Abstract
Interleukin-12 (IL-12) may play a central role in the development and progression of rheumatoid arthritis by driving the immune response towards T helper 1 (Th1) type responses characterized by high IFN-γ and low IL-4 production. In this study we investigated the effect of auranofin (AF), an anti-rheumatic gold compound, on IL-12 production in mouse macrophages and dendritic cells, and studied whether AF-mediated inhibition of IL-12 production could regulate a cytokine profile of antigen (Ag)-primed CD4+ Th cells.
Treatment with AF significantly inhibited IL-12 production in lipopolysaccharide (LPS)-stimulated macrophages and also in CD40L-stimulated dendritic cells. AF-pretreated macrophages reduced their ability to induce IFN-γ and increased the ability to induce IL-4 in Ag-primed CD4+ T cells. AF did not influence the cell surface expression of the class II MHC molecule and the costimulatory molecules CD80 and CD86.
Addition of recombinant IL-12 to cultures of AF-pretreated macrophages and CD4+ T cells restored IFN-γ production in Ag-primed CD4+ T cells.
The in vivo administration of AF resulted in the inhibition of IL-12 production by macrophages stimulated in vitro with LPS or heat-killed Listeria monocytogenes (HKL), leading to the inhibition of Th1 cytokine profile (decreased IFN-γ and increased IL-4 production) in Ag-primed CD4+ T cells.
These findings may explain some known effects of AF including anti-rheumatic effects and the inhibition of encephalitogenicity, and point to a possible therapeutic use of AF in the Th1-mediated immune diseases such as autoimmune diseases.
Keywords: Auranofin, rheumatoid arthritis, interleukin-12, macrophage, T helper cell
Introduction
The existence of Th1/Th2 subsets in Th lymphocytes that differ in their cytokine secretion patterns and effector functions provides a framework for understanding normal and pathological immune responses (Mosmann, 1991). The Th1 subset of CD4+ T cells secretes cytokines usually associated with inflammation such as interferon-γ (IFN-γ) and tumour necrosis factor-β (TNF-β), and induces cell-mediated immune responses. The Th2 subset produces cytokines such as IL-4 and IL-5 that help B cells to proliferate and differentiate and is associated with humoral immune responses (Seder & Paul, 1994; Constant & Bottomly, 1997). Recent studies indicate that the ratio of these two Th cell types, Th1 and Th2, is closely correlated with the outcome of many diseases (Romagnani, 1996; D'Elios & Del Prete, 1998). Th1 responses predominate in organ-specific autoimmune disorders, acute allograft rejection, and in some chronic inflammatory disorders (Hayashi et al., 1995; Lafaille, 1998). In contrast, Th2 responses predominate in Omenn's syndrome, transplantation tolerance, chronic graft-versus-host disease, systemic sclerosis, and allergic diseases (Grewe et al., 1998).
The nature of Th1 or Th2 polarizing signals is not yet fully understood. However, the cytokines that are present in the environment of the CD4+ T cell at the time it encounters the antigen significantly regulate the differentiation of Th cells into either Th1 or Th2 subsets (O'Garra, 1998). IL-12 promotes Th1 differentiation, while IL-4 plays a key role in the differentiation of the precursor CD4+ T cells toward a Th2 phenotype (Murphy et al., 2000). Recent evidence points to a critical role for IL-12 in the pathogenesis of rodent models of Th1-mediated autoimmune diseases such as type-1 diabetes, multiple sclerosis, rheumatoid arthritis (RA), inflammatory bowel disease, and acute graft-versus-host disease (Trinchieri, 1998; Adorini, 1999). For example, IL-12 was expressed by infiltrating macrophages and synovial lining cells in RA (Sakkas et al., 1998). The median level of circulating IL-12 was higher in patients with RA than in normal healthy people, and the IL-12 levels in RA patients were higher in the synovial fluid than in sera (Kim et al., 2000b). Exogenous administration of IL-12 exacerbated RA (Peeva et al., 2000). Thus, pharmacological control of IL-12 production may be a key strategy in modulating specific immune-mediated diseases dominated by type-1 cytokine responses including RA (Hasko & Szabo, 1999).
Auranofin [AF; 2,3,4,6-tetra-O-acetyl-1-thio-β-glucopyranosato-S-(triethylphosphosphine) gold] is a lipophilic gold complex with immuno-suppressive and anti-inflammatory properties (Blodgett et al., 1984). For a long time, AF has been widely used for the treatment of RA (Lean et al., 1997; Simon, 2000). A number of studies have demonstrated that AF is a potent inhibitor of the phagocytic activity of macrophages and neutrophils, suggesting that these cells might be primary targets for this agent (Bondeson, 1997; Liu et al., 2000).
In this study we have demonstrated that treatment with AF significantly inhibited IL-12 production in LPS-stimulated macrophages and in CD40L-stimulated dendritic cells. Importantly, inhibition of IL-12 production in AF-pretreated macrophages deviates CD4+ T cells from the Th1 to the Th2 pathway.
Methods
Preparation of splenic macrophages stimulated with either lipopolysaccharide (LPS) or heat-killed Listeria monocytogenes (HKL)
Splenic macrophages were isolated from DBA/2 mice and stimulated, as previously described (Chung et al., 2000). In brief, spleen cells were cultured at 106 cells ml−1 for approximately 3 h in Dulbecco's modified Eagle's medium (DMEM) containing 10% foetal bovine serum at 37°C in a 5% CO2 humidified air atmosphere. The non-adherent cells were removed by washing with warm DMEM until visual inspection revealed a lack of lymphocytes (>98% of the cell population). The adherent cells were removed from plates by incubating for 15 min with ice-cold phosphate-buffered saline solution and rinsing repeatedly. The isolated adherent cell population was stimulated with 5 μg ml−1 LPS in the absence or presence of AF at 0.01, 0.05, 0.1, 1.0 μg m−1 at 1×105 cells per well in 96-well culture plates for 48 h. For some experiments, the cells were stimulated with HKL at 2×106 bacteria per well.
Preparation of splenic dendritic cells (DCs) stimulated with CD40L-CHO
Mouse dendritic cells were prepared from spleens, as previously described (Fuako & Koyasu, 2000). In brief, the isolated adherent cells as mentioned above were incubated for additional 18 h to allow DCs to detach. After this incubation, floating cells were collected, and DCs were positively purified using anti-CD11c MicroBeads and an MACS column (Miltenyl Biotech., Germany). Purified cells were routinely >95% CD11c+. Freshly isolated DCs were induced to maturate by cultivation in the culture medium overnight. The isolated DCs were stimulated with 3×104 cells per well fixed CD40L-CHO in the absence or presence of AF at 0.01, 0.05, 0.1, 1.0 μg m−1 at 1×105 cells per well in 96-well culture plates for 24 h.
