Abstract
We have measured extracellular NO/NO2− concentrations in guinea-pig suprachiasmatic nucleus (SCN) brain slices using fast cyclic voltammetry. A rapid and transient signal equivalent to 2.2±0.2 μM NO/NO2− (mean±s.e.mean, n=13) was detected at 1.26 V, the peak oxidation potential for NO, following local electrical stimulation (five pulses of 0.1 ms duration at 100 Hz, delivered every 5 min).
The NO/NO2− signal was inhibited by the non-selective nitric oxide synthase (NOS) inhibitors L-NAME, L-NMMA and the highly selective type II NOS (iNOS) inhibitor 1400 W (Garvey et al., 1997) in a concentration-dependent manner. IC50 values were 229 μM (65 – 801, n=3, geomean and 95% confidence intervals (C.I.)), 452 nM (88 – 2310, n=5), and 14.2 μM (3.6 – 54.4, n=5), with maximum inhibitions of 82.8±6.7, 46.0±8.1, and 90.6±3.6%, respectively.
Exposure of the slices to the protein synthesis inhibitor cyclohexamide or the inhibitor of type II NOS induction dexamethasone immediately following slice cutting, and for a subsequent 4 – 5 h, did not inhibit the NO/NO2− signal.
The evoked NO/NO2− signal was not reduced following 6 h perfusion in Ca2+-free media, consistent with a Ca2+-independent type II NOS activity.
PCR for type II NOS revealed the presence of this isotype in the SCN, even immediately following removal of the brain.
These studies provide the first evidence to suggest a functional, constitutively-active type II NOS within the brain of normal, healthy adult animals, and add type II NOS to the multiple isotypes of NO synthase playing a role within the mammalian SCN.
Keywords: Nitric oxide, iNOS, type II NOS, suprachiasmatic nucleus, fast cyclic voltammetry, guinea-pig, circadian, brain, PCR
Introduction
The gas nitric oxide (NO) is an important signalling molecule involved in a wide range of physiological processes, including cellular signalling (Garthwaite, 1991; Kiss, 2000), pathophysiological mechanisms (Licinio et al., 1999), host defence (Nussler & Billiar, 1993), and ageing (McCann et al., 1998; Vernet et al., 1998; Uttenthal et al., 1998). It is synthesized by three isoforms of nitric oxide synthase (NOS): type I or nNOS originally identified in neurones (Bredt & Snyder, 1990; Bredt et al., 1991), type III or eNOS first described in endothelial cells (Lamas et al., 1992; Janssens et al., 1992; Sessa et al., 1992; Marsden et al., 1992), and type II or iNOS initially identified in macrophages (Xie et al., 1992; Lyons et al., 1992). Types I and III have been characterized as constitutively expressed, Ca2+-dependent, producing small and rapid increases in NO in response to increases in intracellular Ca2+ (Bredt & Snyder, 1990; Mülsch et al., 1989). In contrast, type II NOS, the mRNA of which is not generally considered to be expressed under normal circumstances, is induced by stimuli such as cytokines or bacterial endotoxins (e.g. lipopolysaccharide, LPS; Lyons et al., 1992), oxygen and glucose deprivation (Cardenas et al., 2000; De Alba et al., 1999) or stress (Olivenza et al., 2000). The result of type II NOS induction is an increase in type II mRNA over a period of hours, and a continuous, Ca2+-independent production of high concentrations of NO (Licinio et al., 1999). However, the above definitions do not hold in all circumstances, as it has been shown that LPS can induce type III NOS (Iwase et al., 2000), a Ca2+-dependent type II NOS has been described in the guinea-pig lung (Shirato et al., 1998), and type II NOS has been found to be constitutively expressed in normal human retina (Park et al., 1994), guinea-pig optic nerve (Qi & Guy, 1996), rat ovary (Jablonka-Shariff & Olson, 1997), mouse and guinea-pig skeletal muscle fibres (Gath et al., 1999). Nonetheless, type II NOS has not previously been found to be constitutively present in the normal, non-aged, adult brain (Licinio et al., 1999).
The suprachiasmatic nuclei (SCN) are the site of the mammalian biological clock, responsible for generating and synchronizing circadian, or daily, rhythms. NO has been shown to play an important role in regulating the timing of circadian rhythms. A NOS inhibitor administered continuously into the SCN region disrupts water intake rhythms (Masutani et al., 1994). Non-selective NOS inhibitors block light- or glutamate agonist-induced phase-shifts of circadian locomotor activity rhythms in vivo (Ding et al., 1994; Wanatabe et al., 1995; Weber et al., 1995). NOS inhibitors also block phase-shifts of neuronal electrical activity cycles in the SCN in vitro, induced by glutamate agonists (Wanatabe et al., 1994; Ding et al., 1994), 5-HT (Starkey, 1996), and melatonin (Starkey, 1996). NO donors can augment the effects of light in vivo (Melo et al., 1997) and mimic the effects of glutamate, 5-HT and melatonin in vitro (Ding et al., 1994, Starkey, 1996). NO-dependent phase-shifts induced by light/glutamate, 5-HT, or melatonin occur during subjective night, subjective day, or the end of subjective day, respectively (Wanatabe et al., 1995, Weber et al., 1995, Ding et al., 1994, Starkey, 1996). Thus, NO plays a role in resetting the clock by different stimuli at different times throughout the circadian period. The mechanism by which the same molecule can mediate temporally-regulated responses over the circadian period is not fully understood.
The identification of NOS within the SCN has not proven to be easy, and has yielded variable results. Type I NOS immunoreactivity has been detected within a small number of neurones in the hamster (Decker & Reuss, 1994; Caillol et al., 2000), rat (Reuss et al., 1995; Wang & Morris, 1996; Chen et al., 1997; Caillol et al., 2000) and mouse SCN (Wang & Morris, 1996). However, in some studies there appear to be more type I NOS-positive cells surrounding the SCN than within the nuclei (Wang & Morris, 1996; Caillol et al., 2000). Nicotinamide adenine dinucleotide phosphate diaphorase (NADPH-d) activity, exhibited by NO synthase, has been used as a putative histochemical marker of NOS. Such NADPH-d activity has been observed within some cells of the hamster SCN (Decker & Reuss, 1994), a small number in the rat SCN (Amir et al., 1995; Caillol et al., 2000), and in the area surrounding the rat SCN (Lupi et al., 1996). More recently type III NOS has been found in rat SCN astrocytes (Caillol et al., 2000). The distribution of type III NOS mirrored the NADPH-d staining in the SCN (Caillol et al., 2000). Thus, there may be a contribution by more than one NOS isotype to the regulation of circadian rhythms. Interestingly, mice lacking the gene for type I NOS demonstrated no change in the ability of light to phase-shift or entrain the circadian rhythm of locomotor activity (Kriegsfeld et al., 1999). This raises a question over the role of type I NOS in the regulation of circadian rhythms by light. Whilst there may be developmental mechanisms compensating for a lack of type I NOS, the study does suggest that other forms of the enzyme, or a combination of isoforms, are required for mediating the effects of light on the biological clock. The isoform(s) of NOS contributing to the regulation of clock phase by the modulatory inputs remains to be established.
The aims of the present study were firstly to use the technique of fast cyclic voltammetry (FCV) to see whether NO could be detected in SCN in vitro. Secondly, we wanted to further investigate the identity of the NOS isoform(s) contributing to NO production within a mammalian SCN.
