Abstract
The release of acetylcholine was investigated in the human placenta villus, a useful model for the characterization of the non-neuronal cholinergic system.
Quinine, an inhibitor of organic cation transporters (OCT), reduced acetylcholine release in a reversible and concentration-dependent manner with an IC50 value of 5 μM. The maximal effect, inhibition by 99%, occurred at a concentration of 300 μM.
Procaine (100 μM), a sodium channel blocker, and vesamicol (10 μM), an inhibitor of the vesicular acetylcholine transporter, were ineffective.
Corticosterone, an inhibitor of OCT subtype 1, 2 and 3 reduced acetylcholine in a concentration-dependent manner with an IC50 value of 2 μM.
Substrates of OCT subtype 1, 2 and 3 (amiloride, cimetidine, guanidine, noradrenaline, verapamil) inhibited acetylcholine release, whereas carnitine, a substrate of subtype OCTN2, exerted no effect.
Long term exposure (48 and 72 h) of villus strips to anti-sense oligonucleotides (5 μM) directed against transcription of OCT1 and OCT3 reduced the release of acetylcholine, whereas OCT2 anti-sense oliogonucleotides were ineffective.
It is concluded that the release of non-neuronal acetylcholine from the human placenta is mediated via organic cation transporters of the OCT1 and OCT3 subtype.
Keywords: Human placenta, non-neuronal acetylcholine, organic cation transporter, quinine, corticosterone, - anti-sense oligonucleotides
Introduction
Acetycholine has been demonstrated in the vast majority of human non-neuronal cells, for example epithelial, endothelial, mesothelial and immune cells as well as smooth muscle fibres (Grando, 1997; Wessler et al., 1998; 1999; Kawashima & Fujii, 2000). In addition, nicotinic and muscarinic receptors are widely expressed on these cells (Brunner & Kukovetz, 1986; Grando et al., 1995; Maus et al., 1998; Costa et al., 1988; Kawashima & Fujii, 2000). Non-neuronal acetylcholine can act as a local cell molecule via paracrine and autocrine mechanisms to control basic cell functions such as proliferation, differentiation and cell – cell contact (Grando, 1997; Wessler et al., 1998; 1999; Kawashima & Fujii, 2000).
The release mechanisms by which non-neuronal cells, for example epithelial cells of the placenta, liberate acetylcholine into the extracellular space, are unknown. The human placenta is not innervated by extrinsic or intrinsic cholinergic neurons (Sastry & Sadavongvivad, 1979; Sastry, 1997). Thus, released acetylcholine is not contaminated by neuronal acetylcholine and can be used as a model for studying the release mechanisms of non-neuronal acetylcholine. Acetylcholine, an organic cation, may be a substrate for organic cation transporters (OCT). OCTs are most widely expressed in different cell types including the human placenta (Koepsell, 1998; Dresser et al., 1999). Three organic cation transporters have been cloned which represent high capacity non-neuronal monoamine transporters, OCT subtype 1 (Gründemann et al., 1994; Nagel et al., 1997), subtype 2 (Okuda et al., 1996; Gründemann et al., 1997; 1998) and subtype 3, the latter also being known as extraneuronal monoamine transporter uptake 2 (Gründemann et al., 1998; Kekuda et al., 1998; Wu et al., 1998). To test the hypothesis that non-neuronal acetylcholine is transported via OCTs, the release of acetylcholine from isolated villus of human placenta was measured in the absence and presence of substances known to interfere with OCTs.
Methods
Tissue preparation
Human placentae were obtained after normal vaginal delivery or Caesarean section from women with uncomplicated pregnancies. No differences (ChAT activity, acetylcholine content and release) were found in placentae obtained after normal delivery or Caesarean section (Wessler et al., 2001). Specimens (about 500 mg) were dissected from the whole placenta and vigorously washed (five times for 10 min) in oxygenated salt solution (200 ml) of the following composition given in mM: NaCl 125, NaHCO3 23.8, glucose 5.05, KCl 2.68, CaCl2 1.80, MgCl2 1.04, NaH2PO4 0.415, ascorbic acid 0.0567, and choline chloride 0.001. The physiological salt solution was gassed with carbogen (5% (v v−1) carbon dioxide in oxygen); pH of the gassed solution was 7.3. After this washing small villus pieces (20 – 50 mg) were isolated from the placenta, pierced longitudinally by needles (Tabout 15 mm in length) and then transferred to 1.5 ml organ baths where the preparations gravitated to the bottom. For anti-sense experiments thin strips of villus (about 20 mg) were prepared, vigorously rinsed with sterile phosphate buffered saline and then placed in 2 ml Dulbecco's modified Eagle's medium-Ham's F-12 (DMEM/F12; 24 well plates) medium containing 100 u ml−1 streptomycin and 100 u ml−1 penicillin. Two strips were placed in one well and maintained at 37°C in 5% CO2 for 24, 48 or 72 h in a humified atmosphere. Oligonucleotides (5 μM) were added at the start and remained for 24, 48 or 72 h. Culture medium was not exchanged during the incubation period.