Purification and induction of cytokine synthesis in antigen-primed CD4+ T cells
Draining axillary, popliteal, and inguinal lymph nodes were removed from mice 9 days after priming with 100 μg keyhole limpet haemocyanin (KLH) in complete Freunds adjuvant (CFA) in the footpads as previously described (Kim et al., 1997). Lymph node cells were depleted of B cells by adherence to goat anti-mouse immunoglobulin-coated dishes for 1 h at 4°C. The non-adherent cells were depleted of CD8+ T cells and other antigen-presenting cells by treating the cells with a mixture of anti-CD8 and anti-class II MAbs on ice for 30 min, followed by addition of low toxicity rabbit complement (Pel Freeze, Rogers, AR, U.S.A.) and incubation at 37°C for 45 min. More than 95% of the cells were CD4+ T cells, as demonstrated by cytofluorometric analysis using anti-CD4 MAb (PharMingen). Purified CD4+ T cells were incubated in 96-well plates at 4×105 cells per well with macrophages (1×105 cells per well) and KLH (10 μg ml−1). Culture supernatants were harvested after 2 days (for IL-12 p70 and IL-12 p40) or after 4 days (for IFN-γ and IL-4), and assayed by an enzyme-linked immunosorbent assay (ELISA).
Cytokine assays
The quantities of IFN-γ, IL-4, IL-10, IL-12 p40, and IL-12 p70 in culture supernatants were determined by a sandwich ELISA using MAbs specific for each cytokine as previously described (Na et al., 1999). The MAbs for coating the plates and the biotinylated second MAbs were as follows: for IFN-γ, HB170 and XMG1.2; for IL-4, BVD4-1D11 and BVD6; for IL-10, JES-2A5 and SXC-1; for IL-12 p40, C17.8 and C15.6; for IL-12 p70, anti-mouse IL-12 (p35/p70) (Red-T/G297-289) and rat anti-mouse IL-12 p40 (C17.8). Standard curves were generated using recombinant cytokines (purchased from the PharMingen). The lower limits of detection were 125 pg ml−1 for IFN-γ, 3 pg ml−1 for IL-4, 0.2 ng ml−1 for IL-10, 30 pg ml−1 for IL-12 p40 and 50 pg ml−1 for IL-12 p70, respectively.
Reverse transcription-polymerase chain reaction (RT – PCR)
Total RNA was prepared from the cells and reverse-transcribed into cDNA, and then PCR amplification of the cDNA was performed as previously described (Kim et al., 2000a). The sequences of PCR primers are as follows: mouse IL-12 p40 (sense, 5′-CAGAAGCTAACCATCTCCTGGTTTG-3′; antisense, 5′-TCCGGAGTAATTTGGTGCTTCACAC-3′), IL-6 (sense, 5′-TGAACAACGATGATGCACTT-3′; antisense, 5′-CGTAGAGAACAACATAAGTC-3′), IL-10 (sense, 5′-ATGCAGGACTTTAAGGGTTACTTGGGT-3′; antisense, 5′-ATTTCGGAGAGAGGTACAAACGAGGTTT-3′), TNF-α (sense, 5′-GGCAGGTCTACTTTGGAGTCATTG-3′; antisense, 5′-ACATTCGAGGCTCCAGTGAATTCGG-3′) and β-actin (sense, 5′-TGGAATCCTGTGGCATCCATGAAAC-3′; antisense, 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′). The PCR reactions were run for 35 cycles of 94°C (30 s), 58°C (45 s), 72°C (30 s) using a MJ Thermal Cycler (Watertown, MA, U.S.A.). After the amplification, 6 μl of the RT – PCR products were separated in 1.5% (w v−1) agarose gels and stained with ethidium bromide.
Immunofluorescent staining and cytofluorometric measurements
Quantitative immunofluorescence measurements were performed in an Epic V flow cytofluorograph equipped with a multi-parameter data acquisition and display system (MDADS), as previously described (Kang et al., 2001). Briefly, single cell suspensions were collected from the various cultures and washed twice with ice-cold phosphate buffered saline (PBS, pH 7.4). Afterwards, fluorescein isothiocyanate-conjugated anti-H-2I-Ad, anti-CD80, or anti-CD86 MAbs were added and incubated at 4°C for 1 h. After incubation, the cells were washed with PBS and were fixed in PBS containing 1% paraformaldehyde, and cytofluorometric analysis was performed. Background staining was determined by staining cells with fluorescein isothiocyanate-conjugated isotype control mAbs. One parameter fluorescence histograms were generated by analysing at least 1×104 cells.
Statistical analysis
Student's t-test and one-way analysis of variance (ANOVA) were used to determine the statistical significance of differences between values for various experimental and control groups. P-values <0.05 were considered significant.
Materials
Auranofin (2,3,4,6-tetra-O-acetyl-l-thio-β-glucopyranosato-S-[triethylphosphosphine] gold), LPS (from E. coli 0111:B4) and KLH were purchased from the BIOMOL Research Lab., Inc. (Plymouth Meeting, PA, U.S.A.), Sigma Chemical Co. (St. Louis, MO, U.S.A.) and Calbiochem (San Diego, CA, U.S.A.), respectively. Anti-mIL-4 (BVD4 and BVD6) and anti-mIFN-γ MAbs (R46A2 and XMG1.2) were purified from ascitic fluids by ammonium sulphate precipitation followed by diethylaminoethyl (DEAE)-Sephacel chromatography (Sigma). Anti-mouse IL-12 (p35/p70) (Red-T/G297-289), anti-IL-10 MAbs JES-2A5 and SXC-1, anti-CD80 MAb, anti-CD86 MAb, and anti-H-2I-Ad MAb were obtained from the PharMingen (San Diego, CA, U.S.A.), and rat anti-mouse IL-12 p40 MAbs C17.8 and 15.6 were kindly donated by Dr G. Trinchieri (Wistar Institute, Philadelphia, PA, U.S.A.). MAb-secreting hybridomas were obtained from the ATCC (American Type Culture Collection, Rockville, MD, U.S.A.). Chinese hamster ovary cells transfected with murine CD40L (CD40L-CHO) (Inaba et al., 1995) were kindly provided by Dr H. Yagita (Tokyo, Japan), and used after fixation with 1% paraformaldehyde in PBS. The cells were maintained at 37°C in a humidified 5% CO2 in RPMI 1640 or Dulbecco's modified Eagle's medium (DMEM) containing 10% foetal bovine serum and antibiotics (Gibco BRL, Grand Island, NY, U.S.A.). Six- to eight-week-old female DBA/2 mice were obtained from the SLC Japan (Tokyo, Japan), and maintained in pathogen-limited conditions. The mice were maintained and treated according to National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Results
Treatment with AF inhibited IL-12 production from LPS-stimulated mouse macrophages and CD40L-stimulated dendritic cells in a dose-dependent manner
To determine whether AF could affect the production of T cell cytokine indirectly via an effect on IL-12 production by antigen-presenting cells (APCs) such as macrophages and dendritic cells, we first investigated the effect of AF on IL-12 production in mouse macrophages. As shown in Figure 1, LPS readily induced production of IL-12 p70 heterodimer as well as the p40 subunit, as expected. Treatment of macrophages with AF significantly inhibited this induced IL-12 production in a dose-dependent manner (Figure 1A) (P<0.05 at 0.5 and 1.0 μg ml−1 AF, relative to an untreated group). In contrast, treatment with AF did not influence IL-10 production from LPS-stimulated macrophages, suggesting that the AF effects were not the result of a general dampening of cellular activation.
