Abstract
The effects of phosphodiesterase (PDE)4 and TNF-α inhibition were assessed on the local and remote injuries following intestinal ischaemia and reperfusion (I/R) injury in rats.
The PDE4 inhibitor rolipram dose-dependently (1 – 10 mg kg−1) suppressed the local (intestine) and remote (lung) increases in vascular permeability and neutrophil recruitment following mild I/R injury. SB207499 (ariflo), a structurally-distinct PDE4 inhibitor, also suppressed the injuries following mild I/R injury.
In a severe model of I/R injury, treatment with rolipram (10 mg kg−1) partially reversed the local and remote increases in vascular permeability, neutrophil recruitment, intestinal haemorrhage and intestinal LTB4 concentrations. The anti-TNF-α anti-serum was more effective than rolipram at inhibiting local and remote injuries and prevented the lethality associated with severe I/R.
Rolipram and anti-TNF-α prevented the increase in the concentrations of TNF-α in the lung and intestine, but rolipram only partially inhibited the elevation of this cytokine in serum. Rolipram had little effect on the increases of IL-1ß concentrations in lung and serum, whereas treatment with anti-TNF-α markedly increased the concentration of this cytokine. Concentrations of IL-10 rose significantly in the lung and serum and these increases were blocked by rolipram or anti-TNF-α.
The capacity of PDE4 inhibitors to block the recruitment of neutrophils into tissues, the production of LTB4 and of the pro-inflammatory cytokines TNF-α, IL-1ß and IL-6 appear to underlie their anti-inflammatory effects in our model of I/R injury. Overall, PDE4 inhibition was less effective than inhibition of TNF-α for protection against I/R injury.
Keywords: PDE4 inhibitors, TNF-α, rolipram, ischaemia and reperfusion injury, IL-10, IL-1ß
Introduction
Drugs which inhibit phosphodiesterase type 4 (PDE4) enzymes have received a great deal of attention in the last few years because of their inhibitory effects in various models of acute and chronic inflammation (Teixeira et al., 1997; Torphy, 1998; Souness et al., 2000). PDE4 inhibitors work via the elevation of intracellular cyclic AMP, which then activates protein kinase A with subsequent phosphorylation of protein kinase A-specific substrates (Souness et al., 2000). In vivo, there are several possible mechanisms which appear to be involved in the anti-inflammatory actions of PDE4 inhibitors, including direct inhibition of leukocyte recruitment, inhibition of leukocyte activation, inhibition of cytokine production (especially TNF-α) and enhancement of the production of anti-inflammatory cytokines (especially IL-10) (Teixeira et al., 1997; Souness et al., 2000). The capacity of PDE4 inhibitors to suppress TNF-α production and enhance IL-10 production may be beneficial in the treatment of acute inflammatory conditions where these cytokines appear to play a major role. For example, blockade of TNF-α or administration of IL-10 have been shown to limit the inflammatory injuries following reperfusion of several ischaemic tissues (Gilmont et al., 1996; Frangogiannis et al., 2000; Huber et al., 2000).
We have recently described the local and remote reperfusion injuries which occur following ischaemia of the superior mesenteric artery (SMA) in rats (Souza et al., 2000a,2000b). Following ischaemia and reperfusion (I/R) of the SMA, we observed significant local (intestine and mesentery) and remote (lung) oedema and neutrophil accumulation, and marked systemic alterations which included hypotension, neutropaenia and death, especially after more prolonged I/R periods (Souza et al., 2000a,2000b). Also, we detected significant TNF-α production in tissues and serum after reperfusion of the ischaemic SMA (Souza et al., 2000b). A number of studies have described the beneficial effects of the inhibition of phosphodiesterases in models of I/R injury. Nevertheless, most published studies have been conducted using ex-vivo I/R and transplant models (Chang-Chun et al., 1991; Barnard et al., 1994; Bleiweis et al., 1999; Featherstone et al., 1999) or have used non-specific phosphodiesterase inhibitors, such as pentoxifylline or theophylline (Carter et al., 1995; Peng et al., 1995; Müller et al., 1997). Thus, a detailed analysis of the effects of PDE4 inhibitors in models of I/R in vivo is warranted.
Here, we have assessed the effects of PDE4 inhibition on the mild and severe reperfusion injuries following ischaemia of the superior mesenteric artery. Initial experiments evaluated the dose-dependent effects of the PDE4 inhibitors rolipram and SB207499 on the mild I/R model. We then investigated the effects of rolipram in the more severe I/R model with particular emphasis on the effects of this drug on lethality, systemic injuries and cytokine (IL-1ß, IL-6, IL-10 and TNF-α) concentrations in tissue and serum. Since inhibition of TNF-α production may underlie some of the anti-inflammatory actions of PDE4 inhibitors in vivo (Teixeira et al., 1997; Souness et al., 2000), we also evaluated the effects of an anti-TNF-α antiserum on the injuries following severe I/R.
Methods
Animals
Male Wistar rats (200 – 220 g) obtained from the Bioscience unit of our Institution were housed in standard conditions and had free access to commercial chow and water. All procedures described here had prior approval from local animal ethics committee.
Ischaemia and reperfusion injury
Rats were anaesthetized with urethane (140 mg kg−1, ip.) and laparotomy was performed. The superior mesenteric artery (SMA) was isolated and ischaemia was induced by totally occluding the SMA for 30 or 120 min. After ischaemia, reperfusion was initiated by removal of the occlusion. Animals made ischaemic for 30 or 120 min were allowed to reperfuse for 30 (mild I/R) or 120 (severe I/R) min, respectively. The durations of I/R were based upon previous experiments (Souza et al., 2000a,2000b) and were optimal for mild and severe reperfusion injuries. Sham-operated animals or animals only made ischaemic were used as controls for the reperfusion-induced injury.
Initial dose-response experiments were carried out in the mild reperfusion injury model to determine the optimal dose of the PDE4 inhibitor, rolipram, to be used in subsequent experiments. In these experiments, rolipram was administered s.c. in two equally divided doses at 60 min and 15 min prior to reperfusion of the superior mesenteric artery. Total administered doses of rolipram were 1 – 10 mg kg−1. Because of its short half-life, rolipram was given in two doses to guarantee sufficient circulating levels of the drug during the whole experiment (Teixeira et al., 1994). For comparison, we also tested the effects of the PDE4 inhibitor SB207499 (0.5 – 5.0 mg kg−1) administered s.c. 15 min prior to reperfusion. We then tested the effects of the administration of rolipram (10 mg kg−1, s.c. in two equally divided doses at 60 min and 15 min prior to reperfusion) in the more severe I/R model. None of the drugs used in the present study had any significant effects on basal parameters (data not shown) and to simplify the graphs presented, basal data obtained in vehicle or drug treated animals have been pooled for presentation.