Experimental protocol
For equilibration the preparations were superfused for 30 min at 37°C with 2.0 ml min−1 of warmed and oxygenated physiological salt solution (see above). Thereafter superfusion was stopped and the bath medium (1 ml) was exchanged in 10 min periods. The first three samples were discarded and the subsequent 10 samples were collected for measurement of acetylcholine release (t=0 – 100 min). The first five samples were obtained under control conditions, test substances being present from t=50 min of incubation onwards. At the end of the collection period the wet weight of the tissue was determined and the release of acetylcholine was related to 1 g. In some experiments the reversibility of the inhibitory effects of quinine and corticosterone was tested by washing the test substances out of the organ bath. Thus at t=100 min of incubation bath medium was exchanged five times by fresh salt solution in 1 min intervals and thereafter five or six additional samples were collected (see Figures 1 and 3).
Figure 1.
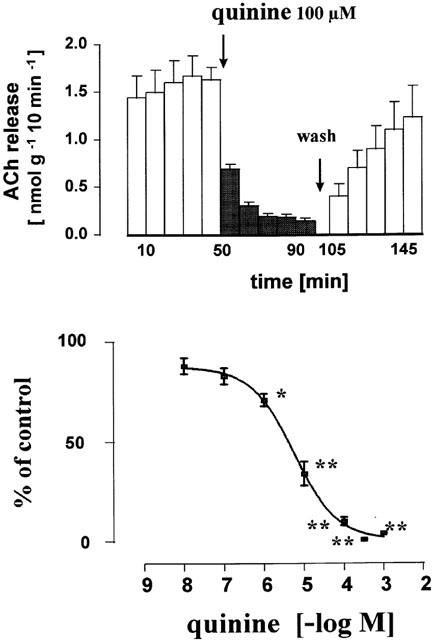
Inhibitory effect of quinine on the release of acetylcholine from the human placenta, villus region. Villus preparations were incubated (155 min) in organ baths to measure the release of acetylcholine in 10 min periods. Quinine was added from t=50 min onwards (filled columns). Upper part: effect of 100 μM quinine. At t=100 – 105 min the bath medium was exchanged five times and thereafter five subsequent 10 min samples were collected. Lower part: concentration response curve for quinine. Given are the mean±s.e.mean of 4 – 8 experiments. The individual control release B1 varied between 1.18±0.12 nmol−1 g−1 10 min−1 (n=7, experiments with 100 μM quinine) and 2.10±0.31 nmol−1 g−1 10 min−1 (n=4, experiments with 300 μM). Significance of differences from the control as shown in Figure 2 and Table 1: * P<0.05, ** P<0.01.
Figure 3.