Methods
Preparation and incubation of slices
Male Dunkin Hartley guinea-pigs (200 – 450 g; 2 – 6 weeks old) were housed in groups of 10 – 20 in floor pens, with constant access to food and water, and a plentiful supply of straw and bedding. Care was taken not to startle the animals whilst in the room or during pen cleaning. Animals were exposed to the same 12 h:12 h light:dark cycle (LD) for at least 3 weeks, to enable entrainment to the lighting conditions. Animals were killed and the brains carefully removed at zeitgeber time 4 – 6 (ZT, where ZT0=time of lights on). Coronal SCN brain slices (500 μm thick) were cut in ice-cold artificial cerebrospinal fluid (ACSF) using a vibratome. The ACSF was of composition (mM): NaCl (124), KCl (2), NaHCO3 (25), MgSO4 (2), KH2PO4 (1.25), D-(+)-glucose (11), CaCl2 (2), dissolved in de-ionized Milli-Q water, gassed with 95% O2 /5% CO2, pH 7.4. Slices were then transferred to a slice chamber and maintained at 32°C, perfused and submerged with ACSF at the rate of 1.2 ml min−1. Voltammetric recordings were made from 1 h after slice cutting.
Fast cyclic voltammetry
Bipolar carbon fibre stimulating electrodes (tip separation 50 μm) were placed in the mediodorsal region of the SCN. Electrical stimulation was five 0.1 ms pulses at 100 Hz, 0.2 – 3 mA amplitude, applied every 5 min. A carbon fibre recording electrode (tip diameter 7 μm, exposed tip length 30 – 50 μm) was positioned approximately 50 μm from either pole of the stimulating electrode, within the slice. Fast cyclic voltammetry (Millar Signal Analyser, PD systems) was used to detect a signal in response to the electrical stimulation, due to the oxidation of a substance on the surface of the recording electrode (sampled at 1.26 V) in response to an applied voltage ramp. The voltage ramp was 1.5 cycles of a 100 Hz triangular waveform, scanning between −1.0 and +1.4 V every 500 ms, returning to 0 V after each scan. The voltage was relative to a silver – silver chloride reference electrode. The voltammetric detection of NO/NO2− was similar to that described by Iravani et al. (1993, 1998). Calibration of the electrodes was achieved by perfusing solutions containing either NO, NO2− or NO3− over the recording electrode and measuring the displacement in the electrochemical signal (V). Solutions of NO were made from ACSF which had been bubbled with nitrogen gas to de-oxygenate it for 45 min, followed by NO for 30 min (in a fume hood). A saturated solution of NO was approximately 2 mM, and serial dilutions were made in de-oxygenated ACSF. NO2− or NO3− solutions were made by dissolving NaNO2 or NaNO3 in de-oxygenated ACSF. Care was taken to minimize the risk of NO volatizing into gas phase, by all solutions being made in appropriately sized bottles, thereby minimizing the gas space. Lids were fitted at all times, except when the solutions were quickly removed for serial dilutions and perfusion over the electrode. For calibration of the electrodes, solutions were in sealed, airtight syringes.
Drug applications
Drugs were applied to the slice via bath perfusion for a minimum of 15 min, and until equilibrated. Where Ca2+-free ACSF was used, the Ca2+ was omitted from the ACSF. The protein synthesis inhibitor or type II NOS induction inhibitor were tested for their effects on the NO/NO2− detected, by placing some slices into a solution containing either 10 μM cyclohexamide or 1 μM dexamethasone in oxygenated (95%O2/5%CO2) ACSF immediately following slice cutting (within 5 min of the removal of the brain from the skull). The slices were maintained in the presence of the inhibitors within the slice chamber for 4 – 5 h.
Tissue for RT – PCR
Male Dunkin Hartley guinea-pigs (200 – 350 g) were kept in 12 h:12 h light:dark cycle for 2 weeks before being killed and their brains removed (ZT8.30 – ZT9). Blocks of tissue containing the two SCN and some surrounding hypothalamus (approximately 1.5 mm3) were dissected, homogenized in Ultraspec RNA isolation reagent (Ambion) and frozen on dry ice. Alternatively, SCN slices 500 μm thick were cut in ice-cold ACSF using a vibratome (as for the voltammetry experiments), the SCNs dissected out, homogenized in Ultraspec RNA isolation reagent and frozen. Some SCN from slices were homogenized and frozen 1.5 h post dissection, after being kept in oxygenated ACSF at 25°C or after having been electrically stimulated (as for the voltammetry) in the slice chamber at 32°C. As a control, tissue samples of lung, kidney, spleen or whole brain were taken from DH guinea-pigs either 6 h following treatment with 4 mg/kg i.v. LPS or age-matched untreated animals. These tissue samples were similarly homogenized in Ultraspec RNA isolation reagent and snap frozen.
RT – PCR
The mouse, rat, human and guinea-pig type II NOS sequences were aligned by ClustalW (Accession numbers M87039, U03699, X73029 and AF027180. PCR primers made were (GP2F) 5′-GTATGACCAGAGGCCCC-3′ (Exon 5) and (GP1R) 5′-GCTGGCTACCAGATGCC-3′ (Exon 8). The gene structure was obtained from Xu et al., 1996. Expected size for type II NOS was 450 base pairs cDNA, and genomic six K-base pairs. Total RNA was prepared using Ultraspec RNA isolation reagent. Briefly, one SCN tissue block, SCN slice or piece of other tissue was homogenized in 2 ml Ultraspec reagent. The aqueous phase was removed and the RNA precipitated with isopropanol. RNA was resuspended in 20 μl of DEPC water. Ten μl RNA was used for the first strand cDNA synthesis using random hexamer primers (Clontech). cDNA was resuspended in 100 μl and 10 μl was used for PCR. Quality of cDNA was checked by PCR with control GAPDH primers (Clontech). Conditions for PCR for type II NOS were: 1×Taq buffer (Promega), 200 μM dNTPs, 0.5 pmoles each primer, 10 μl of cDNA, 1U Advantage Taq polymerase (Perkin Elmer) in a 25 μl volume. Cycling conditions were 94°C for 30 s, 60°C for 1 min, 72°C for 1 min for 35 cycles. Results for time points 0, 0 s (sliced) hours and 1.5 (sliced) and 1.5 s (sliced and stimulated) hours are shown in Figure 6. The clear band at 480 base pairs corresponds to type II NOS (iNOS) cDNA, confirmed by Southern blotting (data not shown) and also sub-cloning of the product and sequencing (data not shown). Some non-specific bands appeared in the PCR: these were sub-cloned and shown to be non-homologous to any known sequence by Blast analysis against European molecular biology laboratory (EMBL) and EMBL new databases.
Figure 6.
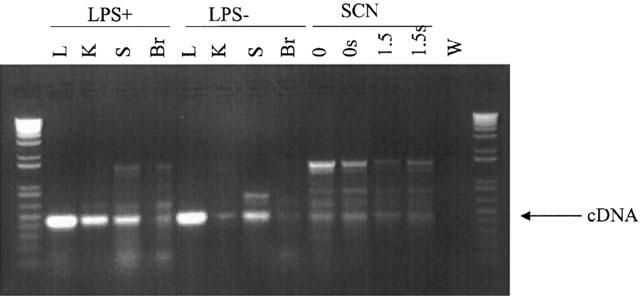
PCR amplification of reverse-transcribed RNA from guinea-pig tissue using a primer for type II NOS. The expected cDNA of 450 base pairs for type II NOS is indicated by the arrow. Lanes 2 – 5 were using guinea-pig lung (L), kidney (K), spleen (S) and whole brain (Br), with tissue samples taken 6 h following in vivo treatment with LPS. Lanes 6 – 9 were with similar guinea-pig tissue, without LPS treatment. There was a much weaker signal in the spleen and virtually no signal in the whole brain without LPS treatment. Lanes 10 – 13 were using SCN tissue. Lane 10 (0) was from a small block of hypothalamus containing the SCN, approximately 1.5 mm3. For lanes 11, 12 and 13 the SCN were cut from a SCN slice, either immediately following removal of the brain (lane 11, 0 s), 1.5 h following removal of the brain (lane 12, 1.5 s) or 1.5 h following removal of the brain where the SCN were stimulated as for a voltammetry experiment (lane 13, 1.5 s). A positive signal was detected in all four SCN samples, with the strongest signal immediately following removal of the brain. Lane 14 was a water negative control. Lanes 1 and 15 contain DNA size standards.
