Abstract
We have recently demonstrated that chronic infusion of Angiotensin II into apoE−/− mice promotes the development of abdominal aortic aneurysms. To determine the involvement of specific Angiotensin II receptors in this response, we co-infused Angiotensin II (1000 ng kg−1 min−1 for 28 days) with losartan (30 mg kg−1 day−1) or PD123319 (3 mg kg−1 day−1) to antagonize AT1 and AT2 receptors, respectively.
Infusion of Angiotensin II promoted the development of abdominal aortic aneurysms in 70% of mature female apoE−/− mice. The formation of aortic aneurysms was totally inhibited by co-infusion of Angiotensin II with losartan (30 mg kg−1 day−1; P=0.003). In contrast, the co-infusion of Angiotensin II with PD123319 resulted in a marked increase in the incidence and severity of aortic aneurysms.
To determine whether AT2 antagonism also promoted Angiotensin II-induced atherosclerosis, Angiotensin II was infused into young female apoE−/− mice that had little spontaneous atherosclerosis. In these mice, co-infusion of PD123319 led to a dramatic increase in the extent of atherosclerosis. This increase was associated with no change in plasma lipid concentrations and only transient and modest increases in blood pressure during co-infusion with PD123319.
While antagonism of AT1 receptors totally prevented the formation of aneurysms, antagonism of AT2 receptors promoted a large increase in the severity of Angiotensin II-induced vascular pathology.
Keywords: Aneurysms, atherosclerosis, angiotensin II, receptors
Introduction
Recent studies in our laboratory, and others, examined whether chronic exposure to elevated Angiotensin II directly influences the atherogenic process (Daugherty & Cassis, 1999; Daugherty et al., 2000; Weiss et al., 2001). Infusion of Angiotensin II for 28 days into apoE−/− or LDL receptor−/− mice resulted in an increase in lesion size. These rapidly developing lesions were characterized by lipid-laden macrophages and large numbers of T lymphocytes. Unexpectedly, pronounced abdominal aortic aneurysms were also present in apoE−/− and LDL receptor−/− mice infused with Angiotensin II (Daugherty & Cassis, 1999; Daugherty et al., 2000). These results demonstrated that a relatively short term exposure to elevated plasma concentrations of Angiotensin II was sufficient to markedly potentiate vascular pathology. Furthermore, they provided additional rationale for inhibiting the renin-angiotensin system with angiotensin converting enzyme inhibitors or Angiotensin II receptor antagonists to limit the progression of abdominal aortic aneurysms and atherosclerosis.
It is well established that Angiotensin II binds with high affinity to two receptor subtypes, AT1 and AT2 (Timmermans et al., 1993). The use of specific antagonists of the AT1 receptor has demonstrated that Angiotensin II acts through this receptor to regulate vascular contractility, aldosterone synthesis and secretion, cell growth and proliferation, and the elaboration of cytokines and matrix proteins. Studies with AT1 receptor antagonists have been performed in atherosclerosis models with equivocal results, demonstrating either no effect in cholesterol-fed rabbits treated with SC-51316 (Schuh et al., 1993), or a reduction in the extent of atherosclerosis in apoE−/− mice treated with losartan (Keidar et al., 1997). More recently, infusion of losartan into male cynomolgus monkeys prevented the formation of early lesions that consist of lipid-laden macrophages (Strawn et al., 2000). Results from these studies implicate endogenous Angiotensin II, through effects at the AT1 receptor, in the atherogenic process. Currently, there have been no reports of the effect of AT2 receptor blockade on the development of atherosclerosis. Furthermore, neither receptor has been defined as the mediator of Angiotensin II induced formation of abdominal aortic aneurysms.
In this study, we determined the Angiotensin II receptor responsible for the vascular pathologies observed in apoE−/− mice during infusion with Angiotensin II. Our results demonstrate that AT1 receptor antagonism abolished the Angiotensin II-dependent formation of abdominal aortic aneurysms. In contrast, AT2 receptor blockade also influenced Angiotensin II induced abdominal aortic aneurysms by increasing the incidence and most strikingly, the complexity of the formed aneurysms. AT2 receptor blockade also markedly increased the atherogenic properties of Angiotensin II infusions.