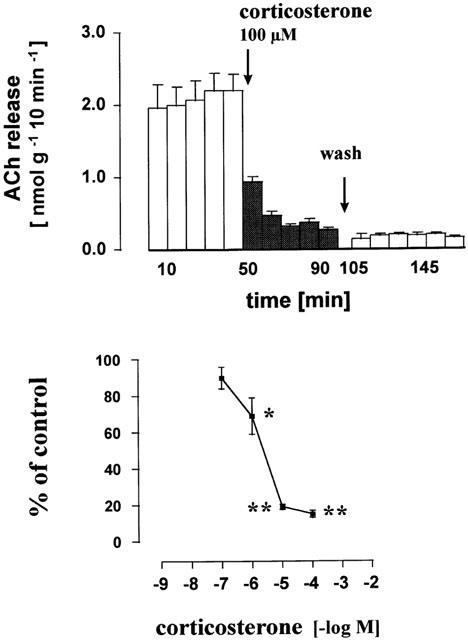
Inhibitory effect of corticosterone on the release of acetylcholine from the human placenta, villus region. Villus preparations were incubated (165 min) in organ baths to measure the release of acetylcholine in 10 min periods. Corticosterone was added from t=50 min onwards (filled columns). Upper part: effect of 100 μM corticosterone. At t=100 – 105 min the bath medium was exchanged five times and thereafter six subsequent 10 min samples were collected. Lower part: concentration response curve for corticosterone. Given are the mean±s.e.mean of 6 – 9 experiments. The individual control release B1 varied between 1.20±0.19 nmol−1 g−1 10 min−1 (n=9, experiments with 10 μM corticosterone) and 4.41±0.89 nmol−1 g−1 10 min−1 (n=6, experiments with 1 μM). Significance of differences from the control as shown in Figure 2 and Table 1: * P<0.05, ** P<0.01.
In long term experiments (culture conditions) villus preparations were virgorously washed with oxgenated salt solution at the end of the 24, 48 or 72 h incubation period and then placed in the organ bath as described above. The preparations were first superfused (30 min, see above) and thereafter incubated in 10 min periods in 1 ml salt solution. The first three samples were discarded. Acetylcholine release was measured in the subsequent three samples (t=0 – 30) and the mean of these samples was calculated.
Measurement of acetylcholine
Acetylcholine was measured by cationic exchange high-pressure liquid chromatography (HPLC) combined with bioreactors and electrochemical detection as described in detail previously (Reinheimer et al., 1996; Klapproth et al., 1997). A microbore HPLC system was used (BAS, Lafayette, U.S.A.). The specificity of the acetylcholine peak detected in the incubation medium was checked by using an analytical column packed with 40 U acetylcholinesterase (not shown). This application specifically eliminates the acetylcholine peak but the choline peak remains (Klapproth et al., 1997). Detection limit was 10 fmol acetylcholine per injection (20 μl).
Calculations and statistics
Results were expressed as mean value±s.e.mean of n experiments. The first five samples were collected under control conditions, test substances were added at t=50 min of incubation. The mean of the three samples before the application of test substances (t=20 – 50 min) was regarded as 100% (control release; B1, see Table 1). For the evaluation of the test substances the mean (B2) of the last three samples (maximum of the effect; see Figures 1 and 3) was related to B1. Several villus preparations could be isolated from a single placenta. Therefore, the number of placenta used in one series of experiments is additionaly indicated in Tables 1 and 2. Statistical analysis of the results was performed by Student's t-test (effect of anti-sense oligonucleotides; Table 2) or ANOVA using GraphPad Prism® (multiple use of single control).
Table 1.
The effect of test compounds on the acetylcholine release from human placenta, villus region
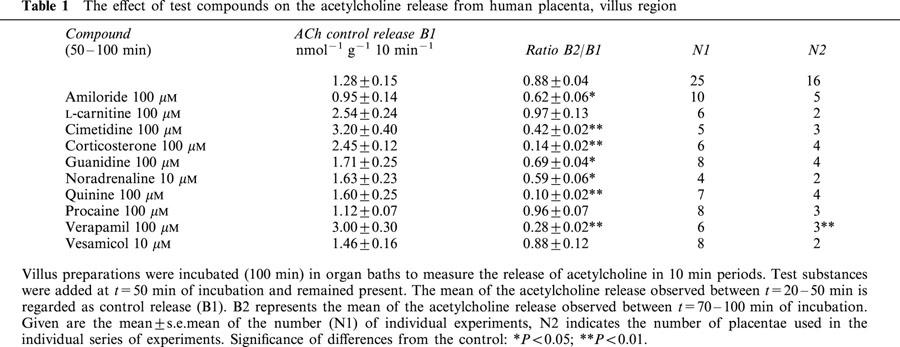
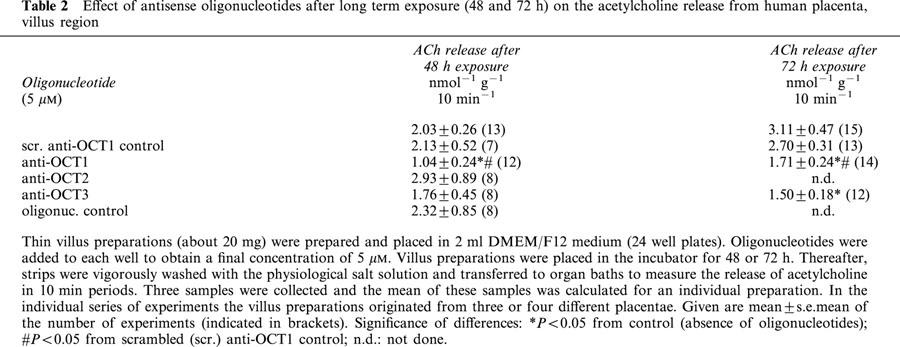
Table 2.
