Abstract
Vascular effects of diadenosine polyphosphates (ApnAs), adenosine polyphospho guanosines (ApnGs) and guanosine polyphospho guanosines (GpnGs), novel families of naturally-occurring signalling molecules, were investigated in methoxamine preconstricted rat isolated perfused mesenteric arterial beds.
Three different types of response were elicited by ApnAs and ApnGs. Those with a short polyphosphate chain (n=2 – 3) elicited vasorelaxation. Ap3A was more potent than Ap2A, and both were more potent than the corresponding ApnG. Relaxations to Ap3A and Ap3G, but not to Ap2A and Ap2G, were blocked by endothelium removal and pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS), a P2 receptor antagonist.
Longer polyphosphate chain ApnAs and ApnGs (n=4 – 6) elicited dose-dependent vasoconstriction followed by prolonged vasorelaxation, with a potency order for both types of response of Ap5A⩾Ap6A>Ap4A. A similar order and potency was observed for ApnGs. Contractions and prolonged relaxations were blocked by PPADS and P2X1 receptor desensitization with α,β-methylene ATP (α,β-meATP), and were largely endothelium-independent.
In the presence of α,β-meATP rapid relaxations to contractile ApnAs and ApnGs (n=4 – 6) were revealed.
GpnGs were virtually inactive, except for Gp2G which elicited vasoconstriction via PPADS- and α,β-meATP-sensitive smooth muscle P2X1-like receptors.
These data show that, as with ApnAs, the length of the polyphosphate chain (n) is an important determinant of the activity of ApnGs at P2 receptors in the rat mesenteric arterial bed. When the chain is short (n=2 – 3) the purines elicit rapid vasorelaxation, which for Ap3A and Ap3G is mediated via endothelial P2Y1-like receptors. When the chain is long (n=4 – 6) ApnAs and ApnGs elicit vasoconstriction via P2X1-like receptors, followed by prolonged endothelium-independent vasorelaxation. Rapid relaxation to contractile dinucleotides (n=4 – 6) is revealed by block of vasoconstriction. Regarding the purine moiety, one adenine is crucial and sufficient for vasoactivity as GpnGs were largely inactive, and ApnAs and ApnGs approximately equipotent.
Keywords: P2 purine receptors, diadenosine polyphosphates, adenosine polyphospho guanosines, guanosine polyphospho guanosines, rat mesenteric arterial bed
Introduction
The diadenosine polyphosphates (ApnAs), adenosine polyphospho guanosines (ApnGs) and guanosine polyphospho guanosines (GpnGs) have attracted considerable recent interest as novel families of extracellular signalling molecules (Hoyle, 1990; Schlüter et al., 1994; Ralevic et al., 1995; van der giet et al., 1997; Lewis et al., 2000; Hoyle et al., 2001). They are released from a number of different cell types, but are found in particularly high concentrations in platelets (Flodgaard & Klenow, 1982; Lüthje & Ogilvie, 1983; Schlüter et al., 1994; 1998; Jankowski et al., 1999; 2001; Luo et al., 1999a,1999b). ApnAs, ApnGs and GpnGs are endogenous ligands at P1 and P2 purine receptors (Ralevic & Burnstock, 1998), although in the central nervous system distinct receptors for these compounds have been proposed (Pintor & Miras-Portugal, 1995).