Methods
Mice
Female apoE−/− mice, (bred 10 times into a C57BL/6 background) obtained either from Monsanto or the Jackson Laboratory, were maintained under barrier conditions. Water and normal laboratory diet were available ad libitum. All drugs were administered subcutaneously by Alzet osmotic mini-pumps (Model 2004) which were implanted into the interscapular space of anaesthetized (ketamine/xylazine) mice at either 2 or 11 months of age. Angiotensin II (500 or 1000 ng kg−1 min−1), losartan (30 mg kg−1 day−1) or PD123319 (3 mg kg−1 day−1) were administered for 28 days as described previously (Daugherty et al., 2000). All procedures were performed with the prior approval of the University of Kentucky Institutional Animal Care and Use Committee.
Determination of blood pressure
Systolic blood pressure was measured in conscious mice using a tail cuff apparatus that was coupled either to a Kent Scientific RTBP1007 data acquisition system or a Visitech system.
Lipid and lipoproteins
Serum total cholesterol and triglyceride concentrations were determined with enzymatic assay kits (Wako Chemical Co.). Lipoprotein cholesterol distributions were evaluated in individual serum samples (50 μl) from four mice in each group after fractionation by size exclusion chromatography on a single Superose 6 column (Daugherty et al., 2000). Fractions were collected and cholesterol concentrations were determined with an enzymatic based assay kit (Wako Chemical Co.).
Quantification of atherosclerosis and characterization of aneurysms
The quantification of the extent of atherosclerosis covering the intimal surface of the aorta was as described previously (Daugherty et al., 2000) Quantification of aneurysms was previously based on the per cent incidence (Daugherty et al., 2000). However, in this study, striking differences in aneurysm appearance were also observed between groups. Therefore, a scale based on the gross appearance of the aorta was devised to represent the different forms of aneurysms. Aneurysms were scored based on the following scale: Type I, dilated lumen in the supra-renal region of the aorta with no thrombus. Type II, remodeled tissue in the supra-renal region that frequently contains thrombus. Type III, a pronounced bulbous form of type II that contains thrombus. Type IV, a form in which there are multiple aneurysms containing thrombus, some overlapping, in the suprarenal area of the aorta. Aneurysmal tissue was categorized independently by two unblinded observers. There was complete concordance in the designation by the two observers.
Statistics
The null hypothesis for the variables measured in the groups was initially tested by ANOVA. If a group difference was noted, post-hoc testing was performed using parametric testing for multiple groups, if the data fitted the constraints of this form of analysis. Non parametric data were analysed using Wilcoxon Rank-Sum tests. The significance of the incidence of aneurysms was tested by Chi squared analysis with a Yates correction factor. All statistical analyses were performed with SigmaStat (SSPS) and a P⩽0.05 was considered to be statistically significant.
Results
In preliminary studies, there appeared to be an increase in the formation of Angiotensin II induced abdominal aortic aneurysms with age in apoE−/− mice (data not shown). Therefore, in the first series of studies the effect of specific receptor antagonists on the development of aortic abdominal aneurysms were examined in mature female apoE−/− mice (11 month of age). Six groups (n=10/group) of mice were implanted with osmotic mini-pumps for 28 day drug delivery as follows: 1. saline; 2. losartan (30 mg kg−1 day−1); 3. PD123319 (3 mg kg−1 day−1); 4. Angiotensin II (1000 ng kg−1 min−1); 5. Angiotensin II and losartan; 6. Angiotensin II and PD123319. In agreement with our previous studies (Daugherty & Cassis, 1999; Daugherty et al., 2000) infusion of Angiotensin II, alone or in the presence of receptor antagonists, did not lead to measurable changes in arterial blood pressure in these mature mice (data not shown).
