Abstract
The cannabinoid arachidonyl ethanolamide (anandamide) caused concentration-dependent relaxation of 5-HT-precontracted, myograph-mounted, segments of rat left anterior descending coronary artery.
This relaxation was endothelium-independent, unaffected by the fatty acid amide hydrolase inhibitor, arachidonyl trifluoromethyl ketone (10 μM), and mimicked by the non-hydrolysable anandamide derivative, methanandamide.
Relaxations to anandamide were attenuated by the cannabinoid receptor antagonist, SR 141716A (3 μM), but unaffected by AM 251 (1 μM) and AM 630 (1 μM), more selective antagonists of cannabinoid CB1 and CB2 receptors respectively. Palmitoylethanolamide, a selective CB2 receptor agonist, did not relax precontracted coronary arteries.
Anandamide relaxations were not affected by inhibition of sensory nerve transmission with capsaicin (10 μM) or blockade of vanilloid VR1 receptors with capsazepine (5 μM). Nevertheless capsaicin relaxed coronary arteries in a concentration-dependent and capsazepine-sensitive manner, confirming functional sensory nerves were present. In contrast, capsazepine and capsaicin did inhibit anandamide relaxations in methoxamine-precontracted rat small mesenteric arteries.
Relaxations to anandamide were inhibited by TEA (1 mM) or iberiotoxin (50 nM), blockers of large conductance, Ca2+-activated K+ channels (BKCa). Gap junction inhibition with 18α-glycyrrhetinic acid (100 μM) did not affect anandamide relaxations.
This study shows anandamide relaxes the rat coronary artery by a novel mechanism. Anandamide-induced relaxations do not involve the endothelium, degradation into active metabolites, or activation of cannabinoid CB1 or CB2 receptors, but may involve activation of BKCa. Vanilloid receptor activation also has no role in the effects of anandamide in coronary arteries, even though functional sensory nerves are present.
Keywords: Coronary artery (rat), cannabinoids, anandamide, vanilloid receptors, cannabinoid receptors, capsaicin, sensory nerves
Introduction
The endogenous cannabinoid, arachidonyl ethanolamide (anandamide) is a potent vasorelaxant in many isolated blood vessel types, including mesenteric (Randall et al., 1996; White & Hiley, 1997), cerebral (Ellis et al., 1995; Gebremedhin et al., 1999), renal (Deutsch et al., 1997) and coronary (Pratt et al., 1998) arteries. Whilst the cardiovascular actions of cannabinoids in anaesthetized whole animals are largely mediated by CB1 receptors (Lake et al., 1997; Járai et al., 1999), there have been relatively few studies reporting cannabinoid relaxation of isolated blood vessels through cannabinoid CB1 receptors (Gebremedhin et al., 1999); indeed, there is evidence that CB1 receptors may be present on vascular smooth muscle but not stimulate vasorelaxation (Holland et al., 1999). Metabolism of anandamide into arachidonic acid, and thence to other vasodilator eicosanoids, has been shown to underpin relaxations induced by the cannabinoid in some preparations (Ellis et al., 1995; Pratt et al., 1998), whilst endothelium-derived factors (Járai et al., 1999) and gap junction communication (Chaytor et al., 1999) have also been implicated.
Zygmunt et al. (1999) provided a major breakthrough in understanding of the effects of anandamide, by showing that it could cause vasorelaxation by activating vanilloid VR1 receptors, thereby stimulating CGRP release from perivascular sensory nerves. Indeed, vanilloid receptor activation represents the predominant mechanism for anandamide relaxation in the rat mesenteric, rat hepatic and guinea-pig basilar arteries (Zygmunt et al., 1999; Ralevic et al., 2000). Interestingly, however, Járai et al. (1999) found that anandamide had no effect on mean arterial blood pressure when administered to cannabinoid CB1 receptor knockout mice, even though anandamide was still able to evoke vanilloid receptor-dependent relaxation in mesenteric beds from the same mice. These results suggest that the vanilloid receptor-mediated effects of anandamide may be important in regulating regional blood flow without affecting blood pressure, hence the effects of anandamide may show pronounced regional as well as species variation.
Interest in the effects of cannabinoids on the heart has recently been stimulated by Lagneux & Lamontagne (2001), who showed that endogenous cannabinoids might mediate cardiac preconditioning induced by lipopolysaccharide in rat hearts. Previous work has shown that anandamide caused vasorelaxation in isolated rat hearts (Randall & Kendall, 1997; Fulton & Quilley, 1998) which was sensitive to the cannabinoid receptor antagonist, SR 141716A and therefore attributed to activation of cannabinoid receptors. However we have demonstrated that the effects of SR 141716A on anandamide relaxation are not consistent with an action at cannabinoid receptors, and that this agent may have non-specific effects in vascular smooth muscle (White & Hiley, 1998a,1998b), thus casting some doubt as to whether the coronary effects of anandamide actually involve cannabinoid receptor activation. Arachidonic acid did not mimic the actions of anandamide in the rat coronary circulation (Randall & Kendall, 1997; Fulton & Quilley, 1998), hence it seems unlikely that metabolism of anandamide to vasoactive arachidonic acid derivatives is involved in the relaxant effect of the cannabinoid. Activation of vanilloid receptors, however, represents a possible mechanism for the coronary effects of anandamide, since capsaicin-induced neuropeptide release from sensory nerve endings has been demonstrated in the heart and may play a role in the modulation of regional blood flow (Franco-Cereceda et al., 1989; Kallner et al., 1999).