Interestingly, the potency and type of response of the ApnAs has been shown to be largely determined by the number of phosphates (n) in the polyphosphate chain (Ralevic et al., 1995; van der giet et al., 1997; Lewis et al., 2000). In general, ApnAs with a phosphate chain length of n=2 – 3 are vasodilators, whilst ApnAs with a phosphate chain length of n=4 – 6 are vasoconstrictors. Ap3A is typically the most potent vasorelaxant, mediating its effect via P2Y receptors on the endothelium and smooth muscle (for example in rat and rabbit mesenteric arteries respectively) (Busse et al., 1988; Ralevic et al., 1995), or via A2 receptors (in rat kidney) (van der giet et al., 1997). However, smooth muscle vasorelaxation mediated by Ap4A and Ap5A in porcine coronary arteries (Sumiyoshi et al., 1997), and endothelium-dependent vasorelaxation mediated by Ap4A in rabbit mesenteric arteries (Busse et al., 1988) has also been described. Vasocontractile actions of ApnAs (n=4 – 6) in rat mesenteric arteries are mediated by ionotropic P2X1-like receptors on the smooth muscle (Ralevic et al., 1995; Lewis et al., 2000). A recent study has show that longer chain length ApnGs (n=4 – 6) can also elicit vasoconstriction via smooth muscle P2X1-like receptors (Lewis et al., 2000). However, whether ApnGs can mediate vasorelaxation is unclear. Most recently, GpnGs were isolated (Schlüter et al., 2000), but their cardiovascular actions have been investigated only in the rat isolated kidney and dissociated smooth muscle cells (Schlüter et al., 1998).
The aim of this study was to investigate the vascular effects of ApnGs and GpnGs, compared to responses mediated by the ApnAs, in the rat isolated mesenteric arterial bed. The complement of purine receptors in rat mesenteric arteries includes vasorelaxant P2Y1- and P2Y2-like receptors on the endothelium (Ralevic et al., 1995; Ralevic & Burnstock, 1988; 1996), vasorelaxant A2B receptors on the smooth muscle (Rubino et al., 1995), and vasocontractile P2X1 receptors on the smooth muscle (Lewis & Evans, 2000). The actions of ApnAs, ApnGs and GpnGs were characterized using pyridoxalphosphate-6-azophenyl 2′,4′-disulphonic acid (PPADS), a selective P2 receptor antagonist (Lambrecht et al., 1992; Ziganshin et al., 1994; Windscheif et al., 1994), and by desensitization of smooth muscle P2X1 receptors with α,β-methylene ATP (α,β-meATP).
Methods
Male Wistar rats (250 – 300 g) were killed by exposure to CO2 and decapitation. Mesenteric beds were isolated and perfused, via the superior mesenteric artery, as described previously (Ralevic et al., 1995). In brief, the abdomen was opened and the superior mesenteric artery exposed and cannulated with a hypodermic needle. The superior mesenteric vein was cut, blood flushed from the preparation with 0.5 ml of Krebs' solution and the gut dissected carefully away from the mesenteric vasculature. The preparation was mounted on a stainless steel grid (7×5 cm) in a humid chamber and perfused at a constant flow rate of 5 ml min−1 using a peristaltic pump (model 7554-30, Cole-Parmer Instrument Co., Chicago, IL, U.S.A.). The perfusate was Krebs'-Bülbring solution of the following composition (mM): NaCl 133, KCl 4.7, NaH2PO4 1.35, NaHCO3 16.3, MgSO4 0.61, CaCl2 2.52 and glucose 7.8, gassed with 95% O2-5% CO2 and maintained at 37°C. Preparations were allowed to equilibrate for 30 min prior to experimentation. Responses were measured as changes in perfusion pressure (mmHg) with a pressure transducer (model P23XL, Viggo-Spectramed, Oxnard, CA, U.S.A.) on a side arm of the perfusion cannula, and recorded on a polygraph (model 7D, Grass Instrument Co., Quincy, MA, U.S.A.).
Experimental protocol
After 30 min equilibration, a submaximal concentration of methoxamine (10 – 50 μM) was added in order to increase the perfusion pressure of the preparations (by 40 – 80 mmHg) above baseline, and responses to doses of the ApnAs, ApnGs and GpnGs (50 μl bolus injections; 0.05 – 50 nmol) were investigated. Preliminary experiments showed that the GpnGs were relatively weak or inactive and thereafter this family was always tested first. One or both of the families of ApnGs and ApnAs were tested in the same preparations. When both were tested the order in which they were applied was alternated. The smaller polyphosphate chain length ApnAs and ApnGs (n=2 and 3) were typically tested first, but the greater chain length members (n=4 – 6) were applied in a randomized order. Antagonists/inhibitors were added to the perfusate during equilibration, 30 min before construction of the dose-response curves. In a group of mesenteric beds the endothelium was removed by perfusing the preparations with water (for 7 – 8 min). Endothelial damage was confirmed by testing with acetylcholine (ACh; 50 nmol), which produced a relaxation of 12.84±2.2% (n=10). This is less than 20% of the normal relaxation response (80 – 90% relaxation) to 50 nmol ACh in endothelially-intact preparations.