Small dilations have been reported previously in the abdominal aorta from apoE−/− mice (Tangirala et al., 1995; Carmeliet et al., 1997) although we did not observe discernable aneurysms in the aorta from saline-infused mice. In contrast, infusion of Angiotensin II generated large aneurysms in the abdominal aorta (distal to the renal arteries) that were characterized by dilations and perimedial remodelling (Daugherty et al., 2000). Aneurysms were generated in 70% of the mice infused with Angiotensin II in this study. Co-infusion of losartan completely abolished the aneurysms generated by Angiotensin II (P=0.003; Figure 1). In contrast, co-infusion of Angiotensin II and PD123319 increased the incidence of aneurysm formation to 100%. In addition, the aneurysms had a more complex appearance. To characterize the complexity of aneurysm formation, we categorized the aneurysms formed in these studies into four classes as described in Methods. Examples of aneurysms in each class are shown in Figure 2. In mice infused with Angiotensin II alone, the majority of aneurysms were categorized in class II (Figure 3A). Strikingly, the majority of the aneurysms formed during co-infusion of Angiotensin II and PD123319 fit the categorization of class IV, with a complex polymorphic appearance (Figure 3B).
Figure 1.
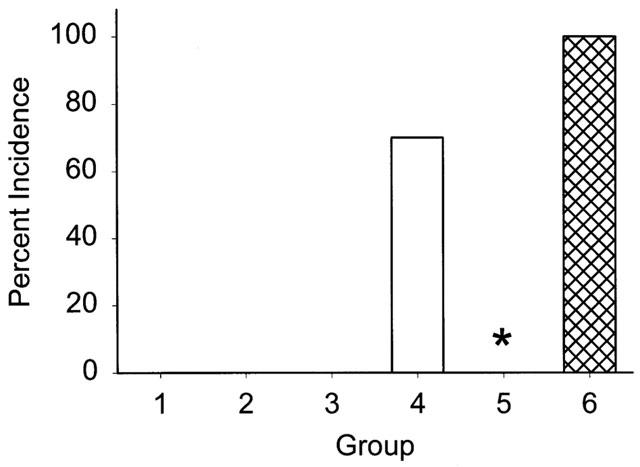
The incidence of aortic abdominal aneurysms during infusion of: (1) Saline; (2) losartan, (30 mg kg−1 day−1); (3) PD123319 (3 mg kg−1 day−1); (4) Angiotensin II (1000 ng kg−1min−1); (5) Angiotensin II and losartan; (6) Angiotensin II and PD123319. (n=10 for each group.) *Denotes P=0.003 for Angiotensin II infusion alone compared to the group co-infused with Angiotensin II and losartan.
Figure 2.
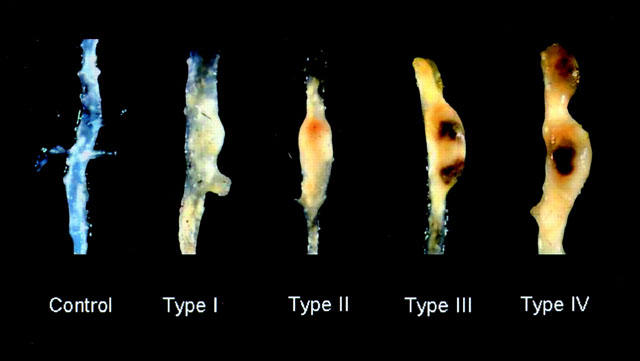
A classification of abdominal aortic aneurysms formed in response to Angiotensin II, alone or during co-infusion with PD123319 in apoE−/− mice.
Figure 3.