The aim of the present study was to examine the mechanism by which anandamide causes vasorelaxation of rat isolated coronary arteries. The possible roles of endothelium-derived factors, activation of cannabinoid receptors, activation of vanilloid receptors on sensory nerves, and metabolism in anandamide-induced relaxation were investigated in myograph-mounted rat isolated left anterior descending coronary arteries. The effectiveness of capsaicin and capsazepine as inhibitors of sensory nerve-mediated actions of anandamide was assessed in rat isolated small mesenteric arteries in order to confirm the effectiveness of the protocols and the results obtained by Zygmunt et al. (1999).
Methods
Myograph studies
Male Wistar rats (250 – 350 g; Tucks, Rayleigh, Essex, U.K.) were killed with an overdose of sodium pentobarbitone (120 mg kg−1, i.p., Sagatal, Rhône Mérieux, Harlow, U.K.). The left anterior descending coronary or small (third generation) mesenteric arteries were then removed and cleaned of adherent tissue (Otley et al., 1996). Segments (2 mm) were mounted in a Mulvany-Halpern type wire myograph and normalized as described previously (White & Hiley, 1997) in gassed (95% O2/5% CO2) Krebs-Henseleit solution of the following composition (mM): NaCl, 118; KCl 4.7; MgSO4, 1.2; KH2PO4 1.2; NaHCO3, 25; CaCl2, 2.5; D-glucose, 5.5 (mesenteric arteries) or 11 (coronary arteries); except where stated, all experiments were carried out in the presence of 10 μM indomethacin (Sigma). In all vessels, the presence of a functional endothelium was tested by precontracting with either 5-HT (coronary arteries) or methoxamine (mesenteric arteries), and adding 10 μM carbachol; relaxations >65% (coronary) or >90% (mesenteric) denoted endothelium-intact vessels. Where the endothelium was not required, vessels were denuded by rubbing the intimal surface with a human hair, and successful endothelium removal was confirmed by a lack of vasodilator response (<10%) to carbachol.
Experimental protocols
After 30 min equilibration, vessels were precontracted submaximally with either 5-HT (coronary arteries) or methoxamine (mesenteric arteries). When a stable level of tone was achieved, concentration-response curves were generated by cumulative addition of the agent under investigation. In experiments where the effect of a vasorelaxant was investigated in the presence of antagonists (SR 141716A, AM 251, AM 630, capsazepine) or other agents (capsaicin, arachidonyl trifluoromethyl ketone), these were added to the organ bath 30 min before, and were present during, construction of the concentration-response curve; some experiments with capsaicin used a 60 min preincubation followed by a 20 min washout before determination of a concentration/response curve.
In all experiments where vessels were incubated with putative inhibitors, the level of tone for precontraction was normalized to the amount of tone obtained in the test for endothelial integrity, by lowering or increasing the concentration of methoxamine (mesenteric) or 5-HT (coronary arteries). Indeed, preliminary experiments revealed that 10 μM capsazepine inhibited arterial contraction in both mesenteric and coronary arteries which could not be controlled for by increasing the concentration of precontracting agent (methoxamine or 5-HT). Hence this agent was used at the highest possible concentration which did not cause insurmountable inhibition of contraction (5 μM in coronary vessels; 3 μM in mesenteric arteries). The overall mean tension developed by rat coronary arteries in response to 5-HT in the test for endothelium was 3.3±0.2 mN as compared with 3.3±0.2 mN (n=97) after preincubation with inhibitors. Similarly, the mean contraction to methoxamine in the rat mesenteric segments was 15.3±1.0 mN in the test for endothelium as compared with 16.8±1.1 mN (n=35) when putative inhibitors were present.
Control responses to anandamide throughout the study generally exhibited a potency in the submicromolar range (pEC50 approximately 7). However for two separate periods during the study, animals exhibited either no response to anandamide or responded with reduced sensitivity (pEC50 approximately 6). Non-responder animals were discarded from the data analysis, whilst experiments performed on rats exhibiting reduced sensitivity (anandamide/methanandamide comparison) were carried out in paired fashion, with control and test experiments both being carried out on segments from the same artery. In all cases where paired experiments were not possible, control values were obtained in vessels obtained from the same batch of animals as those vessels used for the treatment under investigation.
Drugs
All solutions were prepared on the day of the experiment. Arachidonyl ethanolamide (anandamide; Tocris Cookson, Bristol, U.K.) was supplied as a water soluble emulsion. Methoxamine hydrochloride, 5-hydroxytryptamine (5-HT) creatinine sulphate, carbachol, tetraethylammonium chloride (TEA) and iberiotoxin (Sigma Chemical Company, Poole, Dorset, U.K.) were dissolved in distilled water. AM 251 (N-(piperidin - 1 - yl) - 5 -(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide; Tocris Cookson), arachidonyl trifluoromethyl ketone (ATFMK; Alexis Corporation, Nottingham, U.K.), 18α-glycyrrhetinic acid (Sigma) and AM 630 (6-iodo-2-methyl-1-[2-(4-morpholinyl) ethyl]-1H-indol-3-yl (4-methoxyphenyl)methanone; Tocris) were dissolved in dimethylsulphoxide (Sigma). SR 141716A (N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride; Research Biochemicals International, Natick, MA, U.S.A.), R-(+)-methanandamide (Tocris Cookson; supplied as 100% ethanol solution), capsaicin (Sigma) and palmitoylethanolamide (Tocris Cookson) were dissolved in 100% ethanol. Capsazepine (Sigma) was dissolved in 100% methanol. All dilutions were made in distilled water.
Statistical analysis
Relaxation responses in myograph experiments are expressed as the percentage relaxation of the tone induced by 5-HT (coronary artery) or methoxamine (mesenteric artery). Data are given as the mean±s.e.mean. Emax represents the maximum effect and pEC50 the negative logarithm of the concentration of relaxant giving half the maximal relaxation; these values were determined directly from individual concentration-response curves. Statistical comparisons of concentration-response curves were made by two-way ANOVA of the whole data set, followed by the Bonferroni/Dunn post-hoc test for determining significant differences between treatment groups. n indicates the number of animals used. P values less than 0.05 were considered to be statistically significant.
