Abstract
This study exploited established immunoneutralization protocols and an N-terminal annexin 1 peptide (annexin 1Ac2 – 26) to advance our knowledge of the role of annexin 1 as a mediator of acute glucocorticoid action in the rat neuroendocrine system in vivo.
Rats were treated with corticosterone (500 μg kg−1, i.p.) or annexin 1Ac2 – 26 (0.1 – 10 ng rat−1, i.c.v.) and 75 min later with interleukin 1β (IL-1β, 10 ng rat−1, i.c.v. or 500 μg kg−1, i.p). Blood was collected 1 h later for hormone immunoassay. Where appropriate, anti-annexin 1 polyclonal antiserum (pAb) was administered subcutaneously or centrally prior to the steroid challenge.
Corticosterone did not affect the resting plasma corticotrophin (ACTH) concentration but suppressed the hypersecretion of ACTH induced by IL-1β (i.p. or i.c.v.). Its actions were quenched by anti-annexin 1 pAb (s.c. or i.c.v) and mimicked by annexin 1Ac2 – 26.
By contrast, corticosterone provoked an increase in serum growth hormone (GH) which was ablated by central but not peripheral administration of anti-annexin 1 pAb. IL-1β (i.c.v. or i.p.) did not affect basal GH but, when given centrally but not peripherally, it abolished the corticosterone-induced hypersecretion of GH. Annexin 1Ac2 – 26 (i.c.v.) also produced an increase in serum GH which was prevented by central injection of IL-1β.
The results support the hypothesis that the acute regulatory actions of glucocorticoids on hypothalamo-pituitary-adrenocortical function require annexin 1. They also provide novel evidence that the positive influence of the steroids on GH secretion evident within this timeframe is effected centrally via an annexin 1-dependent mechanism which is antagonized by IL-1β..
Keywords: Annexin 1 (lipocortin 1), glucocorticoids, corticosterone, growth hormone, corticotrophin (ACTH)
Introduction
Glucocorticoid hormones and their synthetic analogues exert multiple effects on neuroendocrine function. The most obvious and best documented of these is suppression of the hypothalamo-pituitary-adrenocortical (HPA) axis but the steroids also exert significant effects on the secretion of other pituitary hormones which vary with dose and time. The impact of endogenous and exogenous glucocorticoids (GCs) on the secretion of growth hormone (GH) has attracted particular interest in recent years, partly because growth suppression is a common complication of long-term systemic GC therapy in children and partly because growing importance is attached to the maintenance of normal GH levels in adulthood. It is generally agreed that prolonged elevations in serum GCs, such as those caused by pharmacological treatments or Cushing's disease, depress the GH response to various provocative stimuli both in experimental animals and in man. This is due largely to actions of the steroids at the hypothalamic level which increase the expression and release of somatostatin (Papachristou et al., 1994; Fife et al., 1996; Lam & Srivastava, 1997) and decrease the production of GH releasing hormone (GHRH, Fernandez-Vazquez et al., 1995; Fife et al., 1996; Lam & Srivastava, 1997). Paradoxically, the impact of the consequent change in the hypothalamic drive to the somatotrophs is attenuated by concomitant actions of the steroids at the pituitary level which increase GHRH receptor (Tamaki et al., 1996; Miller & Mayo, 1997) and GH (Oosterom et al., 1983; Evans et al., 1992; Nogami et al., 1997) expression and thereby augment basal and GHRH-stimulated GH release (Vale et al., 1983). Interpretation of the actions of GCs on the growth hormone axis is further complicated by findings in man that acute administration of GCs causes a transient (3 – 4 h), but marked, increase in serum GH (Buguera et al., 1990; Casanueva et al., 1990; Muruais et al., 1991; Pineda et al., 1994; Pinto et al., 1997; Pellini et al., 1998). The mechanism by which the GCs evoke this secretory response is unclear. Casanueva et al. (1990) argued against an action at the pituitary level, a view which is supported by observations that, in stark contrast to their longer term actions, GCs inhibit GHRH-induced GH release from rodent pituitary tissue in vitro within this short time-frame (Vale et al., 1983; Ceda et al., 1987; Taylor et al., 2000a). Other data favour a transient action at the hypothalamic level which augments GHRH release (Fernandez-Vazquez et al., 1995) and/or depresses the secretion of somatostatin (Muruais et al., 1991).
Our previous studies have shown that the acute regulatory actions of GCs on the hypothalamo-pituitary-adrenocortical (HPA) axis are dependent upon the generation of a 37 kDa protein, annexin 1 (also known as lipocortin 1, Buckingham & Flower, 1997). Annexin 1, which was first identified as a potential mediator of the therapeutically important anti-inflammatory actions of GCs, is a well characterized member of the annexin family of Ca2+ and phospholipid binding proteins. It is found in abundance in the anterior pituitary gland (Smith et al., 1993; Taylor et al., 1993), particularly in the S100-positive folliculo-stellate cells (Traverso et al., 1999), and also in specific loci in the hypothalamus, including the median eminence and the paraventricular, arcuate and periventricular nuclei (Smith et al., 1993). Moreover, its expression and cellular disposition in these tissues are regulated by glucocorticoids (Loxley et al., 1993b; Taylor et al., 1993; Philip et al., 1997; Christian et al., 1999). To date, the evidence that annexin 1 fulfils a functional role as a mediator of the early inhibitory actions of the GCs on HPA function (i.e. those apparent 1 – 4 h after a GC challenge) has been derived mainly from in vitro studies. These have shown that the inhibitory effects of GCs on the evoked secretion of corticotrophin releasing hormone (CRH) and corticotrophin (ACTH) from hypothalamic and anterior pituitary tissue respectively are mimicked by recombinant annexin 1 (annexin 11 – 346) and various N-terminal peptides derived from it and reversed specifically by neutralizing anti-annexin 1 antisera and by an antisense oligodeoxynucleotide (ODN) directed against a sequence unique to annexin 1 mRNA (Loxley et al., 1993b; Taylor et al., 1993; 1995a; 1997). The limited in vivo data available accord with these findings in that they demonstrate that annexin 11 – 346 suppresses the HPA response to a cytokine challenge when injected intracerebroventricularly (i.c.v., Loxley et al., 1993a,1993b) and that passive immunization of rats against annexin 1 reverses the inhibitory actions of dexamethasone on the HPA response to an intraperitoneal injection of interleukin-1β (IL-1β, Taylor et al., 1995b). Further studies have shown that annexin 1 also contributes to the regulatory actions of GCs on prolactin release (Taylor et al., 1995a; 2000b) but it is not yet known whether it has a further role in effecting the acute stimulatory actions of GCs on the release of GH in vivo. The experiments described in this study aimed to address this question and also to explore further the role of annexin 1 in modulating the HPA responses to IL-1β by using established immunoneutralization strategies (Taylor et al., 2000b) and a synthetic N-terminal annexin 1 peptide (annexin 1Ac2 – 26). Parallel measures of serum luteinizing hormone (LH) were also made for control purposes.
Methods
Animals
Adult male Sprague-Dawley rats weighing 150±20 g were purchased from Harlan Olac (Banbury, U.K.) and housed in pairs in a quiet room with controlled lighting (lights on 08 00 h – 20 00 h), temperature (21 – 23°C) and humidity (50%). Food and water were available ad libitum. The surgical procedures, drug treatments and procedures for sample collection described below were carried out under licence, in accord with the U.K. Animals (Scientific Procedures) 1986 Act.