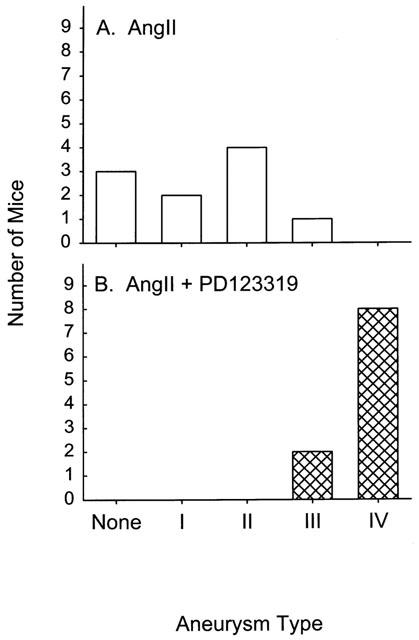
Comparison of the incidence of specific forms of aneurysms described in Figure 2 that were present in apoE−/− mice infused with Angiotensin II alone (A) or in combination with PD123319 (B).
The extent of atherosclerosis was defined in these mice by the en face quantification of area of atherosclerotic lesions that covered the intimal surface of the aorta. No differences were observed in the extent of atherosclerosis during infusion of Angiotensin II, alone or in combination with receptor antagonists (data not shown). However, as expected by the age of the mice, (Nakashima et al., 1994) even the group infused with saline had extensive atherosclerotic lesions and, therefore, the relatively brief infusion of Angiotensin II for 28 days did not produce a measurable increase in atherosclerosis. To determine whether AT2 receptor antagonism also enhanced the development of atherosclerosis, a second study was performed in young apoE−/− mice that were 2 months of age. Using the same dose of antagonist as described above, Angiotensin II or Angiotensin II with PD123319 (n=15 per group) were infused for 28 days. Also, we decreased the dose of Angiotensin II (500 ng kg−1 min−1) to enhance any potential difference during co-infusion with PD123319. Similar to results obtained in 11 month apoE−/− mice, Angiotensin II infusion did not influence systolic blood pressure at 2 months of age (Figure 4). However, in these young mice, co-infusion of Angiotensin II and PD123319 resulted in an initial increase in blood pressure which was not sustained. Infusion of Angiotensin II and PD123319 did not significantly alter the serum lipid concentrations (261±7 vs 280±9 mg/dl for Angiotensin II and Angiotensin II-PD123319 infused, respectively; n.s.). As shown in Figure 5, co-infusion of Angiotensin II and PD123319 promoted a greater than 3 fold increase in lesions covering the intimal surface, markedly potentiating the effect of Angiotensin II to promote the atherogenic process (P<0.001).
Figure 4.
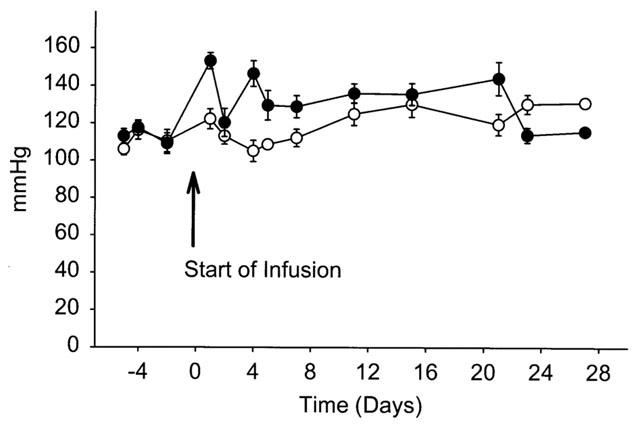
Systolic blood pressure in apoE−/− mice infused with Angiotensin II alone (500 ng kg−1 min−1; ○) or in combination with PD123319 (3 mg kg−1 day−1; •). Symbols represent the mean±s.e.mean of 15 mice.
Figure 5.