Drugs
NG-nitro-L-arginine-methyl ester.HCl (L-NAME) and NG-monomethyl-L-arginine.monoacetate (L-NMMA) were obtained from Alexis Biochemicals; N-(3-(aminomethyl)benzyl)acetamidine.2HCl (1400 W) was synthesized by Medicinal Chemistry, GlaxoWellcome; dexamethasone, cyclohexamide, 5-HT, NaNO2, NaNO3, L-citrulline and LPS (Escherichia coli, serotype 055:B5) were from Sigma. All were dissolved in distilled and de-ionized water, with the exception of dexamethasone and cyclohexamide which were dissolved in ethanol, and LPS in 0.9% saline. Stock solutions for all but LPS were diluted in ACSF.
Data analysis
The voltammetric signal from the Signal Analyser was stored and analysed using Spike 2 (Cambridge Electronic Design). The amplitude of the signal was taken as the difference between the peak height following electrical stimulation and that immediately before stimulation (V). This was expressed either as concentration of NO from the calibration curve or as per cent change from pre-drug values throughout the experiment. IC50 values for inhibition of the signal are the geometric mean (95% confidence interval; Excel). Statistical significance was calculated using a two-tailed Student's t-test, with significance defined as P<0.05.
Results
Voltammetric signal
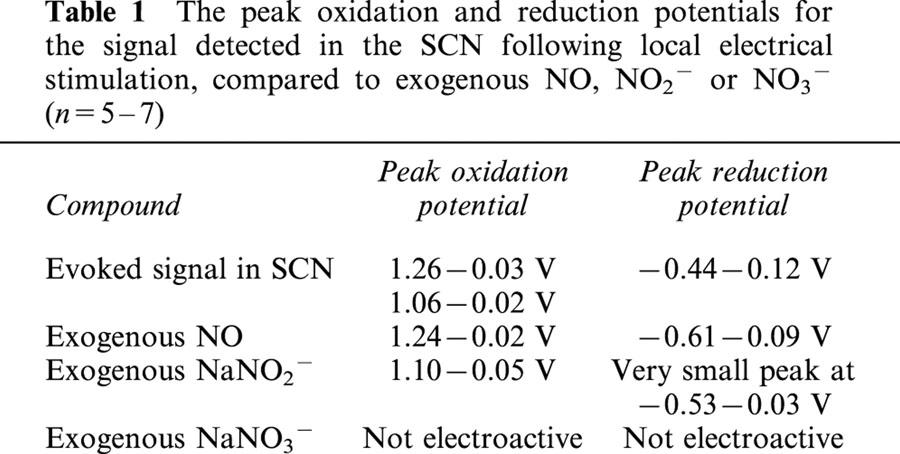
Fast cyclic voltammetry was used to detect a rapid and transient oxidation signal in response to local electrical stimulation in guinea-pig SCN slices (Figure 1A). The evoked signal could be detected at the beginning of the experiment (from 1 h post slice cutting), and remained at a stable amplitude for at least 4 h. Two oxidation signals were detected, one at 1.26 V and one at 1.06 V (Table 1). The oxidation peaks were very similar to those obtained with NO and NO2−, respectively (Table 1). Whilst a reduction peak was detected for NO at −0.44 V, only a very slight signal was seen for NO2− (Table 1). The oxidative product of NO2−, NO3−, was not electroactive (Table 1). The by-product from NO synthesis, L-citrulline (1 mM), had no effect on the electrochemical signal sampled at 1.26 V. Thus the oxidation signals detected in the SCN in response to stimulation suggested that the substance was NO or NO2−. The oxidation peaks were at a much higher potential than is seen for biogenic amines (5-HT 0.61 V, Starkey & Skingle, 1994; dopamine 0.59 V, Bull et al., 1990; noradrenaline 0.69 V, Palij & Stamford, 1992). Indeed, 1 μM 5-HT perfused over the electrode, whilst sampling at 1.26 V produced no change in the electrochemical signal. It was not possible to determine the relative proportions of NO and NO2− present, as the two oxidation peaks overlap. However, the presence of a reduction peak (Table 1) suggests the presence of NO. Any NO2− present is likely to be the oxidized product of NO, and thus the signal should reflect any increases in extracellular NO. Calibration curves for NO and NO2− showed similar sensitivities to the two molecules, both of which demonstrated increased response (nA) to increasing concentrations (Figure 1B,C).
Figure 1.
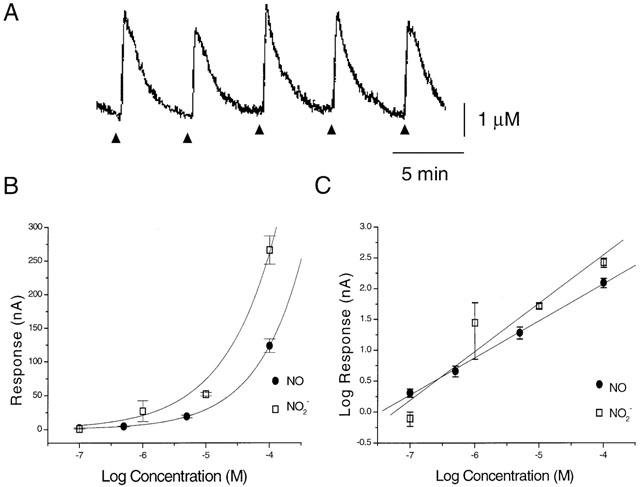
(A) Example sample and hold output trace measuring a rapid and transient increase in an oxidation signal in response to local electrical stimulation in a guinea-pig SCN slice, using fast cyclic voltammetry. The oxidation current was sampled at 1.26 V on each cyclic voltage sweep. Triangles indicate the time of electrical stimulation. (B) and (C) Calibration curves from exogenous NO and NO2− (n=3; B is mean±s.e.mean, C is geometric mean±90% C.I.) rapidly perfused over the recording electrode.
Table 1.
The peak oxidation and reduction potentials for the signal detected in the SCN following local electrical stimulation, compared to exogenous NO, NO2− or NO3− (n=5 – 7)
NOS inhibitors
To confirm whether the evoked signal in the SCN was NO/NO2−, the effects of NOS inhibitors were tested. The signal was reversibly inhibited, in a concentration-dependent manner, by the non-selective NOS inhibitor L-NAME (Figure 2). Maximum inhibition at 1 mM was 82.8±6.7%, and the IC50 was 229 μM (65 – 801 μM, 95% confidence intervals, n=3). Whilst L-NAME inhibits all three isoforms of NOS, its potency here is closest to its reported inhibition of type II (type II IC50 rat macrophages, 1000 μM, Chlopicki et al., 1999; type II rat lung, 41 μM, Stratman et al., 1996) and greater than for types I or III (type I IC50 rat cerebellum, 3 μM, Stratman et al., 1996; type III, porcine aorta, 3 μM, Rees et al., 1990). However, the exact potencies of the compounds will vary with the concentration of the substrate, L-arginine, present in the tissue or assay. L-NMMA inhibited the signal to a maximum of only 46±8.1% at 1 mM, with an IC50 of 452 nM (88 – 2310 nM; n=5). L-NMMA is not selective for the isotypes of NOS (type II, rat aorta, 6 μM; type III, rat aorta, 17 μM, Garvey et al., 1997; type III porcine aorta 3 μM, Rees et al., 1990; type I, rat cerebellum, 3 μM, Garthwaite et al., 1989). The selective type II NOS inhibitor 1400 W inhibited the signal in a concentration-dependent manner, with a maximum inhibition of 90.6% at 1 mM and an IC50 of 14.2 μM (3.6 – 54.4; n=5). This is closest to its reported functional inhibition of type II NOS (0.8 μM, Garvey et al., 1997), as the compound has no functional inhibition of type III until >300 μM (Garvey et al., 1997) and an IC50 for inhibition of type I NOS of 428 μM (342 – 536; n=4, rat cortical slices, C. Craig and R. Knowles, unpublished data). The signal did not recover following 1 h of washout of 1400 W (Figure 3A). 1400 W is a very slow reversible inhibitor of type II NOS, but its weak effects on type I or III are rapidly reversed (Garvey et al., 1997). Thus, although the IC50s are not identical to those reported previously in different tissues (not surprisingly), they have the rank order and pharmacology consistent with type II NOS.