Anti-sera and drugs
A neutralizing polyclonal anti-annexin 1 anti-serum (anti-annexin 1 pAb) of proven efficacy and specificity was used (Taylor et al., 1995b; 2000b). It was raised in sheep against human recombinant annexin 1 and purified before injection with an Immmunopure (A/G) IgG purification kit (Pierce, Illinois, U.S.A.); a similarly purified non-immune sheep serum (NSS) was used as a control; the final protein concentration of the serum was 0.434 mg ml−1. An N-terminal annexin 1 peptide, annexin 1Ac2 – 26, was synthesized in-house (Dr I. Moss, Advanced Biotechnology Centre, Imperial College School of Medicine) by a solid phase method and purified by high performance liquid chromatography. It was dissolved in 1 M (NH4)2HCO3 (Sigma Chemical Co., Poole, Dorset, U.K.) and diluted in sterile saline (0.9% sodium chloride solution, Pharmacy, Hammersmith Hospitals Trust) immediately before use to a maximum concentration of 20 mM ammonium bicarbonate. Rat recombinant interleukin 1β (IL-1β), generously donated by Dr Mauro Perretti (William Harvey Research Institute, London) or purchased from ICN Pharmaceuticals Ltd (Basingstoke, Hants, U.K.) and corticosterone-21-acetate (Sigma) were each dissolved and diluted in sterile saline immediately before use.
Surgery
The rats were handled daily for 2 weeks by the same person before being subjected to a preliminary operation, performed under surgical anaesthesia [0.125 mg kg−1 fentanyl citrate, 4 mg kg−1 fluanisone (Hypnorm, 0.4 ml kg−1, i.p. Janssen-Ciba Ltd, High Wycombe Buckinghamshire, U.K.) and 2 mg kg−1 midazolam (Hypnovel, 0.4 ml kg−1, i.p., Roche Ltd, Welwyn Garden City, Hertfordshire, U.K.)] in which a stoppered guide cannula was implanted stereotaxically in the third ventricle (Loxley et al., 1993a). After surgery, the animals were handled daily, again by the same person, for 7 – 10 days and then subjected to one of the experimental protocols described below; body weight and behaviour were closely monitored throughout this period and any animal which appeared unwell was destroyed immediately (<3%). At the end of the experiment the position of the indwelling cannula was verified histologically.
Experimental protocols
Experiments 1 – 3 examined the effects of anti-annexin 1 pAb, given centrally or peripherally, on the changes in plasma ACTH and serum GH concentrations induced by injections of corticosterone and/or rat IL-1β (i.c.v. or i.p.); parallel measurements of serum LH were also made. Details of the protocols and doses are provided in Figure 1. Briefly, the rats were treated at t=0 min with corticosterone (500 μg kg−1 in a volume of 1 ml kg−1, i.p.) and 75 min later with rat IL-1β (10 ng rat−1 in a volume of 3 μl rat−1, i.c.v. or 500 μg kg−1 in a volume of 1 ml kg−1, i.p.); controls received appropriate volumes of the sterile saline vehicle. The rats were killed 1 h later and blood was collected for hormone immunoassay. When required, anti-annexin 1 pAb or non-immune sheep serum (NSS, controls) was given centrally (3 μl rat−1, i.c.v. via the guide cannula) or subcutaneously (1 ml kg−1, s.c.) 15 min or 24 h respectively before the steroid challenge.
Figure 1.
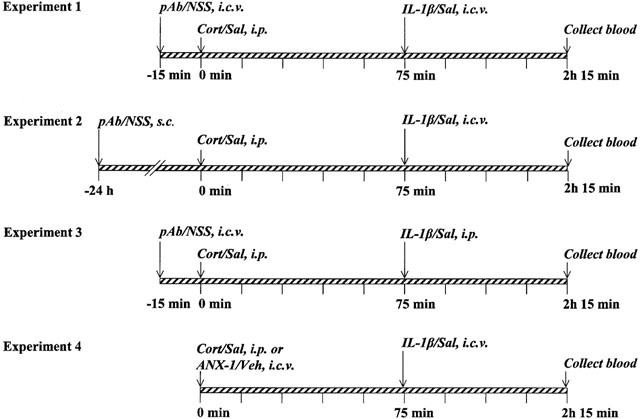
Experimental protocols illustrating the times and routes of antiserum and drug adminstration. i.c.v.=intracerebroventicularly; i.p.=intraperitoneally; s.c. subcutaneously. pAb=anti-annexin 1 polyclonal antiserum (3 μl rat−1 i.c.v. or 1 ml kg−1, s.c); NSS=non-immune sheep serum (3 μl rat−1, i.c.v. or 1 ml kg−1, s.c); Cort=corticosterone (500 μg kg−1, i.p. in a volume of 1 ml kg−1); IL-1β=interleukin 1β (10 ng rat−1 in a volume of 3 μl, i.c.v. or 500 μg kg−1, i.p. in a volume of 1 ml kg−1); ANX-1=annexin 1Ac2 – 26 (0.1 – 10 ng rat−1 in a volume of 3 μl, i.c.v.). Corresponding volumes of saline (Sal) or vehicle (Veh) were administered as controls where appropriate.
A fourth experiment (Figure 1) compared the effects of annexin 1Ac2 – 26 on the secretion of ACTH, GH and LH in control and IL-1β-treated animals with those of corticosterone. Rats were injected at t=0 min with either annexin 1Ac2 – 26 (0.1 – 10 ng rat−1, i.c.v.) or corticosterone (500 μg kg−1, i.p.); controls received appropriate volumes of the vehicle (3 μl, i.c.v. or 1 ml kg−1, i.p.). Seventy-five minutes later (t=75 min) rats from each group were treated with IL-1β (10 ng rat−1, i.c.v.) or a corresponding volume of saline (3 μl rat−1 i.c.v.); blood was collected 1 h later for hormone assay.
The doses of rIL-1β and anti-annexin 1 pAb administered centrally or peripherally and of corticosterone (injected i.p.) were selected on the basis of previous studies (Loxley et al., 1993b; Taylor et al., 1995b; 2000b) as too were the intervals between drug treatments and further manipulations. Doses of annexin 1Ac2 – 26, were calculated on the basis of our previous in vivo experience with annexin 11 – 188 and annexin 11 – 346, (Loxley et al., 1993a) and of evidence from in vitro studies that annexin 1Ac2 – 26 is 100 – 1000 times less potent than these molecules (Harris et al., 1995; Croxtall et al., 1998; John et al., 1998).
Collection and storage of plasma and serum samples
Rats were killed by decapitation. Trunk blood from each rat was divided between chilled heparinized and non-heparinized plastic tubes for centrifugal separation (2000 × g, 10 min, 4°C) of plasma and serum respectively. Sample collection was always completed by 13 00 h so as to avoid circadian-dependent fluctuations in hormone levels. The samples were then stored at −80°C for subsequent determination of ACTH, GH and LH.
Hormone assays
ACTH
Plasma ACTH was determined in duplicate by a highly specific immunoradiometric assay method (Hodgkingson et al., 1984) using kits purchased from Europath (Dorset, U.K.). The sensitivity of this direct assay was 0.04 pg ml−1 with inter and intra- assay coefficients of variation of 5.0 and 2.5% respectively (n=8).
Growth hormone
Serum GH was measured in duplicate by an enzyme linked immunosorbent assay (ELISA) using a modification of the method of Farrington & Hymer (1987). The antiserum (monkey anti-rat GH coded anti-GH-S5) and standard preparation (rat GH, coded rGH-B13) were supplied by the National Hormone and Pituitary Programme (Ogden Bioservices, Rockville, U.S.A.). The sensitivity of the assay was 2 ng ml−1 with inter- and intra-assay coefficients of variation of 15.6 and 9.4% respectively (n=8).