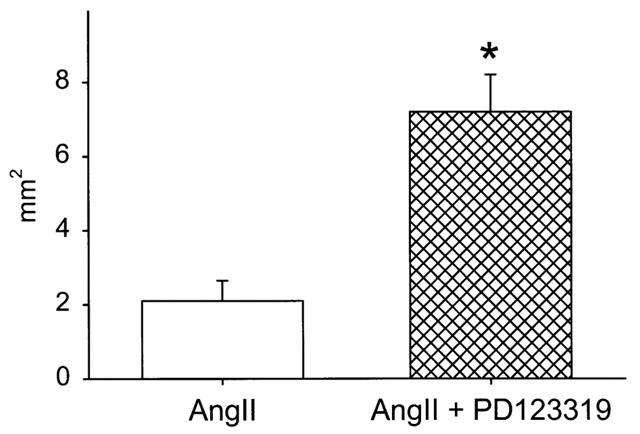
Extent of intimal area covered by grossly discernable atherosclerotic lesions in 2-month-old apoE−/− mice infused with Angiotensin II alone (500 ng kg−1 min−1; open histobars) or in combination with PD123319 (3 mg kg−1 day−1; crosshatched histobars). Histobars represent the mean±s.e.mean of 15 mice. *Denotes significant differences compared to mice infused with Angiotensin II alone, P<0.001.
Discussion
In agreement with our previous findings, infusion of Angiotensin II for one month into apoE−/− mice resulted in the generation of large abdominal aortic aneurysms in hyperlipidemic mice (Daugherty et al., 2000). Results of the present study demonstrate that blockade of the AT1 receptor attenuated the Angiotensin II induced abdominal aortic aneurysm formation in apoE−/− mice. However, unexpectedly, AT2 receptor blockade markedly increased the severity of both abdominal aortic aneurysms and atherosclerosis. These data are indicative of a protective role of the AT2 receptor in these Angiotensin II induced vascular pathologies.
Angiotensin II is assumed to exert many of its effects through interactions with AT1 receptors. In agreement with this tenet, losartan ablated the development of Angiotensin II induced aortic abdominal aneurysms. In previous studies, losartan decreased the extent of atherosclerosis in apoE−/− mice (Keidar et al., 1997) and cynomolgus monkeys (Strawn et al., 2000) even in the absence of exogenously administered Angiotensin II. AT1 receptors have been identified on macrophages (Scheidegger et al., 1997; Keidar et al., 1999; Okamura et al., 1999), a cell type heavily implicated in the development of atherogenic lesions. The pro-atherogenic effects of Angiotensin II, mediated via AT1 receptors, may be through several mechanisms including stimulation of 15-lipoxygenase, (Scheidegger et al., 1997), increased cholesterol biosynthesis (Keidar et al., 1999) and increased oxidized LDL uptake (Hayek et al., 2000). In contrast, potential mechanisms by which stimulation of AT1 receptors leads to the development of abdominal aortic aneurysms remain largely undefined. Based on previous results suggesting enhanced release of matrix metalloproteases (Pyo et al., 2000; Carmeliet et al., 1997) in aneurysm models, we are currently investigating whether Angiotensin II activates enzymes involved in extracellular matrix degradation.
While AT1 receptor antagonists abolished the development of aortic abdominal aneurysms, the co-infusion of Angiotensin II and an AT2 receptor antagonist had a striking effect to enhance the development of abdominal aortic aneurysms. A consistent finding in the present study was the presence of pronounced aneurysms in every mature mouse co-infused with Angiotensin II and PD123319. Not only was the incidence of aneurysm formation increased, but there was a marked difference in the appearance of the aortic tissue. As presented in Figure 2, we arbitrarily defined aneurysms based largely on the visual characteristics. It was noteworthy that the co-infusion of PD123319 with Angiotensin II generated polymorphic aneurysms with more progressive pathology. We have not previously observed this extensive severity of aneurysm formation during infusion of Angiotensin II alone.
Our studies are the first to examine the effect of AT2 receptor antagonism on atherogenesis. We infused PD123319, the AT2 receptor antagonist, at a dose previously utilized for chronic infusion by osmotic mini-pump. (Kuizinga et al., 1998; Cao et al., 1999) To determine whether AT2 antagonism affected Angiotensin II-induced atherosclerosis in the absence of established disease, these drugs were co-infused into 2 month old mice in which the disease would have been minimal (Nakashima et al., 1994) We used a lower dose of Angiotensin II to attempt to maximize the differences during co-infusion. Under these conditions, there was a striking enhancement of the development of atherosclerosis.