Drugs
α,β-methylene ATP (lithium salt), acetylcholine (chloride), methoxamine (hydrochloride) and suramin (sodium salt) were from Sigma. Pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) was from TOCRIS Cookson Ltd. 8-(p-sulphophenyl)theophylline (8-PSPT) was from Research Biochemicals International. ApnAs, ApnGs and GpnGs were synthesized and HPLC purified by H Schlüter. All drugs were dissolved in distilled water.
Data analysis
Vasocontractile and vasorelaxant responses of the mesenteric arterial beds were measured as changes in perfusion pressure (mmHg) and vasorelaxant responses expressed as percentage relaxation of the methoxamine-induced increase in tone above baseline. Data are expressed as mean±s.e.mean. Data were analysed by analysis of variance followed by Tukey's multiple comparison or Student's t-test. pD30 is the dose that would cause an increase in perfusion pressure of 30 mmHg, and was calculated as the mean of individual pD30 values obtained from graphs of dose-response relationships for each experiment. A value of P<0.05 was taken to indicate a statistically significant difference.
Results
Effects of ApnGs and ApnAs in the rat isolated mesenteric arterial bed
Three different types of response to the ApnGs and ApnAs were observed (Figures 1 and 2). Short chain purine compounds (n=2 – 3) elicited dose-dependent vasorelaxation and longer chain purine compounds (n=4 – 6) elicited dose-dependent vasoconstriction followed by prolonged vasorelaxation.
Figure 1.
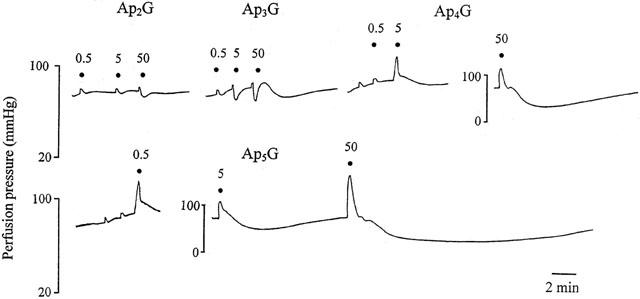
Representative trace showing responses to ApnGs (n=2 – 5) in the rat isolated perfused methoxamine-preconstricted mesenteric arterial bed. Ap2G and Ap3G elicited only vasorelaxation, whereas Ap4G and Ap5G elicited vasoconstriction followed by slow and prolonged vasorelaxation. Responses to Ap6G (not shown) were similar to those to Ap5G. ApnGs were applied at the doses (nmol) indicated. The very small increases in perfusion pressure observed at low doses of all agonists are injection artefacts. Note the different vertical scale for responses to 50 nmol Ap4G and 5 and 50 nmol Ap5G.
Figure 2.
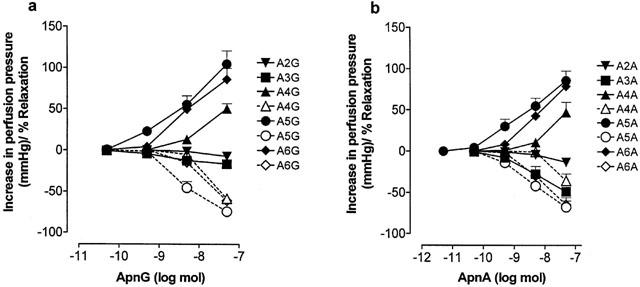
Dose response curves to (a) ApnGs and (b) ApnAs in the rat isolated perfused methoxamine-preconstricted mesenteric arterial bed. Solid lines indicate constrictions (increase in perfusion pressure, mmHg) or rapid relaxations (% of tone) and dashed lines indicate prolonged relaxations (% of tone). n=5 – 6 for each point. Data are shown as mean+s.e.mean.
