Abstract
Endostatin is a potent endogenous inhibitor of angiogenesis that was recently shown to be stored in platelets and released in response to thrombin, but not ADP. In the present study, we have tested the hypothesis that thrombin-induced endostatin release from rat platelets is mediated via proteinase-activated receptor-4 (PAR4). Immunoprecipitation and Western blotting confirmed that endostatin is contained within rat platelets. Aggregation and release of endostatin could be elicited by thrombin (0.5 – 1.0 U ml−1) or by specific PAR4 agonist (AYPGKF-NH2; AY-NH2; 15 – 50 μM). Significant release of endostatin could be induced by a dose of thrombin below that necessary for induction of aggregation. An adenosine diphosphate (ADP) scavenger, apyrase, inhibited the platelet aggregation induced by thrombin, but not the release of endostatin. In contrast, a selective PAR4 antagonist (trans-cinnamoyl-YPGKF-NH2; tcY-NH2) prevented endostatin release and aggregation induced by thrombin or by AY-NH2. We conclude that thrombin-induced endostatin release from rat platelets is PAR4-mediated via an ADP-independent mechanism that can occur independently of platelet aggregation.
Keywords: Platelet endostatin release, thrombin, PAR4
Introduction
The activation of platelets by thrombin is mediated at least in part by cleavage of proteinase-activated receptors (PARs). Four distinct PARs have been cloned, with PAR1, PAR3 and PAR4 being activated by thrombin. Human platelets express PAR1 and PAR4 and activation of either is sufficient to trigger platelet aggregation and secretion (Vu et al., 1991; Xu et al., 1998; Kahn et al., 1999), whereas murine platelets express PAR3 and PAR4, and thrombin signalling is PAR4-dependent (Nakanishi-Matsui et al., 2000). PAR4 is activated when thrombin cleaves its amino-terminal exodomain to unmask a tethered ligand (Kahn et al., 1998). GYPGKF represents the first six amino acids of the new amino terminus unmasked when thrombin cleaves murine PAR4. A selective PAR4 agonist, AY-NH2, is 10 times more potent in activating platelets than a peptide with the sequence of the natural amino terminus (Faruqi et al., 2000; Hollenberg & Saifeddine, 2001). Recently, a selective PAR4 antagonist (tcY-NH2) was described which inhibits thrombin- and AY-NH2-induced rat platelet aggregation (Hollenberg & Saifeddine, 2001).
A variety of bioactive substances, including growth factors and chemokines (Linder et al., 1979; Maloney et al., 1998; von hundelshausen et al., 2001), are stored in platelets and released during activation. Endostatin, a potent inhibitor of angiogenesis (O'reilly et al., 1997), has been shown recently to be contained within platelets and to be released in response to thrombin but not to ADP (Ma et al., 2001). Moreover, endostatin released from platelets was found to play a significant role in modulating gastric ulcer healing in the rat (Ma et al., 2001). However, the mechanism through which thrombin produces this effect, and in particular the PAR receptor involved, are unknown. In the present study, we have demonstrated, using the selective PAR4 receptor antagonist tcY-NH2, that in rat platelets thrombin-induced endostatin release is mediated through PAR4 and occurs independently of thrombin-induced aggregation and of ADP release.
Methods
Reagents were obtained from the following sources: AY-NH2 and tcY-NH2 were prepared (>95% purity) by solid phase synthesis at The Peptide Synthesis Facility of the University of Calgary. Stock peptide solutions were prepared in 2.5 mM HEPES buffer, pH 7.4. Thrombin (from human plasma, activity of 3,270 NIH U/mg protein) was obtained from Calbiochem (La Jolla, CA, U.S.A.); apyrase and the protease inhibitor cocktail were from Sigma Chemical Co. (St. Louis, MO, U.S.A.); anti-endostatin antibody and ELISA kits for measurement of endostatin were from Chemicon International (Temecula, CA, U.S.A.); protein A agarose was from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.).