Anti-TNF-α polyclonal antibodies were raised in sheep as previously described (Rees et al., 1999b). Hyperimmune anti-TNF-α antiserum was administered s.c. 60 min prior to reperfusion. Control animals received non-immune sheep serum. As non-immune serum or vehicle had no effect on injuries following reperfusion of the ischaemic SMA (data not shown), results in non-immune- and vehicle-treated animals were pooled for presentation.
Evaluation of changes in vascular permeability
The extravasation of Evans blue dye into the tissue was used as an index of increased vascular permeability (de matos et al., 1999). Evans blue (20 mg kg−1) was administered i.v. (1 ml kg−1) via a femoral vein 2 min prior to reperfusion of the ischaemic artery. Thirty min (in the mild model) or 120 min (in the severe model) after reperfusion, segments of the duodenum (10 cm) were cut open and allowed to dry in a petri dish for 24 h at 37°C. The dry weight of the tissue was calculated and Evans blue extracted using 3 ml of formamide (24 h at room temperature). The amount of Evans blue in the tissue was obtained by comparing the extracted absorbance with that of a standard Evans blue curve read at 620 nm in an ELISA plate reader. Results are presented as the amount of Evans blue per μg per 100 mg of tissue. The mesentery was also extracted en bloc, halved and a similar extraction procedure was performed. The right ventricle was flushed with 20 ml of phosphate buffered saline to wash the intravascular Evans blue in the lungs. The left lung was then excised and used for Evans blue extraction. The right lung was used for the determination of myeloperoxidase as described below.
Myeloperoxidase concentrations
The extent of neutrophil accumulation in the intestine, mesentery and right lung tissue was measured by assaying myeloperoxidase activity as previously described (de matos et al., 1999). Briefly, a portion of duodenum, half the mesentery and the flushed right lungs of animals that had undergone I/R injury were removed and snap frozen in liquid nitrogen. Upon thawing, the tissue (1 g of tissue per 19 ml of buffer) was homogenized in pH 4.7 buffer (NaCl 0.1 M, NaPO4 0.02 M, NaEDTA 0.015 M), centrifuged at 260 × g for 10 min and the pellet subjected to hypotonic lysis (15 ml of 0.2% NaCl solution followed 30 s later by addition of an equal volume of a solution containing NaCl 1.6% and glucose 5%). After a further centrifugation, the pellet was resuspended in 0.05 M NaPO4 buffer (pH 5.4) containing 0.5% hexadecyltrimethylammonium bromide (HTAB) and re-homogenized. One ml aliquots of the suspension were transferred into 1.5 ml-Eppendorf tubes followed by three freeze-thaw cycles using liquid nitrogen. The aliquots were then centrifuged for 15 min at 10,000 × g, the pellet was resuspended to 1 ml and samples of intestine, mesentery and lung were diluted prior to assay. Myeloperoxidase activity in the resuspended pellet was assayed by measuring the change in optical density (O.D.) at 450 nm using tetramethylbenzidine (1.6 mM) and H2O2 (0.5 mM). Results were expressed as total number of neutrophils by comparing the O.D. of tissue supernatant with the O.D. of rat peritoneal neutrophils processed in the same way. To this end, neutrophils were induced in the peritoneum of rats by injecting 3 ml of casein 5%. A standard curve of neutrophil (> 95% purity) numbers versus O.D. was obtained by processing purified neutrophils as above and assaying for MPO activity.
Determination of the concentrations of circulating leukocytes
The total number of circulating leukocytes and neutrophils were evaluated in blood samples obtained via a cannula in the femoral artery. Samples were collected prior to ischaemia (time 0), 120 min after ischaemia and 30 and 120 min after reperfusion. The number of total circulating leukocytes was determined by counting leukocytes in a modified Neubauer chamber after staining with Turk's solution and differential counts by evaluating the percentage of each leukocyte on blood films stained with May-Grunwald-Giemsa.
Measurement of haemoglobin concentrations
The determination of the concentrations of haemoglobin in tissue was used as an index of tissue haemorrhage. After washing and perfusing the intestines to remove excess blood in the intravascular space, a sample of approximately 100 mg of duodenum was removed and homogenized in Drabkin's colour reagent according to instructions of the manufacturer (Analisa, Belo Horizonte, Brazil). The suspension was centrifuged for 15 min at 3000 × g and filtered using 0.2 μm filters. The resulting solution was read using an ELISA plate reader at 520 nm and compared against a standard curve of haemoglobin.
Measurement of cytokine concentrations in serum, intestine and lungs
TNF-α, IL-1ß, IL-6 and IL-10 concentrations were measured in serum and intestine of animals using ELISA techniques previously described (Rees et al., 1999a,1999b; Hagan et al., 1993; Francischi et al., 2000). Serum was obtained from coagulated blood (15 min at 37°C, then 30 min at 4°C) and stored at −20°C until further analysis. Serum samples were analysed at a 1 : 1 dilution in PBS. One hundred mg of duodenum or lung of sham-operated and reperfused animals were homogenized in 1 ml of PBS (NaCl 0.4 m and NaPO4 10 mM) containing anti-proteases (PMSF 0.1 mM, benzethonium chloride 0.1 mM, EDTA 10 mM and 20 KI aprotinin A) and 0.05% Tween 20. The samples were then centrifuged for 10 min at 3000 × g and the supernatant immediately used for ELISA assays at a 1 : 5 dilution in PBS. ELISA plates (Nunc MaxiSorb) were coated with sheep anti-rat TNF-α/IL-1ß/IL-6 or IL-10 polyclonal antibodies (1 – 2 μg ml−1) overnight. The plates were washed thrice and then blocked with 1% bovine serum albumin. After a further wash, plates were incubated with samples or recombinant rat cytokine and incubated overnight. The biotinylated polyclonal antibodies were used at a 1 : 1000 to 1 : 2000 dilution and the assays had a sensitivity of 16 pg ml−1.
Drugs and reagents
The following drugs were obtained from Sigma (U.S.A.): urethane, Evans blue, fucoidin, hexadecyltrimethylammonium bromide. Rolipram was purchased from Calbiochem (U.S.A.) and SB207499 (Ariflo, Barnette et al., 1998) was a gift of Chiroscience Limited (Cambridge).
Statistical analysis
Results are shown as means±s.e.mean. Per cent inhibition was calculated by subtracting the background concentrations of Evans blue extravasation or myeloperoxidase (obtained in sham-operated animals) from control and treated animals. Differences were compared by using analysis of variance (ANOVA) followed by Student-Newman-Keuls post-hoc analysis. Results with a P<0.05 were considered significant.