Figure 1.
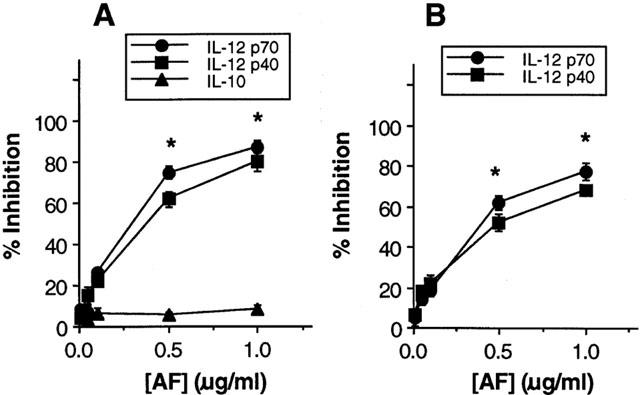
Treatment with auranofin (AF) inhibits IL-12 production in mouse macrophages and dendritic cells. Mouse macrophages were stimulated with LPS (5 μg ml−1) for 48 h in the absence or presence of varying concentrations of AF (A). Mouse DCs (1×105 cells well−1) were stimulated with 3×104 cells well−1 CD40L-CHO (B). Culture supernatants were harvested and cytokine levels were evaluated by ELISA. The results are presented as the mean±s.e.mean (n=3) of the percentage response of cytokine production of AF-treated cells compared with untreated control cells stimulated with LPS for macrophages or CD40L-CHO for DCs. Mean cytokine levels in the absence of AF were as follows: IL-12 p70, 650 pg ml−1 for macrophages and 870 pg ml−1 for DCs; IL-12 p40, 2.8 ng ml−1 for macrophages and 3.9 ng ml−1 for DCs; IL-10, 1.2 ng ml−1 for macrophages. *P<0.05, relative to an untreated group.
We also investigated whether IL-12 production by in vitro-generated dendritic cells (DCs) is affected by AF. DCs are the professional effective APC and their secretion of immunoregulatory and proinflammatory cytokines plays a crucial role during T cell priming (Peters et al., 1996). As shown in Figure 1B, the stimulation of DCs with CD40L-CHO induced production of IL-12 p70 heterodimer as well as the p40 subunit. Treatment with AF resulted in a dose-dependent inhibition of IL-12 production. The stimulation with untransfected CHO as a control cell-line did not induce detectable amounts of IL-12 production.
IL-12-inhibition by AF did not result from a general cytotoxic effect since cells' viability in all cultures remained constant throughout the incubation period in the presence of AF concentrations used in the experiment, as demonstrated by trypan blue exclusion test (data not shown).
Furthermore, to determine whether the inhibition of IL-12 production by AF is the results of decreased IL-12 mRNA expression, the effect of AF on the expression of IL-12 p40 and other cytokines (IL-6, IL-10, TNF-α) mRNA was analysed in LPS-stimulated macrophages. An IL-12 p40 subunit was known as the highly inducible and tightly regulated component of IL-12 (Trinchieri, 1998). As shown in Figure 2A, AF significantly inhibited mRNA levels of IL-12 p40 gene in a dose-dependent manner, indicating that the inhibition of IL-12 production by AF occurred at the transcriptional level. In contrast, treatment with AF did not influence mRNA levels of IL-6, IL-10, and TNF-α in LPS-stimulated macrophages, suggesting that the inhibition of IL-12 production by AF was not the result of a general dampening of cellular activation.
Figure 2.
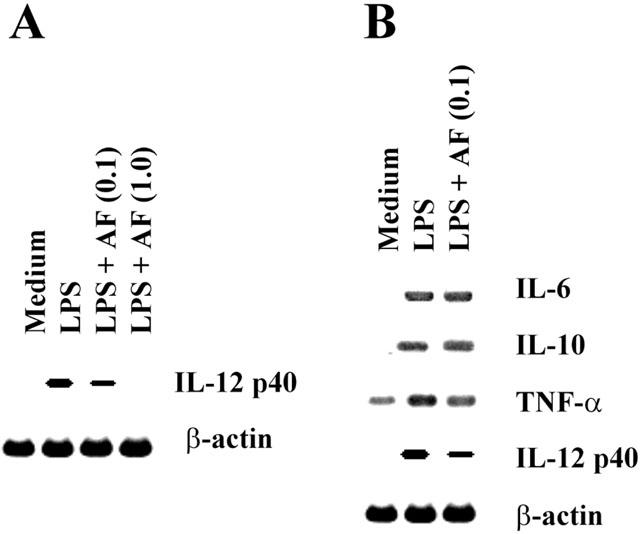
Effect of AF on the expression of IL-12 gene at the mRNA level. Macrophages were stimulated with LPS (5 μg ml−1) in the absence or presence of AF (0.1 and 1.0 μg ml−1) for 6 h and total RNA was prepared from the cells. RT – PCR products for IL-12 p40 and β-actin (A), or for IL-6, IL-10, TNF-α, IL-12 p40 and β-actin (B) were analysed in 1.5% agarose gels.
Pretreatment of macrophages with AF inhibited IFN-γ production and enhanced IL-4 production by antigen-primed CD4+ T cells
Since IL-12 has been known to potently enhance IFN-γ and inhibit IL-4 in CD4+ T cells, we asked if the cytokine profiles of CD4+ T cells responding to Ag presented by AF-treated macrophages would be altered. Direct effects of AF on CD4+ T cells were eliminated by pretreatment of macrophages in vitro with AF for 4 h and washing them to remove AF, before culture with syngeneic CD4+ T cells purified from lymph nodes of KLH-primed mice and the Ag KLH. Figure 3A shows that IL-12 production in cultures of AF-treated macrophages was significantly decreased in comparison with untreated macrophages. In the absence of AF treatment, stimulation with KLH resulted in the development of T cells producing high levels of IFN-γ. However, pretreatment of macrophages with AF for 4 h greatly inhibited their capacity to induce IFN-γ production by KLH-primed CD4+ T cells (Figure 3B) and significantly increased IL-4 production (Figure 3C). No cytokine production by CD4+ T cells was detected in the absence of macrophages, demonstrating that the AF-treated macrophages regulated the cytokine production by KLH-primed CD4+ T cells. Thus, pretreatment of macrophages with AF enhances their capacity to inhibit Th1 and enhance Th2 cytokine synthesis.
Figure 3.