Figure 2.
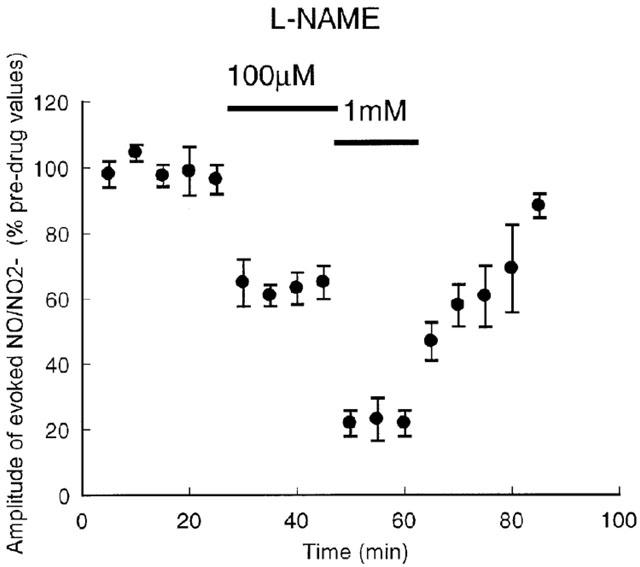
Effect of L-NAME on the amplitude of the NO/NO2− signal in the SCN, evoked every 5 min. Points are mean±s.e.mean, n=3.
Figure 3.
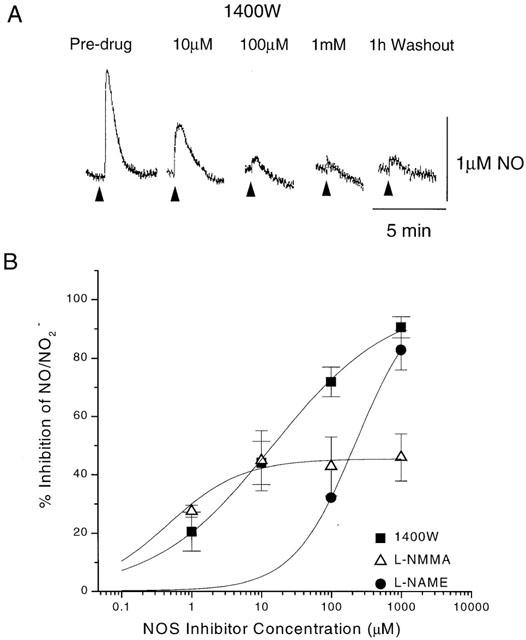
The effect of NOS inhibitors on the evoked NO/NO2− signal in the guinea-pig SCN. (A) example traces obtained in the presence of increasing concentrations of 1400 W. Triangles indicate the time of electrical stimulation. (B) Concentration – response curves for effect of the NOS inhibitors 1400 W, L-NMMA and L-NAME on the evoked NO/NO2− signal in the guinea-pig SCN. The points are mean±s.e.mean, with n values of 5, 5 and 3, respectively.
Protein synthesis inhibitors
Type II NOS has not previously been shown to be present in normal, healthy, adult brain, but is induced between 2 – 48 h following a stimulus (Wong et al., 1996; Moro et al., 1998; McCann et al., 1998). Although the signal was detectable at 1 h post-slice cutting, there was the possibility that the dissection and slicing procedure may have resulted in a very rapid induction of type II NOS mRNA synthesis. Protein synthesis inhibitors were able to prevent the induction of type II NOS in cortical slices following oxygen and glucose deprivation (Moro et al., 1998). In a similar manner, slices in this study were placed directly into ACSF containing the protein synthesis inhibitor cyclohexamide (10 μM) or a glucocorticoid inhibitor of type II NOS induction, dexamethasone (1 μM), immediately following slice cutting (within 5 min of the removal of the brain). The slices were then transferred to the slice chamber, where they remained perfused with the induction/protein synthesis inhibitors for 4 – 5 h. 1.5 h or 4 h post-slice cutting the electrically evoked NO/NO2− was still detected in the slices (Ca2+-free ACSF, n=3, Figure 4). This signal could still be inhibited by 100 μM 1400 W, after 4 h in the presence of the induction/protein synthesis inhibitors. The NO/NO2− signal did not recover following 1 h washout with 1400 W, consistent with the compound being a very slowly reversible inhibitor of type II NOS.
Figure 4.
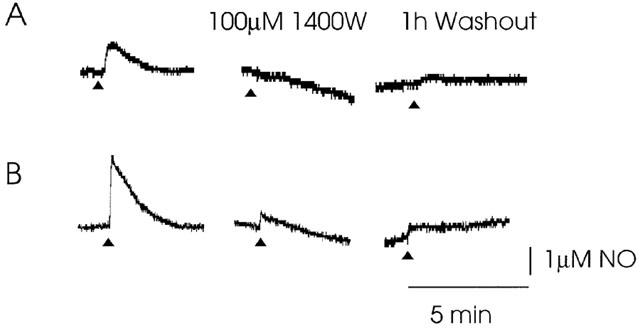
Incubation of SCN slices in ACSF containing 1 μM dexamethasone or 10 μM cycloheximide immediately following slice cutting (within 5 min of the removal of the brain) did not inhibit the evoked NO/NO2− signal. (A) Example traces of evoked NO/NO2−, recorded after 4 h in the presence of dexamethasone (A) or cycloheximide (B). 1400 W was still able to inhibit the signal, indicating a functional type II NOS. The signal did not recover following 1 h of wash-out of 1400 W, consistent with a slowly reversible compound. Triangles indicate the time of electrical stimulation.
Ca2+-dependency
Since type II NOS activity is not dependent upon Ca2+, the effect of Ca2+-free ACSF was tested on the signal in the SCN. The evoked NO/NO2− was not significantly attenuated following 5 h perfusion in Ca2+-free ACSF (P>0.05, first 0.5 h NO/NO2− concentrations compared to last 0.5 h, Figure 5). In contrast, in the same system, the release of 5-HT was completely abolished following 10 min perfusion with Ca2+-free ACSF (Starkey & Skingle, 1994). It is therefore highly unlikely that the NO/NO2− signal is the result of a Ca2+-dependent neurotransmitter release or a Ca2+-dependent NO synthase. It does however offer support for the signal being produced by the activation of a Ca2+-independent NO synthase. Interestingly, switching from Ca2+-free ACSF to solution containing 2 mM CaCl, produced a small decrease in the height of the evoked NO/NO2− signal (data not shown).
Figure 5.
