Abstract
We analysed the effect of dantrolene (Dan) and five newly synthesized derivatives (GIFs) on Ca2+ release from the sarcoplasmic reticulum (SR) of mouse skeletal muscle.
In intact muscles, GIF-0185 reduced the size of twitch contraction induced by electrical stimulation to the same extent as Dan. GIF-0082, an azido-functionalized Dan derivative, also inhibited twitch contraction, although the extent of inhibition was less than that of Dan and of GIF-0185.
In skinned fibres, Dan inhibited Ca2+-induced Ca2+ release (CICR) under Mg2+-free conditions at room temperature. In contrast, GIF-0082 and GIF-0185 showed no inhibitory effect on CICR under the same conditions.
Dan-induced inhibition of CICR was not affected by the presence of GIF-0082, whereas it was diminished in the presence of GIF-0185.
GIF-0082 and GIF-0185 significantly inhibited clofibric acid (Clof)-induced Ca2+ release, as did Dan.
Several Dan derivatives other than GIF-0082 and GIF-0185 showed an inhibitory effect on twitch tension but not on the CICR mechanism. All of these derivatives inhibited Clof-induced Ca2+ release.
The magnitudes of inhibition of Clof-induced Ca2+ release by all Dan derivatives were well correlated with those of twitch inhibition. This supports the notion that the mode of Clof-induced opening of the RyR-Ca2+ release channel may be similar to that of physiological Ca2+ release (PCR).
These results indicate that the difference in opening modes of the RyR-Ca2+ release channel is recognized by certain Dan derivatives.
Keywords: Calcium release, clofibric acid, dantrolene
Introduction
Ryanodine receptor (RyR)-mediated Ca2+ release from the sarcoplasmic reticulum (SR) is an essential step in excitation-contraction coupling (E-C coupling) in skeletal and cardiac muscles. Purified RyR proteins incorporated into lipid bilayers show properties of the Ca2+-induced Ca2+ release (CICR) channel (Meissner, 1994). While CICR is thought to be the mechanism of Ca2+ release in cardiac E-C coupling (Näbauer et al., 1989; Bers & Perez-Reyes, 1999), it is not considered to be the mechanism of physiological Ca2+ release (PCR) in skeletal muscles E-C coupling (Endo, 1985; Schneider, 1994), because, for example, inhibitors of CICR such as procaine and adenine (in the presence of ATP) do not inhibit PCR (Thorens & Endo, 1975; Ishizuka et al., 1983). However, since mice with a targeted mutation of the skeletal muscle RyR (RyR1) gene lack E-C coupling (Takeshima et al., 1994), it must be concluded that PCR takes place through RyR1. This means that RyR1 can open in two different modes, PCR and CICR. In order to elucidate a molecular or submolecular mechanism of PCR that is distinct from CICR, specific inhibitors of PCR at the level of RyR1 would be very useful. However, no agents have been shown to inhibit PCR without also affecting CICR. Some specific inhibitors of CICR mentioned above, and other agents that inhibit both PCR and CICR, such as tetracaine or ruthenium red, are known (Almers & Best, 1976; Ohnishi, 1979; Smith et al., 1986).
Dantrolene sodium (Dan) was originally reported to inhibit E-C coupling of skeletal muscle (Ellis & Bryant, 1972; Ellis & Carpenter, 1972). It was then found to be an effective agent for the treatment of porcine and human malignant hyperthermia (MH), a pathophysiological state in susceptible individuals caused by an excessive release of Ca2+ through the RyR1 activated by inhalation anaesthetics (Harrison, 1975; Kolb et al., 1982; MacLennan & Phillips, 1992). MH was shown to be a consequence of an excessive CICR (Endo et al., 1983), and it was demonstrated that Dan, in addition to inhibiting PCR, can effectively inhibit CICR at 37°C (Ohta & Endo, 1986; Ohta et al., 1989). Interestingly, it was then found that at room temperature, Dan does not inhibit CICR, although it inhibits E-C coupling at 37°C and at room temperature equally (Ohta & Endo, 1986; Kobayashi & Endo, 1988; Ohta et al., 1990). The results described in this study are somewhat different from these reports. However, they do indicate that under a certain experimental condition, Dan somehow preferentially inhibits PCR at room temperature.
In the present study, we examined the effects of several newly synthesized Dan derivatives with the goal of finding some agents that more clearly distinguish between PCR and CICR than Dan. We tested these agents for inhibition of twitch tension of intact muscle fibres and inhibition of CICR in saponin-treated skinned muscle fibres. We also tested for their effects on clofibric acid (Clof)-induced Ca2+ release, which has certain characteristics similar to those of PCR (Ikemoto & Endo, 2001, accompanying study). We found that some Dan derivatives indeed distinguish PCR from CICR more clearly than Dan itself. In addition, the data demonstrate further similarity of the mode of opening of RyR-Ca2+ release channels activated by Clof to the mode of PCR.
