Abstract
The multidrug resistance protein 2 (MRP2) has been shown to play an important role in the transport of glutathione conjugates in the liver. Its importance in renal excretion, however, is still uncertain and other organic anion transporters may be involved. The objective of the present study was to characterize glutathione conjugate efflux from rat kidney proximal tubule cells (PTC), and to determine the contribution of Mrp2.
We used isolated PTC in suspension, as well as grown to monolayer density. For comparison, transport characteristics were also determined in the human intestinal epithelial cell line Caco-2, an established model to study MRP2-mediated transport. The cells were loaded with monochlorobimane (MCB) at 10°C. MCB enters the cells by simple diffusion and is conjugated with glutathione to form the fluorescent glutathione-bimane (GS-B).
In primary cultures of rat PTC, no indications for a transporter-mediated mechanism were found. The efflux of GS-B from Caco-2 cells and freshly isolated PTC was time- and temperature-dependent. Furthermore, GS-B transport in both models was inhibited by chlorodinitrobenzene (CDNB), with an inhibitory constant of 46.8±0.9 μM in freshly isolated PTC. In Caco-2 cells, the inhibitory potency of CDNB was approximately 20 fold higher. Finally, efflux of GS-B from freshly isolated PTC from Mrp2-deficient (TR−) rats was studied. As compared to normal rat PTC, transport characteristics were not different.
We conclude that in freshly isolated rat PTC glutathione conjugate excretion is mediated by other organic anion transporters rather than by Mrp2.
Keywords: Multidrug resistance protein 2, organic anions, monochlorobimane, renal proximal tubular cells, Caco-2 cells, glutathione conjugates
Introduction
Many lipophilic endogenous and xenobiotic compounds are metabolized in epithelial cells of liver, kidney or intestine by glutathione S-transferase-mediated conjugation. In general, these anionic conjugates are actively transported out of the cells into bile, urine or intestinal lumen. In this way, the body protects itself against potentially harmful compounds. An organic anion transporter involved in the efflux of glutathione conjugates is the multidrug resistance protein 2 (MRP2; König et al., 1999). This transporter belongs to the superfamily of ATP-Binding Cassette (ABC) proteins, and couples ATP hydrolysis to the transport of bulky organic anions. MRP2 is located predominantly in liver canalicular membranes (Büchler et al., 1996; Paulusma et al., 1996), and also in luminal membranes of intestine (van aubel et al., 2000) and kidney proximal tubular cells (PTC; Schaub et al., 1997). The Eisai hyperbilirubinemic rat (EHBR) and the transport deficient (TR−) Wistar mutant rat strain have an hereditary defect in Mrp2, leading to the absence of the transport protein (Büchler et al., 1996; Paulusma et al., 1996; Ito et al., 1997). These rats are well-characterized by an impaired canalicular transport of anionic conjugates, but little is known about the influence of Mrp2 absence on renal and intestinal organic anion transport. This is because Mrp2-mediated transport can be studied only in isolated liver canalicular membrane vesicles. Vesicles isolated from the brush-border membrane of PTC and intestinal cells are unsuitable, because they are predominantly orientated right-side out, which makes the ATP-binding site inaccessible for extravesicular ATP (Haase et al., 1978). Therefore, intact cell systems are needed to study organic anion efflux from intestinal and renal cells. The objective of this study was to characterize the efflux of glutathione conjugates from renal PTC, and to determine the contribution of Mrp2. We used the organic anion, glutathione-bimane (GS-B), as a model substrate. This compound is formed after preloading cells with its hydrophobic, non-fluorescent precursor monochlorobimane (MCB). MCB easily enters the cells by passive diffusion and is conjugated with glutathione into a fluorescing adduct. In isolated perfused liver GS-B is rapidly secreted into bile, and this secretion is strongly reduced in TR− rats, indicating that GS-B is a substrate for Mrp2 (Oude Elferink et al., 1993).
GS-B efflux was characterized in primary cultures of rat PTC and freshly isolated PTC from normal and TR− rats, and compared with transport from monolayers of the human colon adenocarcinoma Caco-2 cell line, an established model for MRP2-mediated efflux. In contrast to the results obtained in liver, our results indicate that Mrp2 is not involved in the efflux of GS-B from rat renal PTC.
Methods
Materials
MCB was purchased from Molecular Probes (Eugene, OR, U.S.A.). Collagenase, bovine serum albumin (BSA) and 4-(2-hydroxyethyl)-1-piperazine ethanesulphonic acid (HEPES) were obtained from Boehringer Mannheim (Mannheim, Germany). Heparin was obtained from Leo Pharmaceutical Products (Weesp, The Netherlands). [3H]-leukotriene C4 (LTC4) (165 Ci mmol−1) was purchased from NEN Life Science Products (Hoofddorp, The Netherlands). Mouse monoclonal antibodies directed against cell surface antigens specific for the apical membrane of the proximal tubule (101E12) were obtained as described previously (Rose et al., 1993). Rabbit polyclonal antibodies directed against Mrp2 (k78mrp2) were obtained as described previously (van aubel et al., 1998) and fluorescein-labelled anti-rabbit IgG were derived from Kirkegaard & Perry Lab. Inc. (Gaithersburg, MD, U.S.A.). DMEM, gentamicin, glutamax and Hank's Balanced Salt Solution (HBSS) were purchased from Gibco-BRL (Breda, The Netherlands). Foetal bovine serum and non-essential amino acids were from ICN (Costa Mesa, CA, U.S.A.). All other chemicals were of analytical grade and purchased from Sigma (St. Louis, MO, U.S.A.). The TR− rat strain was kindly provided by Dr P.L.M. Jansen (Groningen, The Netherlands; de vries et al., 1989) and bred in Nijmegen.
