., PAGANI M. Function of afferents in cardiovascular sympathetic nerves. J. Auton. Nerv. Syst. 1981;3:231–236. doi: 10.1016/0165-1838(81)90065-5. [DOI] [PubMed] [Google Scholar]
- MARSHALL J.M. Peripheral chemoreceptors and cardiovascular regulation. Physiol. Rev. 1994;74:543–594. doi: 10.1152/physrev.1994.74.3.543. [DOI] [PubMed] [Google Scholar]
- MATSUDA L.A., LOLAIT S.J., BROWNSTEIN M.J., YOUNG A.C., BONNER T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- MCQUEEN D.S., BOND S.M., MOORES C., CHESSELL I., HUMPHREY P.P.A., DOWD E. Activation of P2X receptors for adenosine triphosphate evokes cardiorespiratory reflexes in anaesthetised rats. J. Physiol. 1998;507.3:843–855. doi: 10.1111/j.1469-7793.1998.843bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MECHOULAM R., FRIDE E., DI MARZO V. Endocannabinoids. Eur. J. Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- MOSKOWITZ M.A. Basic mechanisms in vascular headache. Neurol. Clin. 1990;8:801–815. [PubMed] [Google Scholar]
- MUNRO S., THOMAS K.L., ABU-SHAAR M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Cannabinoid receptor ligands: clinical and neuropharmacological considerations, relevant to future drug discovery and development. Exp. Opin. Invest. Drugs. 2000;9:1–19. doi: 10.1517/13543784.9.7.1553. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., KENDALL D.A., RANDALL M.D., ZYGMUNT P.M., MOVAHED P., HOGESTATT E.D. Vanilloid receptors on capsaicin-sensitive sensory nerves mediate relaxation to methanandamide in the rat isolated mesenteric arterial bed and small mesenteric arteries. Br. J. Pharmacol. 2000;130:1483–1488. doi: 10.1038/sj.bjp.0703456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSS R.A., GIBSON T.M., BROCKIE H.C., LESLIE M., PASHMI G., CRAIB S.J., DI MARZO V., PERTWEE R.G. Structure-activity relationship for the endogenous cannabinoid, anandamide, and certain of its analogues at vanilloid receptors in transfected cells and vas deferens. Br. J. Pharmacol. 2001;132:631–640. doi: 10.1038/sj.bjp.0703850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARIA A., SKOFITCH G., LEMBECK F. Distribution of capsaicin in rat tissues after systemic administration. J. Pharm. Pharmacol. 1982;34:273–275. doi: 10.1111/j.2042-7158.1982.tb04245.x. [DOI] [PubMed] [Google Scholar]
- SHEPHERD J.T. The lungs as receptor sites for cardiovascular regulation. Circulation. 1981;63:1–10. doi: 10.1161/01.cir.63.1.1. [DOI] [PubMed] [Google Scholar]
- SMART D., GUNTHORPE M.J., JERMAN J.C., NASIR S., GRAY J., MUIR A.I., CHAMBERS J.K., RANDALL A.D., DAVIS J.B. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br. J. Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TALLARIDA G., BALDONI F., PERUZZI G., BRINDISI F., RAIMONDI G., SANGIORGI M. Cardiovascular and respiratory chemoreflexes from the hindlimb sensory receptors evoked by intra-arterial injection of bradykinin and other chemical agents in the rabbit. J. Pharmacol. Exp. Ther. 1979;208:319–329. [PubMed] [Google Scholar]
- TAN C.H., ONSIONG M.K. Pain on injection of propofol. Anaesthesia. 1998;53:468–476. doi: 10.1046/j.1365-2044.1998.00405.x. [DOI] [PubMed] [Google Scholar]
- TUCKER R.C., KAGAYA M., PAGE C.P., SPINA D. The endogenous cannabinoid agonist, anandamide stimulates sensory nerves in guinea-pig airways. Br. J. Pharmacol. 2001;132:1127–1135. doi: 10.1038/sj.bjp.0703906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARGA K., LAKE K.D., HUANGFU D., GUYENET P.G., KUNOS G. Mechanism of the hypotensive action of anandamide in anesthetized rats. Hypertension. 1996;28:682–686. doi: 10.1161/01.hyp.28.4.682. [DOI] [PubMed] [Google Scholar]
- WAGNER J.A., VARGA K., ELLIS E.F., RZIGALINSKI B.A, , MARTIN B.R., KUNOS G. Activation of peripheral CB1 cannabinoid receptors in haemorrhagic shock. Nature. 1997;390:518–521. doi: 10.1038/37371. [DOI] [PubMed] [Google Scholar]
- WOOLLARD H.H. The innervation of blood vessels. Heart. 1927;13:321–336. [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H.H., SORGARD M., DI MARZO V., JULIUS D., HOGESTATT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