Luteinizing hormone
Serum LH was measured in duplicate by a direct radioimmunoassay method (Kilpatrick et al., 1976) using reagents supplied by the National Hormone and Pituitary Programme. The reference preparation, antibody and tracer (labelled with 125I) were coded NIADDK-rat LR-RP3, NIADDK-anti-rat-LH-s9 and NIADDK-rat-I-9 respectively. The sensitivity of the assay was 0.2 ng ml−1 with inter- and intra-assay coefficients of variance of 10.7 and 9.0% respectively (n=8).
Data analysis
Preliminary analysis by the Shapiro and Wilks test confirmed that the data were normally distributed. Subsequent analysis was done using ANOVA with post hoc comparisons by Scheffé's test (Experiments 1 – 3) or Duncan's test (Experiment 4) which respectively permit comparisons between multiple groups where number of observations per group (i.e. n) varies or is constant. Differences were considered to be significant if P<0.05.
Results
Immunoneutralization studies
Figures 2, 3 and 4a and b show the results from experiments 1 – 3 which examined the effects of the neutralizing anti-annexin 1 antiserum, given centrally or peripherally, on the acute regulatory actions of corticosterone on the secretion of ACTH (a) and GH (b) in the rat; parallel measures of serum LH are shown in Figures 2, 3 and 4c. The antiserum did not affect the resting concentrations of ACTH, GH and LH in the blood per se. Thus, in all three experiments the plasma ACTH and serum GH and LH concentrations of rats treated centrally or peripherally with anti-annexin 1 pAb did not differ significantly (P>0.05) either from those given the control serum (NSS) or from those of rats subjected to surgery only.
Figure 2.
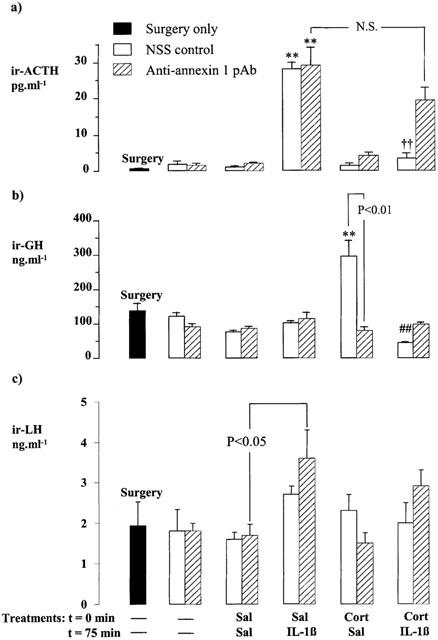
Effects of corticosterone (Cort, 500 μg kg−1, i.p.) and/or rat interleukin 1β (IL-1β, 10 ng rat−1, i.c.v.) on the plasma ACTH (a), serum GH (b) and serum LH (c) concentrations in rats pretreated 15 min before the steroid injection with anti-annexin 1 pAb (3 μl rat−1, i.c.v.) or an equal volume of non-immune sheep serum (NSS, i.c.v.). Values represent the mean±s.e.mean (n=6 – 7). **P<0.01 vs corresponding Sal-Sal control; ††P<0.01 vs Sal- IL-1β-treated group; ##P<0.01 vs Cort-Sal-treated group; N.S.=not significant (P>0.05), (ANOVA plus Scheffé's test).
Figure 3.
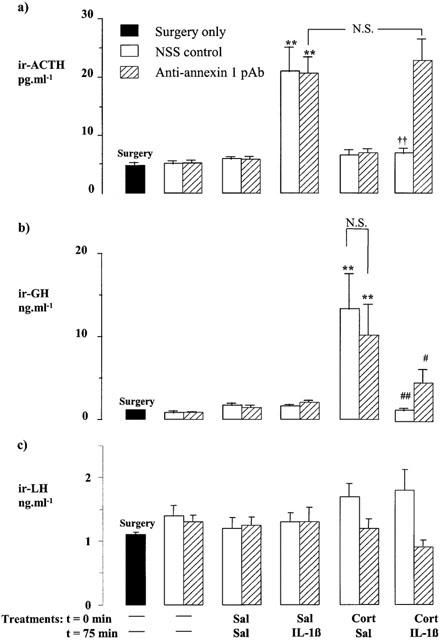
Effects of corticosterone (Cort, 500 μg kg−1, i.p.) and/or interleukin 1β (IL-1β, 10 ng rat−1, i.c.v.) on the plasma ACTH (a), serum GH (b) and serum LH (c) concentrations in rats pretreated 24 h before the steroid injection with anti-annexin 1 pAb (1 ml kg−1, s.c.) or an equal volume of non-immune sheep serum (NSS, s.c.). Sal=saline. Values represent the mean±s.e.mean (n=6 – 7). **P<0.01 vs Sal-Sal-treated control; ††P<0.01 vs Sal-IL-1β-treated group; #P<0.05, ##P<0.01 vs Cort-Sal-treated group; N.S.=not significant (P>0.05), (ANOVA plus Scheffé's test).
Figure 4.
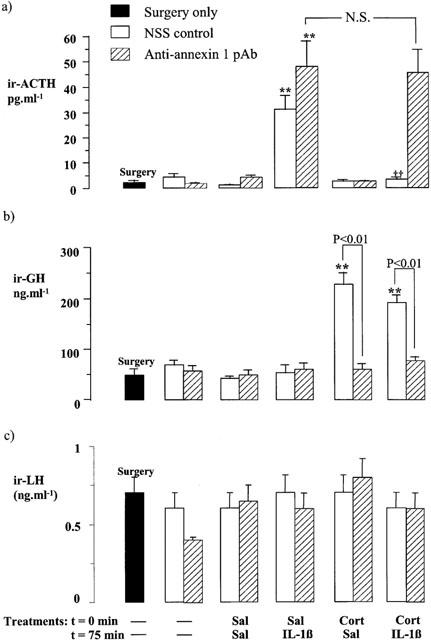
Effects of corticosterone (Cort, 500 μg kg−1, i.p.) and/or interleukin 1β (IL-1β, 500 μg kg−1, i.p.) on the plasma ACTH (a), serum GH (b) and serum LH (c) concentrations in rats pretreated 15 min before the steroid injection with anti-annexin 1 pAb (3 μl rat−1, i.c.v.) or an equal volume of non-immune sheep serum (NSS, i.c.v.). Sal=saline. Values represent the mean±s.e.mean (n=6 – 7). **P<0.01 vs Sal-Sal-treated control. ††P<0.01, vs corresponding Sal-IL-1β-treated group; N.S.=not significant (P>0.05), (ANOVA plus Scheffé's test).
Experiment 1 (Figure 2)
Administration of IL-1β (10 ng rat−1, i.c.v.) caused within 1 h a highly significant increase in plasma ACTH (P<0.01 vs corresponding saline-treated control) in rats pretreated with the control serum (NSS, 3 μl rat−1, i.c.v.). The corticotrophic response to the cytokine was inhibited (P<0.01) by pre-treatment with corticosterone (500 μg kg−1, i.p., 75 min before the cytokine challenge) which alone had no discernible effect on the plasma ACTH concentration. Rats treated centrally with anti-annexin 1 pAb (3 μl rat−1, i.c.v.) also responded to IL-1β (10 ng rat−1, i.c.v.) with a marked rise in plasma ACTH concentration (P<0.01 vs corresponding saline-treated control). However, the antiserum effectively quenched the inhibitory influence of corticosterone on the secretory response to IL-1β; thus, in rats treated with anti-annexin 1 pAb 15 min prior to the steroid, corticosterone failed to suppress the significant IL-1β-induced rise in plasma ACTH concentration (Figure 2a).