Caco-2 cell culture and preparation of brush-border membrane vesicles
Caco-2 cells, passage 26 – 33, were cultured in DMEM which was supplemented with 10% foetal bovine serum, 1% non-essential amino acids, and 0.05 mg ml−1 gentamicin and were grown in a humidified atmosphere of 5% CO2 at 37°C. Cells were subcultured until 80% confluency. For transport experiments Caco-2 cells were plated on 24-well plates (Costar, Cambridge, MA, U.S.A.) at a density of 1.0×105 cells well−1 and were used 8 to 21 days post seeding.
Brush-border membrane vesicles from Caco-2 cells were isolated by the CaCl2 precipitation method as described previously by Iseki et al. (1989). The enrichment of the preparation was determined by assaying the activity of alkaline phosphatase using p-nitrophenylphosphate as substrate (Mircheff & Wright, 1976). Alkaline phosphatase showed an enrichment of 12.5±0.9 fold (n=4) indicating that the vesicle preparation was predominantly of brush-border membrane origin.
Uptake of [3H]-LTC4 into membrane vesicles was measured as described previously (van aubel et al., 1998), with some modifications. Briefly, 10 μl of membrane vesicle fraction (8 – 12 mg protein ml−1) was added to 40 μl of experimental buffer (37°C) supplemented with an ATP-regeneration mix (0.1 mg ml−1 creatine phosphokinase, 10 mM creatine phosphate and 4 mM ATP) and 1 nM [3H]-LTC4. Uptake was stopped after the indicated time periods by the addition of 2.5 ml of ice-cold stop buffer containing 250 mM mannitol, 10 mM NaCl and 10 mM Tris-HCl, pH 7.4. Membrane vesicles and buffer were quickly separated by using rapid vacuum filtration through Whatman GF/F glassfibre filters (Omnilabo International, Breda, The Netherlands). Incubation tubes and filters were washed twice with 2.5 ml ice-cold stop buffer. To the filters, 4 ml of scintillation fluid (Aqualuma Plus, Lumac, Schaersberg, The Netherlands) was added.
Isolation and primary culture of rat PTC
Rat kidney PTC were isolated as described previously (Masereeuw et al., 1994), with some modifications. Briefly, male WH rats or TR− rats (200 – 300 g) were anaesthetized intraperitoneally with pentobarbitone (60 mg kg−1). Heparin (300 U 100 g−1) was administered in the spleen. The aorta was cannulated above the renal arteries and the kidneys were perfused at a flow rate of 7.5 ml min−1 with 150 ml Hank's HEPES (HH) buffer containing 137 mM NaCl, 5 mM KCl, 0.8 mM MgSO4, 0.33 mM Na2HPO4, 0.44 mM KH2PO4, 26 mM NaHCO3, and 25 mM HEPES at pH 7.4, supplemented with 0.5 mM EGTA. The perfusion fluid was carbogen-saturated and kept at 37.5°C. Subsequently, the kidneys were perfused with 50 ml HH-buffer to washout the EGTA and finally perfused with 30 ml of HH-buffer containing 0.13% (w v−1) collagenase B and 4 mM CaCl2 in a recirculating system for 20 min. After perfusion, the cortex was suspended in ice-cold HH-buffer with 2.5% (w v−1) BSA and 1.8 mM CaCl2 and filtered twice, first through a 200 μm nylon gauze filter followed by a filter with 80 μm pore size. PTC were purified using a nycodenz density gradient and finally suspended in incubation buffer containing 117.5 mM NaCl, 4 mM KCl, 1.2 mM MgSO4, 0.95 mM KH2PO4, 22.5 mM NaHCO3, 11.1 mM glucose, and 2.5 mM CaCl2, adjusted to pH 7.4. Cell yield was 20 – 60×106 cells rat−1 and viability was higher than 90% as judged by Trypan blue exclusion and lactate dehydrogenase release.
For immunohistochemistry, freshly isolated PTC were washed in 10 mM PBS and fixed for 10 min at room temperature in 2% (v v−1) formaldehyde/0.1% (v v−1) glutaraldehyde. After washing in PBS, cells were permeabilized in 1% (v v−1) Triton X-100 in PBS, washed and incubated for 90 min at 37°C in PBS with the primary antibody, followed by a second incubation for 60 min at 37°C in PBS with a fluorescein-labelled secondary antibody. For determination of polarity, PTC were immunostained with mouse monoclonal antibodies recognizing antigens specific for the apical membrane of the proximal tubule, and were subsequently viewed under fluorescence microscope as described previously (Rose et al., 1993). Mrp2 was detected with the polyclonal antibody k78mrp2 and cells were examined with an MRC 1000 confocal microscope (Bio-Rad, Hertfordshire, U.K.), coupled to a Nikon microscope equipped with a 60× objective exhibiting a numerical aperture of 1.4.
For cell monolayers, rat renal PTC were seeded on 24-well plates coated with rat tail collagen (50 μl cm2−1) at a density of 2.0×105 cells well−1 in DMEM supplemented with 5% FCS, 1% non-essential amino acids, and 0.05 mg ml−1 gentamicin and were grown in a humidified atmosphere of 5% CO2 at 37°C. After approximately 7 days of culture the cells reached confluency and transport experiments were performed.
Transport studies
Before the experiment, Caco-2 cells and primary cultured rat PTC were washed twice with ice-cold HBSS supplemented with 10 mM HEPES at pH 7.4. The cells were preloaded with 5 μM MCB in 300 μl of HBSS for 60 min at 10°C in the presence or absence of chlorodinitrobenzene (CDNB). After this period a steady state in cellular fluorescence intensity was reached (data not shown). The cells were subsequently washed twice with cold HBSS. The Caco-2 cell monolayers were incubated in 500 μl of HBSS at 37°C or at 10°C in the presence or absence of CDNB for the specified time periods. The apical medium was taken and the cells were homogenized in water.