Results
Dose-dependent effects of PDE4 inhibitors in a mild model of I/R injury
Initial experiments were carried out in the mild model of I/R to assess the ideal doses of rolipram to use in further experiments. Systemic treatment of animals with rolipram (1 – 10 mg kg−1) induced a dose-dependent inhibition of the vascular permeability changes in the intestine and lung of reperfused animals (Figure 1). At 10 mg kg−1 rolipram the increase in vascular permeability in the intestine and lungs were inhibited by 95 and 100%, respectively (Figure 1A,C). Similarly, rolipram inhibited in a dose-dependent manner the accumulation of neutrophils, as assessed by concentrations of MPO, in the intestine and lungs after mild I/R (Figure 1C,D). Maximal inhibition of neutrophil accumulation in the intestine and lungs occurred at 10 mg kg−1 and reached 93 and 97%, respectively (Figure 1). For comparison, experiments were also carried out with a newer generation, structurally distinct PDE4 inhibitor, SB207499. Similarly to rolipram, SB207499 dose-dependently inhibited both the increase in vascular permeability and accumulation of neutrophils following mild I/R injury (Table 1). Maximum inhibition occurred in most cases with a dose of 1.5 mg kg−1 of SB207499. In all further experiments, rolipram was used at the dose of 10 mg kg−1.
Figure 1.
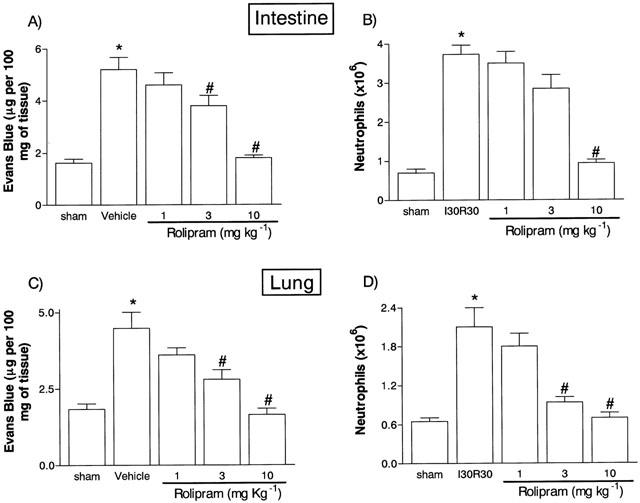
Dose-dependent effects of the treatment with rolipram on the increase in vascular permeability and recruitment of neutrophils in the intestine (A,B) and lung (C,D) following mild ischaemia (30 min) and reperfusion (30 min) of the superior mesenteric artery. Changes in vascular permeability (A,C) were assessed by evaluating the extravasation of Evans blue dye and neutrophil recruitment (B,D) was assessed by evaluating the tissue levels of myeloperoxidase. Rolipram (1 – 10 mg kg−1) was given in two equally divided doses s.c. 60 and 15 min prior to reperfusion. Control animals (Vehicle) received phosphate buffered saline. Results are shown as μg Evans blue or as number of neutrophils per 100 mg of tissue and are the mean±s.e.mean of 4 – 6 animals in each group. *P<0.01 when compared to sham-operated animals and #P<0.05 when compared to mild I/R animals.
Table 1.
Dose-dependent effects of SB207499 on the injuries to the intestine and lung following mild ischaemia (30 min) and reperfusion (30 min) of the superior mesenteric artery

Comparative effects of the treatment with rolipram or an anti-TNF-α serum on the local and remote tissue injuries in a model of severe I/R injury
The next series of experiments was carried out in a model of severe I/R injury in which, in addition to changes in vascular permeability and neutrophil accumulation, we could observe tissue haemorrhage, leucopoenia, changes in cytokine concentrations in tissue and blood and lethality (Souza et al., 2000b). The treatment with rolipram, 45 min prior to reperfusion virtually abolished the increase in vascular permeability in the intestine and lungs following severe I/R injury (Figure 2). Likewise, rolipram inhibited the accumulation of neutrophils in the intestine and lungs by 60 and 80%, respectively, following severe I/R injury (Figure 2). Intestinal haemorrhage, as assessed by the extravasation of haemoglobin, was inhibited by 60% in rolipram-treated animals (Figure 3). For comparison, post-ischaemic treatment with an anti-TNF-α polyclonal antiserum inhibited by at least 90% the increase in vascular permeability (Figure 2) and neutrophil accumulation (Figure 2) in intestine and lungs and the intestinal haemorrhage (Figure 3) following severe I/R injury.
Figure 2.
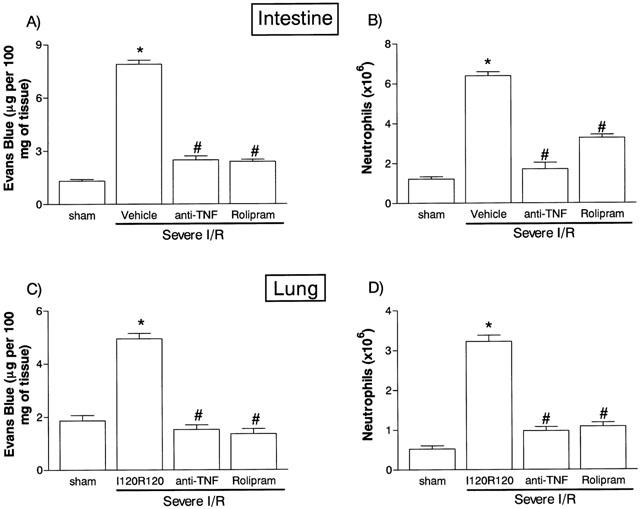
Effects of the treatment with rolipram or anti-TNF-α on the increase in vascular permeability and recruitment of neutrophils in the intestine (A,B) and lung (C,D) following severe ischaemia (120 min) and reperfusion (120 min) of the superior mesenteric artery. Changes in vascular permeability (A,C) were assessed by evaluating the extravasation of Evans blue dye and neutrophil recruitment (B,D) was assessed by evaluating the tissue levels of myeloperoxidase. Rolipram (10 mg kg−1) was given s.c. in two equally divided doses 60 and 15 min prior to reperfusion and anti-TNF-α antiserum (5 μl g−1) was given s.c. 60 min prior to reperfusion. Control animals (Vehicle) received phosphate buffered saline or control sheep serum (results in control animals are pooled for presentation). Results are shown as μg Evans blue or as number of neutrophils per 100 mg of tissue and are the mean±s.e.mean of five animals in each group. *P<0.01 when compared to sham-operated animals and #P<0.01 when compared to mild I/R animals.
Figure 3.