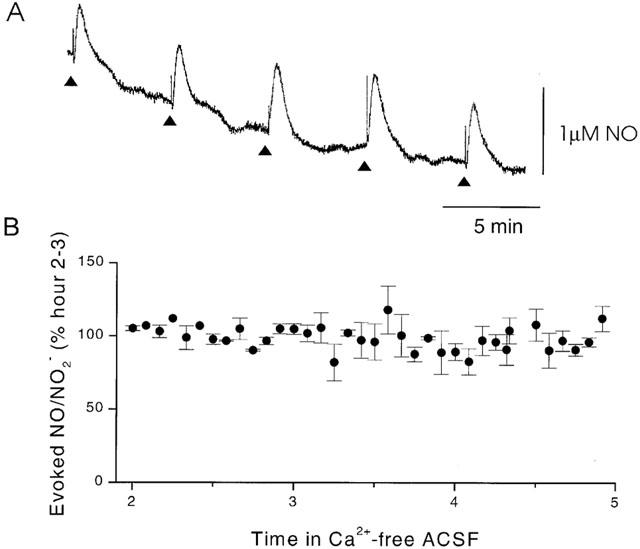
(A) An example trace of evoked NO/NO2− in a SCN slice following 4 h in the presence of Ca2+-free ACSF. Triangles indicate the time of electrical stimulation. (B) Mean amplitude of the NO/NO2− signal in Ca2+-free ACSF, evoked in the SCN every 5 min over a 3 h period. Points are mean±s.e.mean, n=4.
RT – PCR
Reverse transcription PCR was used to test for the presence of type II NOS mRNA within the guinea-pig SCN. Type II NOS primers detected a cDNA signal (ZT9.30) 6 h following LPS treatment (ZT3.30), in the kidney, spleen, lung and some in the whole brain (Figure 6). In LPS untreated animals, type II NOS was detected in the lung, some in the spleen, with virtually none in the whole brain, or the kidney (Figure 6). However, SCN tissue taken from LPS-untreated guinea-pigs at ZT9 demonstrated the presence of type II NOS mRNA (Figure 6). Type II NOS mRNA was present from hypothalamic blocks and SCN slices immediately following dissection, and to a lesser extent in SCN slices 1.5 h post dissection, with or without electrical stimulation. Thus, although the band was not as strong as for the lung, kidney or spleen following LPS treatment, it was present in the SCN immediately following removal of the brain.
Discussion
Fast cyclic voltammetry has been used to detect rapid and transient increases in NO/NO2− in SCN slices in response to local electrical stimulation. The detection of NO in the SCN supports previous studies which have demonstrated the presence of NOS in or around the nuclei and identified the importance of NO in the regulation of circadian rhythms (see Introduction). Histological and molecular studies have identified type I and type II NOS in rat SCN, and type I in hamster and mouse SCN (see Introduction). Whilst it was expected that the NO/NO2− signal in the guinea-pig SCN might be synthesized by type I NOS, the effects of NOS inhibitors pointed to the presence of a functional type II NO synthase. The high concentration of L-NAME needed to inhibit the signal, and the potent and irreversible attenuation by the highly selective type II inhibitor 1400 W, is consistent with activity of a type II NOS. High concentrations of NO/NO2− (mean of 2 μM) were detected following a very brief electrical stimulation in the SCN. Such concentrations might conventionally be expected from type II NOS, rather than from the Ca2+-dependent isoforms (Licinio et al., 1999). The signal was not affected by 5 h in Ca2+-free ACSF, strongly suggesting the involvement of a Ca2+-independent enzyme. Since type II NOS is usually induced, we tested whether the signal could be prevented by the type II NOS induction or protein synthesis inhibitors dexamethasone or cyclohexamide. Neither compound inhibited the signal, suggesting that the process of brain slice preparation had not induced the synthesis of a type II NOS protein. This contrasts with the induction of type II NOS protein following oxygen and glucose deprivation in brain slices (Moro et al., 1998). The guinea-pigs were healthy, young adults, housed in groups, handled with care, and with disturbances kept to a minimum. Thus, the presence of type II NOS is unlikely to be the result of stress, pathological states, or ageing. We suggest that type II NOS is constitutively expressed in the SCN of the guinea-pig. The RT – PCR demonstrated the presence of a type II NOS mRNA in the guinea-pig SCN. Whilst the PCR signal was virtually undetectable in the untreated whole brain, there was an enriched signal in the isolated nuclei. Type II NOS mRNA was present even immediately following removal of the brain, further supporting a constitutive expression. Weaker bands were seen 1.5 h following removal of the brain, and following stimulation of the SCN with electrodes, as for the voltammetry experiments. Therefore the presence of type II NOS mRNA was not dependent upon electrical stimulation of the slice.
The NOS inhibitors, L-NAME and 1400 W almost totally inhibited the NO/NO2− signal, with maximum inhibitions of 83 and 91%, respectively. However, L-NMMA only inhibited the signal by a maximum of 46%. One possible reason may be that L-NMMA can be converted to L-citrulline (dimethylarginase or NO synthase) and then to L-arginine (arginosuccinate synthase). The decrease in L-NMMA concentrations and/or a competition for the L-NMMA binding site by L-arginine may account for the smaller maximal response (Feldman et al., 1993). The mechanism by which fairly rapid, transient, Ca2+-independent increases in type II NOS activity were evoked following a brief electrical stimulation is not known.
The functional presence of type II NOS in the guinea-pig SCN adds another isotype of NO synthase to the forms already identified within mammalian circadian pacemakers. We do not yet know whether the guinea-pig also expresses type I or III NOS in the SCN, or whether the rat or hamster also express type II. However, the rat SCN does express multiple isotypes of NOS (I and III, Caillol et al., 2000). Interestingly, elderly rats express type II NOS in the cortex, hypothalamus and cerebellum, although isolated SCN were not examined (Uttenthal et al., 1998; Vernet et al., 1998). The multiple isoforms of NOS may each contribute to the control of circadian rhythms. Possible mechanisms by which glutamate, 5-HT and melatonin phase-shift circadian rhythms via NO but with different phase – response relationships (Wanatabe et al., 1994; Ding et al., 1994; Starkey, 1996) are discussed below. It is possible that the different stimuli are only capable of activating NO synthesis to produce required concentrations of NO, at restricted times of the day. Administration of NO donors to SCN slices showed that higher concentrations of a NO donor were required to induce phase shifts during subjective day (ZT6.5, ZT10) than subjective night (ZT18; Starkey, 1996). Alternatively, NO could be synthesized in response to each stimulus within a subregion of the SCN, with NO diffusing to modulate transduction pathways in a subset of cells. This could occur via differential distribution of the receptors, and/or NOS isotypes. Current information suggests some variation in the location of the NOS isoforms. In the rat, type III NOS was found in astrocytes in the rostral part of the SCN, particularly above the optic chiasm (Caillol et al., 2000). Type I has been found in neurones, either predominantly in the caudal SCN (Caillol et al., 2000), rostral SCN (Reuss et al., 1995), throughout the SCN (Chen et al., 1997), in the ventrolateral part of the nucleus (Decker & Reuss, 1994; Wang & Morris, 1996) or the mediodorsal region (Hashida-Okumura et al., 1999). There are clearly differences in type I NOS distribution between studies. The electrodes for the detection of NO by voltammetry in the present study were positioned within the mediodorsal region of the guinea-pig SCN. Another possibility why NO may mediate different phase-resetting stimuli at different times of day is that the stimulus, pathway or NOS which produces NO may modulate other receptor subtypes or pathways to enable temporally-restricted responses of downstream targets.
Circadian changes in Ca2+-dependent NOS activity have been measured in the hamster SCN (Ferreyra et al., 1998) and the rat hypothalamus (Ayers et al., 1996) in animals housed in a light:dark (LD) cycle. Higher NOS activity was detected during the dark than the light period. One study found no change in Ca2+-dependent NOS activity over 24 h (LD, Chen et al., 1997). The NOS activity was not under the direct control of the circadian clock, as in constant darkness (DD), there was no circadian change in Ca2+-dependent NOS activity (Ferreyra et al., 1998). The circadian variation in NOS activity was not due to changes in type I NOS protein concentrations, which appeared to be constant over 24 h in the rat and hamster, in the absence of phase-shifting stimuli (Chen et al., 1997; Ferreyra et al., 1998). Whether there are circadian changes in type II NOS protein or function, or the relative contribution of type I or III to the Ca2+-dependent NOS has yet to be clarified.