Methods
Preparation of muscle fibres
Male ICR mice (5 – 9 weeks) were anaesthetized with 50 – 80 mg kg−1 sodium pentobarbitone (i.p.) and decapitated. The extensor digitorum longus muscles (EDL) were excised. One of the four heads of EDL was carefully isolated in a physiological saline solution (PSS, in mM: NaCl 150; KCl 4; CaCl2 1.8; MgCl2 1; 2-[4-(2-hydroxyethyl)-1-piperazinyl]ethanesulphonic acid (HEPES) 5; glucose 5.6; pH 7.4). The bundles were tied at both ends with silk threads and mounted between a pair of stainless steel rods that attached to a force transducer (AE801, Akers, Norway or UL-10GR, NMB, Japan) and a micromanipulator (Narishige, Japan). The muscle preparation was immersed in PSS.
Preparation of skinned fibres was described previously (Ikemoto & Endo, 2001, accompanying study). All experiments were carried out at room temperature (21 – 23°C).
Measurement of tension in intact fibres
Supramaximal field electrical stimulation (20 V, 0.5 ms duration) was delivered by a pair of platinum wires placed on both sides of the preparation. After twitch tension became constant in PSS containing D-tubocurarine (25 μM) and 0.2% dimethyl sulphoxide (DMSO), the external solution was changed to a PSS containing dantrolene (Dan) or its derivatives, stock solutions of which were made by dissolving the agents in DMSO as described below. After the twitch tension became constant during a 15 – 30 min treatment period, it was compared with that measured before treatment. D-tubocurarine was present throughout these experiments. Statistical significance was tested by Dunnett's test for multiple comparisons (Figure 2a).
Figure 2.
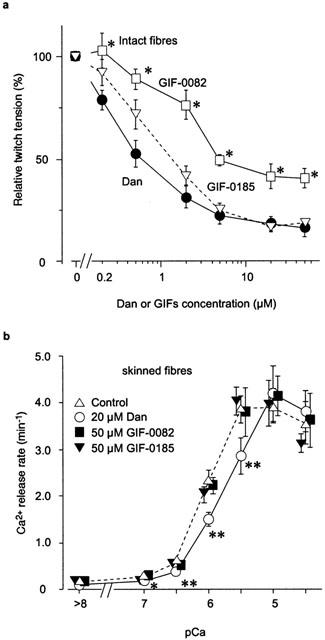
Effects of Dan, GIF-0082, and GIF-0185 on Ca2+ release. (a) In intact fibres, after treatment with dantrolene (Dan), GIF-0082, or GIF-0185 in the concentration range of 0.2 – 50 μM, the size of twitch contraction was compared with that before the treatment. Relative tensions were plotted against the concentrations. (mean±s.e.mean, n=9 for Dan, n=3 – 6 for GIF-0082, n=3 – 6 for GIF-0185). *P<0.05 (Dunnett's test). (b) The rates of Ca2+ release were obtained in saponin-skinned fibres with 20 μM Dan (n=5), 50 μM GIF-0082 (n=3 – 7) or 50 μM GIF-0185 (n=3 – 4) in the absence of Mg2+ and adenine nucleotide at pCa>8 – 4.5. Open triangles show the rates without these agents (control, n=11 – 16). The symbols for GIF-0082 and GIF-0185 were slightly offset horizontally for clarity (mean±s.e.mean). *P<0.05, **P<0.01 (vs control). Error bars smaller than the symbols are not shown.
Measurement of Ca2+ release in skinned fibres
The Ca2+ released from the skinned fibres was quantified using Fura-2 as described previously (Ikemoto & Endo, 2001, accompanying study). Briefly, the protocol for the measurement of the Ca2+ release rate consisted of (1) loading the SR with Ca2+, (2) test (Ca2+-releasing stimuli are given), and (3) determination of the Ca2+ remaining in the SR. During the test period, various concentrations of Ca2+ buffered with 10 mM EGTA, sometimes together with Clof or CICR activators (caffeine or AMP), were given, and the effects of further addition of Dan or its derivatives were examined. The activity of Ca2+ release was expressed in terms of the decay rate constants based on the amount of Ca2+ in the SR. Stock solutions of Dan or its derivatives were dissolved in DMSO to obtain concentrations of 0.1 – 25 mM (Dan, GIF-0082, and GIF-0185) or 25 mM (other derivatives). All test solutions with or without these agents were adjusted to contain 0.2% DMSO except for those used in the experiments performed to determine the effects of GIFs on Dan-induced inhibition of CICR activity; in those experiments, the concentration of DMSO in the test solution was kept at 0.4%. Statistical significance was tested using the paired t-test.