To study efflux in freshly isolated PTC, approximately 2×106 cells were preloaded with 5 μM MCB in 250 μl of incubation buffer for 30 min at 10°C. After 30 min of incubation a steady state in the intracellular concentration of GS-B was reached (data not shown). Subsequently, the cells were washed twice with washing buffer containing 140 mM NaCl, 4 mM KCl, 1.2 mM MgSO4, 0.95 mM KH2PO4, 11.1 mM glucose, and 2.5 mM CaCl2, pH 7.4. Cells were incubated at 37°C or 10°C in 250 μl of incubation buffer for the indicated time in the presence or absence of CDNB. Incubations were stopped by the addition of 250 μl ice-cold washing buffer and cells were centrifuged rapidly. Supernatant was collected and the cell pellet was washed twice with washing buffer after which cells were lysed in 500 μl 0.1% Triton X-100 in water.
Transport analysis
For the measurement of [3H]-LTC4 uptake in Caco-2 brush-border membrane vesicles, the radioactivity remaining on the filters was counted in a Beckman LS 6000 LL liquid scintillation counter. Corrections were made for non-specific filter binding in absence of vesicles. Uptake is expressed as fmol mg−1 protein. In intact cell experiments, the GS-B content in the samples was measured by determining the fluorescence intensity using a Perkin-Elmer LS50 luminescence spectrophotometer with excitation wavelength set to 393 nm and emission wavelength of 475 nm. For both wavelengths slit widths of 2.5 nm were used in primary cultures of PTC. In the efflux measurements with freshly isolated PTC and Caco-2 cells, slit widths of 5 nm were used. Data are presented with the measured fluorescence intensity shown in arbitrary units.
Data analysis
All data are expressed as mean±s.e.mean; n is the number of different experiments performed in duplo. Statistical differences between means were assessed by one-way analysis of variance, followed by Student's t-test with Bonferroni correction or followed by Dunnett's multiple comparison test. Values were considered statistically different when P<0.05. Nonlinear regression analysis was performed using GraphPad Prism 3.0 for Windows 95 (GraphPad Software Inc., San Diego, CA, U.S.A.). The log-concentration inhibition curves were analysed according to a one-site competition model and the concentration at 50% inhibition (IC50) of GS-B efflux was determined.
Results
LTC4 uptake in Caco-2 brush-border membrane vesicles
Several recent reports have demonstrated that the human colon adenocarcinoma, Caco-2, cells functionally express MRP2 at the apical membrane (Gutmann et al., 1999; Walle et al., 1999; Hirohashi et al., 2000; Walgren et al., 2000). To confirm this finding, we studied the uptake of a model substrate for MRP2, LTC4 (van aubel et al., 1998), in isolated Caco-2 brush-border membrane vesicles. Time- and ATP-dependency of LTC4 uptake was determined by incubating the vesicles with 1 nM [3H]-LTC4 for different time periods in the presence and absence of ATP. The uptake of LTC4 was clearly induced in the presence of ATP (Figure 1), confirming the presence of MRP2 at the apical membrane of Caco-2 cells.
Figure 1.
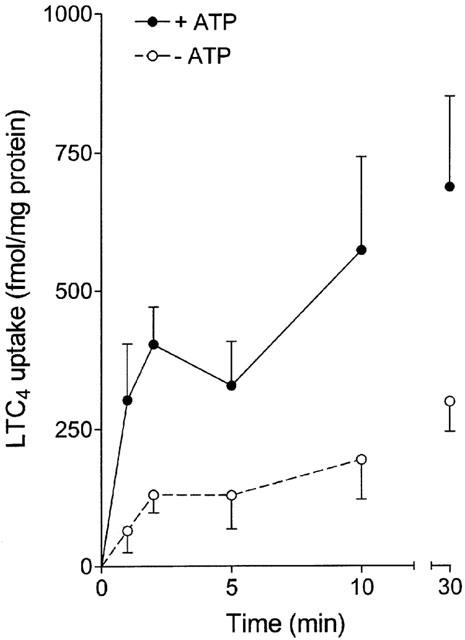
Time-dependent uptake of 1 nM [3H]-LTC4 in Caco-2 brush-border membrane vesicles in the presence (closed circles) and absence (open circles) of 4 mM ATP. Values are mean±s.e.mean of two different isolations.
GS-B efflux from Caco-2 cells
The time-dependency of the efflux of the fluorescent conjugate, GS-B, from monolayers of Caco-2 cells was measured after preloading cells with 5 μM of non-fluorescent MCB followed by an incubation at 37°C in HBSS for different time periods (Figure 2). The efflux of GS-B increased during the first 2.5 min, whereafter equilibrium was reached. The increase in the extracellular amount of GS-B was paralleled by a decrease in the cellular fluorescence. Lowering the temperature to 10°C during incubation strongly reduced the efflux of the glutathione conjugate, suggesting a transporter-mediated mechanism (Figure 2). The effect of metabolic degradation of GS-B was evaluated using acivicin (0.5 mM), an inhibitor of γ-glutamyl transferase. However, no differences in fluorescence intensity of cells or supernatant were observed as compared to control values. Furthermore, the efflux of GS-B was studied in presence of CDNB (Figure 3). Similar to MCB, CDNB enters the cell through diffusion and is conjugated to glutathione within the cells into a potent inhibitor of MRP2-mediated transport, dinitrophenyl-glutathione DNP-GS (Oude Elferink et al., 1990; Evers et al., 1998). In presence of CDNB, the fluorescence intensity of the supernatant decreased in a concentration-dependent manner, indicating an inhibition of efflux. Simultaneous with the decrease in the supernatant, an increase in the cellular fluorescence was observed at a concentration of 2.5 μM CDNB. However, as compared to the 2.5 μM concentration, the cellular amount of GS-B decreased with increasing concentrations of CDNB, although the cellular fluorescence remained significantly higher than the control values, except for 25 μM CDNB. This finding indicates that CDNB, besides inhibiting GS-B efflux, also reduced the intracellular production of GS-B. Concentrations lower than 2.5 μM CDNB had no effect on the efflux of GS-B (fluorescence intensity after incubation for 2.5 min at 37°C of the supernatant of control Caco-2 cells and after addition of 1 μM CDNB was 160±28 and 164±22, respectively; n=4).