All experiments were approved by the University of Calgary Animal Care Committee and carried out in accordance with the guidelines of the Canadian Council on Animal Care. Male Wistar rats (170 – 200 g) were fed standard laboratory chow and tap water and were kept in a room with controlled temperature (22±1°C), humidity (65 – 70%), and light cycle (12 h light/dark).
Rats were anaesthetized with sodium pentobarbital (65 mg kg−1 i.p.) and blood was collected from the descending aorta with a 10 ml syringe that contained 3.4% sodium citrate (8 : 1, v v−1). The blood was centrifuged at 200×g for 15 min at room temperature. The PRP was then removed by aspiration. Some of the PRP was further centrifuged at 400×g for 10 min at room temperature to obtain platelet poor plasma (PPP). The number of platelets in the PRP was counted and adjusted to 2.5×108 ml−1 with PPP.
Platelet aggregation and endostatin release was studied in response to thrombin and PAR4 activating peptide ex vivo (Wallace et al., 1995; Ma et al., 2001). Aliquots (0.4 ml) of the PRP were placed in the cuvette of a Payton platelet aggregometer. The PRP was maintained at 37°C and was continuously stirred at 800 r.p.m. Three minutes later, platelet agonists (thrombin at 0.5 – 1.0 u ml−1) or AY-NH2 at 15 – 50 μM) were added to the platelet suspension in the absence or presence of the PAR4 antagonist (tcY-NH2 at 400 μM) and aggregation was monitored for 5 min. In another set of experiments, thrombin was added to the platelet suspension in the absence or presence of apyrase (5 u ml−1), an ADP scavenger (Lau et al., 1994). The resulting aggregate of platelets was centrifuged, and the supernatant was collected and stored at −70°C until enzyme-linked immunosorbent assay for endostatin was performed.
Washed platelets were obtained as previously described (Wallace et al., 1995) and lysed with lysis buffer (10 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, protease inhibitor cocktail, 100 μg ml−1 phenyl-methylsulfonyl fluoride). Lysates were collected by centrifugation (16,000×g, 4°C) for 15 min, and then precleared by incubation with 30 μl protein A agarose with lysis buffer on a rotating incubator for 3 h at 4°C, followed by centrifugation. Supernatants were incubated with 30 μl of antibody conjugated protein A agarose for 3 h at 4°C. Beads were washed three times with buffer (20 mM Tris- HCl pH 7.4, 150 mM NaCl, 0.1% Tween 20), precipitated and resuspended in 25 μl of electrophoresis sample buffer and boiled for 5 min. Immunoprecipitated proteins were analysed by 12.5% SDS – PAGE gel electrophoresis followed by immunoblotting using an anti-endostatin antibody (Chemicon).
All data are expressed as means±s.e.mean, with sample sizes of at least five per group. Comparisons of data among groups were performed with one-way analysis of variance followed by the Student-Newman-Keuls test. An associated probability (P value) of less than 5% was considered significant.
Results
Thrombin (1.0 u ml−1) and AY-NH2 (15 – 50 μM) caused significant release of endostatin from platelets (Figure 1). A specific rat PAR4 antagonist, tcY-NH2, at a concentration previously shown to inhibit thrombin- and AY-NH2-induced rat platelet aggregation (Hollenberg & Saifeddine, 2001), completely blocked endostatin release induced by these two agonists (Figure 1). The PAR4 antagonist (tcY-NH2), alone had no effect on endostatin release.
Figure 1.