Synthesis of Dan derivatives
Dan derivatives, which we referred to as GIF-0082 (Figure 1b) and GIF-0163 (Figure 1e), were prepared by coupling Dan (Figure 1a) with (3-azido-5-iodo)benzyl bromide or benzyl bromide, respectively. GIF-0146 (Figure 1c), which is an iodo-congener of dantrolene, was prepared from 4-iodoaniline by the conventional method reported by Snyder et al. (1967). The methoxy analogue of Dan, GIF-0185 (Figure 1c), was synthesized by the Stille reaction (Stille, 1986) of 4-iodoanisole and 5-(tri-n-butylstannyl)-2-furaldehyde (Denat et al., 1992), followed by condensation with 1-aminohydantoin (Traube & Hoffa, 1898). GIF-0149-R (Figure 1d) was obtained by condensation of 5-(4-nitrophenyl)-2-furaldehyde with racemic 1-amino-5-(6-methoxycarbonylhexyl)hydantoin (Barraclough et al., 1994). All of the newly synthesized GIF compounds were purified by recrystallization (>99% purity) and fully characterized by 1H- and 13C-NMR (nuclear magnetic resonance) spectroscopy, IR (infrared) spectroscopy, UV (ultraviolet) spectroscopy, and combustion analysis. The details of the synthesis of GIFs will be reported elsewhere (manuscript in preparation).
Figure 1.
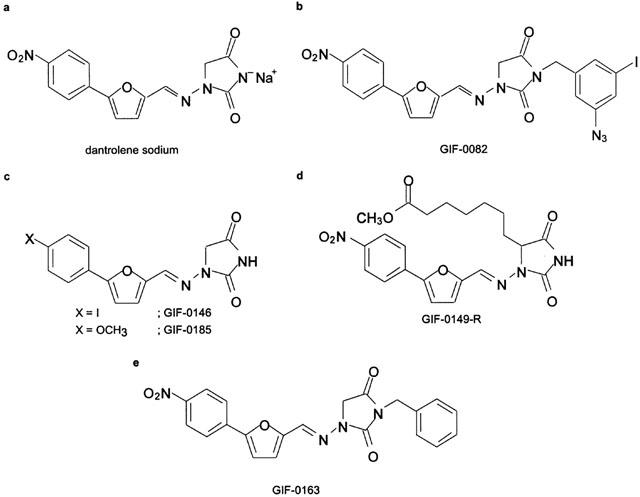
Structures of dantrolene and its derivatives. (a) The structure of dantrolene sodium (Dan). (b) N-(3-azido-5-iodo)benzylated Dan derivative, which is referred to as GIF-0082, was designed as a photoaffinity probe by incorporation of azido group as a photoreactive unit and iodine. (c) GIF-0146 and GIF-0185 are Dan analogues, in which the substituent on the phenyl ring is changed from an electron-withdrawing nitro group to electron-donating iodo and methoxy groups, respectively. (d) GIF-0149-R is another type of Dan derivative in which an alkyl chain is anchored on the hydantoin ring. (e) GIF-0163, N-benzyldantrolene, is the structurally more simplified compound of GIF-0082.
Other materials
Dantrolene sodium was a gift from Yamanouchi Pharmaceutical Co., Ltd. (Japan) or purchased from Nacalai Tesque, Inc. (Japan). D-tubocurarine chloride was purchased from Nacalai Tesque, Inc. All the other chemicals were as described in the previous paper (Ikemoto & Endo, 2001, accompanying study).
Results
Effects of Dan and GIFs on Ca2+ release in intact fibres and on CICR in skinned fibres
We designed and synthesized N-(3-azido-5-iodo)benzylated dantrolene, referred to as GIF-0082, which carries an azido group as a photoreactive unit (Kotzyba-Hibert et al., 1995). As shown in Figure 2a, GIF-0082 inhibited the twitch tension of intact skeletal muscle in a dose-dependent manner (0.2 – 50 μM, open squares), although the extent of inhibition was less than that produced by Dan (Figure 2a, filled circles). GIF-0185, another Dan derivative, also inhibited the twitch tension to the same extent as Dan (Figure 2a, open triangles). Since GIF-0082 and GIF-0185 showed no inhibitory effect on the Ca2+ uptake activity in the SR or on the relationship between pCa and tension in skinned fibres (data not shown) as was the case with Dan (Ohta et al., 1990), these data indicate that both of these GIFs inhibit the physiological Ca2+ release (PCR) from the SR in intact muscles.
Figure 2b shows the effects of Dan (open circles), GIF-0082 (filled squares), and GIF-0185 (filled triangles) on the Ca2+-induced Ca2+ release (CICR) in skinned fibres of mouse skeletal muscles. The rates of Ca2+ release were significantly decreased by the application of Dan, although it exhibited little effect on Ca2+ release at high Ca2+ concentrations (open triangles vs open circles). The rates of Ca2+ release at pCa 6.0 were decreased by approximately 50% in the presence of high concentrations of Dan (20 – 50 μM, Figure 4a, open circles). These data indicated that Dan had an inhibitory effect on CICR even at room temperature, in apparent disagreement with a previous report (Ohta et al., 1990). In contrast, the rates of CICR were not significantly changed by the GIFs (50 μM) at any Ca2+ concentration (Figure 2b, open triangles vs filled squares and filled triangles). Thus, these GIFs can distinguish CICR from PCR much more clearly than Dan.