Results
Rat isolated left anterior descending coronary artery
Anandamide-induced relaxation of rat isolated coronary arteries
Anandamide caused concentration-dependent relaxation of endothelium-intact 5-HT-precontracted coronary arteries (pEC50=6.8±0.4, Emax=45±5%, n=5). A trace from a typical experiment is shown in Figure 1a, where it can be seen that the time course of the effect is broadly similar to the actions of anandamide in rat isolated mesenteric arteries. Anandamide-induced relaxations were not significantly affected by removal of the endothelium (pEC50=6.0±0.2, Emax=56±8%, n=6).
Figure 1.

(a) Original recording showing anandamide-induced relaxation of methoxamine-precontracted rat mesenteric arteries (top panel) and 5-HT-precontracted rat coronary arteries (lower panel). Vertical lines denote addition of drugs at the concentrations indicated. AEA represents anandamide. (b) Concentration-response curves for the relaxation of 5-HT-precontracted rat isolated coronary arteries by anandamide in the absence or presence of indomethacin (10 μM). pEC50 and Emax values are given in the text. Data are presented as the mean with the vertical bars showing the s.e.mean; n=5 for both.
Figure 1b shows that relaxations to anandamide were not significantly different in the presence of 10 μM indomethacin (pEC50=6.8±0.4, Emax=56±8%, n=5) or in its absence (pEC50=6.5±0.3, Emax=63±9%, n=5).
Effects of cannabinoid receptor antagonists and inhibition of anandamide breakdown on anandamide-induced relaxation
Figure 2a shows that relaxation of 5-HT-precontracted coronary arteries by anandamide (pEC50=7.3±0.2, Emax=65±11%, n=9) was significantly (P<0.001) attenuated by SR 141716A (3 μM; anandamide pEC50=6.6±0.3, Emax=47±9%, n=5). However the CB1 receptor antagonist, AM 251 (1 μM) had no significant effect (anandamide pEC50=7.3±0.2, Emax=62±13%, n=4; Figure 2a).
Figure 2.
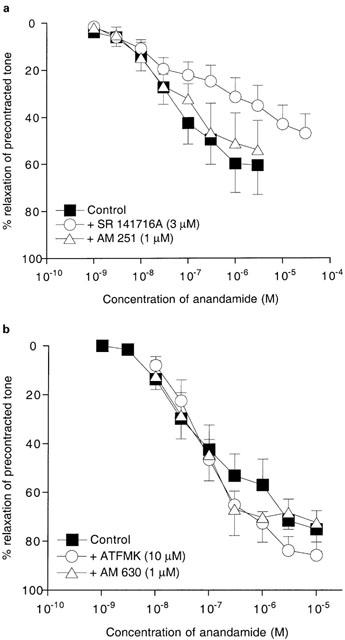
(a) Concentration-response curves for the relaxation of 5-HT-precontracted rat isolated coronary arteries by anandamide in the absence or presence of SR 141716A (3 μM) or AM 251 (1 μM). (b) Concentration-response data for anandamide relaxation of precontracted rat isolated coronary arteries in the absence or presence of ATFMK (10 μM) or AM 630 (1 μM). pEC50 and Emax values are given in the text. Data are presented as the mean with the vertical bars showing the s.e.mean. In (a), control, n=9; with SR 141716A, n=5; with AM 251, n=4. In (b), control, n=5; with ATFMK, n=4; with AM 630, n=5.
Figure 2b shows that anandamide-induced relaxation (pEC50=6.9±0.3, Emax=77±8%, n=6) was also unaffected by AM 630 (1 μM), which is an inverse agonist at CB2 receptors (anandamide pEC50=7.1±0.2, Emax=72±11%, n=5). ATFMK (10 μM), which inhibits anandamide hydrolysis, similarly had no significant effect on relaxation to anandamide (pEC50=7.1±0.2, Emax=87±5%, n=4).
Effect of capsaicin and capsazepine on relaxations induced by anandamide and capsaicin
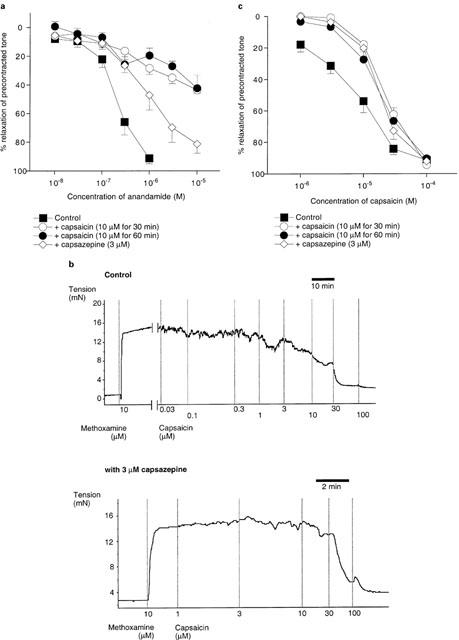
Figure 3a shows that anandamide-induced relaxations were not significantly affected by inhibition of sensory nerve transmission with capsaicin (10 μM for 30 min; anandamide pEC50=7.1±0.6, Emax=60±9%, n=6), or by the vanilloid receptor antagonist, capsazepine (5 μM; anandamide pEC50=6.1±0.7, Emax=76±10%, n=4). Increasing the preincubation time with capsaicin (to 60 min, with 20 min washing out) did not reveal any inhibitory effect (anandamide pEC50=7.5±0.2, Emax=61±10%, n=4).