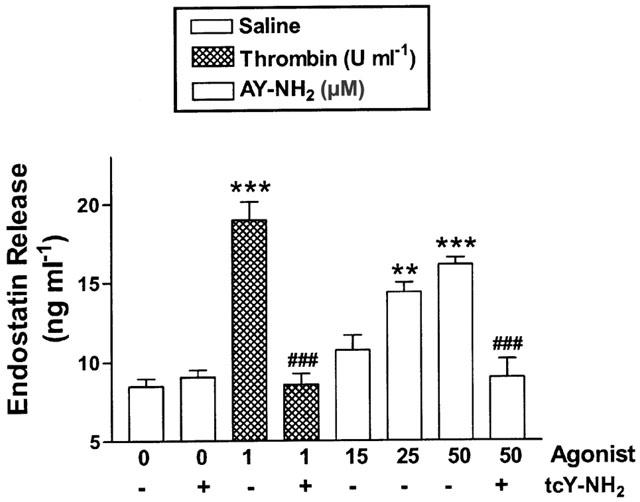
Platelet endostatin release induced by thrombin or a PAR4 agonist (AY-NH2) and the effects of a selective PAR4 antagonist (tcY-NH2; 400 μM). Platelet aggregation was monitored in response to thrombin (1 u ml−1) or AY-NH2 (15 – 50 μM) in the absence or presence of tcY-NH2. The concentration of endostatin in supernatants of the platelet suspension was measured by enzyme-linked immunosorbent assay. **P<0.01, ***P<0.001 vs saline group; ##P<0.01, ###P<0.001 vs corresponding thrombin or AY-NH2 group.
Thrombin dose-dependently increased the extent of platelet aggregation (Figure 2A), and this was significantly attenuated in the presence of the ADP scavenger, apyrase. Thrombin caused significant release of endostatin, with no significant difference in the amount of such release with the three doses of thrombin tested (Figure 2B). Thus, there was a lack of correlation between the aggregation and endostatin release stimulated by thrombin. Apyrase had no effect on thrombin-induced endostatin release. Apyrase alone did not significantly affect either platelet aggregation or endostatin release.
Figure 2.
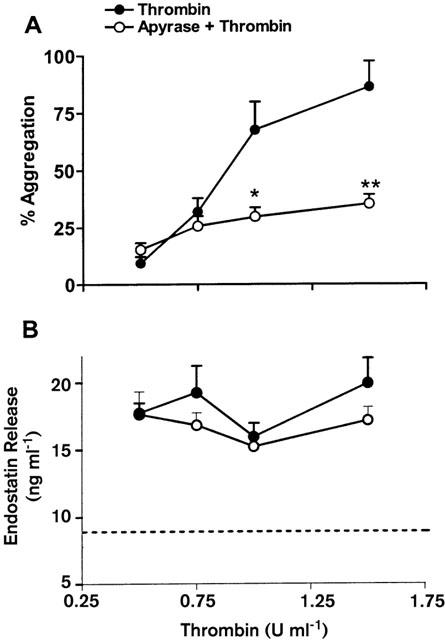
Effects of apyrase (5 u ml−1) on thrombin-induced platelet aggregation (A) and endostatin release (B). The dotted line shows the basal level of endostatin in platelet rich plasma not exposed to an agonist. *P<0.05, **P<0.01 vs corresponding thrombin alone group.
Immunoprecipitation of platelet lysates followed by Western blotting demonstrated that immunoreactive rat endostatin exhibited a 20 kDa band (Figure 3).
Figure 3.

Detection of endostatin protein by immunoprecipitation and Western blotting in rat platelet lysates. Immunoreactive endostatin from rat platelets shows a 20 kDa band similar to mouse endostatin. Lane A: 5 ng of mouse endostatin standard (from Chemicon International); Lanes B – D: Immunoprecipitate of rat platelet endostatin from 500, 1000 and 2000 μg of total platelet lysate protein; Lane E: Immunoprecipitate of 10 ng of mouse endostatin standard.