Figure 4.
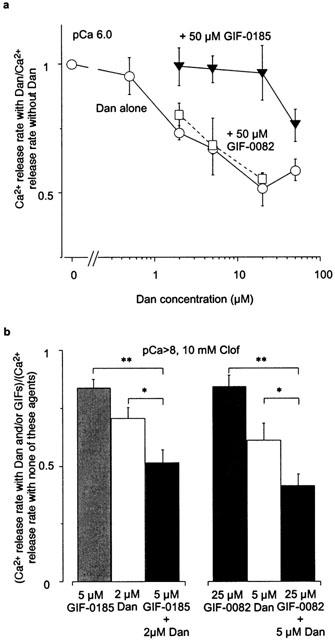
Different effect of GIF-0082 and GIF-0185 on Ca2+ release. (a) In skinned fibres, the rates of Ca2+ release measured with Dan (0 – 50 μM) at pCa 6.0 under Mg2+-free conditions were normalized to the control value (without Dan) obtained in the same preparation in the absence (Dan alone, mean±s.e.mean, n=3 – 6) or presence of 50 μM GIF-0082 (n=3) or GIF-0185 (n=5). (b) The rates of Ca2+ release by 10 mM Clof in the presence of Dan (2 or 5 μM, mean±s.e.mean) and/or either GIF-0185 (5 μM) or GIF-0082 (25 μM, n=4) under Ca2+, Mg2+-free conditions (buffered with 10 mM EGTA) were normalized to the control value (without these agents) within each preparation. *P<0.05, **P<0.01.
Effect of Dan and GIF-0082 on Clof-induced Ca2+ release in skinned fibres
We have reported that the opening mode of the RyR channel activated by Clof in skinned fibres has some features similar to those of PCR, but different from those of CICR (Ikemoto & Endo, 2001, accompanying study). If the similarity applies also to GIF-0082 and GIF-0185, they may inhibit Clof-induced Ca2+ release as well. Therefore, in skinned fibres, we examined the effect of GIF-0082 on Clof-induced Ca2+ release at pCa>8. As shown in Figure 3a, the rates of Clof (>10 mM)-induced Ca2+ release were significantly decreased by GIF-0082 (50 μM). On the other hand, GIF-0082 exerted no effect on the Ca2+ release rate in the presence or absence of caffeine (Figure 3b), in accordance with the absence of effects on CICR (Figure 2b), while Dan significantly decreased the Ca2+ release rate under the same conditions (Figure 3b). The results were made clearer by normalizing the rate with GIF-0082 or Dan by the rates without these inhibitors in the same fibres (Figure 3c). Although the AMP-stimulated Ca2+ release rate was not significantly inhibited by Dan, its normalized rate may suggest that it is reduced by Dan. It is also seen that the extent of inhibition by GIF-0082 was greater, at higher Clof concentrations. GIF-0185 also inhibited Clof-induced Ca2+ release, as described in the next section.
Figure 3.
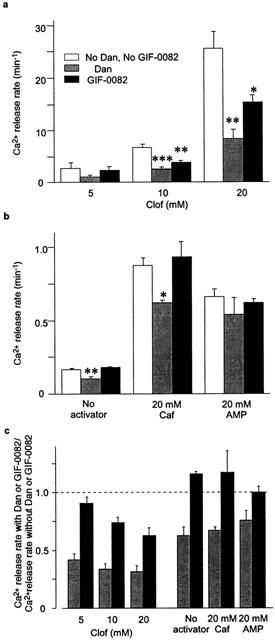
Effects of Dan and GIF-0082 on caffeine- and clofibric acid-induced Ca2+ release in skinned fibres. The rates of Ca2+ release with 20 μM Dan or 50 μM GIF-0082 were measured under Mg2+- and Ca2+-free conditions (buffered with 10 mM EGTA) with 5 – 20 mM clofibric acid (Clof, Figure 3a, mean±s.e.mean, n=3 – 6) or with or without CICR activators (caffeine, Caf; AMP; Figure 3b, n=5 – 7). Note the difference in the scales. (c) The results in a and b were normalized to the control value (without Dan or GIF-0082) within each preparation. *P<0.05, **P<0.01, ***P<0.001.
Effects of GIF-0082 and GIF-0185 on Dan-induced inhibition of Ca2+ release
It is not surprising that while GIF-0082 and GIF-0185 do not inhibit CICR at all, they inhibit PCR, because it is thought that the PCR from the SR is not mediated by the CICR mechanism (Endo, 1985; Schneider, 1994). On the other hand, it is possible that in preparations of skinned fibres, molecule(s) responsible for the action of GIFs may have been lost. This is very unlikely, however, since Clof-induced Ca2+ release in skinned fibres is well affected by GIFs as just described. The following experiments also provide support that this theory is unlikely.