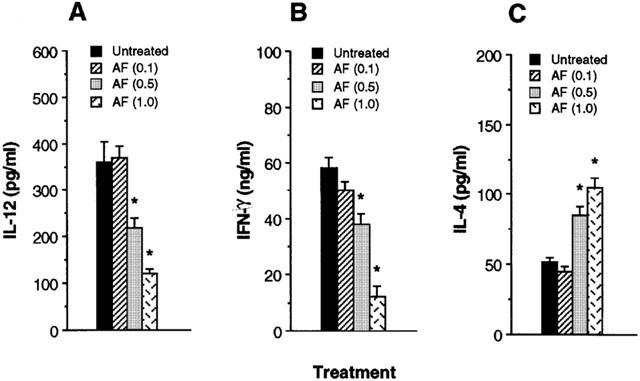
Macrophages pretreated with AF inhibit IFN-γ and enhance IL-4 production by Ag-primed CD4+ T cells. Macrophages (1×105 cells well−1) were pretreated with medium alone or 1.0 μg ml−1 AF. After 4 h, the cells were washed and incubated with KLH-primed CD4+ T cells (5×105 cells well−1) and KLH (10 μg ml−1). Supernatants were harvested after 2 days for IL-12 (A) or after 4 days for IFN-γ (B) and IL-4 (C), and assayed by cytokine-specific ELISA. The results are presented as the mean±s.e.mean (n=3). *P<0.01, relative to an untreated group.
Pretreatment with AF does not affect the expression of surface molecules on macrophages
To determine whether pretreatment of macrophages with AF affects their cell surface expression of the class II MHC molecule H-2I-Ad and the costimulatory molecules CD80 and CD86, mouse macrophages were treated with medium alone (control) or 1.0 μg ml−1 AF for 24 h and the expression of the cell surface molecules was determined by cytofluorometric analysis. A shown in Figure 4, treatment with AF did not affect the expression of surface molecules when compared with the control cells.
Figure 4.
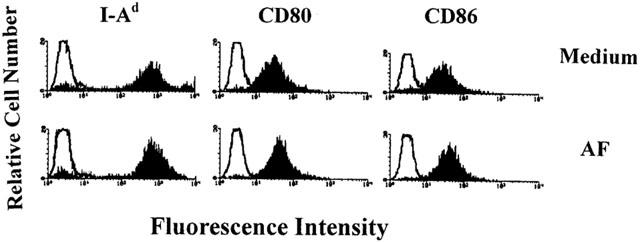
Cytofluorometric analysis of cell surface molecules on AF-treated macrophages. Mouse macrophages were treated for 24 h with medium alone or 1.0 μg ml−1 AF and analysed for the expression of cell surface molecules by flow cytometry. Data are representative of three independent experiments.
Addition of recombinant IL-12 (rIL-12) to cultures of AF-pretreated macrophages and CD4+ T cells restored the levels of IFN-γ production in CD4+ T cells
To determine whether the reduced ability of AF-pretreated macrophages to induce IFN-γ synthesis in CD4+ T cells was a result of their diminished production of IL-12, we reconstituted cultures of AF-pretreated macrophages and CD4+ T cells with rIL-12 (10 pg ml−1). Figure 5 shows that addition of rIL-12 to cultures of AF-pretreated macrophage and CD4+ T cells significantly increased IFN-γ and reduced IL-4 production to levels seen in untreated control cultures. These results suggest that reduction of IL-12 synthesis by AF-treated macrophages was a major effect that affected the ability of macrophages to regulate cytokine synthesis in CD4+ T cells.
Figure 5.
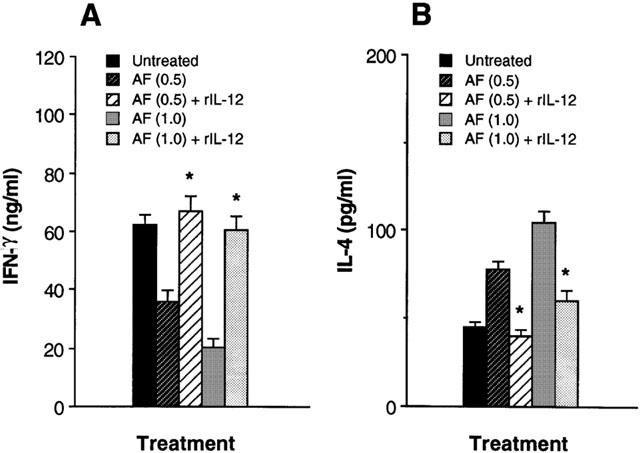
Addition of recombinant IL-12 restores the decreased IFN-γ production of T cells in cultures of AF-pretreated macrophages and Ag-primed CD4+ T cells. KLH-primed CD4+ T cells were cultured with AF (1.0 μg ml−1)-pretreated macrophages in the presence of rIL-12 (10 pg ml−1) and KLH (10 μg ml−1). Culture supernatants were harvested 4 days later and assayed for IFN-γ (A) and IL-4 (B) levels by ELISA. The results are presented as the mean±s.e.mean (n=3). *P<0.01, relative to each AF-treated group in the absence of rIL-12.
Macrophages from mice treated in vivo with AF also inhibited Th1 cytokine profile by Ag-primed CD4+ T cells
To demonstrate that AF had a consequential effect on macrophages in an in vivo system, mice were injected intraperitoneally (i.p.) with 200 μg AF per mouse. After 24 h, splenic macrophages were purified from the AF-treated mice, or from saline-injected control mice. Figure 6 shows that macrophages from AF-treated mice induced significantly lower amounts of IL-12 in response to either LPS or HKL than macrophages from the control mice (P<0.001).
Figure 6.
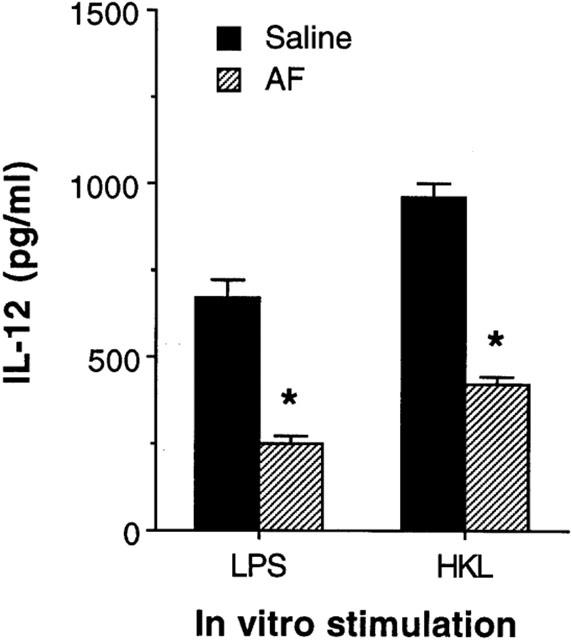
Macrophages exposed to AF in vivo decrease levels of IL-12 production. Mice were injected in vivo with AF (200 μg per mouse, i.p.). After 24 h, macrophages were purified and stimulated with either LPS (5 μg ml−1) or HKL (2×106 bacteria well−1). Culture supernatants were harvested 48 h later and IL-12 levels were determined by ELISA. The results are presented as the mean±s.e.mean (n=4). *P<0.001, relative to a saline-injected group.