Figure 3.

(a) Concentration-response curves for the relaxation of 5-HT-precontracted rat isolated coronary arteries by anandamide in the absence or presence of capsaicin (10 μM pretreatment for 30 or 60 min) or capsazepine (5 μM). Control data are reproduced from Figure 2a for clarity. (b) Concentration-response data for the relaxation of precontracted rat coronary arteries by capsaicin in the absence or presence of capsazepine (5 μM). pEC50 and Emax values are given in the text. (c) Original recording from a vessel exhibiting no response to methanandamide (methAEA), anandamide (AEA) or palmitoylethanolamide (PalmitoylEA). Nevertheless the ability of capsaicin to cause relaxation indicates the presence of functional sensory nerves in this preparation. Vertical lines denote addition of drugs at the concentrations indicated. In (a) and (b) data are presented as the mean with the vertical bars showing the s.e.mean.; with capsaicin (30 min), n=6; with capsaicin (60 min), n=4; with capsazepine, n=4. In (b), control, n=6; with capsazepine, n=4.
The effectiveness of capsaicin and capsazepine was then tested. Figure 3b shows that capsaicin caused concentration-dependent relaxation of 5-HT-precontracted coronary arteries (pEC50=5.1±0.1, Emax=95±7%, n=6). Figure 3b also shows that capsaicin-induced relaxations were significantly (P<0.001) inhibited by preincubation of vessels with 5 μM capsazepine (capsaicin pEC50=4.7±0.1, Emax=64±8%, n=4).
Crucially, we were able to test the effectiveness of capsaicin in vessels which were unresponsive to anandamide or methanandamide. Figure 3c shows a representative trace of such an experiment. In all instances, capsaicin caused concentration-dependent relaxation even though previous addition of anandamide, methananamide or palmitoylethanolamide had not evoked relaxation in the same vessel (n=4).
Effect of anandamide, methanandamide and palmitoylethanolamide on 5-HT-precontracted rat coronary arteries
The relaxant effects of anandamide and methanandamide, a stable analogue of anandamide, were compared in matched vessels taken from the same animal. Methanandamide caused relaxation of rat coronary arteries (pEC50=5.1±0.2, Emax=53±6%, n=8) but was significantly (P<0.001) less potent when compared with anandamide (pEC50=6.2±0.3, Emax=56±6%, n=8; Figure 4).
Figure 4.

Concentration-response data for the relaxation of 5-HT-precontracted rat isolated coronary arteries by anandamide, methanandamide and palmitoylethanolamide. pEC50 and Emax values are given in the text. Data are presented as the mean with the vertical bars showing the s.e.mean. Anandamide and methanandamide responses were evaluated in paired segments taken from the same animals, n=8. Palmitoylethanolamide never exhibited vasorelaxant effects (n=5).
Palmitoylethanolamide, which is an agonist at CB2 cannabinoid receptors, elicited no appreciable relaxation of precontracted coronary arteries (n=5; Figure 4).
Effect of K+ channel blockers and gap junction inhibition on anandamide-induced relaxation of rat coronary arteries
Figure 5 shows that anandamide-induced relaxation of rat coronary arteries (pEC50=6.8±0.4, Emax=56±8%, n=5) was significantly (P<0.001) attenuated in the presence of 1 mM TEA (Emax=23±13%, n=4) and further inhibited by 10 mM TEA (Emax=6±1%; n=4). Iberiotoxin (50 nM), a selective inhibitor of large conductance Ca2+-activated K+ channels (BKCa), also inhibited anandamide relaxations (P<0.001) to the same extent as 1 mM TEA (anandamide Emax=23±7%; n=4).
Figure 5.

Concentration-response data for the relaxation of 5-HT-precontracted rat isolated coronary arteries by anandamide in the presence of TEA (1 mM or 10 mM), iberiotoxin (50 nM) or 18α-glycyrrhetinic acid (100 μM). pEC50 and Emax values are given in the text. Data are presented as the mean with the vertical bars showing the s.e.mean. Control anandamide responses, and responses in the presence of 18α-glycyrrhetinic acid, n=5; anandamide responses in the presence of TEA or iberiotoxin, n=4.
It can also be seen from Figure 5 that inhibition of gap junction function with 18α-glycyrrhetinic acid (100 μM) did not significantly affect relaxation to anandamide (pEC50=6.8±0.3, Emax=44±7%, n=5).
Rat isolated mesenteric artery
Effect of capsaicin and capsazepine on relaxations induced by anandamide and capsaicin
Anandamide-induced relaxations of precontracted mesenteric arteries (pEC50=6.7±0.1, Emax=91±4%, n=6) were significantly inhibited (P<0.001) after incubation of vessels for 30 min with either 10 μM capsaicin (anandamide pEC50=5.8±0.3, Emax=63±1%, n=3) or 3 μM capsazepine (pEC50=6.1±0.2, Emax=88±3%, n=4; Figure 6a). Figure 6a also shows that increasing the incubation time with capsaicin (to 60 min, with 20 min washing out) did not produce further inhibition of the anandamide response (anandamide pEC50=5.2±0.3, Emax=68±8%, n=4).
Figure 6.