Figure 2.
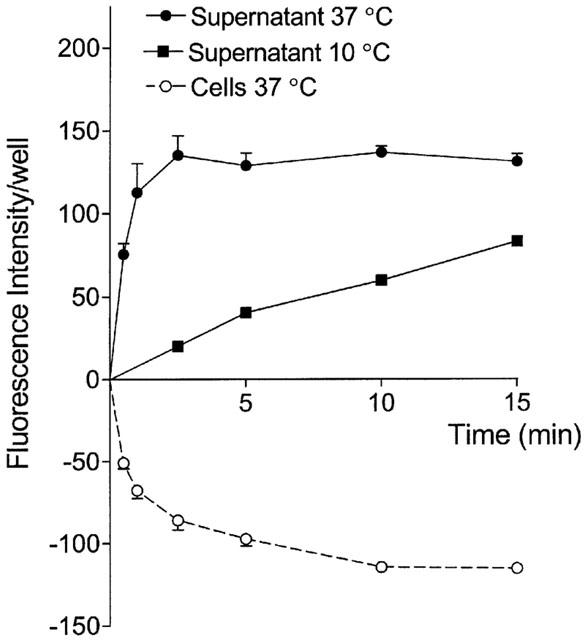
Time-dependent efflux of GS-B from Caco-2 cell monolayers at 37°C (circles) or 10°C (squares). At 37°C, extracellular (closed symbols) and intracellular (open symbols) fluorescence intensities are shown. The cellular values minus the mean fluorescence intensity at time 0 (158±24) are shown. Cells were preloaded with 5 μM of the non-fluorescent precursor MCB for 60 min and, subsequently, the efflux of fluorescent GS-B was measured. Values are mean±s.e.mean of 3 – 8 different experiments.
Figure 3.
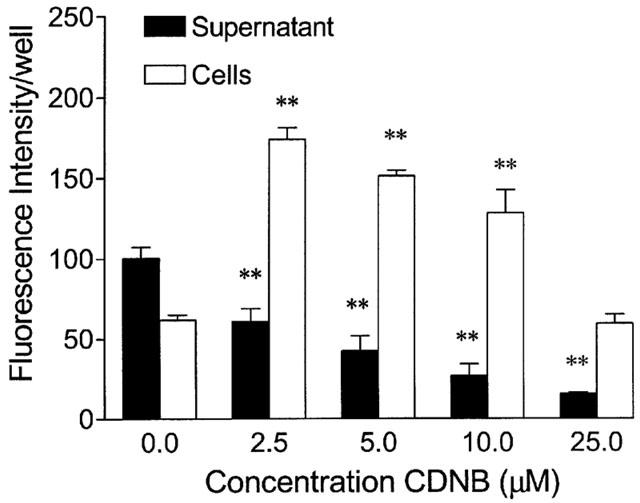
Concentration-dependent inhibition of GS-B efflux by CDNB in Caco-2 cell monolayers. Caco-2 cells were preloaded with 5 μM MCB and 0 – 25 μM CDNB for 60 min and subsequently incubated for 2.5 min at 37°C. Values are mean±s.e.mean of 2 – 5 different experiments. **P<0.01 vs respective controls.
GS-B efflux from primary cultures of rat PTC
Primary cultures were used when confluency was reached after 7 days of culture. Figure 4 presents the time-dependent efflux of GS-B from primary cultured PTC. Efflux was very slow as compared to that measured from Caco-2 cells, as can be concluded from the fact that after 60 min of efflux equilibrium was not yet reached. To determine whether the transport measured was mediated by a carrier or pump, cells were preloaded and incubated in the presence of 1 and 5 μM CDNB. To correct for the decrease in GS-B production caused by 5 μM CDNB, the ratio of fluorescence intensity in supernatant over cells was used. For both concentrations of CDNB no difference in this ratio was observed as compared to control values (0.17±0.03, 0.19±0.02 and 0.19±0.02 for control WH, WH PTC exposed to 1 μM CDNB, and WH PTC exposed to 5 μM CDNB, respectively). Together, these data suggest that the transport system for anionic conjugates is either lost or impaired during culture. Finally, there was no difference in efflux between primary cultures of WH and TR− rat PTC (0.17±0.03 and 0.16±0.03, respectively), confirming the absence of Mrp2-mediated transport in cultured PTC.
Figure 4.
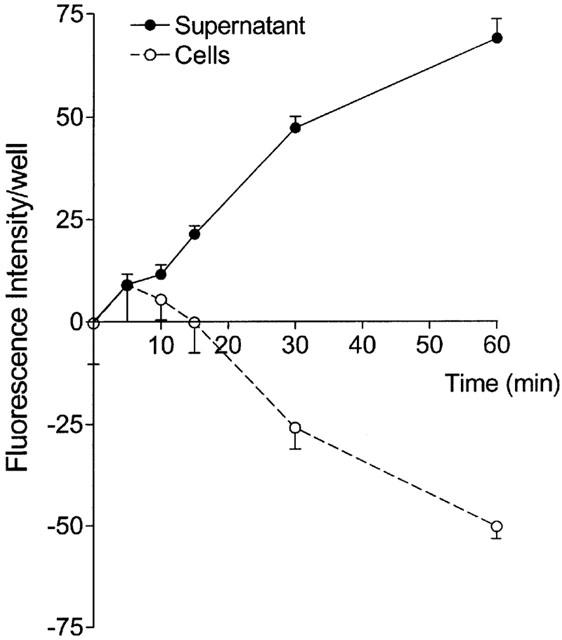
Time-dependent efflux of GS-B from primary cultured rat PTC at 37°C. The closed circles represent the fluorescence intensities in the medium, the open circles the intracellular fluorescence intensities. The cellular values minus the mean fluorescence intensity at time 0 (85±10) are shown. Cells were preloaded with 5 μM MCB for 60 min and subsequently the efflux of GS-B was measured. Values are mean±s.e.mean of 5 – 6 different experiments.