In addition to effects on aneurysms and atherogenic lesions, the coinfusion of Angiotensin II and PD123319 provided several unexpected results. In younger mice, co-infusion of PD123319 with Angiotensin II resulted in a modest hypertensive response. These results are in agreement with previous studies demonstrating a greater hypertensive response during infusion of Angiotensin II in AT2 receptor deficient mice compared to mice that were wild type for the receptor (Siragy et al., 1999). A reduction in vascular responsiveness with aging (Kihara et al., 2000) may explain the lack of change in systolic blood pressure in mature mice co-infused with Angiotensin II and PD123319.
The AT2 receptor is widely expressed in foetal tissue, with more restricted expression in tissues from adult animals (Gallinat et al., 2000). Of note, the AT2 receptor is upregulated in cardiovascular tissues during remodelling, including vascular injury and atherosclerosis (Masaki et al., 1998). While our results do not indicate the cell type involved in the protective effects of Angiotensin II mediated through the AT2 receptor, both atherogenic lesions and aneurysms were negatively influenced by AT2 receptor blockade. We have preliminary evidence that Angiotensin II induced aneurysm formation is due to effects on infiltrating leukocytes (Manning et al., 2001). Therefore, future studies will define the effect of modulation of the AT2 receptor in specific leukocyte populations on the development of Angiotensin II induced vascular pathology.
Evidence in the literature suggests that in general, Angiotensin II effects at the AT1 receptor are opposed by actions at the AT2 receptor (Carey et al., 2000). For example, overexpression of the AT2 receptor in the heart resulted in an attenuated pressor and chronotropic response to Angiotensin II (Masaki et al., 1998). Also, these two receptors have opposing effects on Angiotensin II induced cell growth (Nakajima et al., 1995) and angiogenesis (Fujiyama et al., 2001). Results from this study are the first to demonstrate opposing effects of Angiotensin II at AT1 versus AT2 receptors to exacerbate atherogenesis and the formation of aneurysms. Based on these results, we suggest that a specific AT2 receptor agonist may have beneficial and protective advantages in the treatment of atherosclerosis and aortic aneurysms.
In summary, we have previously demonstrated that infusion of Angiotensin II into hyperlipidemic mice leads to the formation of abdominal aortic aneurysms and augmented atherosclerosis (Daugherty & Cassis, 1999; Daugherty et al., 2000). The present study demonstrates that the aneurysms are ablated by the co-infusion of the AT1 receptor antagonist, losartan. Unexpectedly, co-infusion of the AT2 receptor antagonist enhanced the incidence and severity of aneurysms. In addition, co-infusion of PD123319 also augmented Angiotensin II induced atherosclerosis. These data suggest that drugs targeted against the renin-angiotensin system, specifically AT1 receptor antagonists or AT2 receptor agonists, may be of benefit in the treatment of abdominal aortic aneurysms and atherosclerosis.
Acknowledgments
This work was supported by the National Institutes of Health (HL 62846) and a Medical School Grant from Merck & Co. We appreciate the gift of apoE−/− mice from Monsanto and PD123319 from Parke Davis. We are grateful for the technical assistance of Marc Helton.