(a) Concentration-response data for the relaxation of methoxamine-precontracted rat isolated mesenteric arteries by anandamide in the absence or presence of capsaicin (10 μM, for 30 or 60 min) or capsazepine (3 μM). pEC50 and Emax values are given in the text. (b) Original recording showing capsaicin-induced relaxation of rat mesenteric arteries in the absence (top panel) or presence (bottom panel) of 3 μM capsazepine. Owing to the desensitizing effects of capsaicin, the responses were obtained from different vessels. Vertical lines denote addition of drugs at the concentrations indicated. (c) Concentration-response data for the relaxation of precontracted rat mesenteric arteries by capsaicin in the absence or presence of capsaicin (10 μM, pretreatment for 30 or 60 min) or capsazepine (3 μM). pEC50 and Emax values are given in the text. Data in (a) and (c) are presented as the mean with the vertical bars showing the s.e.mean. In (a), control, n=6; with capsaicin (30 min pretreatment), n=3; with capsaicin (60 min pretreatment), n=4; with capsazepine, n=4. In (c), control, n=8; with capsaicin (30 min pretreatment), n=4; with capsaicin (60 min pretreatment), n=4; with capsazepine, n=4.
Capsaicin caused concentration-dependent relaxation of methoxamine-precontracted mesenteric arteries (pEC50=5.2±0.2, Emax=91±1%, n=8; Figure 6b). Figure 6c shows that the vasorelaxant effects of capsaicin were significantly (P<0.01) inhibited after preincubation of vessels for 30 min with either capsaicin (10 μM; capsaicin pEC50=4.7±0.1, Emax=94±1%, n=4) or capsazepine (3 μM; capsaicin pEC50=4.8±0.1, Emax=92±1%, n=4). A longer preincubation period with capsaicin (60 min, with 20 min wash out) did not produce further inhibition of the capsaicin response (pEC50=4.8±0.1, Emax=90±2%, n=4).
Discussion
The major finding of the present study is that anandamide causes relaxation of rat isolated small coronary arteries through a mechanism distinct from those which have previously been ascribed to this cannabinoid. Thus, anandamide-induced relaxation does not involve endothelium-derived factors, metabolism of the cannabinoid into vasoactive derivatives, cannabinoid CB1 or CB2 receptors, or activation of vanilloid receptors on sensory nerves.
Our initial experiments showed that anandamide caused concentration-dependent relaxations of 5-HT-precontracted rat isolated coronary arteries that were independent of the presence of an intact endothelium. These results contrast markedly with the effects of anandamide in the bovine coronary circulation (Pratt et al., 1998), where relaxation to the cannabinoid was shown to be entirely endothelium-dependent and due to the metabolism of anandamide (by fatty acid amide hydrolase, FAAH) into arachidonic acid and thence into vasodilator eicosanoids. Convincing evidence that metabolic products of anandamide are not important in its vasorelaxant effects in rat coronary arteries is provided by our observation that methanandamide, a non-hydrolysable analogue of anandamide (Abadji et al., 1994), also caused relaxation of precontracted vessels. Moreover, ATFMK, which inhibits anandamide hydrolysis by FAAH (Koutek et al., 1994), did not affect anandamide-induced relaxation, in marked contrast with the ability of diazomethylarachidonyl ketone, another FAAH inhibitor, to abolish anandamide relaxations in bovine coronary arteries (Pratt et al., 1998). The lack of endothelium-dependence in the anandamide responses rules out any role of endothelium-derived factors, which have been implicated in anandamide relaxation in rat mesenteric arteries (Járai et al., 1999), and our finding that anandamide relaxations were unaffected by 18α-glycyrrhetinic acid also precludes any role for gap junction communication in the anandamide response (Chaytor et al., 1999). Moreover, anandamide relaxation was unchanged in the absence or presence of indomethacin, ruling out any major involvement of prostanoid synthesis, which underpins the effects of anandamide in rabbit mesenteric arteries (Fleming et al., 1999).
We have previously examined the actions of anandamide in rat isolated mesenteric arteries (White & Hiley, 1997; 1998a). Our results showed that anandamide-induced vasorelaxation was sensitive to the cannabinoid receptor antagonist, SR 141716A, but only at micromolar concentrations, and that this, in addition to the relative potencies of synthetic cannabinoid receptor agonists, was not consistent with anandamide activating either classical CB1 or CB2 receptors (White & Hiley, 1998a). In the present study, SR 141716A was used at a concentration (3 μM) below those at which we have shown it to cause non-selective effects in mesenteric arteries (White & Hiley, 1998b). SR 141716A antagonized the effects of anandamide in rat isolated coronary arteries, results which are consistent with previous findings in the mesenteric (Randall et al., 1996; White & Hiley, 1997) and coronary (Randall & Kendall, 1997) circulations.
Nevertheless our finding that micromolar concentrations of SR 141716A were required to antagonize anandamide responses argues against the involvement of CB1 receptors (Ki of SR 141716A=11.8 nM; Felder et al., 1995). We therefore tested the effects of another CB1 receptor antagonist, AM 251, which is a potent (Ki=7.49 nM) and more selective (306 fold over CB2 receptors) antagonist at CB1 receptors (Lan et al., 1999). AM 251 did not antagonize anandamide-induced relaxation in rat isolated coronary arteries, indicating that the effect of SR 141716A does not involve antagonism of CB1 receptors.
The effectiveness of SR 141716A at micromolar concentrations would seem consistent with an action at CB2 receptors (Ki of SR 141716A=702 nM; Showalter et al., 1996). However the selective CB2 inverse agonist, AM 630 (Hosohata et al., 1997; Ross et al., 1999) did not affect anandamide responses even at a concentration of 1 μM, which is more than 30 fold higher than its affinity for CB2 receptors (Ki 31.2 nM; Ross et al., 1999). Moreover the CB2 receptor agonist, palmitoylethanolamide (Facci et al., 1995), was found to be ineffective at causing relaxation of 5-HT-precontracted rat coronary arteries in the present study. Taken together, these findings are convincing evidence against involvement of CB2 receptors in the effects of anandamide in rat coronary arteries.