GS-B efflux from rat PTC
To check the polarity of PTC in suspension, we immunostained freshly isolated cells with antibodies against alkaline phosphatase. The microscopic image in Figure 5A shows a clear staining of only one part of the cell membrane, the luminal membrane, indicating that PTC retain their morphological polarity after isolation. Furthermore, when freshly isolated PTC are immunostained with antibodies to Mrp2, again only the luminal side of the cells is stained confirming the presence of the transport protein (Figure 5B).
Figure 5.
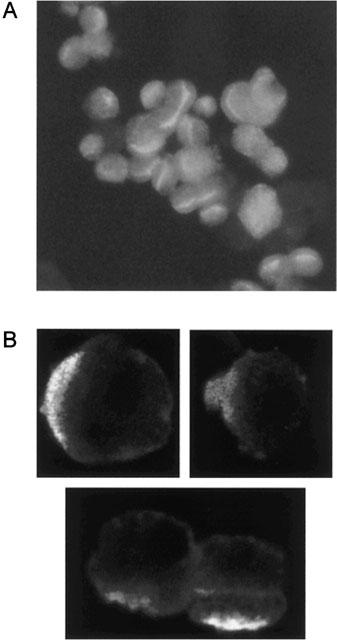
(A) A representative microscopic image of rat kidney PTC after staining with an antibody against alkaline phosphatase and a fluorescent secondary antibody. Staining is strongest along the luminal membrane of the PTC, confirming the maintenance of cell polarity. (B) Immunolocalization of Mrp2 in rat kidney PTC. Cells were immunostained with polyclonal antibodies against Mrp2 and a fluorescent secondary antibody. Mrp2-specific immunofluorescence is observed at the luminal membrane, confirming the apical localization of the transporter.
The time-dependent efflux of GS-B from PTC was determined by preloading the cells with 5 μM MCB followed by incubation at 37°C for different time periods. The efflux of the fluorescent GS-B increased in time until equilibrium was reached after 5 min (Figure 6A). In parallel with an increase in the amount of extacellular GS-B, a decrease in the cellular fluorescence was observed in time. Diffusion was determined by incubation at 10°C. Almost no efflux occurred at this temperature (Figure 6A). Coincubation with acivicin (0.5 mM) showed that further metabolism of GS-B has no effect on the fluorescence intensities measured. Furthermore, we studied the efflux of GS-B from freshly isolated PTC of TR− rats. The absence of Mrp2 in the kidney of TR− rats was confirmed using immunohistochemistry and Western blot of brush-border membrane vesicles (data not shown). Remarkably, the time-dependent efflux of the glutathione conjugate was comparable to the efflux measured in WH rats (Figure 6B). The effect of CDNB on GS-B efflux from WH PTC was investigated by measuring the concentration-dependent inhibition. To this end, different concentrations of CDNB were added during preloading as well as during efflux measurements of the PTC. Similar to the inhibition of GS-B efflux from Caco-2 cells by CDNB, the fluorescence intensity in the supernatant decreases in parallel with an increase in cellular fluorescence intensity (Figure 7A). Figure 7B shows the log-concentration CDNB plotted against the ratio supernatant : cells to correct for variation between the different isolations and the possible effect of CDNB on the synthesis of GS-B. The inhibition curve was described best according to a one-site competition model and the concentration of CDNB causing 50% inhibition (−log IC50 value) was determined. Nonlinear regression analysis revealed a −log IC50 value of 4.33±0.06 (46.8±0.9 μM). In addition, inhibition of GS-B efflux by CDNB in PTC isolated from TR− rats was determined. The concentration-dependency was not different from the effect of CDNB on GS-B efflux in WH PTC (Figure 7B), with an inhibitory constant (−log IC50) of 4.27±0.09. These findings indicate that there is no difference in efflux characteristics of GS-B between freshly isolated PTC from WH and TR− rats.
Figure 6.
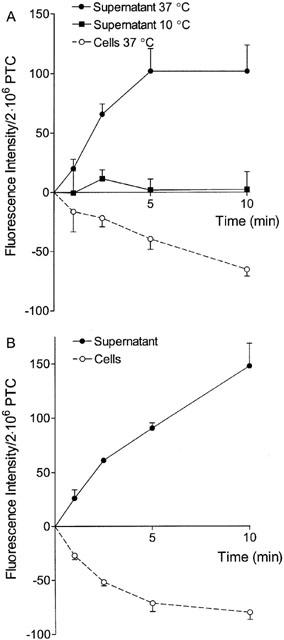
Time-dependent efflux of GS-B from freshly isolated PTC from WH (A) and TR− rats (B) at 37°C (circles) or 10°C (squares). At 37°C, extracellular (closed symbols) and intracellular (open symbols) fluorescence intensities are shown. The cellular values minus the mean fluorescence intensity at time 0 (116±10 and 141±25 for WH and TR− rats, respectively) are shown. Cells were preloaded with 5 μM MCB for 30 min and subsequently efflux of GS-B was measured. Values are mean±s.e.mean of 2 – 4 different isolations.
Figure 7.