Dan decreased the rates of CICR in skinned fibres in a dose-dependent manner (2 – 50 μM, Figure 4a, open circles). Although GIF-0082 and GIF-0185 could not inhibit the CICR as shown in Figure 2b, the inhibitory action of low concentrations of Dan (2 – 20 μM) were completely abolished by the presence of GIF-0185 (Figure 4a, filled triangles). On the other hand, Dan-induced inhibition of CICR was not affected by the presence of GIF-0082 (Figure 4a, open squares). The abolition of the Dan effect on CICR by GIF-0185 in skinned fibres clearly shows that CICR in skinned fibres retains some sensitivity to GIF-0185.
We further studied the effect of co-applications of GIF-0082 or GIF-0185 and Dan on Clof-induced Ca2+ release. As shown in Figure 4b, both GIF-0082 (25 μM) and GIF-0185 (5 μM) significantly inhibited Clof-induced Ca2+ release. We confirmed that these GIFs showed no inhibitory effect on CICR at the same concentrations (data not shown). Dan (2 and 5 μM) also inhibited Clof-induced Ca2+ release (Figure 4b). Simultaneous applications of GIF-0082 or GIF-0185 and Dan showed additive inhibitory effects (Figure 4b, filled column).
Effect of other Dan derivatives on the Ca2+ release
Next we examined the effect of Dan derivatives other than GIF-0082 and GIF-0185. Among the many derivatives we tested, GIF-0146 and GIF-0149-R inhibited twitch tension in intact fibres, while GIF-0163 had little effect (Figure 5a). The magnitude of inhibitory action of these derivatives and Dan on twitch tension was in the following sequence: Dan (20 μM)=GIF-0185 (50 μM)>GIF-0082 (50 μM)>GIF-0146 (50 μM)⩾GIF-0149-R (50 μM)>GIF-0163 (50 μM) (Figures 2a and 5a). On the other hand, in skinned fibres, the rates of CICR at pCa 6 were not decreased with any of these derivatives (Figure 5b).
Figure 5.
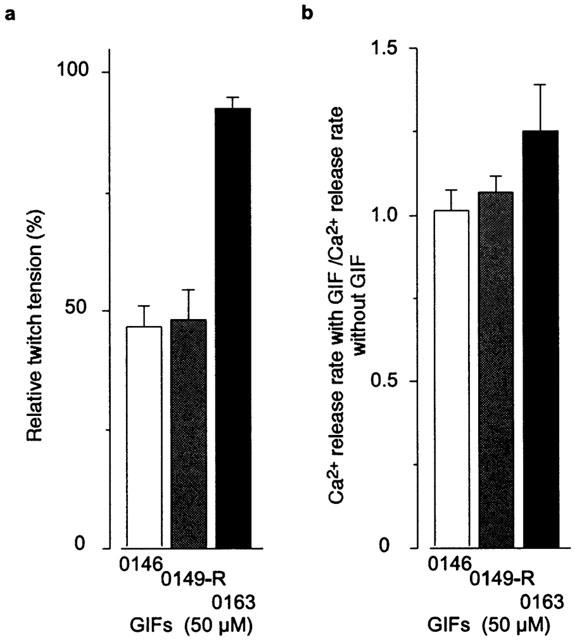
Effects of some Dan derivatives on Ca2+ release. (a) The sizes of twitch contraction after treatment of GIF-0146, GIF-0149-R, GIF-0163, or GIF-0185 (50 μM) were compared with those before treatment in intact muscle. (b) In skinned fibres, the rates of Ca2+ release measured with these derivatives at pCa 6.0 in the absence of Mg2+ were normalized to the control value (without the derivatives) within each preparation (mean±s.e.mean, n=3 – 4 for a, n=3 for b).
We also tested the effect of these derivatives on the Clof-induced Ca2+ release in skinned fibres. In order to mimic the physiological condition, Clof was applied at pCa 7 in the presence of 0.5 mM Mg2+ and 10 mM AMP. As shown in Figure 6a, all of the derivatives that showed the inhibitory effect on twitch contraction decreased the rates of Clof (20 mM)-induced Ca2+ release (Figure 6a). On the other hand, caffeine-induced Ca2+ release (Caf, 20 mM) was not significantly inhibited by these derivatives, although the rates appeared slightly reduced (Figure 6b).
Figure 6.
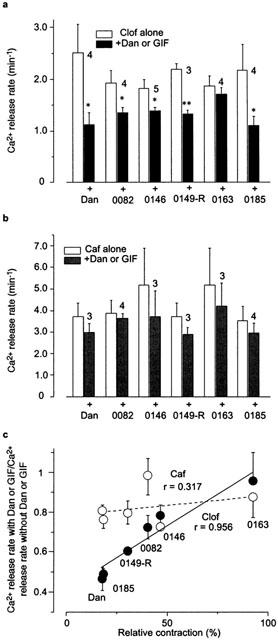
Correlation between the rates of Ca2+ release in skinned fibres and twitch contraction in the presence of Dan derivatives. In skinned fibres, the rates of Ca2+ release with Dan (20 μM) or GIFs (50 μM) were measured at pCa 7.0 with 20 mM Clof (a) or caffeine (Caf, b) in the test solution containing 0.5 mM Mg2+ and 10 mM AMP (mean±s.e.mean, n=3 – 5 for a, n=3 – 4 for b). (c) These results were normalized to the control value (without Dan or its derivatives) and plotted against the mean value of relative twitch contraction at 20 μM Dan or 50 μM other derivatives from Figures 2a and 5a. The data for Caf or Clof were fitted by a least square method, and the correlation coefficients were 0.317 and 0.956, respectively. *P<0.05, **P<0.01.