In rat mesenteric arteries, most of the vasorelaxant effects of anandamide can be explained by its ability to stimulate release of CGRP from capsaicin-sensitive sensory nerves through the activation of vanilloid VR1 receptors (Zygmunt et al., 1999; Ralevic et al., 2000). These results were confirmed in the present study, where anandamide-induced relaxation of endothelium-denuded rat mesenteric arteries was sensitive either to inhibition of sensory nerves by prior treatment (10 μM) with capsaicin, or to vanilloid receptor antagonism with capsazepine (3 μM). Moreover, the presence of a functional capsaicin-sensitive sensory nervous system in the isolated mesenteric arteries was confirmed by the ability of capsaicin to cause vasorelaxation which was sensitive to capsazepine, and was inhibited after prior exposure of the vessels to capsaicin. It should be noted that the potency of capsaicin as a vasorelaxant in the present study was much lower than was found by Zygmunt et al. (1999), yet consistent with earlier work in isolated mesenteric arteries (Ahluwalia & Vallance, 1996). The reasons for these discrepancies are not clear, however it is unlikely to be due to a lack of functional sensory nerves in the tissue, since responses to anandamide in the present study were very similar to those obtained by Zygmunt et al. (1999). The inability of capsazepine or capsaicin pretreatment to inhibit relaxations induced by high concentrations (>10 μM) of capsaicin is most likely to reflect the ability of this agent to induce relaxation through sensory nerve-independent pathways at such concentrations (Yeon et al., 2001).
Strikingly, neither capsaicin nor capsazepine affected anandamide-induced relaxation of isolated coronary arteries, even though the effectiveness of these agents was confirmed in our own experiments on rat mesenteric arteries. Nevertheless, capsaicin caused concentration-dependent relaxation of 5-HT-precontracted coronary arteries which was sensitive to vanilloid receptor inhibition by capsazepine. Moreover, capsaicin was able to evoke relaxation in vessels which had previously shown no response to anandamide or methanandamide. These results provide strong evidence that the vasorelaxant effects of anandamide in rat isolated coronary arteries do not involve the activation of vanilloid receptors on sensory nerves, even though a functional sensory nervous system is present in these arteries. The exact reasons for this are not clear. However, recent work suggests that anandamide activates vanilloid receptors by acting at an intracellular site, hence a functional membrane transporter for the cannabinoid is required for it to act (de petrocellis et al., 2001). It may therefore be possible that there is no membrane transporter present in rat coronary arteries, preventing access of anandamide to the vanilloid receptors.
In considering the possible mechanisms by which anandamide might cause relaxation of coronary arteries, it is important to note that the sensitivity of preparations to the cannabinoid showed some variation between individual animals over the course of the study. Thus, of a total of 60 animals tested, 18 did not respond to anandamide or methanandamide at all, even though the viability of the preparations was confirmed by their ability to contract normally in response to 5-HT, and relax in response to the addition of carbachol or capsaicin. It therefore seems unlikely that the vasorelaxant effects of the cannabinoids are underpinned by a non-selective action to inhibit the contractile mechanism (for example, inhibition of voltage-activated Ca2+ channels, or inhibition of Ca2+ sensititization mechanisms), since this mechanism is common to all of the vessels under investigation. Differential metabolism of anandamide, for example due to varying degrees of expression of hydrolase enzymes such as FAAH in different vessels, can also be ruled out as a possible explanation, since preparations which did not respond to anandamide were also insensitive to methanandamide, the non-hydrolysable derivative of anandamide (Abadji et al., 1994). Moreover, the lack of response to anandamide in coronary arteries was not correlated with a similar lack of response in mesenteric vessels from the same animal, providing further circumstantial evidence that the respective relaxant mechanisms are not the same in the two blood vessel types. We hypothesize that the most likely explanation for the effects of anandamide is that it acts at a site which is antagonized by SR 141716A, but is distinct from the existing CB1 and CB2 receptor subtypes. Indeed, Járai et al. (1999) showed that just such an ‘anandamide receptor' might explain the effects of anandamide and SR 141716A in the rat perfused mesenteric bed, whilst Ralevic & Kendall (2001) have also recently demonstrated effects of SR 141716A which could not be mimicked by selective CB1 or CB2 receptor antagonists. The observed variability in the effects of anandamide could be due to differences in the level of expression of the receptor between different animals. Alternatively, since cannabinoid receptors present in some tissues are functional, but do not couple to vasorelaxant pathways (Holland et al., 1999), it may be that the degree of coupling of the putative receptor varies between animals.
Given that previous studies have shown a possible role for K+ channel activation in the relaxant mechanism of anandamide (Randall et al., 1996; White & Hiley, 1997; Randall & Kendall, 1997), we determined if this might be an important in the effects of the cannabinoid in rat coronary arteries. Indeed TEA, used at a concentration (1 mM) where it acts as a relatively selective inhibitor of BKCa (Langton et al., 1991), significantly inhibited anandamide relaxations. A higher concentration of TEA (10 mM), which blocks K+ channels non-selectively, essentially abolished the effects of anandamide. Furthermore the more selective BKCa inhibitor, iberiotoxin (Galvez et al., 1990) inhibited anandamide relaxation to a similar extent to 1 mM TEA. It therefore seems reasonable to conclude that K+ channel activation, and in particular BKCa activation, represents an important step in the relaxant process of anandamide in rat coronary arteries.
In summary, the present study has demonstrated that anandamide-induced relaxations of rat isolated coronary arteries are endothelium-independent, are unlikely to involve breakdown of anandamide into other vasoactive products, and do not involve activation of cannabinoid CB1 or CB2 receptors or gap junctional communication. Moreover, anandamide relaxation of coronary vessels does not involve activation of vanilloid receptors on sensory nerves, even though a functional sensory nervous system is present in these arteries. Anandamide relaxation of rat coronary arteries may involve K+ channel activation, however the mechanism by which this occurs remains to be determined.