To determine whether cytokine production by Ag-primed CD4+ T cells would differ in the presence of Ag presented by macrophages from mice treated in vivo with AF, splenic macrophages were purified from DBA/2 mice 24 h following i.p. injection with AF (200 μg per mouse), and cultured with CD4+ T cells from KLH-primed mice, and KLH. Figure 7 shows that production of IL-12 in cultures containing KLH-primed CD4+ T cells and macrophages from AF-treated mice was greatly reduced compared with those from saline-injected control mice. Moreover, macrophages from mice injected with AF significantly induced lower amounts of IFN-γ and higher amounts of IL-4 than macrophages from the control mice. These results show that in vivo treatment with AF regulates the ability of macrophages to control IFN-γ and IL-4 production in CD4+ T cells.
Figure 7.
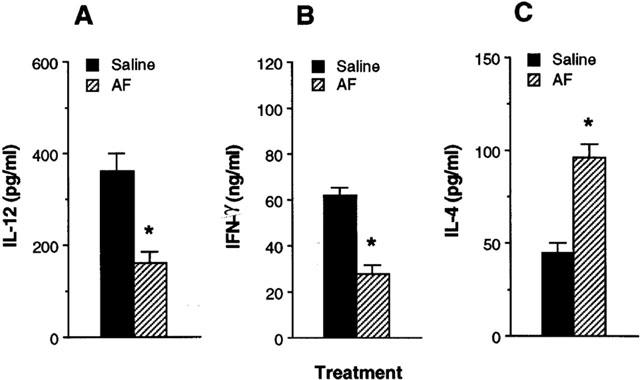
Macrophages purified from mice treated in vivo with AF regulate cytokine production in Ag-primed CD4+ T cells. DBA/2 mice were injected in vivo with either AF (200 μg per mouse, i.p.) or saline. After 24 h, macrophages were purified and incubated with KLH-primed CD4+ T cells and KLH (10 μg ml−1). After 4 days, culture supernatants were harvested and assayed for IL-12 (A), IFN-γ (B) and IL-4 (C) levels by ELISA. The results are presented as the mean±s.e.mean (n=4). *P<0.001, relative to a saline-injected group.
Discussion
Inhibiting the action of IL-12 has been shown to prevent development and progression of disease in experimental models of autoimmunity (Caspi, 1998). These findings have raised great interest in identifying inhibitors of IL-12 production for the treatment of Th1-mediated diseases such as type-1 diabetes, multiple sclerosis, rheumatoid arthritis, inflammatory bowel disease and acute graft-versus-host disease. In this study we demonstrated that treatment of macrophages with AF, an anti-rheumatic gold compound, significantly inhibited IL-12 production from macrophages, resulting in a reduced ability to induce IFN-γ and an increased ability to induce IL-4 in Ag-primed CD4+ T cells. These results suggest that AF-mediated inhibition of IL-12 production led to the inhibition of Th1 and enhancement of Th2 cytokine synthesis in CD4+ T cells. Since the cytokine profile of Th cells plays an important role to determine the outcome of many diseases, AF may have therapeutic potential to treat Th1-mediated diseases. Conversely, AF may trigger or enhance Th2-mediated diseases because of their ability to increase IL-4 production in CD4+ T cells.
Cytokines from synovium and inflammatory cells are thought to be important in the initiation and perpetuation of RA. In particular, macrophage-derived cytokines, including TNF-α and IL-1, have been detected at high levels and many of these cytokines function as potent inflammatory molecules in the joints, and reflect the disease activity (Maini & Taylor, 2000). Recently IL-12 has been shown to play a critical role in the regulation of autoimmune disease models including RA and type 1 diabetes (Caspi, 1998). Administration of rIL-12 and type II collagen was reported to induce severe arthritis in DBA/1 mice (Germann et al., 1995). In patients with RA, IL-12 p70 induces IFN-γ dominant cytokine production by infiltrating T cells into chronic arthritic joints, suggesting that IL-12 may play an important role in a shift toward rheumatoid T cells with Th1 cytokine profiles in RA. Furthermore, IL-12 synergizes with IL-1 and IL-18 in the production of IFN-γ and other proinflammatory cytokines (Skeen & Ziegler, 1995; Yoshimoto et al., 1998), while IL-12-induced IFN-γ production is down-regulated by IL-4 and IL-10 (Lester et al., 1995). Recent report showed that 3′ UTR alleles of the IL-12p40 gene in type 1 diabetic patients led to variation in IL-12 p40 production which may influence T-cell responses crucial for either mediating or protecting against autoimmune diseases (Morahan et al., 2001).
Although AF may affect cytokine production in CD4+ T cells in several ways, we believe that inhibition of IL-12 production in macrophages is a major mechanism by which AF affects cytokine production in CD4+ T cells, particularly since IL-12 is extremely potent in enhancing IFN-γ and inhibiting IL-4 in CD4+ T cells (Marshall et al., 1995; Gerosa et al., 1996; Umetsu et al., 1996). In our cultures, the effect of AF on cytokine production in CD4+ T cells was indirect since the CD4+ T cells in these cultures were never directly exposed to the AF.
Similar studies showed that β2-adrenergic compounds including salbutamol inhibited IL-12 production from human monocytes or dendritic cells by increasing intracellular cyclic AMP levels, leading to the inhibition of Th1 development while promoting Th2 cell differentiation (Panina-Bordignon et al., 1997). Corticosteroids and retinoids have been known to enhance the capacity of macrophages to induce IL-4 synthesis in CD4+ T cells by inhibiting IL-12 production (Dekruyff et al., 1998; Kang et al., 2000). 1,25-dihydroxyvitamin D3 inhibited IL-12 production and IL-12-dependent Th1 cell development without inducing a deviation to the Th2 phenotype (Mattner et al., 2000). In this report, we added AF to the lists of compounds that inhibit IL-12 production in macrophages and dendritic cells. However, AF may also directly affect the cytokine production of CD4+ T cells. Harth et al. (1990) reported that AF inhibited IFN-γ production by concanavalin A-stimulated peripheral blood mononuclear cells in human and suggested that gold therapy might affect IFN-γ production in RA.
Inhibition of IL-12 production in AF-treated macrophages may be accompanied by a block of their maturation and antigen-presenting capacity. Gold salts inhibited peptide-induced responses of a peptide-specific T cell clone (Romagnoli et al., 1992). Glucocorticoids and 1,25-dihydroxyvitamin D3 were known to interfere with the cell surface expression of molecules involved in costimulation and antigen presentation (Russo-Marie, 1992; Penna & Adorini, 2000). However, our data show that the expression of the class II MHC molecule H-2I-Ad and the costimulatory molecules CD80 and CD86 remained unaffected after the preincubation of macrophages with AF. In addition, AF may indirectly suppress IL-12 production via the up-regulation of IL-10, which is known to reduce IL-12 synthesis (Aste-Amezaga et al., 1998). Although this inhibitory pathway may be relevant, the levels of IL-10 expression in AF-treated and untreated macrophages were approximately the same (Figures 1 and 2B), suggesting that the inhibitory effect of AF on IL-12 production by mouse macrophages cannot be explained by the indirect effect of up-regulation of IL-10.