Discussion
Thrombin is known to cleave at least three PARs (PAR1, PAR3, and PAR4), but there is considerable interspecies variability in terms of which PARs mediate thrombin-induced secretion and aggregation. PAR1 and PAR4 (mRNA and protein) are expressed by human platelets. Activation of either receptor is sufficient to trigger secretion and aggregation (Coughlin, 2000). In the mouse, however, PAR3 and PAR4 act coordinately as thrombin receptors on platelets. PAR3 itself does not mediate transmembrane signalling; rather, it functions as a co-factor for the cleavage and activation of PAR4 by thrombin (Coughlin, 2000). In rat platelets, the PAR4 activating peptide, AY-NH2, stimulates platelet aggregation in a similar manner as thrombin (Hollenberg & Saifeddine, 2001). The specific antagonist tcY-NH2 completely abolished both thrombin- and AY-NH2-induced rat platelet aggregation (Hollenberg & Saifeddine, 2001), indicating that PAR4 plays a significant role in rat platelet function.
Endostatin inhibits endothelial cell proliferation and migration, inhibits angiogenesis-dependent tumour growth and can promote endothelial cell apoptosis (O'reilly et al., 1997; Dhanabal et al., 1999). Recently, endostatin was shown to be contained within platelets and released in response to thrombin (Ma et al., 2001). The platelet plays a key role in wound healing, including gastric ulcer healing (Ma et al., 2001), in part through the release of pro- and anti-angiogenic factors, endostatin being an example of the latter. In the present study, we confirmed that rat platelet endostatin is a 20 KDa protein. A variety of other growth factors, including vascular endothelial growth factor, epidermal growth factor and platelet-derived growth factor, are stored in platelet alpha granules and released during platelet aggregation in response to different agonists (Linder et al., 1979; Maloney et al., 1998). It is most likely that the release of those factors is due to degranulation during platelet activation and therefore parallels the extent of aggregation (Linder et al., 1979). However, although thrombin and ADP induced a similar extent of platelet aggregation in our previous study (Ma et al., 2001), only thrombin stimulated platelet endostatin release, suggesting that endostatin is unlike those growth factors that are stored in alpha granules and released in accordance with the extent of platelet aggregation. Furthermore, it has been found that in rat platelets, which do not possess PAR1, the PAR4 activating peptide AY-NH2 stimulated platelet aggregation (Hollenberg & Saifeddine, 2001) and endostatin release in the same manner as thrombin. The selective PAR4 antagonist tcY-NH2 markedly inhibited thrombin- and AY-NH2-induced platelet aggregation (Hollenberg & Saifeddine, 2001) and also completely blocked the associated endostatin release from platelets, suggesting that in the rat, PAR4 is involved in both platelet aggregation and platelet endostatin release. The fact that apyrase did not inhibit thrombin-induced endostatin release confirms that endostatin release occurs independently of platelet aggregation. This separation of platelet aggregation and platelet endostatin release is consistent with our previous finding that ticlopidine, an ADP receptor antagonist, significantly inhibited thrombin-induced platelet aggregation but dramatically enhanced platelet endostatin release induced by thrombin (Ma et al., 2001).
In conclusion, this study reveals a novel function of PAR4 in rat platelets that may be important in the context of wound healing and angiogenesis.
Acknowledgments
This work was supported by a grant from the Canadian Institutes of Health research (CIHR; to J.L. Wallace and M.D. Hollenberg). L Ma is supported by a fellowship jointly sponsored by AstraZeneca, the Canadian Association of Gastroenterology and the CIHR. J.L. Wallace is a CIHR Senior Scientist and an Alberta Heritage Foundation for Medical Research Senior Scientist. The authors are grateful to Mahmoud Saifeddine for his assistance with these studies.