Guinea-pigs exhibit very weak diurnal circadian rhythms, often with crepuscular patterns of activity (increases at dawn and dusk; King, 1956; Fuchs, 1980; Tobler & Jaggi, 1993). The weak rest-activity rhythms are reflected in multiple peaks of electrical activity in suprachiasmatic slices over a 24 h period (Starkey et al., 1995). In contrast, rats express distinct nocturnal behaviour and a clear single peak in electrical activity in the SCN during the day (Redman et al., 1983; Green & Gillette, 1982; Groos & Hendricks, 1982; Shibata et al., 1982; Starkey et al., 1995). It is possible that type II NOS may contribute to the weak circadian rhythms observed in the guinea-pig. Interestingly, elderly rats, which also express type II NOS (cortex, hypothalamus and cerebellum, Uttenthal et al., 1998; Vernet et al., 1998) exhibit a breakdown in circadian rhythms and lose the distinct single peak of electrical activity in the SCN (Satinoff et al., 1993). It would be interesting to see whether type II NOS is present in the SCN of old rats, and whether type II NOS inhibitors could improve disrupted circadian rhythms in the elderly. The presence of type II NOS in the SCN may affect the Ca2+-dependent isoforms, if, as in rat forebrain slices, an increase in NO from type II NOS decreases Ca2+-dependent NOS protein (de alba et al., 1999).
The present study has identified a constitutively active, functional type II NOS within the guinea-pig circadian pacemaker. Thus, all three isotypes of NO synthase have been identified within normal, adult, mammalian SCN. This is to our knowledge, the first description of a type II NOS in the CNS of healthy young, adult animals, where there has been no known inducing agent. The mechanism by which the type II NOS activity was rapidly evoked following electrical stimulation remains to be identified.
Acknowledgments
We would like to thank Graham Hagger for technical assistance, and Caroline Craig for the data on 1400 W in a functional type I NO synthase assay. We thank R. Knowles for his comments on the manuscript.
Abbreviations
- C.I.
confidence intervals
- DD
constant darkness
- EMBL
European molecular biology laboratory
- FCV
fast cyclic voltammetry
- LD
12 h:12 h light dark cycle
- LPS
lipopolysaccharide
- NADPH-d
nicotinamide adenine dinucleotide phosphate diaphorase
- NO
nitric oxide
- NOS
nitric oxide synthase
- SCN
suprachiasmatic nuclei
- ZT
zeitgeber time
References
- AMIR S., ROBINSON B., EDELSTEIN K. Distribution of NADPH-diaphorase staining and light-induced fos expression in the rat suprachiasmatic nucleus region supports a role for nitric oxide in the circadian system. Neurosci. 1995;69:545–555. doi: 10.1016/0306-4522(95)00252-e. [DOI] [PubMed] [Google Scholar]
- AYERS N.A., KAPAS L., KRUEGER J.M. Circadian variation of nitric oxide synthase and cytosolic protein levels in rat brain. Brain Res. 1996;707:127–130. doi: 10.1016/0006-8993(95)01362-8. [DOI] [PubMed] [Google Scholar]
- BULL D.R., PALIJ P., SHEEHAN M.J., MILLAR J., STAMFORD J.A., KRUK Z.L., HUMPHREY P.P. Application of fast cyclic voltammetry to measurement of electrically evoked dopamine overflow from brain slices in vitro. J. Neurosci. Meth. 1990;32:37–44. doi: 10.1016/0165-0270(90)90069-r. [DOI] [PubMed] [Google Scholar]
- BREDT D.S., HWANG P.M., GLATT C.E., LOWENSTEIN C., REED R.R., SNYDER S.H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature (London) 1991;351:714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- BREDT D.S., SNYDER S.H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc. Natl. Acad. Sci. U.S.A. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAILLOL M., DEVINOY E., LACROIX M.-C., SCHIRAR A. Endothelial and neuronal nitric oxide synthases are present in the suprachiasmatic nuclei of Syrian hamsters and rats. Eur. J. Neurosci. 2000;12:649–661. doi: 10.1046/j.1460-9568.2000.00961.x. [DOI] [PubMed] [Google Scholar]
- CARDENAS A., MORO M.A., HURTADO O., LEZA J.C., LORENZO P., CASTRILLO A., BODELON O.G., BOSCA L., LIZASOAIN I. Implication of glutamate in the expression of inducible nitric oxide synthase after oxygen and glucose deprivation in rat forebrain slices. J. Neurochem. 2000;74:2041–2048. doi: 10.1046/j.1471-4159.2000.0742041.x. [DOI] [PubMed] [Google Scholar]
- CHEN D., HURST W.J., DING J.M., FAIMAN L.E., MAYER B., GILLETTE M.U. Localization and characterization of nitric oxide synthase in the rat suprachiasmatic nucleus: evidence for a nitrergic plexus in the biological clock. J. Neurochem. 1997;68:855–861. doi: 10.1046/j.1471-4159.1997.68020855.x. [DOI] [PubMed] [Google Scholar]
- CHLOPICKI S., OLSZANECKI R., JAKUBOWSKI A., LOMNICKA M., GRYGLEWSKI R.J. L-NIL but not S-methylisothiourea sulphate displays selectivity towards NOS-2. Pol. J. Pharmacol. 1999;51:443–447. [PubMed] [Google Scholar]
- DE ALBA J., CARDENAS A., MORO M.A., LEZA J.C., LORENZO P., LIZASOAIN I. Use of brain slices in the study of pathogenic role of inducible nitric oxide synthase in cerebral ischaemia-reperfusion. Gen. Pharmacol. 1999;32:577–581. doi: 10.1016/s0306-3623(98)00280-8. [DOI] [PubMed] [Google Scholar]
- DECKER K., REUSS S. Nitric oxide-synthesising neurons in the hamster suprachiasmatic nucleus: a combined NOS- and NADPH- staining and retinohypothalamic tract tracing study. Brain Res. 1994;666:284–288. doi: 10.1016/0006-8993(94)90785-4. [DOI] [PubMed] [Google Scholar]
- DING J.M., CHEN D., WEBER E.T., FAIMAN L.E., REA M.A., GILLETTE M.U. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science. 1994;266:1713–1717. doi: 10.1126/science.7527589. [DOI] [PubMed] [Google Scholar]
- FELDMAN P.L., GRIFFITH O.W., HONG H., STUEHR D.J. Irreversible inactivation of macrophage and brain nitric oxide synthase by L-NG-methylarginine requires NADPH-dependent hydroxylation. J. Med. Chem. 1993;36:491–496. doi: 10.1021/jm00056a009. [DOI] [PubMed] [Google Scholar]
- FERREYRA G.A., CAMMAROTA M.P., GOLOMBEK D.A. Photic control of nitric oxide synthase activity in the hamster suprachiasmatic nuclei. Brain Res. 1998;797:190–196. doi: 10.1016/s0006-8993(98)00376-x. [DOI] [PubMed] [Google Scholar]
- FUCHS S. Spacing patterns in a colony of guinea-pigs: predictability from environmental and social factors. Behav. Ecol. Sociobiol. 1980;6:265–276. [Google Scholar]