Abbreviations
- AT1
Angiotensin receptor type 1
- AT2
Angiotensin receptor type 2
- apoE
apolipoprotein E
- LDL
low-density lipoprotein
References
- CAO Z., DEAN R., WU L., CASLEY D., COOPER M.E. Role of angiotensin receptor subtypes in mesenteric vascular proliferation and hypertrophy. Hypertension. 1999;34:408–414. doi: 10.1161/01.hyp.34.3.408. [DOI] [PubMed] [Google Scholar]
- CAREY R.M., WANG Z.Q., SIRAGY H.M. Role of the angiotensin type 2 receptor in the regulation of blood pressure and renal function. Hypertension. 2000;35:155–163. doi: 10.1161/01.hyp.35.1.155. [DOI] [PubMed] [Google Scholar]
- CARMELIET P., MOONS L., LIJNEN R., BAES M., LEMAITRE V., TIPPING P., DREW A., EECKHOUT Y., SHAPIRO S., LUPU F., COLLEN D. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nature Genet. 1997;17:439–444. doi: 10.1038/ng1297-439. [DOI] [PubMed] [Google Scholar]
- DAUGHERTY A., CASSIS L. Chronic angiotensin II infusion promotes atherogenesis in low density lipoprotein receptor −/− mice. Proc. N.Y. Acad. Sci. 1999;892:108–118. doi: 10.1111/j.1749-6632.1999.tb07789.x. [DOI] [PubMed] [Google Scholar]
- DAUGHERTY A., MANNING M.W., CASSIS L.A. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J. Clin. Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJIYAMA S., MATSUBARA H., NOZAWA Y., MARUYAMA K., MORI Y., TSUTSUMI Y., MASAKI H., UCHIYAMA Y., KOYAMA Y., NOSE A., IBA O., TATEISHI E., OGATA N., JYO N., HIGASHIYAMA S., IWASAKA T. Angiotensin AT(1) and AT(2) receptors differentially regulate angiopoietin-2 and vascular endothelial growth factor expression and angiogenesis by modulating heparin binding-epidermal growth factor (EGF)-mediated EGF receptor transactivation. Circ. Res. 2001;88:22–29. doi: 10.1161/01.res.88.1.22. [DOI] [PubMed] [Google Scholar]
- GALLINAT S., BUSCHE S., RAIZADA M.K., SUMNERS C. The angiotensin II type 2 receptor: an enigma with multiple variations. Am. J. Physiol. Endocrinol. Metab. 2000;278:E357–E374. doi: 10.1152/ajpendo.2000.278.3.E357. [DOI] [PubMed] [Google Scholar]
- HAYEK T., AVIRAM M., HEINRICH R., SAKHNINI E., KEIDAR S. Losartan inhibits cellular uptake of oxidized LDL by monocyte-macrophages from hypercholesterolemic patients. Biochem. Biophys. Res. Comm. 2000;273:417–420. doi: 10.1006/bbrc.2000.2963. [DOI] [PubMed] [Google Scholar]
- KEIDAR S., ATTIAS J., HEINRICH R., COLEMAN R., AVIRAM M. Angiotensin II atherogenicity in apolipoprotein E deficient mice is associated with increased cellular cholesterol biosynthesis. Atherosclerosis. 1999;146:249–257. doi: 10.1016/s0021-9150(99)00145-8. [DOI] [PubMed] [Google Scholar]
- KEIDAR S., ATTIAS J., SMITH J., BRESLOW J.L., HAYEK T. The angiotensin-II receptor antagonist, losartan, inhibits LDL lipid peroxidation and atherosclerosis in apolipoprotein E-deficient mice. Biochem. Biophys. Res. Comm. 1997;236:622–625. doi: 10.1006/bbrc.1997.6844. [DOI] [PubMed] [Google Scholar]
- KIHARA M., NAKASAKA Y., MITSUI Y., TAKAHASHI M., SCHMELZER J.D. Aging differentially modifies sensitivity of nerve blood flow to vasocontractile agents (endothelin-1, noradrenaline and angiotensin II) in sciatic nerve. Mech. Aging Dev. 2000;114:5–14. doi: 10.1016/s0047-6374(99)00115-3. [DOI] [PubMed] [Google Scholar]
- KUIZINGA M.C., SMITS J.F.M., ARENDS J.W., DAEMEN M.J.A.P. AT2 receptor blockade reduces cardiac interstitial cell DNA synthesis and cardiac function after rat myocardial infarction. J. Mol. Cell. Cardiol. 1998;30:425–434. doi: 10.1006/jmcc.1997.0607. [DOI] [PubMed] [Google Scholar]