In the reconstitution experiment of AF-treated macrophages with rIL-12, a small amount of rIL-12 (10 pg ml−1) could effectively reverse cytokine production by the activated CD4+ T cells if present at the initiation of cultures. Addition of 5 pg ml−1 rIL-12 to cultures of dexamethasone-treated macrophages was also known to restore the induction of IFN-γ and inhibit the induction of IL-4 synthesis by T cells (Dekruyff et al., 1998). Delayed addition of rIL-12 to the cultures was significantly less effective because the corresponding T cells became more activated and the capacity to produce IL-4 or IFN-γ became more established in vitro (Dekruyff et al., 1995). Early Th1 cells maintain the ability to signal for some time after they have acquired high IFN-γ synthesis, and addition of IL-4 can reverse the development of the Th1 phenotype. Fully mature Th1 effectors appear to be irreversibly committed and cannot be switched away from the Th1 phenotype (Asnagli & Murphy, 2001). The apparent irreversibility of the Th phenotype at the clonal level does not necessarily, however, need this for immunotherapy approaches aimed at reversing the phenotype of the response at the population level. Numerous studies have established that the cytokine milieu can direct development of newly recruited autoimmune cells to a nonpathogenic phenotype. In chronic autoimmune disease new clones continue to be primed and recruited into the antigen-specific effector pool, and those newly emerging effectors could conceivably be shifted away from the pathogenic phenotype by immunotherapy designed to provide an appropriate cytokine milieu.
Addition of rIL-12 might enhance IL-4 production in CD4+ T cells by increasing IL-10 production in the cultures of the AF-treated macrophages and T cells. IL-12 has been reported to increase IL-10 production by T cells in systems using established T cell lines (Jeannin et al., 1996). However, IL-10 production increased by IL-12 appears to occur mainly when very high concentrations of IL-12 (10 ng ml−1, 1000 fold higher than the amount used in our study) are used (Windhagen et al., 1996). Therefore, reduction of IL-12 production by AF was a major immunoregulatory mechanism that led to the inhibition of Th1 cytokine profile in CD4+ T cells. Furthermore, AF-induced IL-12 inhibition may lead to the reduction of the levels of TNF-α and/or IL-1, which contribute the signs and symptoms of RA. Previous reports showed that small amounts of IL-12 production could induce the proinflammatory cytokines including TNF-α and IL-1 (Aste-Amezaga et al., 1994; Kim et al., 2000b). Conversely, TNF-α and IL-1 were known to require for optimal IL-12-induced development of Th1 cells producing high levels of IFN-γ (Shibuya et al., 1998).
In contrast to the inhibitory effect of AF (0.1 – 1.0 μg ml−1) on IL-12 production as shown in this study, aurothioglucose, a hydrophilic gold compound with anti-rheumatic activity, did not inhibit IL-12 production in mouse macrophages stimulated with LPS at the range of 0.1 – 20 μg ml−1 (data not shown). Although the reason is not clear, other reports also showed that AF inhibited the induction of IL-1β and TNF-α in mouse macrophages stimulated with either zymosan, LPS, or various bacteria while the hydrophilic gold compounds aurothiomalate and aurothioglucose did not (Bondeson & Sundler, 1995).
In conclusion, we have shown that treatment of macrophages with AF inhibited IL-12 production in a dose-dependent manner, leading to the inhibition of Th1 cytokine profile in CD4+ T cells. These results suggest that AF-mediated inhibition of IL-12 production in macrophages may explain some known biological effects of AF including its anti-rheumatic effect and AF may also be useful in the treatment of Th1-mediated immunological disorders.
Acknowledgments
We would like to thank Drs J.W. Lee, I. Choi, S. Wolf and G. Trinchieri for providing valuable reagents and helpful discussion. This work was supported by grants from the KOSEF (HRC 1998G0201) and from the HMP (01-PJ1-PG3-21200-002).
Abbreviations
- AF
auranofin
- Ag
antigen
- APC
antigen-presenting cell
- CHO
Chinese hamster ovary cell
- DC
dendritic cell
- ELISA
enzyme-linked immunosorbent assay
- HKL
heat-killed Listeria monocytogenes
- IFN-γ
interferon-gamma
- IL
interleukin
- KLH
keyhole limpet haemocyanin
- LPS
lipopolysaccharide
- MAb
monoclonal antibody
- RA
rheumatoid arthritis
- RT – PCR
reverse transcription-polymerase chain reaction
- Th
T helper
References
- ADORINI L. Interleukin-12, a key cytokine in Th1-mediated autoimmune diseases. Cell. Mol. Life Sci. 1999;55:1610–1625. doi: 10.1007/s000180050400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASNAGLI H., MURPHY K.M. Stability and commitment in T helper cell development. Curr. Opin. Immunol. 2001;13:242–247. doi: 10.1016/s0952-7915(00)00210-7. [DOI] [PubMed] [Google Scholar]
- ASTE-AMEZAGA M., D'ANDREA A., KUBIN M., TRINCHIERI G. Cooperation of natural killer cell stimulatory factor/interleukin-12 with other stimuli in the induction of cytokines and cytotoxic cell-associated molecules in human T and NK cells. Cell. Immunol. 1994;156:480–492. doi: 10.1006/cimm.1994.1192. [DOI] [PubMed] [Google Scholar]
- ASTE-AMEZAGA M., MA X., SARTORI A., TRINCHIERI G. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. J. Immunol. 1998;160:5936–5944. [PubMed] [Google Scholar]
- BLODGETT R.C., HEUER M.A., PIETRUSKO R.G. Auranofin: a unique oral chrysotherapeutic agent. Semin. Arthritis Rheum. 1984;13:255–273. doi: 10.1016/0049-0172(84)90029-5. [DOI] [PubMed] [Google Scholar]
- BONDESON J. The mechanisms of action of disease-modifying antirheumatic drugs: a review with emphasis on macrophages signal transduction and the induction of proinflammatory cytokines. Gen. Pharmacol. 1997;29:127–150. doi: 10.1016/s0306-3623(96)00419-3. [DOI] [PubMed] [Google Scholar]