The serum GH profile differed markedly (Figure 2b). Rats treated centrally with NSS responded to the injection of corticosterone (500 μg kg−1, i.p.) with an overt increase in serum GH (P<0.01 vs corresponding steroid-free controls). This response, which was observed 2 h 15 min after the steroid injection, was prevented by central administration of anti-annexin 1 pAb (3 μl rat−1, i.v.c.) 15 min before the steroid (P<0.01, NSS+corticosterone vs anti-annexin 1 pAb+corticosterone, Figures 2b and 4b). IL-1β given centrally (10 ng rat−1, i.c.v) 1 h before autopsy did not affect the resting serum GH concentration in rats pretreated with NSS or anti-annexin 1 pAb only. However, it abolished the increase in serum GH concentration provoked by corticosterone in the NSS-treated group (P<0.01).
The serum LH concentrations were largely unaffected by the various treatments. However, in rats treated centrally with anti-annexin 1 pAb, i.c.v. injection of IL-1β produced a small but significant (<0.05) increase in serum LH (Figure 2c).
Experiment 2 (Figure 3)
Rats pre-treated peripherally with NSS (1 ml kg−1, i.p) also responded to a single injection of IL-1β (10 ng rat−1, i.c.v) with a marked increase in plasma ACTH (P<0.01 vs corresponding saline-treated group) which was prevented by injection of corticosterone 1 h 15 min before the cytokine (P<0.01, IL-1β vs rIL-1β plus corticosterone). Administration of anti-annexin 1 pAb (1 ml kg−1, s.c., 24 h prior to the steroid challenge) did not affect the corticotrophic response to IL-1β per se. However, it abolished the capacity of corticosterone to block the secretory response to the cytokine (Figure 3a).
Corticosterone (500 μg kg−1, i.p.) produced within 2 h 15 min an overt increase in serum GH (P<0.01 vs corresponding steroid-free controls, Figure 3b) in NSS-treated rats (1 ml kg−1, s.c.). The response to the steroid was unaffected by peripheral administration of anti-annexin 1 pAb (1 ml kg−1, s.c., P>0.05, NSS+corticosterone vs anti-annexin 1 pAb+corticosterone, Figure 3b) but, as in Experiment 1, it was inhibited by central administration of IL-1β 1 h prior to autopsy (10 ng rat−1, i.c.v.).
The serum LH concentration was unaffected by the various treatments (Figure 3c).
Experiment 3 (Figure 4)
Peripheral administration of IL-1β (500 μg kg−1, i.p.) also caused a highly significant increase in plasma ACTH in rats treated centrally with NSS (3 μl rat−1, i.c.v., P<0.01 vs corresponding saline-treated control). This response, which was evident 1 h after the administration of the cytokine, was readily abolished (P<0.001) by pretreatment (1 h 15 min before the cytokine) with corticosterone (500 μg kg−1, i.p.). A marked rise in plasma ACTH concentration was also observed 1 h after the injection of the cytokine in rats treated with anti-annexin 1 pAb (3 μl rat−1, i.c.v., P<0.001 vs corresponding saline-treated control). However, as in Experiments 1 and 2, the anti-annexin 1 antiserum effectively quenched the inhibitory influence of corticosterone on the secretory responses to IL-1β (Figure 4a).
In line with the data from Experiment 1, central administration of anti-annexin 1 pAb (3 μl rat−1, i.v.c., 15 min before the injection of corticosterone) abolished (P<0.001) the highly significant increase (P<0.01) in serum GH induced by a single injection of corticosterone while the control serum (NSS) was without effect (Figure 4b). However, although in Experiments 1 and 2 central administration of rIL-1β inhibited the rise in serum GH induced by corticosterone, when given peripherally (500 μg kg−1, i.p.), the cytokine was ineffective in this regard (Figure 4b).
The serum LH concentration was unaffected by the various treatments (Figure 3c).
Experiment 4: Neuroendocrine responses to Annexin 1Ac2 – 26
Figure 5a,b,c compare the effects of corticosterone (500 μg kg−1, i.p.) on the circulating levels of ACTH, GH and LH with those of graded doses of annexin 1Ac2 – 26 (0.1 – 10 ng rat−1, i.c.v.). Like corticosterone, annexin 1Ac2 – 26 had no discernible effect on the resting plasma ACTH concentration (P>0.05 vs vehicle-treated control). However, it inhibited, in a dose-dependent manner, the highly significant increase in plasma ACTH concentration induced by IL-1β (10 ng rat−1, i.c.v., administered 1 h 15 min after the injection of corticosterone or annexin 1Ac2 – 26), reducing the response to the cytokine by approximately 60% at the highest dose tested (P<0.01, Figure 5a).
Figure 5.
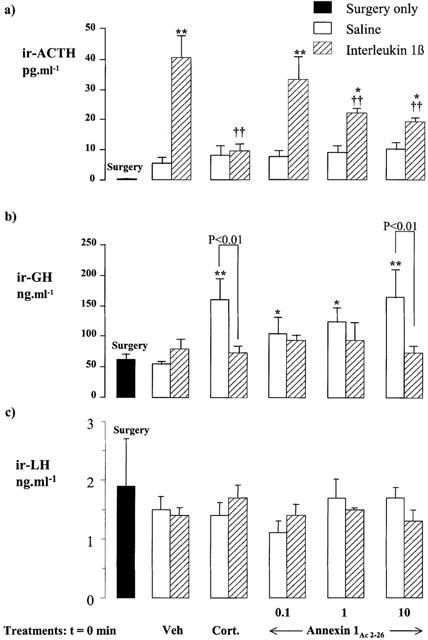
Comparison of the effects of treatment with corticosterone (Cort, 500 μg kg−1, i.p.) and annexin 1Ac2 – 26 (0.1 – 10 ng rat−1, i.c.v.) on the resting plasma ACTH (a), serum GH (b) and serum LH (c) concentrations in rats and on the responses to IL-1β (10 ng rat−1, i.c.v.). Values represent the mean±s.e.mean (n=6). *P<0.05, **P<0.01 vs annexin 1 vehicle alone (Veh, 3 μl rat−1). ††P<0.01 vs IL-1β alone (ANOVA plus Duncan's test).
Annexin 1Ac2 – 26 also mimicked the stimulatory effect of corticosterone on GH release and thus produced, within 2 h 15 min, a dose-dependent increase in serum GH (P<0.01 vs vehicle at the highest dose tested) which, like that evoked by the steroid, was inhibited (P<0.01) by a central injection of IL-1β (10 ng rat−1, i.c.v., administered 1 h before autopsy, Figure 5b).
The serum LH concentration (Figure 5c) was unaffected by injection of annexin 1Ac2 – 26 (0.1 – 10 ng rat−1, i.c.v.), corticosterone (500 μg kg−1, i.p.) and/or IL-1β (10 ng rat−1, i.c.v.).