- GARTHWAITE J. Glutamate, nitric oxide and cell–cell signalling in the nervous system. TiNS. 1991;14:60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- GARTHWAITE J., GARTHWAITE G., PALMER R.M.J., MONCADA S. NMDA receptor activation induces nitric oxide synthesis from arginine in rat brain slices. Eur. J. Pharmacol. 1989;172:413–416. doi: 10.1016/0922-4106(89)90023-0. [DOI] [PubMed] [Google Scholar]
- GARVEY E.P., OPLINGER J.A., FURFINE E.S., KIFF R.J., LASZLO F., WHITTLE J.R., KNOWLES R.G. 1400 W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J. Biol. Chem. 1997;272:4959–4963. doi: 10.1074/jbc.272.8.4959. [DOI] [PubMed] [Google Scholar]
- GATH I., EBERT J., GODTEL-ARMBRUST U., ROSS R., RESKE-KUNZ A.B., FOSTERMANN U. NO synthase II in mouse skeletal muscle is associated with caveolin 3. Biochem. J. 1999;340:723–728. [PMC free article] [PubMed] [Google Scholar]
- GREEN D.J., GILLETTE R. Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Res. 1982;245:198–200. doi: 10.1016/0006-8993(82)90361-4. [DOI] [PubMed] [Google Scholar]
- GROOS G., HENDRICKS J. Circadian rhythms in electrical discharge of rat suprachiasmatic neurones recorded in vitro. Neurosci. Lett. 1982;34:283–288. doi: 10.1016/0304-3940(82)90189-6. [DOI] [PubMed] [Google Scholar]
- HASHIDA-OKUMURA A., OKUMURA N., IWAMATSU A., BUIJS R.M., ROMIJN H.J., NAGAI K. Interaction of neuronal nitric-oxide synthase with a1-Syntrophin in rat brain. J. Biol. Chem. 1999;274:11736–11741. doi: 10.1074/jbc.274.17.11736. [DOI] [PubMed] [Google Scholar]
- IRAVANI M.M., KRUK Z.L., MILLAR J. Electrochemical detection of nitric oxide using fast cyclic voltammetry. J. Physiol. 1993;467:48P. [Google Scholar]
- IRAVANI M., MILLAR J., KRUK Z.L. Differential release of dopamine by nitric oxide in subregions of rat caudate putamen slices. J. Neurochem. 1998;71:1969–1977. doi: 10.1046/j.1471-4159.1998.71051969.x. [DOI] [PubMed] [Google Scholar]
- IWASE K., MIYANAKA K., SHIMIZU A., NAGASAKI A., GOTOH T., MORI M., TAKIGUCHI M. Induction of endothelial nitric-oxide synthase in rat brain astrocytes by systemic lipopolysaccharide treatment. J. Biol. Chem. 2000;275:11929–11933. doi: 10.1074/jbc.275.16.11929. [DOI] [PubMed] [Google Scholar]
- JABLONKA-SHARIFF A., OLSON L.M. Hormonal regulation of nitric oxide synthases and their cell-specific expression during follicular development in the rat ovary. Endocrinology. 1997;138:460–468. doi: 10.1210/endo.138.1.4884. [DOI] [PubMed] [Google Scholar]
- JANSSENS S.P., SHIMOUCHI A., QUERTERMOUS T., BLOCH D.B., BLOCH K.D. Cloning and expression of a cDNA encoding human endothelium-derived relaxing factor/nitric oxide synthase. J. Biol. Chem. 1992;267:14519–14522. [PubMed] [Google Scholar]
- KING J.A. Social relations of the domestic guinea-pig living under semi-natural conditions. Ecology. 1956;37:221–228. [Google Scholar]
- KISS J.P. Role of nitric oxide in the regulation of monoaminergic neurotransmission. Brain Res. Bull. 2000;52:459–466. doi: 10.1016/s0361-9230(00)00282-3. [DOI] [PubMed] [Google Scholar]
- KRIEGSFELD L.J., DEMAS G.E., LEE S.E., DAWSON T.M., DAWSON V.L., NELSON R.J. Circadian locomotor analysis of male mice lacking the gene for neuronal nitric oxide synthase (nNOS−/−) J. Biol. Rhythms. 1999;14:20–27. doi: 10.1177/074873099129000407. [DOI] [PubMed] [Google Scholar]
- LAMAS S., MARSDEN P.A., LI G.K., TEMPST P., MICHEL T. Endothelial nitric oxide synthase: molecular cloning and characterisation of a distinct constitutive enzyme isoform. Proc. Natl. Acad. Sci. U.S.A. 1992;89:6348–6352. doi: 10.1073/pnas.89.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LICINIO J., PROLO P., MCCANN S.M., WONG M.-L. Brain iNOS: current understanding and clinical implications. Mol. Med. Today. 1999;5:225–232. doi: 10.1016/S1357-4310(99)01453-7. [DOI] [PubMed] [Google Scholar]
- LUPI D., DEBERNARDIS D., VALLERGA S., MORGAN P.J., DJAMGOZ M.B.A. NADPH diaphorase activity around the suprachiasmatic nucleus in rat brain. Cell Tissue Res. 1996;283:335–338. doi: 10.1007/s004410050544. [DOI] [PubMed] [Google Scholar]
- LYONS C.R., ORLOFF G.J., CUNNINGHAM J.M. Molecular cloning and functional expression of an inducible nitric oxide synthase from a murine macrophage cell line. J. Biol. Chem. 1992;267:6370–6374. [PubMed] [Google Scholar]
- MARSDEN P.A., SCHAPPERT K.T., CHEN H.S., FLOWERS M., SUNDELL C.L., WILCOX J.N., LAMAS S., MICHEL T. Molecular cloning and characterization of human endothelial nitric oxide synthase. FEBS Lett. 1992;307:287–293. doi: 10.1016/0014-5793(92)80697-f. [DOI] [PubMed] [Google Scholar]
- MASUTANI H., NAGE K., NAKAGAWA H. Possible involvement of nitric oxide in generation of circadian rhythm. Biol. Rhythm Res. 1994;25:433–441. [Google Scholar]
- MCCANN S.M., LICINIO J., WONG M.-L., YU W.H., KARANTH S., RETTORRI V. The nitric oxide hypothesis of ageing. Exp. Gerontol. 1998;33:813–826. doi: 10.1016/s0531-5565(98)00050-3. [DOI] [PubMed] [Google Scholar]
- MELO L., GOLOMBEK D.A., RALPH M.R. Regulation of circadian photic responses by nitric oxide. J. Biol. Rhythms. 1997;12:319–326. doi: 10.1177/074873049701200404. [DOI] [PubMed] [Google Scholar]
- MORO M.A., DE ALBA J., LEZA J.C., LORENZO P., FERNANDEZ A.P., BENTURA M.L., RODRIGO J., LIZASOAIN I. Neuronal expression of inducible nitric oxide synthase after oxygen and glucose deprivation in rat forebrain slices. Eur. J. Neurosci. 1998;10:445–456. doi: 10.1046/j.1460-9568.1998.00028.x. [DOI] [PubMed] [Google Scholar]
- MÜLSCH A., BASSENGE E., BUSSE R. Nitric oxide synthesis in endothelial cytosol: evidence for a calcium-dependent and a calcium-independent mechanism. Naunyn-Schmiederberg's Arch. Pharmacol. 1989;340:767–770. doi: 10.1007/BF00169688. [DOI] [PubMed] [Google Scholar]
- NUSSLER A.K., BILLIAR T.R. Inflammation, immunoregulation, and inducible nitric oxide synthase. J. Leukoc. Biol. 1993;54:171–178. [PubMed] [Google Scholar]
- OLIVENZA R., MORO M.A., LIZASOAIN I., LORENZO P., FERNANDEZ A.P., RODRIGO J., BOSCA L., LEZA J.C. Chronic stress induces the expression of inducible nitric oxide synthase in rat brain cortex. J. Neurochem. 2000;74:785–791. doi: 10.1046/j.1471-4159.2000.740785.x. [DOI] [PubMed] [Google Scholar]
- PALIJ P., STAMFORD J.A. Real-time monitoring of endogenous noradrenaline release in rat brain slices using fast cyclic voltammetry: 1. Characterisation of evoked noradrenaline efflux and uptake from nerve terminals in the bed nucleus of stria terminalis, pars ventralis. Brain Res. 1992;587:137–146. doi: 10.1016/0006-8993(92)91438-k. [DOI] [PubMed] [Google Scholar]