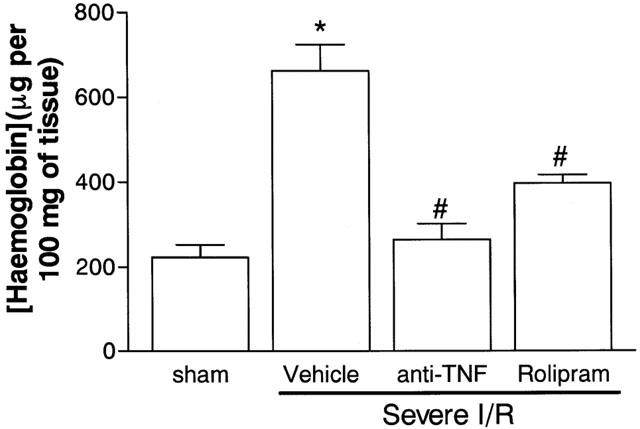
Effects of the treatment with rolipram or anti-TNF-α on the haemorrhage observed in the intestine following severe ischaemia (120 min) and reperfusion (120 min) of the superior mesenteric artery. Tissue haemorrhage was assessed by evaluating the tissue levels of haemoglobin. Rolipram (10 mg kg−1) was given s.c. in two equally divided doses 60 and 15 min prior to reperfusion and anti-TNF-α antiserum (5 μl g−1) was given s.c. 60 min prior to reperfusion. Control animals (Vehicle) received phosphate buffered saline or control sheep serum (results in control animals are pooled for presentation). Results are shown as μg haemoglobin per 100 mg of tissue and are the mean±s.e.mean of five animals in each group. *P<0.01 when compared to sham-operated animals and #P<0.01 when compared to severe I/R animals.
We have previously shown an important role for LTB4 after mild (Souza et al., 2000a) and severe (unpublished results) I/R injury of the SMA artery. Thus, the concentrations of LTB4 in the intestine were measured in control and treated animals following severe I/R injury. There was a significant increase in LTB4 concentrations in the intestine after severe I/R injury (basal, below detection limit; severe I/R 56.1±6.9 pg per 100 mg of intestine, n=5). Treatment with rolipram or anti-TNF-α inhibited the increase in LTB4 concentrations by 78±9.4% and 95±2.5% (n=4 – 5 in each group, P<0.01), respectively.
Comparative effects of the treatment with rolipram or anti-TNF-α on the concentrations of circulating neutrophils in a model of severe I/R injury
In agreement with our previous studies (Souza et al., 2000b), prolonged ischaemia of SMA was accompanied by significant neutrophilia which dropped rapidly and persistently to below background concentrations after reperfusion (Figure 4). In rolipram- and anti-TNF-α-treated animals, the neutrophilia following ischaemia was not different from control animals (Figure 4), as these treatments were administered at the end of the ischaemic period. Rolipram partially reversed the neutropenia at 120 min but not 15 min after the onset of reperfusion (Figure 4). Treatment with anti-TNF-α partially reversed the fall in neutrophil concentrations both early and late after reperfusion and the effects of anti-TNF-α were more pronounced than those of rolipram (Figure 4).
Figure 4.
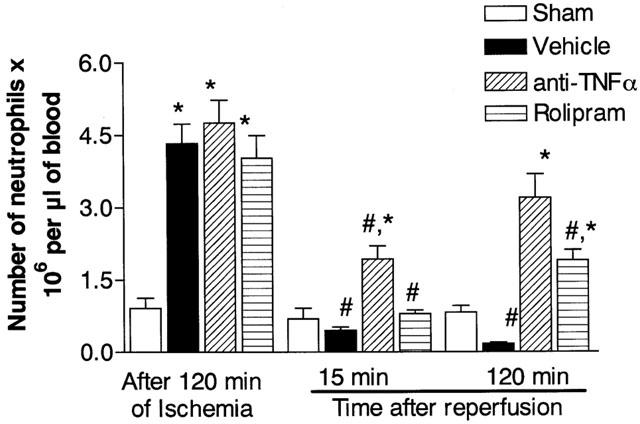
Effects of the treatment with rolipram or anti-TNF-α on the concentration of circulating neutrophils following severe ischaemia (120 min) and reperfusion (120 min) of the superior mesenteric artery. Neutrophils were counted in May-Grunwald-Giemsa-stained blood smears just prior to and at 15 and 120 min after reperfusion. Rolipram (10 mg kg−1) was given s.c. in two equally divided doses 60 and 15 min prior to reperfusion and anti-TNF-α antiserum (5 μl g−1) was given s.c. 60 min prior to reperfusion. Control animals (Vehicle) received phosphate buffered saline or control sheep serum (results in control animals are pooled for presentation). Results are shown as the number of neutrophils×106 per μl of blood and are the mean±s.e.mean of five animals in each group. *P<0.01 when compared to sham-operated animals and #P<0.05 when compared to severe I/R animals.
Comparative effects of the treatment with rolipram or anti-TNF-α on the lethality and cytokine concentrations following severe I/R injury
Figure 5 shows the survival curve of animals after the onset of reperfusion. Of note, animals began to die from around 30 min after reperfusion and approximately 50% were dead by 120 min (Figure 5). Under the same conditions, sham-operated animals did not die. Treatment with rolipram had no significant effect on lethality whereas anti-TNF-α treatment was 100% effective in preventing lethality following severe I/R injury (Figure 5).
Figure 5.

Effects of the treatment with rolipram or anti-TNF-α on the lethality following severe ischaemia (120 min) of the superior mesenteric artery. Rolipram (10 mg kg−1) was given s.c. in two equally divided doses 60 and 15 min prior to reperfusion and anti-TNF-α antiserum (5 μl g−1) was given s.c. 60 min prior to reperfusion. Control animals (Vehicle) received phosphate buffered saline or control sheep serum (results in control animals are pooled for presentation). Results show the number of surviving animals at different times after reperfusion in sham-operated animals (sham, n=5), vehicle- (n=12), rolipram- (n=14), anti-TNF-α- (n=5) treated animals.
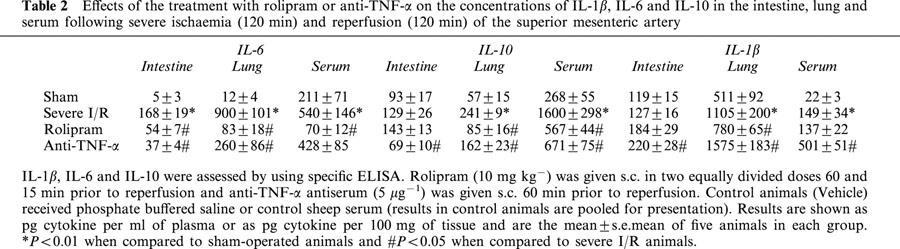
Cytokine concentrations were measured in serum and tissues at the end of the reperfusion period. Both rolipram and anti-TNF-α treatment abrogated the elevation in TNF-α concentrations in intestine and lungs (Figure 6A,B). Anti-TNF-α additionally inhibited by 98% the TNF-α concentrations in serum, whereas these were inhibited by only 57% by rolipram (Figure 6C). IL-6 concentrations in tissues were also markedly inhibited by rolipram or anti-TNF-α, whereas the concentrations of this cytokine in serum was suppressed by rolipram, but not anti-TNF-α (Table 2). There was only a slight increase of IL-10 in the intestine and this increase was blocked by anti-TNF-α, but not rolipram (Table 2). In contrast, IL-10 concentrations were greatly elevated in the lungs and, especially, in serum after severe I/R injury. Both anti-TNF-α and rolipram inhibited the increases in IL-10 concentrations to a similar extent (Table 2). IL-1ß concentrations rose only in the lung and serum after severe I/R injury and treatment with rolipram had no significant effect on the increases observed (Table 2). Surprisingly, treatment with anti-TNF-α enhanced the concentrations of IL-1ß in the intestine, lung and serum after severe I/R injury (Table 2). Of note, IL-1ß in serum of anti-TNF-α-treated animals was at least 20 and three times greater than that found in sham-operated and vehicle-treated severe I/R animals, respectively (Table 2).