Discussion
The present study aimed to advance our understanding of the role of annexin 1 in mediating the acute regulatory actions of glucocorticoids in the neuroendocrine system, i.e. those which emerge within 1 – 3 h of a steroid challenge. Our principal strategy was to examine the effects of exogenous corticosterone on neuroendocrine function in rats in which annexin 1 activity was ablated by neutralizing antisera, using specific immunization protocols which were developed and validated in our laboratory and which allow delineation of the central and peripheral actions of the protein (Taylor et al., 1995b; 2000b). In addition, we compared the effects of corticosterone on hypothalamo-pituitary function with those of annexin 1Ac2 – 26, an N-terminal annexin 1 peptide which mimics the biological activity of the parent molecule (annexin 11 – 346) in several other biological systems, for example in the anterior pituitary gland in vitro (John et al., 1998) and in various models of cell growth (Croxtall et al., 1998) and inflammation (Harris et al., 1995). Our results provide further evidence that annexin 1 fulfils an obligatory role in the processes which effect the acute (‘early delayed') regulatory actions of glucocorticoids on HPA function. In addition, they provide novel evidence to suggest that the stimulatory effects of the steroids on GH secretion, which are manifest over a similar timeframe, are effected at the hypothalamic level by a mechanism which is dependent on annexin 1 and which is antagonized by IL-1β.
The data confirm reports that IL-1β, given i.c.v. or i.p., is a powerful activator of the rat HPA axis (reviewed in Mulla & Buckingham, 1999; Turnbull & Rivier, 1999) and show that, irrespective of the route of cytokine administration, the resultant increases in ACTH release are readily prevented by pretreatment with corticosterone. They thus support the view that the HPA responses to cytokines such as IL-1β are sensitive to the negative feedback actions of GCs (Loxley et al., 1993b; Taylor et al., 1993). Previous studies suggest that the early-delayed inhibitory effects of GCs on cytokine-stimulated ACTH release are exerted at the levels of the pituitary gland, the hypothalamus and possibly elsewhere in the brain (reviewed in Mulla & Buckingham, 1999). In addition, the steroids may disrupt the mechanisms used by IL-1β in the periphery to transmit signals to the brain, possibly by depressing the cytokine-induced prostaglandin-dependent activation of vagal afferents (Laye et al., 1996) or by opposing prostanoid-dependent actions of IL-1β on the modified endothelial cells of the blood brain barrier (van dam et al., 1996). It is evident from the present study that injection of a neutralizing anti-annexin 1 pAb into the third ventricle specifically ablates the inhibitory effects of exogenous corticosterone on the hypersecretion of ACTH provoked by central or peripheral injection of IL-1β. While we cannot exclude the possibility that some of the centrally injected antibody may have diffused into the hypophyseal portal vessels (Turnbull & Rivier, 1998a,1998b), our previous studies strongly suggest that the dose administered would be insufficient to gain access to and produce a measurable effect at the pituitary level although it readily ablates annexin 1 actions within the hypothalamus (Taylor et al., 2000b). The present findings thus suggest that an annexin 1-dependent mechanism within the hypothalamus fulfils a critical role in effecting the early regulatory actions of corticosterone on IL-1β-driven ACTH. Similar conclusions may be drawn from our observation that, when given centrally, annexin 1Ac2 – 26 mimics the inhibitory actions of corticosterone on HPA function, thereby producing a dose-dependent inhibition of the ACTH response to i.c.v. injection of IL-1β, and from previous studies by ourselves and others using annexin 11 – 346 and annexin 11 – 188 (Loxley et al., 1993a; Sudlow et al., 1996). In line with studies on other tissues/systems (Harris et al., 1995; Croxtall et al., 1998; John et al., 1998) the dose of annexin 1Ac2 – 26 required to produce a measurable response was considerably higher than that of either annexin 11 – 346 or annexin 11 – 188 (Loxley et al., 1993b). Moreover, at the highest dose tested annexin 1Ac2 – 26 produced only a partial blockade of the response to IL-1β, a finding that accords with reports that, in contrast with its actions in the host-defence system (Harris et al., 1995) and in models of cell growth (Croxtall et al., 1998), this N-terminal peptide lacks the efficacy of the full length molecule in the neuroendocrine system (John et al., 1998). Further support for a role of annexin 1 within the hypothalamus as a mediator of GC action has been provided by in vitro studies which demonstrated the capacity of annexin 11 – 346 to mimic and anti-annexin 1 antisera to ablate the inhibitory actions of dexamethasone on IL-1β- and IL-6-stimulated CRH release from isolated hypothalamic tissue (Loxley et al., 1993b; Taylor et al., 1995b). Notwithstanding these arguments, many other data point to the pituitary gland as a further important locus of annexin 1-dependent glucocorticoid action within the HPA axis, most notably those derived from in vitro studies (Smith et al., 1993; Taylor et al., 1993; 1995b; 1997; Christian et al., 1999; Traverso et al., 1999). In accord with these findings, we found the subcutaneous route of anti-annexin 1 pAb administration to be as effective as the central route in quenching the inhibitory effects of corticosterone on the hypersecretion of ACTH induced by an i.c.v. injection of IL-1β. Since our studies on GH (discussed below) indicate that the peripherally injected antiserum does not gain access to the ‘central components' of the hypothalamus in sufficient quantities to neutralize the actions of endogenous annexin 1, it seems likely that the antiserum injected via this route exerted its actions at the pituitary level, although we cannot exclude an action at the median eminence to which the antiserum would also have ready access. Complementary studies using dexamethasone, which unlike corticosterone has only limited access to the central nervous system (Meijer et al., 1998), have led to similar conclusions (Taylor et al., 1995b).
Our measurements of serum GH provide novel evidence for complex interplay between corticosterone, annexin 1 and IL-1β in the regulation of the growth hormone axis in the rat. The positive effect of the exogenous corticosterone on the secretion of GH reported here accords with data from a number of clinical studies which have demonstrated prompt increases in serum GH following acute administration of glucocorticoids by mouth or parenterally (Buguera et al., 1990; Casanueva et al., 1990; Muruais et al., 1991; Pineda et al., 1994; Pinto et al., 1997; Pellini et al., 1998) and advocated a role for GCs in the diagnosis of GH deficiency (Pineda et al., 1994; Pellini et al., 1998). Our finding that the hypersecretion of GH provoked by a single injection of corticosterone in the rat is (a) blocked specifically by central but not peripheral administration of anti-annexin 1 pAb and (b) mimicked by a central injection of annexin 1Ac2 – 26 suggests that the steroid exerts its positive influence on the GH axis via an annexin 1-dependent mechanism within the hypothalamus. Although this is the first demonstration of a role for annexin 1 in the central control of GH secretion, other data support the concept that the acute stimulatory actions of exogenous glucocorticoids on GH release are exerted at hypothalamic level and mitigate against an early positive action at the pituitary level. Thus, results from clinical studies suggest that acute GC administration causes a transient increase GHRH secretion (Fernandez-Vazquez et al., 1995) and/or depresses the secretion of somatostatin (Muruais et al., 1991). By contrast, over the same timeframe GCs exert an inhibitory influence on the somatotrophs and thereby suppress GHRH-stimulated GH release (Vale et al., 1983; Ceda et al., 1987; Taylor et al., 2000a). The present study provides no further insight to the locus of corticosterone/annexin 1 action within the hypothalamus although the magnitude of the changes in serum GH suggests that alterations in both GHRH and somatostatin secretion may be involved. The data do however show clearly that the GH responses to exogenous corticosterone and annexin 1Ac2 – 26 are readily inhibited by central but not peripheral administration of IL-1β. There is already substantial published evidence that IL-1β acts within the hypothalamus to suppress GH release (Wada et al., 1995) via mechanisms which involve increased somatostatin release (Honegger et al., 1991) and possibly also decreased secretion of GHRH (McCann et al., 1998). Our finding that central but not peripheral injection of IL-1β inhibits the rise in serum GH provoked by corticosterone or annexin 1Ac2 – 26 accords with this concept although, in contrast to other studies (Peisen et al., 1995; Wada et al., 1995), we found no effects of the cytokine on the basal release of GH. It has been suggested that the positive effects of IL-1β on the somatostatin secretion are secondary to the increase in CRH secretion the cytokine provokes (Peisen et al., 1995). Since GCs inhibit IL-1β-stimulated CRH release from isolated hypothalamic tissue in vitro (Loxley et al., 1993b; Taylor et al., 1995b), it may be argued that such a mechanism would also explain the opposing effects of IL-1β and corticosterone/annexin 1 on GH secretion. The present data mitigate against this view however for the hypersecretion of ACTH induced by central or peripheral administration of IL-1β, which has been shown repeatedly to be driven by CRH (reviewed in Mulla & Buckingham, 1999; Turnbull & Rivier, 1999), was not associated with a concomitant reduction in serum GH. Moreover, while neither corticosterone nor annexin 1Ac2 – 26 influenced basal ACTH secretion, both caused a marked increase in the serum GH concentration. We thus conclude that if CRH mediates the effects of IL-1β and corticosterone on GH secretion, the population of CRH neurones involved must be distinct from those concerned with the regulation of ACTH secretion and subject to differential regulation.