Effect of antisense oligonucleotides after long term exposure (48 and 72 h) on the acetylcholine release from human placenta, villus region
Drugs and special chemicals
The following compounds were purchased from Sigma Chemie, München, Germany: acetylcholine chloride, amiloride hydrochloride, L-carnitine hydrochloride; choline chloride, cimetidine, corticosterone, DMEM/F12 medium, guanidine hydrochloride, (±)-noradrenaline bitartrate, penicillin, procaine hydrochloride, quinine, streptomycin, (±)-verapamil hydrochloride, (±)-vesamicol hydrochloride. Corticosterone and vesamicol were dissolved in DMSO (dimethyl sulphoxide; final concentration 0.02%) which did not modify the basal acetylcholine release from the villus (n=4, not shown).
For the anti-sense experiments the following oligonucleotides (20) were used according to the published gene sequence (first coding exon) and purchased from MWG AG Biotech (München, Germany): anti-OCT1 (GGA-TGA-CAT-TCT-GGA-GCA-GG; cDNA sequence 796 – 815); anti-OCT1 scrambled control (GAG-GTG-GCT-GAG-TCG-AGA-TC); anti-OCT2 (GAT-CAT-GCC-CAC-CAC-CGT-GG; 4031 – 4050); anti-OCT3 (GCA-CCA-TGC-CCT-CCT-TCG-AC; 4691 – 4710); oligonucleotide control (randomized nucleotides: ATG-GAT-CGT-CGG-GTA-TCC-TA).
Results
Effect of quinine, procaine and vesamicol on the release of acetylcholine from human placenta
Under control conditions release of acetylcholine from the villus maintained at a constant rate. B1 amounted to 1.28 nmol−1 g−1 10 min−1 (n=25) and the ratio B2/B1 was 0.88±0.04 (Table 1; Figure 2). Quinine (100 μM) added at t=50 min of incubation caused a rapidly developing inhibition of acetylcholine release by 90% (Figure 1). Acetylcholine release gradually recovered after washing out quinine from the organ bath (Figure 1). The complete concentration response curve is shown in Figure 1; the IC50 value was 5 μM. Procaine (100 μM), an inhibitor of sodium channels, did not reduce acetylcholine release (Table 1). Also vesamicol, the inhibitor of the vesicular acetylcholine transporter failed to modify the acetylcholine release (Table 1).
Figure 2.
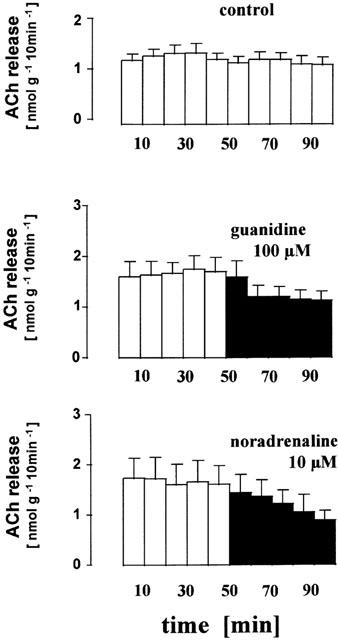
Inhibitory effect of guanidine and noradrenaline on acetylcholine release from the human placenta, villus region. Villus preparations were incubated (t=0 – 100 min) in organ baths to measure the release of acetylcholine in 10 min periods. Guanidine (100 μM) or noradrenaline (10 μM) were present from t=50 min onwards (filled columns). Given are the mean±s.e.mean of 25 (control), eight (guanidine) and four (noradrenaline) experiments.