The extent of reduction of the rates of Clof-induced or caffeine-induced Ca2+ release in skinned fibres by Dan and its derivatives was compared with that of the twitch tension in intact fibres (Figure 6c). The magnitude of inhibition of twitch contraction was in good agreement with that of the inhibition of Clof-induced Ca2+ release (Figure 6c, filled circles), while no correlation was seen between the twitch tension and the rate of caffeine-induced Ca2+ release (Figure 6c, open circles).
In conclusion, we showed that some Dan derivatives specifically inhibited PCR and Clof-induced Ca2+ release without appreciable inhibition of CICR.
Discussion
We analysed the effects of Dan and some newly synthesized derivatives on twitch tension (indicating PCR) of intact fibres and CICR in skinned fibres of mouse skeletal muscle. We demonstrated that several Dan derivatives did not inhibit CICR at all, but definitely inhibited twitch tension (Figures 2 and 4). Since twitch inhibition of these Dan derivatives is most likely to be the result of their action on the Ca2+-release mechanism of the SR, they appear to distinguish PCR and CICR clearly.
The notion that these Dan derivatives directly affect the Ca2+ release mechanism of the SR was supported by the fact that they inhibited Clof-induced Ca2+ release in skinned fibres (Figures 3, 4, and 6). In the accompanying study, we demonstrated that Clof-induced Ca2+ release has some features similar to those of PCR (Ikemoto & Endo, 2001, accompanying study). The results of the present report provide a further similarity: the magnitude of twitch inhibition by Dan and its derivatives that is most likely to indicate the magnitude of PCR inhibition is very closely correlated with the magnitude of inhibition of Clof-induced Ca2+ release, but not with that of CICR inhibition (Figure 6).
Difference of Dan effect on CICR at room temperature between the present and previous results
We showed that the CICR in skinned fibres from mouse skeletal muscle was significantly inhibited by Dan (20 μM) at room temperature, which is in disagreement with the previous report (Ohta et al., 1990). The inhibitory effect of Dan on CICR was reduced by increasing Mg2+ concentrations, and it was abolished in the presence of 0.5 mM Mg2+ (unpublished observation), and some of the previous results were obtained in presence of Mg2+ (>0.5 mM) (Ohta & Endo, 1986; Ohta et al., 1990). However, Ohta et al. (1990) reported that Dan (50 μM) showed no inhibitory effect on CICR in skinned fibres of skeletal muscles from guinea-pigs even in the absence of Mg2+ at room temperature (20°C). The reason for the discrepancy is presently unknown. It may be a consequence of the difference in animal species used.
Usefulness of Dan and its derivatives on the study of Ca2+ release
Recently, Dan has been used in a wide variety of cells other than skeletal muscles as an inhibitor of intracellular Ca2+ release (Hui, 1983; Shoshan-Barmatz et al., 1991; Partridge & Valenzuela, 1999; Seutin et al., 2000). However, the exact mechanism of action of Dan has remained unclear, not only in skeletal muscles, but also in other types of cells. For example, it is not clear whether the inhibitory action of Dan on Ca2+ release results from direct binding to the RyR (Parness & Palnitkar, 1995; Palnitkar et al., 1997; Fruen et al., 1997). Therefore, it is important to elucidate the molecular mechanism of Dan-induced inhibition of the Ca2+ release via the RyR.
Some of the Dan derivatives we synthesized inhibited PCR without an inhibitory effect on CICR. They inhibited Clof-induced Ca2+ release in skinned fibres, an effect that was significantly correlated with their inhibitory effect on twitch tension (Figure 6c). In addition, GIF-0185 diminished the inhibition by Dan on CICR, although it showed no inhibitory effect on CICR (Figures 2b and 3a). These results indicate that the target(s) of Dan and its derivatives are clearly retained in skinned fibres. Among these specific PCR inhibitors at room temperature (21 – 23°C), GIF-0082, with its azido-functionalized and iodinated structure, is applicable to photo-affinity and its binding experiment on target molecule(s). Since GIF-0082 does not interfere with the inhibitory action of Dan on CICR (unlike GIF-0185), it might well be more specific to PCR than GIF-0185. We, therefore, think that this agent may be a useful probe to study PCR of skeletal muscles in various preparations including isolated SR membranes and the purified RyR.