Abbreviations
- ADP
adenosine diphosphate
- PAR
proteinase-activated receptor
- PPP
platelet-poor plasma
- PRP
platelet-rich plasma
- SDS – PAGE
sodium dodecylsulphate-polyacrylamide electrophoresis
References
- COUGHLIN S.R. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- DHANABAL M., RAMCHANDRAN R., WATERMAN M.J.F., LU H., KNEBELMANN B., SEGAL M., SUKHATME V.P. Endostatin induces endothelial cell apoptosis. J. Biol. Chem. 1999;274:11721–11726. doi: 10.1074/jbc.274.17.11721. [DOI] [PubMed] [Google Scholar]
- FARUQI T.R., WEISS E.J., SHAPIRO M.J., HUANG W., COUGHLIN S.R. Structure-function analysis of protease-activated receptor 4 tethered ligand peptides. J. Biol. Chem. 2000;275:19728–19734. doi: 10.1074/jbc.M909960199. [DOI] [PubMed] [Google Scholar]
- HOLLENBERG M.D., SAIFEDDINE M. Proteinase-activated receptor 4 (PAR4): activation and inhibition of rat platelet aggregation by PAR4-derived peptides. Can. J. Physiol. Pharmacol. 2001;79:439–442. [PubMed] [Google Scholar]
- KAHN M.L., NAKANISHI-MATSUI M., SHAPIRO M.J., ISHIHARA H., COUGHLIN S.R. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J. Clin. Invest. 1999;103:879–887. doi: 10.1172/JCI6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAHN M.L., ZHEN Y.W., HUANG W., BIGORNIA V., ZENG D., MOFF S., FARESE R.V., TAM C., COUGHLIN S.R. A dual thrombin receptor system for platelet activation. Nature. 1998;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- LAU L.F., PUMIGLIA K., COTE Y.P., FEINSTEIN M.B. Thrombin-receptor agonist peptides, in contrast to thrombin itself, are not full agonists for activation and signal transduction in human platelets in the absence of platelet-derived secondary mediators. Biochem. J. 1994;303:391–400. doi: 10.1042/bj3030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDER B.L., CHERNOFF A., KAPLAN K.L., GOODMAN D.S. Release of platelet-derived growth factor from human platelets by arachidonic acid. Proc. Natl. Acad. Sci. U.S.A. 1979;76:4107–4111. doi: 10.1073/pnas.76.8.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MA L., ELLIOTT S.N., CIRINO G., BURET A., IGNARRO L.J., WALLACE J.L. Platelets modulate gastric ulcer healing: Role of endostatin and vascular endothelial growth factor release. Proc. Natl. Acad. Sci. U.S.A. 2001;98:6470–6475. doi: 10.1073/pnas.111150798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALONEY J.P., SILLIMAN C.C., AMBRUSO D.R., WANG J., TUDER R.M., VOELKEL N.F. In vitro release of vascular endothelial growth factor during platelet aggregation. Am. J. Physiol. 1998;275:H1054–H1061. doi: 10.1152/ajpheart.1998.275.3.H1054. [DOI] [PubMed] [Google Scholar]
- NAKANISHI-MATSUI M., ZHENG Y.W., SULCINER D.J., WEISS E.J., LUDEMAN M.J., COUGHLIN S.R. PAR3 is a cofactor for PAR4 activation by thrombin. Nature. 2000;404:609–613. doi: 10.1038/35007085. [DOI] [PubMed] [Google Scholar]
- O'REILLY M.S., BOEHM T., SHING Y., FUKAI N., VASIOS G., LANE W.S., FLYNN E., BIRKHEAD J.R., OLSEN B.R., FOLKMAN J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- VON HUNDELSHAUSEN P., WEBER K.S., HUO Y., PROUDFOOT A.E., NELSON P.J., LEY K., WEBER C. RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation. 2001;103:1718–1720. doi: 10.1161/01.cir.103.13.1772. [DOI] [PubMed] [Google Scholar]
- VU T.-K.H., HUNG D.T., WHEATON V.I., COUGHLIN S.R. Molecular cloning of functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., MCKNIGHT W., SOLDATO P.D., BAYDOUN A.R., CIRINO G. Anti-thrombotic effects of a nitric oxide-releasing, gastric-sparing aspirin derivative. J. Clin. Invest. 1995;96:2711–2718. doi: 10.1172/JCI118338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU W.F., ANDERSEN H., WHITMORE T.E., PRESNELL S.R., YEE D.P., CHING A., GILBERT T., DAVIE E.W., FOSTER D.C. Cloning and characterization of human protease-activated receptor 4. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]