- PARK C.-S., PARDHASARADHI K., GIANOTTI C., VILLEGAS E., KRISHNA G. Human retina expresses both constitutive and inducible isoforms of nitric oxide synthase mRNA. Biochem. Biophys. Res. Comm. 1994;205:85–91. doi: 10.1006/bbrc.1994.2633. [DOI] [PubMed] [Google Scholar]
- QI X., GUY J. Localization of NADPH diaphorase/nitric oxide synthase in the optic nerve of the normal guinea pig: a light and electron microscopic study. J. Comp. Neurol. 1996;370:396–404. doi: 10.1002/(SICI)1096-9861(19960701)370:3<396::AID-CNE9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- REDMAN J., ARMSTRONG S., NG K.T. Free-running activity rhythms in the rat: entrainment by melatonin. Science. 1983;219:1089–1091. doi: 10.1126/science.6823571. [DOI] [PubMed] [Google Scholar]
- REES D.D., PALMER R.M.J., SCHULZ R., HODSON H.F., MONCADA S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br. J. Pharmacol. 1990;101:746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REUSS S., DECKER K., RÖßELER L., LAYES E., SCHOLLMAYER A., SPESSERT R. Nitric oxide synthase in the hypothalamic suprachiasmatic nucleus of rat: evidence from histochemistry, immunohistochemistry and Western blot; and colocalization with VIP. Brain Res. 1995;695:257–262. doi: 10.1016/0006-8993(95)00829-f. [DOI] [PubMed] [Google Scholar]
- SATINOFF E., LI H., TCHENG T.K., LIU C., MCARTHUR A.J., MEDANIC M., GILLETTE M.U. Do the suprachiasmatic nuclei oscillate in old rats as they do in young ones. Am. J. Psychol. 1993;265:R1216–R1222. doi: 10.1152/ajpregu.1993.265.5.R1216. [DOI] [PubMed] [Google Scholar]
- SESSA W.C., HARRISON J.K., BARBER C.M., ZENG D., DURIEUX M.E., D'ANGELO D.D., LYNCH K.R., PEACH M.J. Molecular cloning and expression of a cDNA encoding endothelial cell nitric oxide synthase. J. Biol. Chem. 1992;267:15274–15276. [PubMed] [Google Scholar]
- SHIBATA S., OOMURA Y., KITA H., HATTORI K. Circadian rhythmic changes of neuronal activity in the suprachiasmatic nucleus of the rat hypothalamic slice. Brain Res. 1982;247:154–158. doi: 10.1016/0006-8993(82)91041-1. [DOI] [PubMed] [Google Scholar]
- SHIRATO M., SAKAMOTO T., UCHIDA Y., NOMURA A., ISHII Y., IIJIMA H., GOTO Y., HASEGAWA S. Molecular cloning and characterization of Ca2+-dependent inducible nitric oxide synthase from guinea-pig lung. Biochem. J. 1998;333:795–799. doi: 10.1042/bj3330795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STARKEY S.J. Melatonin and 5-hydroxytryptamine phase-advance the rat circadian clock by activation of nitric oxide synthesis. Neurosci. Lett. 1996;211:199–202. doi: 10.1016/0304-3940(96)12756-7. [DOI] [PubMed] [Google Scholar]
- STARKEY S.J., OAKLEY N.R., BERESFORD I.J.M. An unusual rhythm of neuronal discharge activity in the suprachiasmatic nuclei (SCN) brain slice over 24 h. J. Physiol. 1995;483:57P. [Google Scholar]
- STARKEY S.J., SKINGLE M. 5-HTID as well as 5-HTIA autoreceptors modulate 5-HT release in the guinea-pig dorsal raphe nucleus. Neuropharmacol. 1994;33:393–402. doi: 10.1016/0028-3908(94)90069-8. [DOI] [PubMed] [Google Scholar]
- STRATMAN N.C., FICI G.J., SETHY V.H. U-19451A: a selective inducible nitric oxide synthase inhibitor. Life Sci. 1996;59:945–951. doi: 10.1016/0024-3205(96)00393-1. [DOI] [PubMed] [Google Scholar]
- TOBLER I., FRANKEN P., JAGGI K. Vigilance states, EEG spectra, and cortical temperature in the guinea-pig. Am. J. Physiol. 1993;264:R1125–R1132. doi: 10.1152/ajpregu.1993.264.6.R1125. [DOI] [PubMed] [Google Scholar]
- UTTENTHAL L.O., ALONSO D., FERNANDEZ A.P., CAMPBELL R.O., MORO M.A., LEZA J.C., LIZASOAIN I., ESTEBAN F.J., BARROSO J.B., VALDERRAMA R., PEDROSA J.A., PEINADO M.A., SERRANO J., RICHART A., BENTURA M.L., SANTACANA M., MARTINEZ-MURILLO R., RODRIGO J. Neuronal and inducible nitric oxide synthase and nitrotyrosine immunoreactivities in the cerebral cortex of the ageing rat. Microscopy Res. Technique. 1998;43:75–88. doi: 10.1002/(SICI)1097-0029(19981001)43:1<75::AID-JEMT11>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- VERNET D., BONAVERA J.J., SWERDLOFF R.S., GONZALEZ-CADAVID N.F., WANG C. Spontaneous expression of inducible nitric oxide synthase in the hypothalamus and other brain regions of ageing rats. Endocrinology. 1998;139:3254–3261. doi: 10.1210/endo.139.7.6119. [DOI] [PubMed] [Google Scholar]
- WANATABE A., HAMADA T., SHIBATA S., WANATABE S. Effects of nitric oxide synthase inhibitors on N-methyl-D-aspartate-induced phase delay of circadian rhythm of neuronal activity in the rat suprachiasmatic nucleus in vitro. Brain Res. 1994;646:161–164. doi: 10.1016/0006-8993(94)90071-x. [DOI] [PubMed] [Google Scholar]
- WANATABE A., ONO M., SHIBATA S., WANATABE S. Effect of nitric synthase inhibitor, N-nitro-L-arginine methylester, on light-induced phase delay of circadian rhythm of wheel running activity in golden hamsters. Neurosci. Lett. 1995;192:25–28. doi: 10.1016/0304-3940(95)11599-r. [DOI] [PubMed] [Google Scholar]
- WANG H., MORRIS J.F. Presence of neuronal nitric oxide synthase in the suprachiasmatic nuclei of mouse and rat. Neurosci. 1996;74:1059–1068. doi: 10.1016/0306-4522(96)00165-0. [DOI] [PubMed] [Google Scholar]
- WEBER E.T., GANNON R.L., MICHEL A.M., GILLETTE M.U., REA M.A. Nitric oxide inhibitor blocks light-induced phase shifts of the circadian activity rhythm, but not c-fos expression in the suprachiasmatic nucleus of the Syrian hamster. Brain Res. 1995;692:137–142. doi: 10.1016/0006-8993(95)00685-j. [DOI] [PubMed] [Google Scholar]
- WONG M.-L., RETTORI V., AL-SHEKHLEE A., BONGIORNO P.B., CANTEROS G., MCCANN S.M., GOLD P.W., LICINIO J. Inducible nitric oxide synthase gene expression in the brain during systemic inflammation. Nature Med. 1996;2:581–584. doi: 10.1038/nm0596-581. [DOI] [PubMed] [Google Scholar]
- XIE Q.W., CHO H.J., CALAYCAY J., MUMFORD R.A., SWIDEREK K.M., LEE T.D., DING A., TROSO T., NATHAN C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256:225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- XU W., CHARLES I.G., LIU L., MONCADA S., EMSON P. Molecular cloning and structural organization of the human inducible nitric oxide synthase gene (NOS2) Biochem. Biophys. Res. Comm. 1996;219:784–788. doi: 10.1006/bbrc.1996.0311. [DOI] [PubMed] [Google Scholar]