Despite the widespread evidence that LH secretion is modulated by IL-1β and GCs (Brann & Mahesh, 1991; Rivest & Rivier, 1993), we found little effect of IL-1β, corticosterone or annexin 1 on the serum LH concentration. We did however observe an increase in serum LH in rats treated centrally with anti-annexin 1 pAb following i.c.v. injection of IL-1β. The positive influence of the cytokine was unexpected and contrary to many reports in the literature (Brann & Mahesh, 1991; Rivest & Rivier, 1993). Interestingly it was not observed when the antiserum was given peripherally. The significance of these findings is unclear but, nonetheless, they raise the possibility that annexin 1 exerts a tonic inhibitory influence on the responsivity of the gonadotrophin releasing hormone neurones to incoming stimuli and thus support findings from an earlier study which advocated a role for annexin 1 in the regulation of hypothalamo-pituitary-gonadal activity (Loxley et al., 1992).
In conclusion, our results provide further evidence that annexin 1 fulfils a major role in the hypothalamus and the anterior pituitary gland as a mediator of the acute regulatory actions of glucocorticoids on HPA function. In addition, they provide novel evidence to suggest that the stimulatory effects of the steroids on GH secretion, which are manifest over a similar timeframe, are effected at the hypothalamic level by a mechanism which is dependent on annexin 1 and which is antagonized by IL-1β. The physiological significance of the interplay between corticosterone, annexin 1 and IL-1β in the central control of GH secretion requires further study. GH is released in response to certain stressors (e.g. pain, haemorrhage, restraint; reviewed in Buckingham et al., 1997) and it is conceivable that the stress-induced rise in serum glucocorticoids contributes to this response. However, other stressors, for example endotoxin, cold, electric footshock, suppress GH release (reviewed in Buckingham et al., 1997). The mechanisms which determine the stress-specific pattern of GH secretion have yet to be fully elucidated. Our finding that IL-1β effectively counters the stimulatory actions of the adrenal steroids on GH secretion may be particularly relevant to the suppression of serum GH observed in experimental animals following an acute immune insult. In this context, it would be valuable to extend these studies to examine the interplay between corticosterone/annexin 1 and other cytokines, in particular IL-6 and IL-8 whose stimulatory actions on CRH release in vitro are modified by corticosterone and annexin 1 (Loxley et al., 1993b; Taylor et al., 1995b), and also inflammatory stimuli such as endotoxin.
Acknowledgments
We are grateful to the Wellcome Trust (grant no 051887/Z/97/A), Chemodyne SA (Geneva) and the Charing Cross Hospital Trustees for generous financial support.
Abbreviations
- ACTH
corticotrophin
- ANOVA
analysis of variance
- CRH
corticotrophin releasing hormone
- GC
glucocorticoid
- GH
growth hormone
- GHRH
growth hormone releasing hormone
- HPA
hypothalamo-pituitary-adrenocortical
- IL-1β
interleukin I-1β
- IL-6
interleukin 6
- i.c.v.
intracerebroventricularly
- i.p.
intraperitoneal
- LH
luteinizing hormone
- NSS
non-immune sheep serum
- pAb
polyclonal antiserum
References
- BRANN D.W., MAHESH V.B. Role of corticosteroids in female reproduction. FASEB J. 1991;5:2691–2698. doi: 10.1096/fasebj.5.12.1655548. [DOI] [PubMed] [Google Scholar]
- BUCKINGHAM J.C., COWELL A.M., GILLIES G.E., HERBISON A.E., STEEL J.H.The Neuroendocrine System: Anatomy, Physiology and Responses to Stress Stress, Stress Hormones and the Immune System 1997John Wiley & Sons, Chichester, U.K; 9–47.eds. Buckingham J.C., Gillies G.E. & Cowell A.M. pp [Google Scholar]
- BUCKINGHAM J.C., FLOWER R.J. Lipocortin 1: a second messenger of glucocorticoid action in the hypothalamo-pituitary axis. Mol. Med. Today. 1997;3:296–302. doi: 10.1016/S1357-4310(97)88908-3. [DOI] [PubMed] [Google Scholar]
- BUGUERA B., MURUAIS C., PENALVLA A., CASANUEVA Dual and selective actions of glucocorticoids upon basal and stimulated growth hormone release in man. Neuroendocrinol. 1990;51:51–58. doi: 10.1159/000125315. [DOI] [PubMed] [Google Scholar]
- CASANUEVA F.F., BURGUERA B., MURUAIS C., DIEGUEZ C. Acute administration of corticoids: a new and peculiar stimulus of growth hormone secretion in man. J. Clin. Endocrinol. Metab. 1990;70:234–237. doi: 10.1210/jcem-70-1-234. [DOI] [PubMed] [Google Scholar]
- CEDA G.P., DAVIES R.G., HOFFMAN A.R. Glucocorticoid modulation of growth hormone secretion in vitro. Evidence for a biphasic effect on GH-releasing hormone mediated release. Acta Endocrinologica. 1987;114:465–469. doi: 10.1530/acta.0.1140465. [DOI] [PubMed] [Google Scholar]
- CHRISTIAN H.C., FLOWER R.J., MORRIS J.F., BUCKINGHAM J.C. Localisation and semi-quantitative measurement of lipocortin 1 in rat anterior pituitary cells by fluorescence-activated cell analysis/sorting and electron microscopy. J. Neuroendocrinology. 1999;11:707–714. doi: 10.1046/j.1365-2826.1999.00389.x. [DOI] [PubMed] [Google Scholar]
- CROXTALL J.D., CHOUDURY Q., FLOWER R.J. Inhibitory effect of peptides derived from the N-terminus of lipocortin 1 on arachidonic acid release and proliferation in the A549 cell line: identification of E-Q-E-Y-V as a crucial component. Br. J. Pharmacol. 1998;123:975–983. doi: 10.1038/sj.bjp.0701679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS R.M., BIMBERG N.C., ROSENFELD M.G. Glucocorticoids and thyroid hormones transcriptionally regulate growth hormone gene expression. Proc. Natl. Acad. Sci. U.S.A. 1992;79:7659–7663. doi: 10.1073/pnas.79.24.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARRINGTON M.A., HYMER W.C. An enzyme immunoassay for rat growth hormone: application to the study of growth hormone variants. Life Sciences. 1987;40:2479–2488. doi: 10.1016/0024-3205(87)90068-3. [DOI] [PubMed] [Google Scholar]