Figure 6.
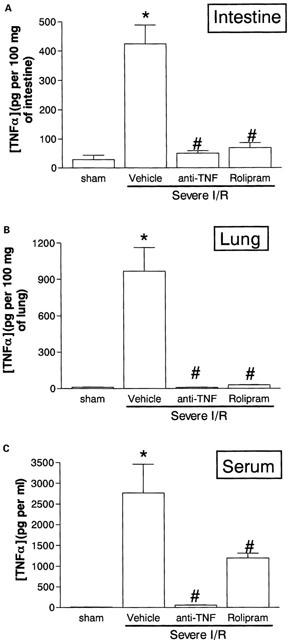
Effects of the treatment with rolipram or anti-TNF-α on the concentrations of TNF-α in the (A) intestine, (B) lung and (C) serum following severe ischaemia (120 min) and reperfusion (120 min) of the superior mesenteric artery. TNF-α was assessed by using a specific ELISA. Rolipram (10 mg kg−1) was given s.c. in two equally divided doses 60 and 15 min prior to reperfusion and anti-TNF-α antiserum (5 μl g−1) was given s.c. 60 min prior to reperfusion. Control animals (Vehicle) received phosphate buffered saline or control sheep serum (results in control animals are pooled for presentation). Results are shown as pg TNF-α per ml of plasma or as pg TNF-α per 100 mg of tissue and are the mean±s.e.mean of five animals in each group. *P<0.01 when compared to sham-operated animals and #P<0.05 when compared to severe I/R animals.
Table 2.
Effects of the treatment with rolipram or anti-TNF-α on the concentrations of IL-1β, IL-6 and IL-10 in the intestine, lung and serum following severe ischaemia (120 min) and reperfusion (120 min) of the superior mesenteric artery
Discussion
The restoration of blood flow of an ischaemic vascular bed, i.e. reperfusion, is a major therapeutic objective following ischaemia of an organ or tissue (Willerson, 1997). However, the reperfusion of an ischaemic bed may lead to inflammation locally and systemically, limiting the potential benefits of blood flow restoration. Thus, strategies which limit the injury caused by the reperfusion process may be a useful adjunct in the treatment of acute ischaemic disorders in various organs (Willerson, 1997). Here, we evaluate the effects of treatment with an important class of anti-inflammatory drugs, PDE4 inhibitors, on the local and systemic injuries following reperfusion of the ischaemic SMA in rats.
Initial experiments were carried out in a mild model of I/R injury. Our results clearly show that the prototypic PDE4 inhibitor rolipram inhibited, in a dose-dependent manner, both the local (intestine) and remote (lung) increase in vascular permeability and neutrophil accumulation observed after mild I/R injury. A structurally-unrelated newer generation PDE4 inhibitor, SB207499 (Barnette et al., 1998), also inhibited, in a dose-dependent manner, the I/R injuries observed in the mild model. SB207499 was as effective as and 3 – 5 times more potent than rolipram. In addition, rolipram markedly inhibited the increase in vascular permeability and neutrophil accumulation in the severe model of I/R injury. In the severe model, the inhibition of neutrophil recruitment into tissues was reflected by the partial capacity of rolipram treatment to reverse the leucopoenia observed during reperfusion. Also, intestinal haemorrhage and histopathological damage observed after severe I/R injury were significantly attenuated by rolipram. These observations are in line with previous studies demonstrating the capacity of PDE4 inhibitors to suppress oedema formation and leukocyte recruitment and activation in acute models of inflammation (reviewed in Teixeira et al., 1997; Souness et al., 2000). As the local influx of neutrophils is a determinant in the development of reperfusion injury following mild or severe ischaemia (Souza et al., 2000a,2000b), the capacity of rolipram to modulate the recruitment of this cell type may underlie most of the beneficial effects of the compound in our models. Previous studies using an ex vivo approach have suggested the potential benefit of PDE4 inhibitors in the treatment of I/R injuries in various models, including the heart (Barnard et al., 1994; Bleiweis et al., 1999). However, to our knowledge only one other study has previously shown a beneficial effect of treatment with PDE4 inhibitors, in an in vivo model of I/R injury (Kato et al., 1995). Thus, it appears that inhibition of PDE4 enzymes is a valid principle in the treatment of I/R injury in diverse organs.
Previously we have shown that LTB4 is an important mediator of the local and remote injuries following mild I/R (Souza et al., 2000a). Antagonists of LTB4 receptors (BLT1/2 receptor) were more effective at inhibiting the increase in vascular permeability than the accumulation of neutrophils, suggesting a role for neutrophil-derived LTB4 in the increase of vascular permeability following I/R injury (Souza et al., 2000a). Inasmuch as PDE4 inhibitors are effective modulators of LTB4 production by neutrophils in vitro, we examined whether inhibition of LTB4 production could account for some of the inhibitory effects of rolipram in our model. Pretreatment of animals with rolipram significantly inhibited the increase in LTB4 concentrations detected in the intestine after severe I/R injury. Thus, in addition to inhibiting the local influx of neutrophils, rolipram may also ameliorate reperfusion injury by inhibiting the release of neutrophil-derived factors, such as LTB4.
PDE4 inhibitors are effective suppressors of TNF-α production by leukocytes and inhibition of TNF-α release may account for their anti-inflammatory action in some in vivo models of inflammatory diseases (Teixeira et al., 1997; Souness et al., 2000). Thus, it was of interest to investigate the role of TNF-α in our model and the possible effects of rolipram on TNF-α concentrations. The local and systemic release and action of TNF-α are key features in the development of the injuries following I/R of several vascular beds, including the gut (Seekamp et al., 1993; Sorkine et al., 1995; Yao et al., 1996; Gurevitch et al., 1997). Our results agree with those of previous studies and confirm the effectiveness of anti-TNF-α treatment in the suppression of the local, remote and systemic injuries following severe I/R of the SMA. Interestingly, whereas the influx of neutrophils was essential for the tissue production of TNF-α (Souza et al., 2000b), anti-TNF-α treatment abrogated the influx of neutrophils in the intestine and lung of reperfused animals. Thus, it appears that there is an initial production of TNF-α by resident cells, possibly mast cells (Frangogiannis et al., 1998), that is sufficient to drive a first wave of incoming neutrophils which in turn induce further TNF-α production and further leukocyte recruitment. In this context, rolipram has been shown to inhibit both neutrophil influx and TNF-α production directly, and both of these actions could account for the anti-inflammatory effects of the drug observed in our model.