Effects of substrate inhibitors of organic cation transporters on the release of acetylcholine
Organic cations (amiloride, cimetidine, verapamil) are known transport inhibitors of the OCTs. The three compounds (100 μM) all significantly reduced the release of acetylcholine (Table 1). Noradrenaline and guanidine are substrates of OCT1, 2 and 3, but not of two new members of the OCT-family, OCTN1 and OCTN2 (Gründemann et al., 1999; Ohashi et al., 1999; Wu et al., 1999). When noradrenaline or guanidine were applied, acetylcholine release declined (Table 1). For example, a stepwise reduction was observed in the presence of noradrenaline (Figure 2). In contrast, L-carnitine, a substrate of OCTN2, did not affect the release of non-neuronal acetylcholine (Table 1).
Effect of corticosterone on the release of acetylcholine
Corticosterone is a known inhibitor of OCT1, 2 and 3. In the present experiments corticosterone reduced the acetylcholine release from the human placenta. A steep concentration-response curve was obtained, the IC50 value being about 2 μM (Figure 3). In contrast to the inhibitory effect of the organic cation quinine the inhibitory effect of the lipophilic compound corticosterone remained even after a washout period of 65 min (Figure 3).
Effects of oligonucleotides on the release of acetylcholine
Anti-sense oligonucleotides (5 μM) directed against OCT1, 2 and 3 mRNA were exposed for 24, 48 and 72 h to villus specimens. In addition, a scrambled anti-OCT1 sequence and a randomized oligonucleotide control were also tested. After long term incubation (24, 48 or 72 h) the control release of acetylcholine was higher than the release obtained from freshly prepared villus preparations (compare control experiments in Tables 1 and 2; the control release after 24 h incubation was period 3.24±0.74 nmol−1 g−1 10 min−1, n=12). None of the anti-sense oligonucleotides caused a significant inhibition after an exposure time of 24 h (values not shown), but inhibitory effects were observed after longer incubation times. Anti-OCT1 oligonucleotides nearly halved acetylcholine release after exposure times of 48 or 72 h (Table 2). Anti-OCT3 oligonucleotides tended to decrease acetylcholine release after 48 h incubation, and a significant inhibition was observed after 72 h incubation period (Table 2). Anti-OCT2 oligonucleotides and both controls (scrambled anti-OCT1 and oligonucleotide control) did not affect the release of acetylcholine (Table 2).
Discussion
Acetylcholine, an organic molecule with a permanent positive net charge at physiological pH, is widely expressed in non-neuronal cells in humans and involved in the regulation of basic cell functions (Grando, 1997; Wessler et al., 1998; 1999; Kawashima & Fujii, 2000). In the present experiments the human placenta was used as a model to investigate the release mechanisms of non-neuronal acetylcholine. The following results demonstrate that acetylcholine, at least in the human placenta, is released into the extracellular space via organic cation transporters: (1) Quinine, an inhibitor of OCTs, caused a concentration-dependent and reversible inhibition. The IC50 value in the present experiments (5 μM) was more or less identical with the IC50 value obtained for the human cloned OCT1 (4.3 μM; Martel et al., 1996). Procaine, an inhibitor of sodium channels, was ineffective. Quinine is also an inhibitor of potassium channels which affects the membrane potential. However, such a mechanism cannot explain the inhibitory effect on acetylcholine release. It has recently shown that high extracellular potassium (54 and 108 mM) causes only a slight increase in the release of acetylcholine from villus preparations (Wessler et al., 2001). (2) Substrate inhibitors of OCTs like amiloride, cimetidine and verapamil reduced acetylcholine release. (3) Noradrenaline and guanidine, substrates for OCT1, 2 and 3, reduced acetylcholine release. (4) Corticosterone, an inhibitor of OCT1, 2 and 3 reduced the basal acetylcholine release with an IC50 value of 2 μM.
All these observations strongly indicate that non-neuronal acetylcholine leaves placental cells via OCTs, which are ubiquitously expressed in humans. This transport mechanism, at least in the human placenta, translocates acetylcholine into the extracellular space and therewith mediates the auto-/paracrine mode of action.