Acknowledgments
R. White is a Junior Research Fellow of Sidney Sussex College, Cambridge. W.-S.V. Ho is supported by the Cambridge Overseas Trust for Hong Kong and New Hall, Cambridge.
Abbreviations
- ATFMK
arachidonyl trifluoromethyl ketone
- BKCa
large conductance Ca2+-activated K+ channels
- Emax
maximum response
- FAAH
fatty acid amide hydrolase
- 5-HT
5-hydroxytryptamine
- pEC50
negative logarithm of the concentration causing 50% of the maximum response
- TEA
tetraethylammonium
References
- ABADJI V., LIN S.Y., TAHA G., GRIFFIN G., STEVENSON L.A., PERTWEE R.G., MAKRIYANNIS A. (R)-methanandamide - a chiral novel anandamide possessing higher potency and metabolic stability. J. Med. Chem. 1994;37:1889–1893. doi: 10.1021/jm00038a020. [DOI] [PubMed] [Google Scholar]
- AHLUWALIA A., VALLANCE P. Interaction between sympathetic and sensory nerves in rat small arteries: Involvement of nitric oxide. Am. J. Physiol. 1996;271:H969–H976. doi: 10.1152/ajpheart.1996.271.3.H969. [DOI] [PubMed] [Google Scholar]
- CHAYTOR A.T., MARTIN P.E.M., EVANS W.H., RANDALL M.D., GRIFFITH T.M. The endothelial component of cannabinoid-induced relaxation in rabbit mesenteric artery depends on gap junctional communication. J. Physiol. 1999;520:539–550. doi: 10.1111/j.1469-7793.1999.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE PETROCELLIS L., BISOGNO T., MACCARRONE M., DAVIS J.B., FINAZZI-AGRO A., DI MARZO V. The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism. J. Biol. Chem. 2001;276:12856–12863. doi: 10.1074/jbc.M008555200. [DOI] [PubMed] [Google Scholar]
- DEUTSCH D.G., GOLIGORSKY M.S., SCHMID P.C., KREBSBACH R.J., SCHMID H.H.O., DAS S.K., DEY S.K., ARREAZA G., THORUP C., STEFANO G., MOORE L.C. Production and physiological actions of anandamide in the vasculature of the rat kidney. J. Clin. Invest. 1997;100:1538–1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIS E.F., MOORE S.F., WILLOUGHBY K.A. Anandamide and delta 9-THC dilation of cerebral arterioles is blocked by indomethacin. Am. J. Physiol. 1995;269:H1859–H1864. doi: 10.1152/ajpheart.1995.269.6.H1859. [DOI] [PubMed] [Google Scholar]
- FACCI L., DALTOSO R., ROMANELLO S., BURIANI A., SKAPER S.D., LEON A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc. Natl. Acad. Sci. U.S.A. 1995;92:3376–3380. doi: 10.1073/pnas.92.8.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDER C.C., JOYCE K.E., BRILEY E.M., MANSOURI J., MACKIE K., BLOND O., LAI Y., MA A.L., MITCHELL R.L. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- FLEMING I., SCHERMER B., POPP R., BUSSE R. Inhibition of the production of endothelium-derived hyperpolarizing factor by cannabinoid receptor agonists. Br. J. Pharmacol. 1999;126:949–960. doi: 10.1038/sj.bjp.0702381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANCO-CERECEDA A., SARIA A., LUNDBERG J.M. Differential release of calcitonin gene-related peptide and neuropeptide Y from the isolated heart by capsaicin, ischaemia, nicotine, bradycardia and oubain. Acta Physiol. Scand. 1989;135:173–187. doi: 10.1111/j.1748-1716.1989.tb08565.x. [DOI] [PubMed] [Google Scholar]
- FULTON D., QUILLEY J. Evidence against anandamide as the hyperpolarizing factor mediating the nitric oxide-independent coronary vasodilator effect of bradykinin in the rat. J. Pharmacol. Exp. Ther. 1998;286:1146–1151. [PubMed] [Google Scholar]
- GALVEZ A., GIMENEZ-GALLEGO G., REUBEN J.P., ROY-CONTANCIN L., FEIGENBAUM P., KACZOROWSKI G.J., GARCIA M.L. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J. Biol. Chem. 1990;265:11083–11090. [PubMed] [Google Scholar]
- GEBREMEDHIN D., LANGE A.R., CAMPBELL W.B., HILLARD C.J., HARDER D.R. Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am. J. Physiol. 1999;276:H2085–H2093. doi: 10.1152/ajpheart.1999.276.6.H2085. [DOI] [PubMed] [Google Scholar]
- HOLLAND M., CHALLISS R.A.J., STANDEN N.B., BOYLE J.P. Cannabinoid CB1 receptors fail to cause relaxation, but couple via Gi/Go to the inhibition of adenylyl cyclase in carotid artery smooth muscle. Br. J. Pharmacol. 1999;128:597–604. doi: 10.1038/sj.bjp.0702842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOSOHATA Y., QUOCK R.M., HOSOHATA K., MAKRIYANNIS A., CONSROE P., ROESKE W.R., YAMAMURA H.I. AM630 antagonism of cannabinoid-stimulated [35S]GTP gamma-S binding in the mouse brain. Eur. J. Pharmacol. 1997;321:R1–R3. doi: 10.1016/s0014-2999(97)00047-2. [DOI] [PubMed] [Google Scholar]