Acknowledgments
We wish to thank Dr M. Iino (University of Tokyo, Japan) for reading the manuscript. This work was supported by a Grant-in-Aid from the Ministry of Education, Science, Sports, and Culture of Japan.
Abbreviations
- CICR
Ca2+-induced Ca2+ release
- Clof
clofibric acid
- Dan
dantrolene
- MH
malignant hyperthermia
- PCR
physiological Ca2+ release
- RyR
ryanodine receptor
- SR
sarcoplasmic reticulum
References
- ALMERS W., BEST P.M. Effects of tetracaine on displacement currents and contraction of frog skeletal muscle. J. Physiol. 1976;262:583–611. doi: 10.1113/jphysiol.1976.sp011611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRACLOUGH P., BROCKWELL M., CALDWELI A.G., DEMAINE D.A., HARRIS C.J., KING W.R., STEPNEY R.J., WHARTON C.J., WHITTLE B.J.R. Synthesis and inhibitory activity on platelet aggregation of 13′-aza and other ω-chain modified BW245C analogs. Arch. Pharmacol. 1994;327:307–317. doi: 10.1002/ardp.19943270508. [DOI] [PubMed] [Google Scholar]
- BERS D.M., PEREZ-REYES E. Ca channels in cardiac myocytes: structure and function in Ca influx and intracellular Ca release. Cardiovasc. Res. 1999;42:339–360. doi: 10.1016/s0008-6363(99)00038-3. [DOI] [PubMed] [Google Scholar]
- DENAT F., GASPARD-ILOUGHMANE H., DUBAC J. An easy one-pot synthesis of group 14 C-metallated 2 (or 3)-furan- and thiophenecarbaldehydes. Synthesis. 1992;1992:954–956. [Google Scholar]
- ELLIS K.O., BRYANT S.H. Excitation-contraction uncoupling in skeletal muscle by dantrolene sodium. Naunyn-Schmiedeberg's Arch. Pharmacol. 1972;274:107–109. doi: 10.1007/BF00501011. [DOI] [PubMed] [Google Scholar]
- ELLIS K.O., CARPENTER J.F. Studies on the mechanism of action of dantrolene sodium. A skeletal muscle relaxant. Naunyn-Schmiedeberg's Arch. Pharmacol. 1972;275:83–94. doi: 10.1007/BF00505069. [DOI] [PubMed] [Google Scholar]
- ENDO M. Ca2+ release from sarcoplasmic reticulum. Curr. Top. Membr. Transp. 1985;25:181–230. [Google Scholar]
- ENDO M., YAGI S., ISHIZUKA T., HORIUTI K., KOGA Y., AMAHA K. Changes in the Ca-induced Ca release mechanism in the sarcoplasmic reticulum of muscle from patient with malignant hyperthermia. Biomed. Res. 1983;4:83–92. [Google Scholar]
- FRUEN B.R., MICKELSON J.R., LOUIS C.F. Dantrolene inhibition of sarcoplasmic reticulum Ca2+ release by direct and specific action at skeletal muscle ryanodine receptors. J. Biol. Chem. 1997;272:26965–26971. doi: 10.1074/jbc.272.43.26965. [DOI] [PubMed] [Google Scholar]
- HARRISON G.G. Control of the malignant hyperpyrexic syndrome in MHS swine by dantrolene sodium. Br. J. Anesth. 1975;47:62–65. doi: 10.1093/bja/47.1.62. [DOI] [PubMed] [Google Scholar]
- HUI C.S. Pharmacological studies of charge movement in frog skeletal muscle. J. Physiol. 1983;337:509–529. doi: 10.1113/jphysiol.1983.sp014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIZUKA T., IIJIMA T., ENDO M. Effects of adenine on twitch and other contractile response of single fibers of amphibian fast skeletal muscle. Proc. Jpn. Acad. 1983;59:97–100. [Google Scholar]
- IKEMOTO T., ENDO M. Properties of Ca2+ release induced by clofibric acid from the sarcoplasmic reticulum of mouse skeletal muscle fibres. Br. J. Pharmacol. 2001;134:719–728. doi: 10.1038/sj.bjp.0704306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOBAYASHI T., ENDO M. Temperature-dependent inhibition of caffeine contracture of mammalian skeletal muscle by dantrolene. Proc. Jpn. Acad. 1988;64:76–79. [Google Scholar]
- KOLB M.E., HORNE M.L., MARTZ R. Dantrolene in human malignant hyperthermia. Anesthesiology. 1982;56:254–262. doi: 10.1097/00000542-198204000-00005. [DOI] [PubMed] [Google Scholar]
- KOTZYBA-HIBERT F., KAPFER I., GOELDNER M. Recent Trends in Photoaffinity Labeling. Angew. Chem., Int. Ed. Engl. 1995;34:1296–1312. [Google Scholar]
- MACLENNAN D.H., PHILLIPS M.S. Malignant hyperthermia. Science. 1992;256:789–794. doi: 10.1126/science.1589759. [DOI] [PubMed] [Google Scholar]