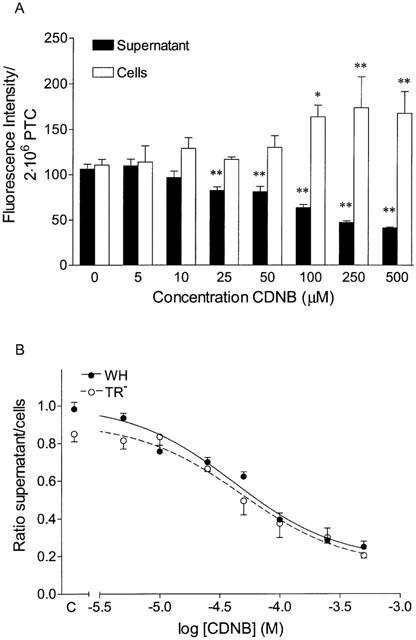
Concentration-dependent inhibition of GS-B efflux by CDNB in freshly isolated PTC of WH (A) and of WH (closed circles) vs TR− rats (open circles; B). Cells were preloaded with 5 μM MCB and 0 – 500 μM CDNB for 30 min and subsequently incubated for 2.5 min at 37°C. (B) The ratio of fluorescence intensities in supernatant over cells was plotted for the different concentrations CDNB. The lines represent the fit according to a one-site competition model. Values are mean±s.e.mean of 2 – 4 different isolations. *P<0.05 and **P<0.01 vs respective controls.
Discussion
The results of this study demonstrate that freshly isolated PTC of rat kidney in suspension are a useful model to study glutathione conjugate efflux. Efflux of the anionic conjugate GS-B was time- and temperature-dependent, suggesting the involvement of a pump or carrier. Furthermore, efflux was susceptible to inhibition by CDNB in a concentration-dependent manner, revealing an IC50 value of approximately 50 μM. In Caco-2 cells the inhibitory constant for CDNB could not be calculated because a maximum inhibition of the ratio of supernatant : cells was observed at 2.5 μM, whereas lower concentrations of CDNB had no effect on the efflux of GS-B. These findings indicate that the potency of CDNB to inhibit efflux in Caco-2 cells was approximately 20 fold higher as compared to the potency in freshly isolated PTC. It may be speculated that the differences in inhibitory potency of CDNB in the different cell models suggest the involvement of different transporters in the efflux of GS-B.
Caco-2 cell monolayers were used as a model system for Mrp2-mediated efflux (Gutmann et al., 1999; Walle et al., 1999; Hirohashi et al., 2000; Walgren et al., 2000). In this study, we confirmed the presence of a functional ATP-dependent glutathione conjugate transporter in the apical membrane of our Caco-2 cells, by uptake of the MRP2 model substrate, LTC4, into brush-border membrane vesicles. Because the Caco-2 cells were grown in wells, basolateral transporters such as MRP1 and MRP3 do not contribute to the efflux of glutathione conjugates. Although Caco-2 cells are a colon derived cell line, the cells show an enterocytic differentiation, which starts directly after confluency is reached. Complete differentiation is obtained within 20 days of culture (Meunier et al., 1995). Hirohashi et al. (2000) demonstrated that expression of MRP2, determined by Northern blotting, was highest after 5 days of confluency. In accordance, we observed that the ability of Caco-2 cells to transport GS-B just after achievement of monolayer density (approximately 4 days post seeding) was less than after 4 to 6 days of confluency. A longer culture period up to 21 days did not influence the transport capacity for GS-B (data not shown).
In contrast to Caco-2 cells, primary cultures of rat PTC appeared to have lost transporter-mediated organic anion transport. The efflux of GS-B was very slow and did not reach equilibrium within 60 min of incubation. Furthermore, the ratio of the fluorescence intensity of supernatant : cells was not affected by concentrations of CDNB up to 5 μM. Higher concentrations were not used, because 5 μM already strongly reduced glutathione conjugation of MCB. Apparently, primary cultured PTC are more sensitive to GSH depletion by CDNB than freshly isolated PTC and Caco-2 cells. This may be the result of a decreased concentration of GSH in PTC during culture. Although primary cultures retain many of their differentiated functions, some transport mechanisms may be lost. Organic anion uptake across the basolateral membrane is lost or impaired in primary cultures of rabbit PTC (Miller, 1991). Our results indicate that, under the culture conditions used, the efflux of glutathione conjugates is impaired, leaving freshly isolated PTC as the best model to study renal efflux of organic anions.
Two mutant rat strains, TR− and EHBR, have been shown to lack functional Mrp2. It was found previously that in the isolated perfused kidney, the excretion of the glucuronide conjugate of 1-naphthol was not affected in TR− rats as compared to normal rats, whereas the hepatobiliary excretion was impaired (de vries et al., 1989). In addition, an in vivo study in EHBR and normal rats showed that biliary excretion of the glucuronide conjugate of 6-hydroxy-5,7-dimethyl-2-methylamino-4-(3-pyridylmethyl)benzothiazole (E3040) was greatly impaired in EHBR rats. In contrast, the renal clearance of the glucuronide conjugate, which exceeded the glomerular filtration rate in the rat, was not affected in EHBR (Takenaka et al., 1995). In agreement with these findings, the present study in freshly isolated PTC showed no difference in efflux characteristics of GS-B between TR− and WH rats. Together, these data suggest that other organic anion transporters than Mrp2 are more important in the excretion of glutathione and glucuronide conjugates in PTC. Another possibility is that (an)other anion transporter(s) may be upregulated in TR− PTC. One possible candidate is the organic anion transporting polypeptide (Oatp1). This transporter is localized to the apical membrane of PTC (Bergwerk et al., 1996) and mediates the transport of several organic anions, among which glutathione and glucuronide conjugates (Li et al., 1998; Eckhardt et al., 1999).
Intracellular glutathione conjugation of MCB is mediated by the cytosolic glutathione S-transferase. Subsequently, the formed GS-B leaves the cell via a transporter-mediated mechanism. Extracellularly, GS-B can be converted to cysteinylglycine-bimane by the aid of γ-glutamyl transferase. This product can be further metabolized to a mercapturate by removal of the glycine residue followed by N-acetylation. However, the molar fluorescence intensity of all four metabolites is the same (Miller et al., 1996). In agreement, in Caco-2 cells as well as freshly isolated PTC, no differences in fluorescence intensity of cells or supernatant were observed in the presence of acivicin, an inhibitor of γ-glutamyl transferase.