- BONDESON J., SUNDLER R. Auranofin inhibits the induction of interleukin-1β and tumor necrosis factor-α mRNA in macrophages. Biochem. Pharmacol. 1995;50:1753–1759. doi: 10.1016/0006-2952(95)02030-6. [DOI] [PubMed] [Google Scholar]
- CASPI R.R. IL-12 in autoimmunity. Clin. Immunol. Immunopathol. 1998;88:4–13. doi: 10.1006/clin.1998.4540. [DOI] [PubMed] [Google Scholar]
- CHUNG S.W., KANG B.Y., KIM S.H., PAK Y.K., CHO D., TRINCHIERI G., KIM T.S. Oxidized low density lipoprotein inhibits interleukin-12 production in lipopolysaccharide-activated macrophages via direct interactions between peroxisome proliferator-activated receptor-γ and nuclear factor-κB. J. Biol. Chem. 2000;275:32681–32687. doi: 10.1074/jbc.M002577200. [DOI] [PubMed] [Google Scholar]
- CONSTANT S.L., BOTTOMLY K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu. Rev. Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- DEKRUYFF R.H., FANG F., UMETSU D.T. Corticosteroids enhance the capacity of macrophages to induce Th2 cytokine synthesis in CD4+ lymphocytes by inhibiting IL-12 production. J. Immunol. 1998;160:2231–2237. [PubMed] [Google Scholar]
- DEKRUYFF R.E., FANG F., WOLF S.E., UMETSU D.T. IL-12 inhibits IL-4 synthesis in keyhole limpet hemocyanin-primed CD4+ T cells through an effect on antigen-presenting cells. J. Immunol. 1995;154:2578–2587. [PubMed] [Google Scholar]
- D'ELIOS M., DEL PRETE G. Th1/Th2 balance in human disease. Transplant Proc. 1998;30:2373–2379. doi: 10.1016/s0041-1345(98)00659-9. [DOI] [PubMed] [Google Scholar]
- FUAKO T., KOYASU S. Expression of functional IL-2 receptors on mature splenic dendritic cells. Eur. J. Immunol. 2000;30:1453–1457. doi: 10.1002/(SICI)1521-4141(200005)30:5<1453::AID-IMMU1453>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- GERMANN T., SZELIGA J., HESS H., STORKEL S., PODLASKI F.J., GATELY M.K., SCHMITT E., RUDE E. Administration of interleukin 12 in combination with type II collagen induces severe arthritis in DBA/1 mice. Proc. Natl. Acad. Sci. U.S.A. 1995;92:4823–4827. doi: 10.1073/pnas.92.11.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEROSA F., PAGANIN C., PERITT D., PAIOLA F., SCUPOLI M.T., ASTE-AMEZAGA M., FRANK I., TRINCHIERI G. Interleukin-12 primes human CD4+ and CD8+ T cell clones for high production of both interferon-γ and interleukin-10. J. Exp. Med. 1996;183:2559–2569. doi: 10.1084/jem.183.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREWE M., BRUIJNZEEL-KOOMEN C.A., SCHOPF E., THEPEN T., LANGEVELD-WILDSCHUT A.G., RUZICKA T., KRUTMANN J. A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol. Today. 1998;19:359–361. doi: 10.1016/s0167-5699(98)01285-7. [DOI] [PubMed] [Google Scholar]
- HARTH M., COUSIN K., MCCAIN G.A. In vitro effects of two gold compounds, and D-penicillamine on the production of interferon-γ. Immunopharmacol. Immunotoxicol. 1990;12:39–60. doi: 10.3109/08923979009006460. [DOI] [PubMed] [Google Scholar]
- HASKO G., SZABO C. IL-12 as a therapeutic target for pharmacological modulation in immune-mediated and inflammatory diseases: regulation of T helper 1/T helper 2 responses. Br. J. Pharmacol. 1999;127:1295–1304. doi: 10.1038/sj.bjp.0702689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI M., MARTINEZ O.M., GARCIA-KENNEDY R., SO S., ESQUIVEL C.O., KRAMS S.M. Expression of cytokines and immune mediators during chronic liver allograft rejection. Transplantation. 1995;60:1533–1538. doi: 10.1097/00007890-199560120-00027. [DOI] [PubMed] [Google Scholar]
- INABA M., INABA K., FUKUDA Y., MORI S., HAUNA H., ADACHI Y., IWAI H., HOSAKA N., HISHA H., YAGITA H., IKEHARA S. Activation of thymic B cells by signals of CD40 molecules plus interleukin-10. Eur. J. Immunol. 1995;25:1244–1248. doi: 10.1002/eji.1830250517. [DOI] [PubMed] [Google Scholar]
- JEANNIN P., DELNESTE Y., SEVESO M., LIFE P., BONNEFOY J.Y. IL-12 synergizes with IL-2 and other stimuli in inducing IL-10 production by human T cells. J. Immunol. 1996;156:3159–3165. [PubMed] [Google Scholar]
- KANG B.Y., CHUNG S.W., KIM S.H., KANG S.N., CHOE Y.K., KIM T.S. Retinoid-mediated inhibition of interleukin-12 production in mouse macrophages suppresses Th1 cytokine in CD4+ T cells. Br. J. Pharmacol. 2000;130:581–586. doi: 10.1038/sj.bjp.0703345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANG S.N., LEE M.H., KIM K.-M., CHO D., KIM T.S. Induction of human promyelocytic leukemia HL-60 cell differentiation into monocytes by silibinin: involvement of protein kinase C. Biochem. Pharmacol. 2001;61:1487–1495. doi: 10.1016/s0006-2952(01)00626-8. [DOI] [PubMed] [Google Scholar]
- KIM T.S., DEKRUYFF R.H., RUPPER R., MAECKER H.T., LEVY S., UMETSU D.T. An ovalbumin-IL-12 fusion protein is more effective than ovalbumin plus free recombinant IL-12 in inducing a T helper cell type 1-dominated immune response and inhibiting antigen-specific IgE production. J. Immunol. 1997;158:4137–4144. [PubMed] [Google Scholar]
- KIM T.S., KIM S.H., HWANG S.Y. Injection with interleukin-4-secreting fibroblasts efficiently induces T helper type 2 cell-dominated immune response. Vaccine. 2000a;18:2832–2837. doi: 10.1016/s0264-410x(00)00075-x. [DOI] [PubMed] [Google Scholar]
- KIM W.U., MIN S.Y., CHO M.L., YOUN J., MIN D.J., LEE S.H., PARK S.H., CHO C.S., KIM H.Y. The role of IL-12 in inflammatory activity of patients with rheumatoid arthritis (RA) Clin. Exp. Immunol. 2000b;119:75–81. doi: 10.1046/j.1365-2249.2000.01095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAFAILLE J.J. The role of helper T cell subsets in autoimmune diseases. Cytokine Growth Factor Rev. 1998;9:139–151. doi: 10.1016/s1359-6101(98)00009-4. [DOI] [PubMed] [Google Scholar]