What subtype of transporter is involved in the release of non-neuronal acetylcholine? The present experiments favour the involvement of the OCT1 and OCT3 subtype for the following reasons: (1) The experiments with anti-sense oligonucleotides support the involvement of the OCT1 and OCT3 subtype. (2) The IC50 value estimated for quinine (5 μM) corresponds excellently with the IC50 value (4.3 μM) obtained in cells expressing OCT1 (Martel et al., 1996; Breidert et al., 1998). (3) Corticosterone inhibits OCT2 and OCT3 with IC50 values below 0.5 μM, whereas a concentration above 1 μM is required to establish an effect on OCT1 (Gründemann et al., 1998; Dresser et al., 1999; Breidert et al., 1998). In the present experiments, however, 1 μM corticosterone caused already 30% inhibition. This observation argues against a poor OCT1 role but corresponds with the additional involvement of OCT3. (4) Guanidine is an excellent substrate for OCT2, whereas its transport by OCT1 is low and that by OCT3 is negligible (Gründemann et al., 1999). In the present experiments a high concentration of 100 μM guanidine merely caused a moderate inhibition, which corresponds with the assumption of the involvement of OCT1 and 3 subtype. (5) Finally, OCTN1 and OCTN2 can be excluded because these transporters are not sensitive to monoamines but to carnitine (OCTN2). In the present study acetylcholine release was inhibited by noradrenaline but not by carnitine.
Taken together, i.e. the inhibitory effects of OCT1 and OCT3 antisense-oligonucleotides, the inhibitory potencies of quinine and corticosterone, the moderate effect of guanidine and the lack of effect with carnitine, the present results indicate that both OCT1 and OCT3 subtypes mediate the release of non-neuronal acetylcholine from the human placenta.
OCT1 represents a most widely expressed transporter, for example in the intestine, kidney, liver, brain, muscle fibres and immune cells (Dresser et al., 1999). In all these tissues or cells the non-neuronal cholinergic system has also been demonstrated (Grando, 1997; Wessler et al., 1998; 1999; Kawashima & Fujii, 2000). It is speculated here that the release mechanism identified in the placenta could represent a more general pathway for the release of acetylcholine, although the hypothesis has to be tested in future experiments. Nevertheless, this transport pathway may occur in the vast majority of human cells and allow non-neuronal acetylcholine to act in an auto-/paracrine manner. It should also be considered that the activity of OCTs is modulated by a variety of drugs and endogenous compounds, like monoamines and steroids which can target the release of non-neuronal acetylcholine under physiological conditions. Particularly, circulating catecholamines may affect the release of non-neuronal acetylcholine via substrate inhibition of OCTs. Thus, adrenergic-cholinergic interactions may also occur at the level of non-neuronal acetylcholine which opens a new view of a highly sophisticated cholinergic-adrenergic cell regulation. In addition, a significant number of clinically used drugs and xenobiotics are substrates of OCTs and therefore may interfere with the release of non-neuronal acetylcholine. For example, the atropine-like effect of quinine may be attributed to the inhibition of the release of non-neuronal acetylcholine.
In conclusion, the present experiments have shown that the release of non-neuronal acetylcholine from the human placenta is mediated via OCTs, most likely of the OCT1 and OCT3 subtype.
Acknowledgments
The present work contains parts of the MD thesis of E. Roth and C. Deutsch The work was supported by the Deutsche Forschungsgemeinschaft (Ki 210/9-1).