- FERNANDEZ-VAZQUEZ G., CACICEDO L., LORENZO M.J., TOLON R., LOPEZ K. Corticosterone modulates growth hormone-releasing factor and somatostatin in fetal rat hypothalamic cultures. Neuroendocrinology. 1995;61:31–35. doi: 10.1159/000126824. [DOI] [PubMed] [Google Scholar]
- FIFE S.K., BROGAN R.S., GUISTINA A., WEHRENBERG W.B. Immunocytochemical and molecular analysis of the effects of glucocorticoid treatment on the hypothalamic-somatotropic axis in the rat. Neuroendocrinology. 1996;64:131–138. doi: 10.1159/000127109. [DOI] [PubMed] [Google Scholar]
- HARRIS J., FLOWER R.J., PERRETTI M. Alteration of neutrophil trafficking by a lipocortin N-terminus peptide. Eur. J. Pharmacol. 1995;279:149–157. doi: 10.1016/0014-2999(95)00145-b. [DOI] [PubMed] [Google Scholar]
- HODGKINGSON S.C., ALLOLIO B., LANDON J., LOWRY P.J. Development of a non-extracted ‘two site' immunoradiometric assay for corticotropin utilising extreme amino- and carboxy-terminally directed antibodies. Biochem. J. 1984;218:703–711. doi: 10.1042/bj2180703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HONEGGER J., SPAGNOLI A., D'URSO R., NAVARRA P., TSAGARAKIS S., BESSER G.M., GROSSMAN A.B. Interleukin 1β modulates the acute release of rat growth hormone-releasing hormone and somatostatin from rat hypothalamus in vitro, whereas tumor necrosis factor and interleukin-6 have no effect. Endocrinology. 1991;129:1275–1282. doi: 10.1210/endo-129-3-1275. [DOI] [PubMed] [Google Scholar]
- JOHN C.D., COVER P.O., MORRIS J.F., FLOWER R.J., BUCKINGHAM J.C. Inhibitory effects of hormones derived from the N-terminus of lipocortin 1 on anterior pituitary hormone secretion evoked in vitro by forskolin. J. Endocrinol. 1998;159:OC12. [Google Scholar]
- KILPATRICK M.J., COLINS W.P., NEWTON J.R. Studies on the release of gonadotrophins during superfusion of isolated rat pituitaries in a continuous flow system. J. Reprod. Fertil. 1976;46:25–30. doi: 10.1530/jrf.0.0460025. [DOI] [PubMed] [Google Scholar]
- LAM K., SRIVASTAVA G. Gene expression of hypothalamic somatostatin and growth hormone releasing hormone in dexamethasone-treated rats. Neuroendocrinology. 1997;66:2–8. doi: 10.1159/000127212. [DOI] [PubMed] [Google Scholar]
- LAYE S., BLUTHE R.M., KENT S., COMBE C., MEDINA P., PARNET P., DANTZER R. Subdiaphragmatic vagotomy blocks induction of IL-1β mRNA in mouse brain in response to peripheral LPS. Am. J. Physiol. 1996;37:R1327–R1331. doi: 10.1152/ajpregu.1995.268.5.R1327. [DOI] [PubMed] [Google Scholar]
- LOXLEY H.D., COWELL A.M., FLOWER R.J., BUCKINGHAM J.C. Effects of lipocortin 1 and dexamethasone on the secretion of corticotrophin releasing factors in the rat: in vitro and in vivo studies. J. Neuroendocrinology. 1993a;5:51–61. doi: 10.1111/j.1365-2826.1993.tb00363.x. [DOI] [PubMed] [Google Scholar]
- LOXLEY H.D., TAYLOR A.D., FLOWER R.J., BUCKINGHAM J.C. Modulation of the hypothalamo-pituitary-gonadal axis by lipocortin-1. Br. J. Pharmacol. 1992;106:109P. [Google Scholar]
- LOXLEY H.D., TAYLOR A.D., FLOWER R.J., BUCKINGHAM J.C. Modulation of the hypothalamo-pituitary-adrenocortical responses to cytokines in the rat by annexin 1 and glucocorticoids: A role for annexin 1 in the feedback inhibition of CRF-41 release. Neuroendocrinology. 1993b;57:801–814. doi: 10.1159/000126439. [DOI] [PubMed] [Google Scholar]
- MCCANN S.M., KIMURA M., KARANTH S., YU W.H., RETTORI V. Role of nitric oxide in the neuroendocrine responses to cytokines. Ann. N. Y. Acad. Sci. 1998;840:174–184. doi: 10.1111/j.1749-6632.1998.tb09561.x. [DOI] [PubMed] [Google Scholar]
- MEIJER O.C., DE LANGE E.C., BREIMER D.D., DE BOER A.G., WORKEL J.O., DE KLOET E.R. Penetration of dexamethasone into brain glucocorticoid targets is enhanced in mdr1A P-glycoprotein knockout mice. Endocrinology. 1998;139:1789–1793. doi: 10.1210/endo.139.4.5917. [DOI] [PubMed] [Google Scholar]
- MILLER T.L., MAYO K.E. Glucocorticoids regulate pituitary growth hormone-releasing hormone receptor messenger ribonucleic acid expression. Endocrinology. 1997;138:2458–2465. doi: 10.1210/endo.138.6.5184. [DOI] [PubMed] [Google Scholar]
- MULLA A., BUCKINGHAM J.C. Regulation of the hypothalamo-pituitary-adreanl axis by cytokines. Balliere's Clinical Endocrinology and Metabolism. 1999;13:503–521. doi: 10.1053/beem.1999.0041. [DOI] [PubMed] [Google Scholar]
- MURUAIS C., CORDIDO F., MORALES M.J., CASNAUEVA F.F., DIEGUEZ C. Corticosteroid-induced growth hormone secretion in normal and obese subjects. Clin. Endocrinol. 1991;35:485–490. doi: 10.1111/j.1365-2265.1991.tb00932.x. [DOI] [PubMed] [Google Scholar]
- NOGAMI H., INOUE K., KAWAMURA K. Involvement of glucocorticoid-induced factor(s) in the stimulation of growth hormone expression in the fetal rat pituitary gland in vitro. Endocrinology. 1997;138:1810–1815. doi: 10.1210/endo.138.5.5124. [DOI] [PubMed] [Google Scholar]
- OOSTEROM R., VERLEUN T., LAMBERTS S.W.J. Human growth hormone-secreting pituitary adenoma cells in long-term culture: effects of dexamethasone and growth hormone releasing factor. J. Endocrinol. 1983;100:353–360. doi: 10.1677/joe.0.1000353. [DOI] [PubMed] [Google Scholar]
- PAPACHRISTOU D.N., LIU J., PATEL Y.C. Glucocorticoids regulate somatostatin peptide and steady state messenger ribonucleic acid levels in normal rat tissues and in a somatostatin-producing islet tumour cell line (1027B2) Endocrinology. 1994;134:2259–2266. doi: 10.1210/endo.134.5.7908873. [DOI] [PubMed] [Google Scholar]
- PEISEN J.N., MCDONNELL K.J., MULRONEY S.E., LUMPKIN M.D. Endotoxin-induced suppression of the somatotrophic axis is mediated by interleukin-1β and corticotropin releasing factor in the juvenile rat. Endocrinology. 1995;136:3378–3390. doi: 10.1210/endo.136.8.7628373. [DOI] [PubMed] [Google Scholar]