In contrast to the complete inhibition of the increases in tissue TNF-α concentrations by pretreatment with rolipram, the drug only partially (by 57%) inhibited the increase in TNF-α concentrations measured in serum. Similarly, strategies (selectin blockade or PAF receptor antagonism) which blocked neutrophil influx into tissues were effective at suppressing tissue (lung and intestine), but not systemic, increases in TNF-α concentrations (Souza et al., 2000b). Together, these data suggest that systemic increases in TNF-α concentrations do not derive from the production of this cytokine in the lung or intestine. Possible sources of the systemic TNF-α were not investigated in the present study, but include the liver (Bathe et al., 1998) and, possibly, endothelial cells in the ischaemic and reperfused intestine (Wada et al., 2001). In the latter case, it is possible that endothelial cells would preferentially release TNF-α into the circulation and not into the reperfused tissue. In contrast to the partial inhibitory effects of rolipram, pretreatment with anti-TNF-α abrogated the increases in TNF-α concentrations in serum and prevented the lethality associated with severe I/R injury. Thus, whereas tissue TNF-α appears to be essential for the influx of neutrophils which in turn promote further TNF-α release and tissue damage, systemic TNF-α is neutrophil-independent. Moreover, as anti-TNF-α treatment prevented the lethality associated with I/R injury, we suggest that systemic levels of TNF-α are largely responsible for the lethality observed in the model. As PDE4 inhibitors are much more effective at inhibiting tissue rather than the systemic concentrations of TNF-α, we suggest that the degree of inhibition of systemic concentrations of TNF-α is not sufficient for the drug to prevent the lethality following I/R injury.
In addition to TNF-α, previous studies have shown that concentrations of IL-1ß and IL-6 are elevated and may have a pathophysiological role following intestinal ischaemia and reperfusion injury (e.g. Seekamp et al., 1993; Yao et al., 1997). Moreover, the production of these cytokines may be under the influence of TNF-α both in vitro and in vivo (e.g. Fong et al., 1989). For example, Yao et al. (1997) described a role for TNF-α in the control of serum IL-6 concentrations following intestinal I/R injury in rats. In our model, pretreatment with anti-TNF-α had no significant effect on systemic IL-6 concentrations, but intestinal and pulmonary production of IL-6 was markedly attenuated. The reasons for the discrepancy between our results and those of Yao et al. (1997) are unclear but differences in methodology (greater ischaemia period and lethality in our model) may account for the differences. In contrast to its effects on IL-6 production, pretreatment with anti-TNF-α antiserum greatly enhanced the systemic and tissue concentrations of IL-1ß. This surprising result – since TNF-α usually stimulates the production of IL-1ß – requires further study. Pretreatment with rolipram effectively inhibited IL-6 production confirming the effectiveness of this drug at inhibiting cytokine production by leukocytes both in vitro and in vivo. The PDE4 inhibitor also suppressed the release of IL-1β, albeit to a lesser extent than its inhibitory effects on TNF-α or IL-6 production. The is in agreement with in vitro studies demonstrating that TNF-α and IL-1β production by macrophages are differentially modulated by cyclic AMP elevating agents (Molnar-Kimber et al., 1993; Verghese et al., 1995).
In contrast to its inhibitory effect on pro-inflammatory cytokine production, PDE4 inhibitors, such as rolipram, and other cyclic AMP-elevating agents may enhance IL-10 production by activated macrophages in vitro (Kambayashi et al., 1995; Eigler et al., 1998; Procópio et al., 1999) or during lipopolysaccharide-induced sepsis in vivo (Strassman et al., 1994). Moreover, the IL-10 produced may mediate some of the anti-inflammatory actions of cyclic AMP elevating agents both in vitro and in vivo (Strassman et al., 1994; Kambayashi et al., 1995; Eigler et al., 1998; Procópio et al., 1999). Since IL-10 may be induced in reperfused tissue and may modulate reaction to injury (Frangogiannis et al., 2000), we measured the concentrations of this cytokine following severe I/R injury and observed a significant elevation of IL-10 concentrations in the serum and lung, but not intestine, following reperfusion of the ischaemic SMA. IL-10 production was partially dependent on TNF-α, as demonstrated by the partial inhibitory effects of anti-TNF-α pretreatment. Pretreatment with rolipram only marginally enhanced IL-10 concentrations in the intestine but significantly abrogated the concentrations of this cytokine in the lung and serum following severe I/R injury. Thus, whereas significant IL-10 is induced following reperfusion of the ischaemic SMA, we could not detect an increase in the concentrations of this cytokine following treatment with rolipram. These results argue that production of IL-10 is not necessary for the anti-inflammatory effects of PDE4 inhibitors in this model of severe I/R injury. However, it is possible that the prevention of IL-10 increase in the lung and serum following rolipram treatment may worsen the physiological recovery after I/R injury.
In conclusion, drugs which inhibit PDE4 enzymes possess significant anti-inflammatory and protective effects in local, remote and systemic injuries following reperfusion of the ischaemic SMA. The capacity of PDE4 inhibitors to block the recruitment of neutrophils into tissues, the production of LTB4 and of the pro-inflammatory cytokines TNF-α, IL-1ß and IL-6 appear to underlie the anti-inflammatory activity observed. Altogether, our experiments suggest that drugs which inhibit PDE4 enzymes may be a useful adjunct therapy for the treatment of the injuries which follow the reperfusion of an ischaemic territory. Nevertheless, these drugs appeared to be less effective than the pretreatment with anti-TNF-α serum and had no effect on the lethality following I/R injury.
Acknowledgments
This work received financial support from FAPEMIG, PADCT and CNPq (Brazil).