The adenosine polyphospho guanosines Ap4G, Ap5G and Ap6G elicited dose-dependent vasoconstriction with an order of potency of: Ap5G⩾Ap6G>Ap4G (Figures 1 and 2a). pD30 values for Ap4G, Ap5G and Ap6G were 7.78±0.1, 8.93±0.18 and 8.71±0.23, respectively (n=6). A similar contractile potency order was observed for the diadenosine polyphosphates, where Ap5A⩾Ap6A>Ap4A (Figure 2b). pD30 values for Ap4A, Ap5A and Ap6A were 7.85±0.14, 8.5±0.15 and 8.2±0.09, respectively (n=5 – 6). pD30 values for the ApnAs were not significantly different to pD30 values that we have reported previously for these compounds in the rat isolated mesenteric arterial bed (Ralevic et al., 1995). There was no significant difference between the contractile responses elicited by any ApnG and its corresponding ApnA.
Vasocontractile responses to ApnAs and ApnGs were followed by slow and prolonged vasorelaxations (Figures 1 and 2). The rank order of potency of the ApnAs and ApnGs in eliciting this response was similar to that of the contractile potency order of the compounds. There was no significant difference between prolonged vasorelaxant responses to Ap5G and Ap5A, and between Ap6G and Ap6A, but Ap4G was significantly more potent than Ap4A (P<0.05) (Figure 2).
Ap2G and Ap3G elicited only modest vasorelaxation and Ap3G was more potent than Ap2G (Figure 1 and 2a). Ap3A was a more potent vasorelaxant than Ap2A and both were significantly more potent as vasorelaxants than the corresponding ApnG (P<0.05 and P<0.001, respectively; n=6) (Figure 2).
Effect of PPADS, endothelium removal and α,β-meATP on contractile responses to ApnGs
Contractile responses to Ap4G and Ap6G were virtually abolished by PPADS (10 μM), and contractions to Ap5G were significantly attenuated (P<0.01; n=4) (Figure 3a). Endothelium removal had no significant effect on contractions to Ap4G, but attenuated slightly contractions to Ap5G (P<0.01) and Ap6G (P<0.05) (n=6) (Figures 3b and 4). In the presence of α,β-meATP (10 μM), contractions to Ap4G, Ap5G and Ap6G were abolished, and rapid vasorelaxations were revealed (n=4) (Figures 3c and 4).
Figure 3.
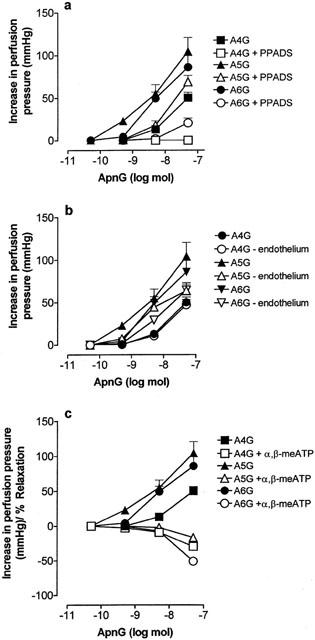
Dose response curves to ApnGs under control conditions (solid symbols; n=6) and (a) in the presence of pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS; 10 μM) (n=4), (b) after endothelium removal (n=6), and (c) in the presence of α,β-methylene ATP (α,β-meATP; 10 μM) (n=4), in the rat isolated perfused methoxamine-preconstricted mesenteric arterial bed. Data are shown as mean+s.e.mean.
Figure 4.