- JÁRAI Z., WAGNER J.A., VARGA K., LAKE K.D., COMPTON D.R., MARTIN B.R., ZIMMER A.M., BONNER T.I., BUCKLEY N.E., MEZEY E., RAZDAN R.K., ZIMMER A., KUNOS G. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc. Natl. Acad. Sci. U.S.A. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALLNER G., OWALL A., FRANCO-CERECEDA A. Myocardial outflow of calcitonin gene-related peptide in relation to metabolic stress during coronary artery bypass grafting without cardiopulmonary bypass. J. Thorc. Cardiovasc. Surg. 1999;117:447–453. doi: 10.1016/s0022-5223(99)70323-5. [DOI] [PubMed] [Google Scholar]
- KOUTEK B., PRESTWICH G.D., HOWLETT A.C., CHIN S.A., SALEHANI D., AKHAVAN N., DEUTSCH D.G. Inhibitors of arachidonyl ethanolamide hydrolysis. J. Biol. Chem. 1994;269:22937–22940. [PubMed] [Google Scholar]
- LAGNEUX C., LAMONTAGNE D. Involvement of cannabinoids in the cardioprotection induced by lipopolysaccharide. Br. J. Pharmacol. 2001;132:793–796. doi: 10.1038/sj.bjp.0703902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAKE K.D, , COMPTON D.R., VARGA K., MARTIN B.R., KUNOS G. Cannabinoid-induced hypotension and bradycardia in rats is mediated by CB1-like cannabinoid receptors. J. Pharmacol. Exp. Ther. 1997;281:1030–1037. [PubMed] [Google Scholar]
- LAN R.X., LIU Q., FAN P.S., LIN S.Y., FERNANDO S.R., MCCALLION D., PERTWEE R., MAKRIYANNIS A. Structure-activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J. Med. Chem. 1999;42:769–776. doi: 10.1021/jm980363y. [DOI] [PubMed] [Google Scholar]
- LANGTON P.D., NELSON M.T., HUANG Y., STANDEN N.B. Block of calcium-activated potassium channels in mammalian arterial myocytes by tetraethylammonium. Am. J. Physiol. 1991;260:H927–H934. doi: 10.1152/ajpheart.1991.260.3.H927. [DOI] [PubMed] [Google Scholar]
- OTLEY C.E., RICHARDSON P.J., HILEY C.R. Adenosine receptors in the coronary artery of the rat. Br. J. Pharmacol. 1996;119:73P. [Google Scholar]
- PRATT P.F., HILLARD C.J., EDGEMOND W.S., CAMPBELL W.B. N-arachidonylethanolamide relaxation of bovine coronary artery is not mediated by CB1 cannabinoid receptor. Am. J. Physiol. 1998;43:H375–H381. doi: 10.1152/ajpheart.1998.274.1.H375. [DOI] [PubMed] [Google Scholar]
- RALEVIC V, , KENDALL D.A., RANDALL M.D., ZYGMUNT P.M., MOHAVED P., HÖGESTÄTT E.D. Vanilloid receptors on capsaicin-sensitive sensory nerves mediate relaxation to methanandamide in the rat isolated mesenteric arterial bed and small mesenteric arteries. Br. J. Pharmacol. 2000;130:1483–1488. doi: 10.1038/sj.bjp.0703456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., KENDALL D.A. Cannabinoid inhibition of capsaicin-sensitive sensory neurotransmission in the rat mesenteric arterial bed. Eur. J. Pharmacol. 2001;418:117–125. doi: 10.1016/s0014-2999(01)00940-2. [DOI] [PubMed] [Google Scholar]
- RANDALL M.D., ALEXANDER S.P.H., BENNETT T., BOYD E.A., FRY J.R., GARDINER S.M., KEMP P.A., MCCULLOCH A.I., KENDALL D.A. An endogenous cannabinoid as an endothelium-derived vasorelaxant. Biochem. Biophys. Res. Commun. 1996;229:114–120. doi: 10.1006/bbrc.1996.1766. [DOI] [PubMed] [Google Scholar]
- RANDALL M.D., KENDALL D.A. Involvement of a cannabinoid in endothelium-derived hyperpolarizing factor-mediated coronary vasorelaxation. Eur. J. Pharmacol. 1997;335:205–209. doi: 10.1016/s0014-2999(97)01237-5. [DOI] [PubMed] [Google Scholar]
- ROSS R.A., BROCKIE H.C., STEVENSON L.A., MURPHY V.L., TEMPLETON F., MAKRIYANNIS A., PERTWEE R.G. Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656, and AM630. Br. J. Pharmacol. 1999;126:665–672. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHOWALTER V.M., COMPTON D.R., MARTIN B.R., ABOOD M.E. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J. Pharmacol. Exp. Ther. 1996;278:989–999. [PubMed] [Google Scholar]
- WHITE R., HILEY C.R. A comparison of EDHF-mediated and anandamide-induced relaxations in the rat isolated mesenteric artery. Br. J. Pharmacol. 1997;122:1573–1584. doi: 10.1038/sj.bjp.0701546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE R., HILEY C.R. The actions of some cannabinoid receptor ligands in the rat isolated mesenteric artery. Br. J. Pharmacol. 1998a;125:533–541. doi: 10.1038/sj.bjp.0702111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE R., HILEY C.R. The actions of the cannabinoid receptor antagonist, SR 141716A, in the rat isolated mesenteric artery. Br. J. Pharmacol. 1998b;125:689–696. doi: 10.1038/sj.bjp.0702127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YEON D., KWON S., LEE Y., LEEM J., NAM T., AHN D. Capsaicin-induced relaxation in rabbit coronary artery. J. Vet. Med. Sci. 2001;63:499–503. doi: 10.1292/jvms.63.499. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H.H., SORGARD M., DI MARZO V., JULIUS D., HOGESTATT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