Abbreviations
- I/R
ischaemia and reperfusion
- MPO
myeloperoxidase
- PDE
phosphodiesterase
- SMA
superior mesenteric artery
References
- BARNARD J.W., SEIBERT A.F., PRASSAD V.R., SMART D.A., STRADA S.J., TAYLOR A.E., THOMPSON W.J. Reversal of pulmonary capillary ischemia-reperfusion injury by rolipram, cAMP phosphodiesterase inhibitor. J. Appl. Physiol. 1994;77:774–781. doi: 10.1152/jappl.1994.77.2.774. [DOI] [PubMed] [Google Scholar]
- BARNETTE M.S., CHRISTENSEN S.B., ESSAYAN D.M., GROUS M., PRABHAKAR U., RUSH J.A., KAGEY-SOBOTKA A., TORPHY T.J. SB 207499 (Ariflo), a potent and selective second-generation phosphodiesterase 4 inhibitor: in vitro anti-inflammatory actions. J. Pharmacol. Exp. Ther. 1998;284:420–426. [PubMed] [Google Scholar]
- BATHE O.F., CHOW A.W., PHANG P.T. Splanchnic origin of cytokines in a porcine model of mesenteric ischemia-reperfusion. Surgery. 1998;123:79–88. [PubMed] [Google Scholar]
- BLEIWEIS M.S., JONES D.R., HOFFMANN S.C., BECKER R.M., EGAN T.M. Reduced ischemia-reperfusion injury with rolipram in rat cadaver lung donors: effect of cyclic adenosine monophosphate. Ann. Thorac. Surg. 1999;67:194–200. doi: 10.1016/s0003-4975(98)01310-1. [DOI] [PubMed] [Google Scholar]
- CARTER M.B., WILSON M.A., WEAD W.B., GARRISON R.N. Pentoxifylline attenuates pulmonary macromolecular leakage after intestinal ischemia-reperfusion. Arch. Surg. 1995;130:1337–1344. doi: 10.1001/archsurg.1995.01430120091014. [DOI] [PubMed] [Google Scholar]
- CHANG-CHUN C., MATSUDA H., SAWA Y., KANEKO M., SALAGOSHI N., NISHIMURA M., KURATANI T., AMEMIYA A., KAWASHIMA Y. Effects of a cyclic adenosine monophosphate phosphodiesterase inhibitor, DN-9693, on myocardial reperfusion injury. Ann. Thorac. Surg. 1991;52:495–499. doi: 10.1016/0003-4975(91)90911-9. [DOI] [PubMed] [Google Scholar]
- DE MATOS I.M., SOUZA D.G., SEABRA D.G., FREIRE-MAIA L., TEIXEIRA M.M. Effects of tachykinin NK1- or PAF-receptor blockade on the lung injury induced by scorpion venom. Eur. J. Pharmacol. 1999;376:293–300. doi: 10.1016/s0014-2999(99)00382-9. [DOI] [PubMed] [Google Scholar]
- EIGLER A., SIEGMUND B., EMMERICH U., BAUMANN K.H., HARTMANN G., ENDRES S. Anti-inflammatory actives of cAMP-elevating agents: enhancement of IL-10 synthesis and concurrent suppression of TNF production. J. Leukoc. Biol. 1998;63:101–107. doi: 10.1002/jlb.63.1.101. [DOI] [PubMed] [Google Scholar]
- FEATHERSTONE R.L., KELLY F., CHAMBERS D.J. Theophylline improves functional recovery of isolated rat lungs after hypothermic preservation. Ann. Thorac. Surg. 1999;67:798–803. doi: 10.1016/s0003-4975(99)00039-9. [DOI] [PubMed] [Google Scholar]
- FONG Y., TRACEY K.J., MOLDAWER L.L., HESSE D.G., MANOGUE K.B., KENNEY J.S., LEE A.T., KUO G.C., ALLISON A.C., LOWRY S.F. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1 beta and interleukin 6 appearance during lethal bacteremia. J. Exp. Med. 1989;170:1627–1633. doi: 10.1084/jem.170.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANCISCHI J.N., YOKORO C.M., CUNHA F.Q., TAFURI Wg.L., TEIXEIRA M.M. Effects of the PDE4 inhibitor rolipram in a rat model of arthritis. Eur. J. Pharmacol. 2000;399:243–249. doi: 10.1016/s0014-2999(00)00330-7. [DOI] [PubMed] [Google Scholar]
- FRANGOGIANNIS N.G., MENDOZA L.H., LINDSEY M.L., BALLANTYNE C.M., MICHAEL L.H., SMITH C.W., ENTMAN M.L. IL-10 induced in the reperfused myocardium and may modulate the reaction to injury. J. Immunol. 2000;165:2798–2808. doi: 10.4049/jimmunol.165.5.2798. [DOI] [PubMed] [Google Scholar]
- FRANGOGIANNIS N.G., LINDSEY M.L., MICHAEL L.H., YOUKER K.A., BRESSLER R.B., MENDOZA L.H., SPENGLER R.N., SMITH C.W., ENTMAN M.L. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation. 1998;98:699–710. doi: 10.1161/01.cir.98.7.699. [DOI] [PubMed] [Google Scholar]
- GILMONT R.R., DARDANO A., ENGLE J.S., ADAMSON B.S., WELSH M.J., LI T., REMICK D.G., SMITH D.J., REES R.S. TNF-α potentiates oxidant and reperfusion-induced endothelial cell injury. J. Surg. Res. 1996;61:175–182. doi: 10.1006/jsre.1996.0101. [DOI] [PubMed] [Google Scholar]
- GUREVITCH J., FROLKIS I., YUHAS Y., LIFSCHITZ-MERCER B., BERGER E., PAZ Y., MATZA M., KRAMER A., MOHR R. Anti -tumor necrosis factor-alpha improves myocardial recovery after ischemia and reperfusion. J. Am. Coll. Cardiol. 1997;30:1554–1561. doi: 10.1016/s0735-1097(97)00328-8. [DOI] [PubMed] [Google Scholar]
- HAGAN P., POOLE S., BRISTOW A.F. Endotoxin-stimulated production of rat hypothalamic interleukin-1 beta in vivo and in vitro, measured by specific immunoradiometric assay. J. Mol. Endocrinol. 1993;11:31–36. doi: 10.1677/jme.0.0110031. [DOI] [PubMed] [Google Scholar]
- HUBER T.S., GAINES G.C., WELBORN M.D. , III, ROSENBERG J.J., SEEGER J.M., MOLDAWER L.L. Anticytokine therapies for acute inflammation and the systemic inflammatory response syndrome: IL-10 and ischemia/reperfusion injury as a new paradigm. Shock. 2000;13:425–434. doi: 10.1097/00024382-200006000-00002. [DOI] [PubMed] [Google Scholar]
- KAMBAYASHI T., JACOB C.O., ZHOU D., MAZUREK N., FONG M., STRASSMANN G. Cyclic nucleotide phosphodiesterase type IV participates in the regulation of IL-10 and in the subsequent inhibition of TNF-alpha and IL-6 release by endotoxin-stimulated macrophages. J. Immunol. 1995;155:4909–4916. [PubMed] [Google Scholar]
- KATO H., ARAKI T., ITOYAMA Y., KOGURE K. Rolipram, a cyclic amp-selective phosphodiesterase inhibitor, reduces neuronal damage following cerebral ischemia in the gerbil. Eur. J. Pharmacol. 1995;272:107–110. doi: 10.1016/0014-2999(94)00694-3. [DOI] [PubMed] [Google Scholar]