In conclusion, we characterized the efflux of a prototypic glutathione conjugate in three different cell models, viz. Caco-2 cells, primary cultures of rat PTC and freshly isolated PTC of normal and TR− rats. Efflux of GS-B from PTC was not impaired in the transport deficient rat, and the inhibitory constants for CDNB determined in the two rat strains were similar. We conclude that organic anion transporter(s) other than Mrp2 contribute to the renal secretion of glutathione conjugates.
Abbreviations
- BSA
bovine serum albumin
- CDNB
chlorodinitrobenzene
- E3040
6-hydroxy-5,7-dimethyl-2-methylamino-4-(3-pyridylmethyl)benzothiazole
- EGTA
ethylene glycol-bis(β-aminoethylether)-N, N, N′, N′-tetraacetic acid
- EHBR
Eisai hyperbilirubinemic rat
- GS-B
glutathione-bimane
- HBSS
Hank's Balanced Salt Solution
- HEPES
4-(2-hydroxyethyl)-1-piperazine ethanesulphonic acid
- HH
Hanks' HEPES
- LTC4
leukotriene C4
- MCB
monochlorobimane
- MRP2
multidrug resistance protein 2
- oat1
organic anion transporter 1
- Oatp1
organic anion transporting polypeptide
- PTC
proximal tubular cells
- TR−
transport deficient rat
- WH
Wistar Hannover
References
- BERGWERK A.J., SHI X., FORD A.C., KANAI N., JACQUEMIN E., BURK R.D., BAI S., NOVIKOFF P.M., STIEGER B., MEIER P.J., SCHUSTER V.L., WOLKOFF A.W. Immunologic distribution of an organic anion transport protein in rat liver and kidney. Am. J. Physiol. 1996;271:G231–G238. doi: 10.1152/ajpgi.1996.271.2.G231. [DOI] [PubMed] [Google Scholar]
- BÜCHLER M., KÖNIG J., BROM M., KARTENBECK J., SPRING H., HORIE T., KEPPLER D. cDNA cloning of the hepatocyte canalicular isoform of the multidrug resistance protein, cMrp, reveals a novel conjugate export pump deficient in hyperbilirubinemic mutant rats. J. Biol. Chem. 1996;271:15091–15098. doi: 10.1074/jbc.271.25.15091. [DOI] [PubMed] [Google Scholar]
- DE VRIES M.H., REDEGELD F.A.M., KOSTER A.S., NOORDHOEK J., DE HAAN J.G., OUDE ELFERINK R.P.J., JANSEN P.L.M. Hepatic, intestinal and renal transport of 1-naphthol-beta-D-glucuronide in mutant rats with hereditary-conjugated hyperbilirubinemia. Naunyn Schmiedeberg's Arch. Pharmacol. 1989;340:588–592. doi: 10.1007/BF00260615. [DOI] [PubMed] [Google Scholar]
- ECKHARDT U., SCHROEDER A., STIEGER B., HÖCHLI M., LANDMANN L., TYNES R., MEIER P.J., HAGENBUCH B. Polyspecific substrate uptake by the hepatic organic anion transporter Oatp1 in stably transfected CHO cells. Am. J. Physiol. 1999;276:G1037–G1042. doi: 10.1152/ajpgi.1999.276.4.G1037. [DOI] [PubMed] [Google Scholar]
- EVERS R., KOOL M., VAN DEEMTER L., JANSSEN H., CALAFAT J., OOMEN L.C.J.M., PAULUSMA C.C., OUDE ELFERINK R.P.J., BAAS F., SCHINKEL A.H., BORST P. Drug export activity of the human canalicular multispecific organic anion transporter in polarized kidney MDCK cells expressing cMOAT (MRP2) cDNA. J. Clin. Invest. 1998;101:1310–1319. doi: 10.1172/JCI119886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTMANN H., FRICKER G., TÖRÖK M., MICHAEL S., BEGLINGER C., DREWE J. Evidence for different ABC-transporters in Caco-2 cells modulating drug uptake. Pharm. Res. 1999;16:402–407. doi: 10.1023/a:1018825819249. [DOI] [PubMed] [Google Scholar]
- HAASE W., SCHAFER A., MURER H., KINNE R. Studies on the orientation of brush-border membrane vesicles. Biochem. J. 1978;172:57–62. doi: 10.1042/bj1720057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIROHASHI T., SUZUKI H., CHU X.Y., TAMAI I., TSUJI A., SUGIYAMA Y. Function and expression of multidrug resistance-associated protein family in human colon adenocarcinoma cells (Caco-2) J. Pharmacol. Exp. Ther. 2000;292:265–270. [PubMed] [Google Scholar]
- ISEKI K., SUGAWARA M., SAITOH H., MIYAZAKI K., ARITA T. Comparison of transport characteristics of amino β-lactam antibiotics and dipeptides across rat intestinal brush border membrane. J. Pharm. Pharmacol. 1989;41:628–632. doi: 10.1111/j.2042-7158.1989.tb06544.x. [DOI] [PubMed] [Google Scholar]
- ITO K., SUZUKI H., HIROHASHI T., KUME K., SHIMIZU T., SUGIYAMA Y. Molecular cloning of canalicular multispecific organic anion transporter defective in EHBR. Am. J. Physiol. 1997;272:G16–G22. doi: 10.1152/ajpgi.1997.272.1.G16. [DOI] [PubMed] [Google Scholar]
- KÖNIG J., NIES A.T., CUI Y., LEIER I., KEPPLER D. Conjugate export pumps of the multidrug resistance protein (MRP) family: localization, substrate specificity, and MRP2-mediated drug resistance. Biochim. Biophys. Acta. 1999;1461:377–394. doi: 10.1016/s0005-2736(99)00169-8. [DOI] [PubMed] [Google Scholar]