- MANNING M.W., SZILVASSY S.J., CASSIS L.A., DAUGHERTY A. Angiotensin AT1a receptors on bone marrow derived cells are critical for angiotensin II-induced aneurysm formation and progression of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2001;21:711. [Google Scholar]
- MASAKI H., KURIHARA T., YAMAKI A., INOMATA N., NOZAWA Y., MORI Y., MURASAWA S., KIZIMA K., MARUYAMA K., HORIUCHI M., DZAU V.J., TAKAHASHI H., IWASAKA T., INADA M., MATSUBARA H. Cardiac-specific overexpression of angiotensin II AT2 receptor causes attenuated response to AT1 receptor-mediated pressor and chronotropic effects. J. Clin. Invest. 1998;101:527–535. doi: 10.1172/JCI1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAJIMA M., HUTCHINSON H.G., FUJINAGA M., HAYASHIDA W., MORISHITA R., ZHANG L., HORIUCHI M., PRATT R.E., DZAU V.J. The angiotensin II type 2 (AT2) receptor antagonizes the growth effects of the AT1 receptor: gain-of-function study using gene transfer. Proc. Natl. Acad. Sci. U.S.A. 1995;92:10663–10667. doi: 10.1073/pnas.92.23.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKASHIMA Y., PLUMP A.S., RAINES E.W., BRESLOW J.L., ROSS R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterio. Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- OKAMURA A., RAKUGI H., OHISHI M., YANAGITANI Y., TAKIUCHI S., MORIGUCHI K., FENNESSY P.A., HIGAKI J., OGIHARA T. Upregulation of renin-angiotensin system during differentiation of monocytes to macrophages. J. Hypertens. 1999;17:537–545. doi: 10.1097/00004872-199917040-00012. [DOI] [PubMed] [Google Scholar]
- PYO R., LEE J.K., SHIPLEY J.M., CURCI J.A., MAO D., ZIPORIN S.J., ENNIS T.L., SHAPIRO S.D., SENIOR R.M., THOMPSON R.W. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J. Clin. Invest. 2000;105:1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEIDEGGER K.J., BUTLER S., WITZTUM J.L. Angiotensin II increases macrophage-mediated modification of low density lipoprotein via a lipoxygenase-dependent pathway. J. Biol. Chem. 1997;272:21609–21615. doi: 10.1074/jbc.272.34.21609. [DOI] [PubMed] [Google Scholar]
- SCHUH J.R., BLEHM D.J., FRIERDICH G.E., MCMAHON E.G., BLAINE E.H. Differential effects of renin-angiotensin system blockade on atherogenesis in cholesterol-fed rabbits. J. Clin. Invest. 1993;91:1453–1458. doi: 10.1172/JCI116350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIRAGY H.M., INAGAMI T., ICHIKI T., CAREY R.M. Sustained hypersensitivity to angiotensin II and its mechanism in mice lacking the subtype-2 (AT2) angiotensin receptor. Proc. Natl. Acad. Sci. U.S.A. 1999;96:6506–6510. doi: 10.1073/pnas.96.11.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAWN W.B., CHAPPELL M.C., DEAN R.H., KIVLIGHN S., FERRARIO C.M. Inhibition of early atherogenesis by losartan in monkeys with diet-induced hypercholesterolemia. Circulation. 2000;101:1586–1593. doi: 10.1161/01.cir.101.13.1586. [DOI] [PubMed] [Google Scholar]
- TANGIRALA R.K., RUBIN E.M., PALINSKI W. Quantitation of atherosclerosis in murine models: Correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J. Lipid Res. 1995;36:2320–2328. [PubMed] [Google Scholar]
- TIMMERMANS P.B., WONG P.C., CHIU A.T., HERBLIN W.F., BENFIELD P., CARINI D.J., LEE R.J., WEXLER R.R., SAYE J.A., SMITH R.D. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol. Rev. 1993;45:205–251. [PubMed] [Google Scholar]
- WEISS D., KOOLS J.J., TAYLOR W.R. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in ApoE-deficient mice. Circulation. 2001;103:448–454. doi: 10.1161/01.cir.103.3.448. [DOI] [PubMed] [Google Scholar]