- PELLINI C., DE ANGELIS R., DI NATALE B., LUKEZIC M., MORA S., CHIUMELLO G. Dexamethasone in the diagnostic work-up of growth hormone deficiency. Clin. Endocrinol. 1998;48:223–228. doi: 10.1046/j.1365-2265.1998.3841202.x. [DOI] [PubMed] [Google Scholar]
- PHILIP J.G., FLOWER R.J., BUCKINGHAM J.C. Glucocorticoids modulate the cellular disposition of lipocortin in the rat brain in vivo and in vitro. NeuroReport. 1997;8:1871–1876. doi: 10.1097/00001756-199705260-00016. [DOI] [PubMed] [Google Scholar]
- PINEDA J., MARTUL P., CASANUEVA F.F., DIEGUEZ C., RICA I., LORIDAN L. Oral dexamethasone administration: new pharmacological test for the assessment of growth hormone secretion. Eur. J. Endocrinol. 1994;131:598–601. doi: 10.1530/eje.0.1310598. [DOI] [PubMed] [Google Scholar]
- PINTO A.C., WEFFORT R.F., DI NINNINO F.B., LENGYEL A.M. Effect of low-dose oral and intravenous dexamethasone administration on growth hormone secretion in children. Horm. Res. 1997;48:5–10. doi: 10.1159/000185356. [DOI] [PubMed] [Google Scholar]
- RIVEST S., RIVIER C. Centrally injected interleukin-1β inhibits hypothalamic LHRH secretion and circulating LH levels via prostaglandins in rats. J. Neuroendocrinol. 1993;5:445–450. doi: 10.1111/j.1365-2826.1993.tb00506.x. [DOI] [PubMed] [Google Scholar]
- SMITH T., FLOWER R.J., BUCKINGHAM J.C. Lipocortins 1, 2 and 5 in the central nervous system and pituitary gland of the rat: selective induction by dexamethasone of annexin 1 in the anterior pituitary gland. Molecular Neuropharmacology. 1993;3:45–55. [Google Scholar]
- SUDLOW A.W., CAREY F., FORDER R., ROTHWELL R.J. Lipocortin 1 inhibits CRH stimulation of plasma ACTH and IL-1β-stimulated hypothalamic CRH release in rats. Am. J. Physiol. 1996;270:R54060. doi: 10.1152/ajpregu.1996.270.1.R54. [DOI] [PubMed] [Google Scholar]
- TAMAKI M., SATO M., MATSUBARA S., WADA Y., TAKAHARA J. Dexamethasone increases growth hormone (GH)-releasing hormone (GHR) receptor mRNA levels in cultured rat anterior pituitary cells. J. Neuroendocrinol. 1996;8:475–480. doi: 10.1046/j.1365-2826.1996.04779.x. [DOI] [PubMed] [Google Scholar]
- TAYLOR A.D., CHRISTIAN H.C., MORRIS J.F., FLOWER R.J., BUCKINGHAM J.C. An antisense oligodeoxynucleotide to lipocortin 1 reverses the inhibitory actions of dexamethasone on the release of ACTH from rat pituitary tissue in vitro. Endocrinology. 1997;138:2909–2918. doi: 10.1210/endo.138.7.5260. [DOI] [PubMed] [Google Scholar]
- TAYLOR A.D., CHRISTIAN H.C., MORRIS J.F., FLOWER R.J., BUCKINGHAM J.C. Evidence from immunoneutralisation and antisense studies that the inhibitory actions of glucocorticoids on growth hormone release in vitro require annexin 1 (lipocortin 1) Br. J. Pharmacol. 2000a;131:1309–1326. doi: 10.1038/sj.bjp.0703694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR A.D., COWELL A-M., FLOWER R.J., BUCKINGHAM J.C. Lipocortin 1 mediates an early inhibitory action of glucocorticoids on the secretion of ACTH by the rat anterior pituitary gland in vitro. Neuroendocrinology. 1993;58:430–439. doi: 10.1159/000126572. [DOI] [PubMed] [Google Scholar]
- TAYLOR A.D., COWELL A.-M., FLOWER R.J., BUCKINGHAM J.C. Dexamethasone suppresses the release of prolactin from the rat anterior pituitary gland by lipocortin 1 dependent and independent mechanisms. Neuroendocrinology. 1995a;62:530–542. doi: 10.1159/000127044. [DOI] [PubMed] [Google Scholar]
- TAYLOR A.D., LOXLEY H.D., FLOWER R.J., BUCKINGHAM J.C. Immunoneutralization of lipocortin 1 reverses the acute inhibitory effects of dexamethasone on the hypothalamo-pituitary-adrenocortical responses to cytokines in the rat in vitro and in vivo. Neuroendocrinology. 1995b;62:19–31. doi: 10.1159/000126984. [DOI] [PubMed] [Google Scholar]
- TAYLOR A.D., PHILIP J.G., JOHN C.D., COVER P.O., MORRIS J.F., FLOWER R.J., BUCKINGHAM J.C. Annexin 1 (lipocortin 1) mediates the glucocorticoid inhibition of 3′,5′ cyclic adenosine phosphate stimulated prolactin secretion. Endocrinology. 2000b;141:2209–2219. doi: 10.1210/endo.141.6.7512. [DOI] [PubMed] [Google Scholar]
- TRAVERSO V., CHRISTIAN H.C., MORRIS J.F., BUCKINGHAM J.C. Lipocortin 1 (annexin 1): a candidate paracrine agent localised in pituitary folliculo-stellate cells. Endocrinology. 1999;140:4311–4319. doi: 10.1210/endo.140.9.7008. [DOI] [PubMed] [Google Scholar]
- TURNBULL A., RIVIER C. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol. Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- TURNBULL A.V., RIVIER C.L. Intracerebroventricular passive immunisation I: The effect of intracerebroventricular administration of an antiserum to tumor necrosis factor-α on the plasma adrenocorticotropin response to lipopolysaccharide in rats. Endocrinology. 1998a;139:119–127. doi: 10.1210/endo.139.1.5642. [DOI] [PubMed] [Google Scholar]
- TURNBULL A.V., RIVIER C.L. Intracerebroventricular passive immunisation II: Intracerebroventricular infusion of neuropeptide antisera can inhibit neuropeptide signalling in peripheral tissues. Endocrinology. 1998b;139:128–136. doi: 10.1210/endo.139.1.5643. [DOI] [PubMed] [Google Scholar]
- VALE W., VAUGHAN J., YAMAMOTO G., SPIESS J., RIVIER J. Effects of synthetic human pancreatic (tumor) GH releasing factor and somatostatin, tri-iodothyronine and dexamethasone on GH secretion in vitro. Endocrinology. 1983;112:1553–1555. doi: 10.1210/endo-112-4-1553. [DOI] [PubMed] [Google Scholar]
- VAN DAM A.-M., DE VRIES H.E., KUIPER J., ZIJLSTRA F.J., DE BOER E.G., TILDERS F.J., BERKENBOSCH F. Interleukin 1 receptors on rat brain endothelial cells: a role in neuroimmune interactions. FASEB J. 1996;10:351–356. doi: 10.1096/fasebj.10.2.8641570. [DOI] [PubMed] [Google Scholar]
- WADA Y., SATO M., NIIMI M., TAMAKI M., ISHDIDA T., TAKAHARA J. Inhibitory effects of interleukin 1 on growth hormone secretion in conscious male rats. Endocrinology. 1995;136:3936–3941. doi: 10.1210/endo.136.9.7649102. [DOI] [PubMed] [Google Scholar]