Abbreviations
- ChAT
choline acetyltransferase
- DMEM/F12
Dulbecco's modified Eagle's medium-Ham's F-12
- DMSO
dimethyl sulphoxide
- HPLC
high pressure liquid chromatography
- OCT
organic cation transporter
References
- BREIDERT T., SPITZENBERGER F., GRÜNDEMANN D., SCHÖMIG E. Catecholamine transport by the organic cation transporter 1 (OCT1) Br. J. Pharmacol. 1998;125:218–224. doi: 10.1038/sj.bjp.0702065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUNNER F., KUKOVETZ W.R. Muscarinic receptors of the vascular bed: radioligand binding studies on bovine splenic veins. J. Cardiovasc. Pharmacol. 1986;8:712–721. [PubMed] [Google Scholar]
- COSTA L.G., KAYLOR G., MURPHY D. Muscarinic cholinergic binding sites on rat lymphocytes. Immunopharmacol. 1988;16:139–149. doi: 10.1016/0162-3109(88)90002-1. [DOI] [PubMed] [Google Scholar]
- DRESSER M.J., ZHANG L., GIACOMINI K.M.Molecular and functional characteristics of cloned human organic cation transporters Membrane transporters as drug targets 1999New York: Kluwe Academic/Plenum Publishers; 441–469.eds. Amidon S., Sadée K. pp [DOI] [PubMed] [Google Scholar]
- GRANDO S.A. Biological functions of keratinocyte cholinergic receptors. J. Invest. Dermatol. Symp. Proc. 1997;2:41–48. doi: 10.1038/jidsymp.1997.10. [DOI] [PubMed] [Google Scholar]
- GRANDO S.A., ZELICKSON B.D., KIST D.A., WEINSHENKER D., BIGLIARDI P.L., WENDELSCHAFER-CRABB G., KENNEDY W.R., DAHL M.V. Keratinocyte muscarinic acetylcholine receptors: immunolocalization and partial characterization. J. Invest. Dermatol. 1995;104:95–100. doi: 10.1111/1523-1747.ep12613582. [DOI] [PubMed] [Google Scholar]
- GRÜNDEMANN D., GORBOULEV V., GAMBARYAN S., VEYHL M., KOEPSELL H. Drug excretion mediated by a new prototype of polyspecific transporter. Nature (Lond.) 1994;372:549–552. doi: 10.1038/372549a0. [DOI] [PubMed] [Google Scholar]
- GRÜNDEMANN D., BABIN-EBELL J., MARTEL F., ÖRDING N., SCHMIDT A., SCHÖMIG E. Primary structure and functional expression of the apical organic cation transporter from kidney epithelial LLC-PK1 Cells. J. Biol. Chem. 1997;272:10408–10413. doi: 10.1074/jbc.272.16.10408. [DOI] [PubMed] [Google Scholar]
- GRÜNDEMANN D., KÖSTER S., KIEFER N., BREIDERT T., ENGELHARDT M., SPITZENBERGER F., OBERMÜLLER N., SCHÖMIG E. Transport of monoamine transmitters by the organic cation transporter type 2, OCT2. J. Biol. Chem. 1998;272:30915–30920. doi: 10.1074/jbc.273.47.30915. [DOI] [PubMed] [Google Scholar]
- GRÜNDEMANN D., LIEBICH G., KIEFER N., KÖSTER S., SCHÖMIG E. Selective substrates for non-neuronal monoamine transporters. Mol. Pharmacol. 1999;56:1–10. doi: 10.1124/mol.56.1.1. [DOI] [PubMed] [Google Scholar]
- KAWASHIMA K., FUJII T. Extraneuronal cholinergic system in lymphocytes. Pharmacol. Ther. 2000;86:29–48. doi: 10.1016/s0163-7258(99)00071-6. [DOI] [PubMed] [Google Scholar]
- KEKUDA R., PRASAD P.D., WU X., WANG H., FEI Y.J., LEIBACH F., GANAPATHY V. Cloning and functional characterization of a potential-sensitive, polyspecific organic cation transporter (OCT3) most abundantly expressed in placenta. J. Biol. Chem. 1998;273:15971–15979. doi: 10.1074/jbc.273.26.15971. [DOI] [PubMed] [Google Scholar]
- KLAPPROTH H., REINHEIMER T., METZEN J., MÜNCH M., BITTINGER F., KIRKPATRICK C.J., HÖHLE K.-D., SCHEMANN M., RACKÉ K., WESSLER I. Non-neuronal acetylcholine, a signalling molecule synthesized by surface cells of rat and man. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;355:515–523. doi: 10.1007/pl00004977. [DOI] [PubMed] [Google Scholar]