- MEISSNER G. Ryanodine receptor/Ca2+ release channels and their regulation by endogenous effectors. Annu. Rev. Physiol. 1994;56:485–508. doi: 10.1146/annurev.ph.56.030194.002413. [DOI] [PubMed] [Google Scholar]
- NÄBAUER M., CALLEWAERT G., CLEEMANN L., MORAD M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science. 1989;244:800–803. doi: 10.1126/science.2543067. [DOI] [PubMed] [Google Scholar]
- OHNISHI S.T. Interaction of metallochromic indicators with calcium sequestering organelles. Biochim. Biophys. Acta. 1979;585:315–319. doi: 10.1016/0304-4165(79)90031-x. [DOI] [PubMed] [Google Scholar]
- OHTA T., ENDO M. Inhibition of calcium-induced calcium release by dantrolene at mammalian body temperature. Proc. Jpn. Acad. 1986;62:329–332. [Google Scholar]
- OHTA T., ENDO M., NAKANO T., MOROHOSHI Y., WANIKAWA K., OHGA A. Ca-induced Ca release in malignant hyperthermia-susceptible pig skeletal muscle. Am. J. Physiol. 1989;256:C358–C367. doi: 10.1152/ajpcell.1989.256.2.C358. [DOI] [PubMed] [Google Scholar]
- OHTA T., ITO S., OHGA A. Inhibitory action of dantrolene on Ca-induced Ca2+ release from sarcoplasmic reticulum in guinea pig skeletal muscle. Eur. J. Pharmacol. 1990;78:11–19. doi: 10.1016/0014-2999(90)94788-y. [DOI] [PubMed] [Google Scholar]
- PALNITKAR S.S., MICKELSON J.R., LOUIS C.F., PARNESS J. Pharmacological distinction between dantrolene and ryanodine binding sites: evidence from normal and malignant hyperthermia-susceptible porcine skeletal muscle. Biochem. J. 1997;326:847–852. doi: 10.1042/bj3260847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARNESS J., PALNITKAR S.S. Identification of dantrolene binding sites in porcine skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 1995;270:18465–18472. doi: 10.1074/jbc.270.31.18465. [DOI] [PubMed] [Google Scholar]
- PARTRIDGE L.D., VALENZUELA C.F. Ca2+ store-dependent potentiation of Ca2+-activated non-selective cation channels in rat hippocampal neurones in vitro. J. Physiol. 1999;521:617–627. doi: 10.1111/j.1469-7793.1999.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEIDER M.F. Control of calcium release in functioning skeletal muscle fibers. Annu. Rev. Physiol. 1994;56:463–484. doi: 10.1146/annurev.ph.56.030194.002335. [DOI] [PubMed] [Google Scholar]
- SEUTIN V., MKAHLI F., MASSOTTE L., DRESSE A. Calcium release from internal stores is required for the generation of spontaneous hyperpolarizations in dopaminergic neurons of neonatal rats. J. Neurophysiol. 2000;83:192–197. doi: 10.1152/jn.2000.83.1.192. [DOI] [PubMed] [Google Scholar]
- SHOSHAN-BARMATZ V., PRESSLEY T.A., HIGHAM S., KRAUS-FRIEDMANN N. Characterization of high-affinity ryanodine-binding sites of rat liver endoplasmic reticulum. Differences between liver and skeletal muscle. Biochem. J. 1991;276:41–46. doi: 10.1042/bj2760041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH J.S., CORONADO R., MEISSNER G. Single channel measurements of the calcium release channel from skeletal muscle sarcoplasmic reticulum: activation by Ca2+ and ATP and modulation by Mg2+ J. Gen. Physiol. 1986;88:573–588. doi: 10.1085/jgp.88.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNYDER H.R., JR, DAVIS C.S., BICKERTON R.K., HALLIDAY R.P. 1-[(5-Arylfurfurylidene)amino]hydantoins. A new class of muscle relaxants. J. Med. Chem. 1967;10:807–810. doi: 10.1021/jm00317a011. [DOI] [PubMed] [Google Scholar]
- STILLE J.K. The palladium-catalyzed cross-coupling reactions of organotin reagents with organic electrophiles. Angew. Chem., Int. Ed. Engl. 1986;25:508–524. [Google Scholar]
- TAKESHIMA H., IINO M., TAKEKURA H., NISHI M., KUNO J., MINOWA O., TAKANO H., NODA T. Excitation-contraction uncoupling and muscular degeneration in mice lacking functional skeletal muscle ryanodine receptor gene. Nature. 1994;369:556–559. doi: 10.1038/369556a0. [DOI] [PubMed] [Google Scholar]
- TRAUBE W., HOFFA E. Ueber die Hydrazinoessigsäure. II. Chem. Ber. 1898;31:162–169. [Google Scholar]
- THORENS S., ENDO M. Calcium-induced calcium release and “depolarization”-induced calcium release: Their physiological significance. Proc. Jpn. Acad. 1975;51:473–478. [Google Scholar]