- LI L., LEE T.K., MEIER P.J., BALLATORI N. Identification of glutathione as a driving force and leukotriene C4 as a substrate for oatp1, the hepatic sinusoidal organic solute transporter. J. Biol. Chem. 1998;273:16184–16191. doi: 10.1074/jbc.273.26.16184. [DOI] [PubMed] [Google Scholar]
- MASEREEUW R., VAN DEN BERGH E.J., BINDELS R.J.M., RUSSEL F.G.M. Characterization of fluorescein transport in isolated proximal tubular cells of the rat: evidence for mitochondrial accumulation. J. Pharmacol. Exp. Ther. 1994;269:1261–1267. [PubMed] [Google Scholar]
- MEUNIER V., BOURRIÉ M., BERGER Y., FABRE G. The human intestinal epithelial cell line Caco-2; pharmacological and pharmacokinetic applications. Cell Biol. Toxicol. 1995;11:187–194. doi: 10.1007/BF00756522. [DOI] [PubMed] [Google Scholar]
- MILLER D.S., LETCHER S., BARNES D.M. Fluorescence imaging study of organic anion transport from renal proximal tubule cell to lumen. Am. J. Physiol. 1996;271:F508–F520. doi: 10.1152/ajprenal.1996.271.3.F508. [DOI] [PubMed] [Google Scholar]
- MILLER J.H. Sodium-sensitive, probenecid-insensitive paminohippuric acid uptake in cultured renal proximal tubule cells of the rabbit. Proc. Soc. Exp. Biol. Med. 1991;199:298–304. doi: 10.3181/00379727-199-43360. [DOI] [PubMed] [Google Scholar]
- MIRCHEFF A.K., WRIGHT E.M. Analytical isolation of plasma membranes of intestinal epithelial cells: identification of Na,K-ATPase rich membranes and the distribution of enzyme activities. J. Membr. Biol. 1976;28:309–333. doi: 10.1007/BF01869703. [DOI] [PubMed] [Google Scholar]
- OUDE ELFERINK R.P.J., BAKKER C.T.M., ROELOFSEN H., MIDDELKOOP E., OTTENHOFF R., HEIJN M., JANSEN P.L.M. Accumulation of organic anion in intracellular vesicles of cultured rat hepatocytes is mediated by the canalicular multispecific organic anion transporter. Hepatology. 1993;17:434–444. [PubMed] [Google Scholar]
- OUDE ELFERINK R.P.J., OTTENHOFF R., LIEFTING W.G.M., SCHOEMAKER B., GROEN A.K., JANSEN P.L.M. ATP-dependent efflux of GSSG and GS-conjugate from isolated rat hepatocytes. Am. J. Physiol. 1990;258:G699–G706. doi: 10.1152/ajpgi.1990.258.5.G699. [DOI] [PubMed] [Google Scholar]
- PAULUSMA C.C., BOSMA P.J., ZAMAN G.J.R., BAKKER C.T.M., OTTER M., SCHEFFER G.L., SCHEPER R.J., BORST P., OUDE ELFERINK R.P.J. Congenital jaundice in rats with a mutation in a multidrug resistance-associated protein gene. Science. 1996;271:1126–1128. doi: 10.1126/science.271.5252.1126. [DOI] [PubMed] [Google Scholar]
- ROSE U.M., BINDELS R.J.M., VIS A., JANSEN J.W.C.M., VAN OS C.H. The effect of L-type Ca2+ channel blockers on anoxia-induced increases in intracellular Ca2+ concentration in rabbit proximal tubule cells in primary culture. Pflügers Arch. 1993;423:378–386. doi: 10.1007/BF00374931. [DOI] [PubMed] [Google Scholar]
- SCHAUB T.P., KARTENBECK J., KÖNIG J., VOGEL O., WITZGALL R., KRIZ W., KEPPLER D. Expression of the conjugate export pump encoded by the mrp2 gene in the apical membrane of kidney proximal tubules. J. Am. Soc. Nephrol. 1997;8:1213–1221. doi: 10.1681/ASN.V881213. [DOI] [PubMed] [Google Scholar]
- TAKENAKA O., HORIE T., SUZUKI H., SUGIYAMA Y. Different biliary excretion systems for glucuronide and sulfate of a model compound; study using Eisai hyperbilirubinemic rats. J. Pharmacol. Exp. Ther. 1995;274:1362–1369. [PubMed] [Google Scholar]
- VAN AUBEL R.A.M.H., VAN KUIJCK M.A., KOENDERINK J.B., DEEN P.M.T., VAN OS C.H., RUSSEL F.G.M. Adenosine triphosphate-dependent transport of anionic conjugates by the rabbit multidrug resistance-associated protein Mrp2 expressed in insect cells. Mol. Pharmacol. 1998;53:1062–1067. [PubMed] [Google Scholar]
- VAN AUBEL R.A.M.H., HARTOG A, , BINDELS R.J.M., VAN OS C.H., RUSSEL F.G.M. Expression and immunolocalization of multidrug resistance protein 2 in rabbit small intestine. Eur. J. Pharmacol. 2000;400:195–198. doi: 10.1016/s0014-2999(00)00391-5. [DOI] [PubMed] [Google Scholar]
- WALGREN R.A., KARNAKY K.J., JR, LINDENMAYER G.E., WALLE T. Efflux of dietary flavonoid quercetin 4′-β-glucoside across human intestinal Caco-2 cell monolayers by apical multidrug resistance-associated protein-2. J. Pharmacol. Exp. Ther. 2000;294:830–836. [PubMed] [Google Scholar]
- WALLE U.K., GALIJATOVIC A., WALLE T. Transport of the flavonoid chrysin and its conjugated metabolites by the human intestinal cell line Caco-2. Biochem. Pharmacol. 1999;58:431–438. doi: 10.1016/s0006-2952(99)00133-1. [DOI] [PubMed] [Google Scholar]