- LEAN W.F., HART L., BUCHANAN W.W. Auranofin. Br. J. Phamacol. 1997;36:560–572. doi: 10.1093/rheumatology/36.5.560. [DOI] [PubMed] [Google Scholar]
- LESTER M.R., HOFER M.F., GATELY M., TRUMBLE A., LEUNG D.Y. Down-regulating effects of IL-4 and IL-10 on the IFN-α response in atopic dermatitis. J. Immunol. 1995;154:6174–6181. [PubMed] [Google Scholar]
- LIU J., AKAHOSHI T., MAMAI R., MATSUI T., KONDO H. Effect of auranofin, an antirheumatic drug, on neutrophil apoptosis. Inflamm. Res. 2000;49:445–451. doi: 10.1007/s000110050615. [DOI] [PubMed] [Google Scholar]
- MAINI R.N., TAYLOR P.C. Anti-cytokine therapy for rheumatoid arthritis. Annu. Rev. Med. 2000;51:207–229. doi: 10.1146/annurev.med.51.1.207. [DOI] [PubMed] [Google Scholar]
- MARSHALL J.D., SECRIST H., DEKRUYFF R.H., WOLF S.F., UMESTU D.T. IL-12 inhibits the production of IL-4 and IL-10 in allergen-specific human CD4+ T lymphocytes. J. Immunol. 1995;155:111–117. [PubMed] [Google Scholar]
- MATTNER F., SMIROLDO S., GALBIATI F., MULLER M., DI LUCIA P., POLIANI P.L., MARTINO G., PANINA-BORDIGNON P., ADORINI L. Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1,25-dihydroxyvitamin D3. Eur. J. Immunol. 2000;30:498–508. doi: 10.1002/1521-4141(200002)30:2<498::AID-IMMU498>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- MORAHAN G., HUANG D., YMER S.I., CANCILLA M.R., STEPHEN K., DABADGHAO P., WERTHER G., TAIT B.D., HARRISON L.C., COLMAN P.G. Linkage disequilibrium of a type 1 diabetes susceptibility locus with a regulatory IL-12B allele. Nat. Genet. 2001;27:218–221. doi: 10.1038/84872. [DOI] [PubMed] [Google Scholar]
- MOSMANN T.R. Cytokine secretion phenotypes of TH cells: how many subsets, how much regulation. Res. Immunol. 1991;142:9–13. doi: 10.1016/0923-2494(91)90003-2. [DOI] [PubMed] [Google Scholar]
- MURPHY K.M., OUYANG W., FARRAR J.D., YANG J., RANGANATH S., ASNAGLI H., AFKARIAN M., MURPHU T.L. Signaling and transcription in T helper development. Annu. Rev. Immunol. 2000;18:451–494. doi: 10.1146/annurev.immunol.18.1.451. [DOI] [PubMed] [Google Scholar]
- NA S.-Y., KANG B.Y., CHUNG S.W., HAN S.-J., MA X., TRINCHIERI G., IM S.-Y., LEE J.W., KIM T.S. Retinoids inhibit interleukin-12 production in macrophages through physical associations of retinoid X receptor and NFκB. J. Biol. Chem. 1999;274:7674–7680. doi: 10.1074/jbc.274.12.7674. [DOI] [PubMed] [Google Scholar]
- O'GARRA A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–283. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- PANINA-BORDIGNON P., MAZZEO D., LUCIA P.D., D'AMBROSIO D., LANG R., FABBRI L., SELF C., SINIGAGLIA F. β2-agonists prevent Th1 development by selective inhibition of interleukin 12. J. Clin. Invest. 1997;100:1513–1519. doi: 10.1172/JCI119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEEVA E., FISHMAN A.D., GODDARD G., WADLER S., BARLAND P. Rheumatoid arthritis exacerbation caused by exogenous interleukin-12. Arthritis Rheum. 2000;43:461–463. doi: 10.1002/1529-0131(200002)43:2<461::AID-ANR29>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- PENNA G., ADORINI L. 1α,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J. Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- PETERS J.H., GIESELER N., THIELE B., STEINBACH Dendritic cells: from ontogenetic orphans to myelomonocytic descendants. Immunol. Today. 1996;17:273–278. doi: 10.1016/0167-5699(96)80544-5. [DOI] [PubMed] [Google Scholar]
- ROMAGNANI S. Th1 and Th2 in human diseases. Clin. Immunol. Immunopathol. 1996;80:225–235. doi: 10.1006/clin.1996.0118. [DOI] [PubMed] [Google Scholar]
- ROMAGNOLI P., SPINAS G.A., SINIGAGLIA F. Gold-specific T cells in rheumatoid arthritis patients treated with gold. J. Clin. Invest. 1992;89:254–258. doi: 10.1172/JCI115569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSSO-MARIE F. Macrophages and the glucocorticoids. J. Neuroimmunol. 1992;40:281–286. doi: 10.1016/0165-5728(92)90144-a. [DOI] [PubMed] [Google Scholar]
- SAKKAS L.I., JOHANSON N.A., SCANZELLO C.R., PLATSOUCAS C.D. Interleukin-12 is expressed by infiltrating macrophages and synovial lining cells in rheumatoid arthritis and osteoarthritis. Cell. Immunol. 1998;188:105–110. doi: 10.1006/cimm.1998.1363. [DOI] [PubMed] [Google Scholar]
- SEDER R.A., PAUL W.E. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu. Rev. Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- SHIBUYA K., ROBINSON D., ZONIN F., HARTLEY S.B., MACATONIA S.E., SOMOZA C., HUNTER C.A., MURPHY K.M., O'GARRA A. IL-1α and TNF-α are required for IL-12-induced development of Th1 cells producing high levels of IFN-γ in BALB/c but not C57BL/6 mice. J. Immunol. 1998;160:1708–1716. [PubMed] [Google Scholar]
- SIMON L.S. DMARDs in the treatment of rheumatoid arthritis: current agents and future development. Int. J. Clin. Pract. 2000;54:243–249. [PubMed] [Google Scholar]
- SKEEN M.J., ZIEGLER H.K. Activation of γδT cells for production of IFN-γ is mediated by bacteria via macrophage-derived cytokines IL-1 and IL-12. J. Immunol. 1995;154:5832–5841. [PubMed] [Google Scholar]
- TRINCHIERI G. Proinflammatory and immunoregulatory functions of interleukin-12. Int. Rev. Immunol. 1998;16:365–396. doi: 10.3109/08830189809043002. [DOI] [PubMed] [Google Scholar]
- UMETSU D.T., GIENI R., DEKRUYFF R.H. Effects of IL-12 in memory CD4+ T lymphocyte responses. Ann. N Y Acad. Sci. 1996;795:88–99. doi: 10.1111/j.1749-6632.1996.tb52658.x. [DOI] [PubMed] [Google Scholar]
- WINDHAGEN A., ANDERSON D.E., CATTIZOSA A., WILLIAMS R.E., HAFLER D.A. IL-12 induces human T cells secreting IL-10 with IFN-γ. J. Immunol. 1996;157:1127–1131. [PubMed] [Google Scholar]
- YOSHIMOTO T., TAKEDA K., TANAKA T., OHKUSU K., KASHIWAMURA S., OKAMURA H., AKIRA S., NAKANISHI K. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-γ. J. Immunol. 1998;161:3400–3407. [PubMed] [Google Scholar]