- KOEPSELL H. Organic cation transporters in intestine, kidney, liver and brain. Annu. Rev. Physiol. 1998;60:243–266. doi: 10.1146/annurev.physiol.60.1.243. [DOI] [PubMed] [Google Scholar]
- MARTEL F., VETTER T., RUSS H., GRÜNDEMANN D., AZEVEDO I., KOEPSELL H., SCHÖMIG E. Transport of small organic cations in the rat liver. Naunyn-Schmiedeberg's Arch. Pharmacol. 1996;354:320–326. doi: 10.1007/BF00171063. [DOI] [PubMed] [Google Scholar]
- MAUS A.D.J., PEREIRA E.F.R., KARACHUNSKI P.I., HORTON R.M., NAVANEETHAM D., MACKLIN K., CORTES W.S., ALBUQUERQUE E.X., CONTI-FINE B.M. Human and rodent bronchial epithelial cells express functional nicotinic acetylcholine receptors. Mol. Pharmacol. 1998;54:779–788. doi: 10.1124/mol.54.5.779. [DOI] [PubMed] [Google Scholar]
- NAGEL G., VOLK C., FRIEDRICH T., ULZHEIMER J.C., BAMBERG E., KOEPSELL H. A reevaluation of substrate specificity of the rat cation transporter rOCT1. J. Biol. Chem. 1997;272:31953–31956. doi: 10.1074/jbc.272.51.31953. [DOI] [PubMed] [Google Scholar]
- OKUDA M., SAITO H., URAKAMI Y., TAKANO M., INUI K. CDNA cloning and functional expression of a novel rat kidney organic cation transporter, OCT2. Biochem. Biophys. Res. Commun. 1996;224:500–507. doi: 10.1006/bbrc.1996.1056. [DOI] [PubMed] [Google Scholar]
- OHASHI R., TAMAI I., YABUUCHI H., NEZU J.I., OKU A., SAI Y., SHIMANE M., TSUJI A. NA+-dependent carnitine transport by organic cation transporter (OCTN2): its pharmacolgical and toxicological relevance. J. Pharmacol. Expt. Ther. 1999;291:778–784. [PubMed] [Google Scholar]
- REINHEIMER T., BERNEDO P., KLAPPROTH H., OELERT H., ZEISKE B., RACKÉ K., WESSLER I. Acetylcholine in isolated airways of rat, guinea-pig, and human: species differences in the role of airway mucosa. Am. J. Physiol. 1996;270:L722–L728. doi: 10.1152/ajplung.1996.270.5.L722. [DOI] [PubMed] [Google Scholar]
- SASTRY B.V.R. Human placental cholinergic system. Biochem. Pharmacol. 1997;53:1577–1586. doi: 10.1016/s0006-2952(97)00017-8. [DOI] [PubMed] [Google Scholar]
- SASTRY B.V.R., SADAVONGVIVAD C. Cholinergic systems in non-nervous tissues. Pharmacol. Rev. 1979;30:65–132. [PubMed] [Google Scholar]
- WESSLER I., KIRKPATRICK C.J., RACKÉ K. Non-neuronal acetylcholine, a locally acting molecule widely distributed in biological systems: expression and function in humans. Pharmacol. Ther. 1998;77:59–79. doi: 10.1016/s0163-7258(97)00085-5. [DOI] [PubMed] [Google Scholar]
- WESSLER I., KIRKPATRICK C.J., RACKÉ K. The cholinergic pitfall: acetylcholine, a universal cell molecule in biological systems including humans. Clin. Exp. Pharmacol. Physiol. 1999;26:198–205. doi: 10.1046/j.1440-1681.1999.03016.x. [DOI] [PubMed] [Google Scholar]
- WESSLER I., ROTH E., SCHWARZE S., WEIKEL W., BITTINGER F., KIRKPATRICK C.J., KILBINGER H. Release of non-neuronal acetylcholine from the human placenta: difference to neuronal acetylcholine. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;364:205–212. doi: 10.1007/s002100100445. [DOI] [PubMed] [Google Scholar]
- WU X., KEKUDA R., HUANG W., FEI Y.J., LEIBACH F.H., CHEN J., CONWAY S.J., GANAPATHY V. Identity of the organic cation transporter OCT3 as the extraneuronal monoamine transporter (uptake 2) and the evidence for the expression of the transporter in the brain. J. Biol. Chem. 1998;273:32776–32786. doi: 10.1074/jbc.273.49.32776. [DOI] [PubMed] [Google Scholar]
- WU X., HUANG W., PRASAD P.D., SETH P., RAJAN D.P., LEIBACH F.H., CHEN J., CONWAY S.J., GANAPATHY V. Functional characteristics and tissue distribution pattern of organic cation transporter 2 (OCTN2), an organic cation/carnitine transporter. J. Pharmacol. Expt. Ther. 1999;290:1482–1492. [PubMed] [Google Scholar]