- MOLNAR-KIMBER K., YONNO L., HEASLIP R., WEICHMAN B. Modulation of TNF alpha and IL-1 beta from endotoxin-stimulated monocytes by selective PDE isozyme inhibitors. Agents Actions. 1993;39:C77–C79. doi: 10.1007/BF01972726. [DOI] [PubMed] [Google Scholar]
- MÜLLER J.M., VOLLMAR B., MENGER M.D. Pentoxifylline reduces venular leukocyte adherence (‘reflow paradox') bur not microvascular ‘no reflow' in hepatic ischemia/reperfusion. J. Surg. Res. 1997;71:1–6. doi: 10.1006/jsre.1997.5132. [DOI] [PubMed] [Google Scholar]
- PENG X.X., CURRIN R.T., THURMAN R.G., LEMASTERS J.J. Protection by pentoxifylline against normothermic liver ischemia/reperfusion in rats. Transplantation. 1995;59:1537–1541. [PubMed] [Google Scholar]
- PROCÓPIO D.O., TEIXEIRA M.M., CAMARGO M.M., TRAVASSO L.R., FERGUSON A.J., ALMEIDA I.C., GAZINELLI R.T. Differential inhibitory mechanism of cyclic AMP on TNF-α and IL-12 synthesis by macrophages exposed to microbial stimuli. Br. J. Pharmacol. 1999;127:1195–1205. doi: 10.1038/sj.bjp.0702624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REES G.S., BALL C., WARD H.L., GEE C.K., TARRANT G., MISTRY Y., POOLE S., BRISTOW A.F. Rat interleukin 6: expression in recombinant Escherichia coli, purification and development of a novel ELISA. Cytokine. 1999a;11:95–103. doi: 10.1006/cyto.1998.0408. [DOI] [PubMed] [Google Scholar]
- REES G.S., GEE C.K., WARD H.L., BALL C., TARRANT G., MISTRY Y., POOLE S., BRISTOW A.F. Rat tumour necrosis factor-alpha: expression in recombinant Pichia pastoris, purification, characterization and development of a novel ELISA. Eur. Cytokine Netw. 1999b;10:383–392. [PubMed] [Google Scholar]
- SEEKAMP A., WARREN J.S., REMICK D.G., TILL G.O., WARD P.A. Requirements for tumor necrosis factor-α and Interleukin-1 in limb ischemia/reperfusion injury and associated lung injury. Am. J. Pathol. 1993;143:453–463. [PMC free article] [PubMed] [Google Scholar]
- SORKINE P., SETTON A., HALPERN P., MILLER A., RUDICK V., MARMOR S., KLAUSNER J.M., GOLDMAN G. Soluble tumor necrosis factor receptors reduce bowel ischemia-induced lung permeability and neutrophil sequestration. Crit. Care Med. 1995;23:1377–1381. doi: 10.1097/00003246-199508000-00011. [DOI] [PubMed] [Google Scholar]
- SOUNESS J.E., ALDOUS D., SARGENT C. Immunosuppressive and anti-inflammatory effects of cyclic AMP phosphodiesterase (PDE) type 4 inhibitors. Immunopharmacology. 2000;47:127–162. doi: 10.1016/s0162-3109(00)00185-5. [DOI] [PubMed] [Google Scholar]
- SOUZA D.G., COUTINHO S.F., SILVEIRA M.R., CARA D.C., TEIXEIRA M.M. Effects of a BLT receptor antagonist on local and remote reperfusion injuries after transient ischemia of the superior mesenteric artery in rats. Eur. J. Pharmacol. 2000a;403:121–128. doi: 10.1016/s0014-2999(00)00574-4. [DOI] [PubMed] [Google Scholar]
- SOUZA D.G., CARA D.C., CASSALI G.D., COUTINHO S.F., SILVEIRA M.R., ANDRADE S.P., POOLE S.P., TEIXEIRA M.M. Effects of the PAF receptor antagonist UK74505 on local and remote reperfusion injuries folloxing ischaemia mesenteric artery in the rat. Br. J. Pharmacol. 2000b;131:1800–1808. doi: 10.1038/sj.bjp.0703756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRASSMAN G., PATIL-KOOTA V., FINKELMAN F., FONG M., KAMBAYASHI T. Evidence for the involvement of interleukin 10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. J. Exp. Med. 1994;180:2365–2370. doi: 10.1084/jem.180.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEIXEIRA M.M., WILLIAMS T.J., HELLEWELL P.G. Effect of phosphodiesterase isoenzyme inhibitors on cutaneous inflammation in the guinea pig. Br. J. Pharmacol. 1994;112:332–340. doi: 10.1111/j.1476-5381.1994.tb13073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEIXEIRA M.M., GRISTWOOD R.W., COOPER N., HELLEWELL P.G. Phosphodiesterase (PDE4) inhibitors: Anti-inflammatory drugs of the future. Trends Pharmacol. Sci. 1997;18:164–171. doi: 10.1016/s0165-6147(97)01049-3. [DOI] [PubMed] [Google Scholar]
- TORPHY T.J. Phosphodiesterase isozymes: molecular targets for novel antiasthma agents. Am. J. Respir. Crit. Care Med. 1998;157:351–370. doi: 10.1164/ajrccm.157.2.9708012. [DOI] [PubMed] [Google Scholar]
- VERGHESE M.W., MCCONNELL R.T., STRICKLAND A.B., GOODING R.C., STIMPSON S.A., YARNALL D.P., TAYLOR J.D., FURDON P.J. Differential regulation of human monocyte-derived TNF alpha and IL-1 beta by type IV cAMP-phosphodiesterase (cAMP-PDE) inhibitors. J. Pharmacol. Exp. Ther. 1995;272:1313–1320. [PubMed] [Google Scholar]
- WADA K., MONTALTO M.C., STAHL G.L. Inhibition of complement C5 reduces local and remote organ injury after intestinal ischemia/reperfusion in the rat. Gastroenterology. 2001;120:126–133. doi: 10.1053/gast.2001.20873. [DOI] [PubMed] [Google Scholar]
- WILLERSON J.T. Pharmacological approaches to reperfusion injury. Adv. Pharmacol. 1997;39:291–312. doi: 10.1016/s1054-3589(08)60074-5. [DOI] [PubMed] [Google Scholar]
- YAO Y.M., BAHRAMI S., REDL H., FUERST S., SCHALAG G. Monoclonal antibody to tumor necrosis factor-α attenuates hemodynamic dysfunction secondary to intestinal ischemia/reperfusion in rats. Crit. Care Med. 1996;24:1547–1553. doi: 10.1097/00003246-199609000-00020. [DOI] [PubMed] [Google Scholar]
- YAO Y.M., BAHRAMI S., REDL H., FUERST S., SCHALAG G. IL-6 release after intestinal ischemia/reperfusion in rats is under partial control of TNF. J. Surg. Res. 1997;70:21–26. doi: 10.1006/jsre.1997.5074. [DOI] [PubMed] [Google Scholar]