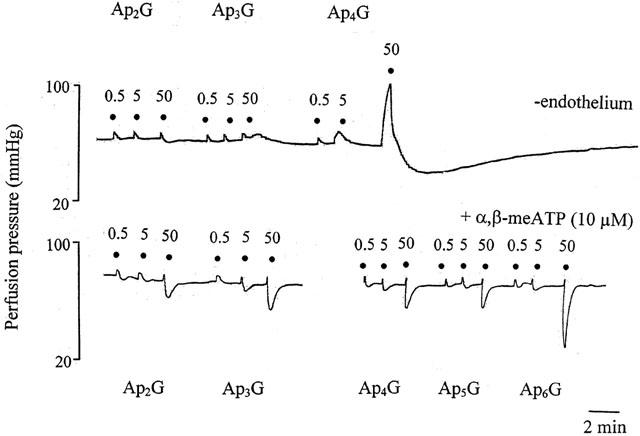
Representative trace showing responses to ApnGs (n=2 – 6) in the rat isolated perfused methoxamine-preconstricted mesenteric arterial bed without endothelium (upper trace) or in the presence of α,β-methylene ATP (α,β-meATP; 10 μM) (lower trace). Rapid vasorelaxations to Ap3G were abolished by endothelium removal, but contraction and prolonged relaxation to Ap4G was unaffected. α,β-meATP blocked contraction and prolonged relaxation to Ap4G, Ap5G and Ap6G and revealed rapid relaxations. ApnGs were applied at the doses (nmol) indicated. The very small increases in perfusion pressure observed at low doses of all agonists are injection artefacts.
Effect of PPADS, endothelium removal and α,β-meATP on contractile responses to ApnAs
Figure 5 (top panel) shows the pivotal role played by the phosphate chain length in determining vasodilator (n=3; Ap3A) and vasoconstrictor (n=4; Ap4A) activity of the purines. Contractile responses to Ap4A were virtually abolished by PPADS (10 μM), and contractions to Ap5A (P<0.05) and Ap6A (P<0.001) were significantly attenuated (n=6) (Figures 5 and 6a). Endothelium removal reduced contractions to Ap4A (P<0.001) but had no significant effect on responses to Ap5A and Ap6A (n=6) (Figure 6b). In the presence of α,β-meATP (10 μM), contractions to Ap4A, Ap5A and Ap6A were abolished, and rapid vasorelaxations revealed (n=6) (Figures 5 and 6c). Rapid vasorelaxations to Ap4A and Ap6A in the presence of α,β-meATP were significantly less potent than rapid relaxations to the corresponding ApnGs under the same conditions (P<0.001) (Figures 3c and 6c). Rapid relaxations to Ap5A and Ap5G in the presence of α,β-meATP were not significantly different (Figures 3c and 6c).
Figure 5.
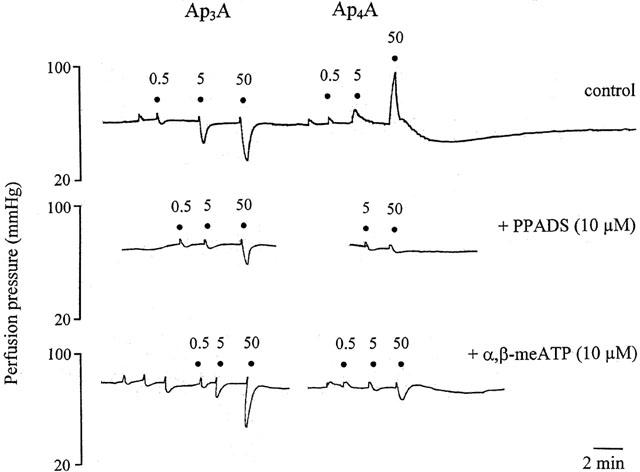
Representative trace showing responses to ApnAs (n=3 and 4) in the rat isolated perfused methoxamine-preconstricted mesenteric arterial bed under control conditions (top trace), in the presence of pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS; 10 μM) (middle trace), and in the presence of α,β-methylene ATP (α,β-meATP; 10 μM) (bottom trace). Note that an increase by a single phosphate of the polyphosphate chain length of Ap3A to produce Ap4A leads to a loss of rapid relaxation and the response is contraction followed by slow relaxation. Rapid vasorelaxations to Ap3A, and contractions and prolonged relaxation to Ap4A were attenuated by PPADS. Rapid relaxation to Ap3A was unaffected by α,β-meATP but contractions and prolonged relaxation to Ap4A was attenuated. ApnAs were applied at the doses (nmol) indicated. The very small increases in perfusion pressure observed at low doses of all agonists are injection artefacts.
Figure 6.
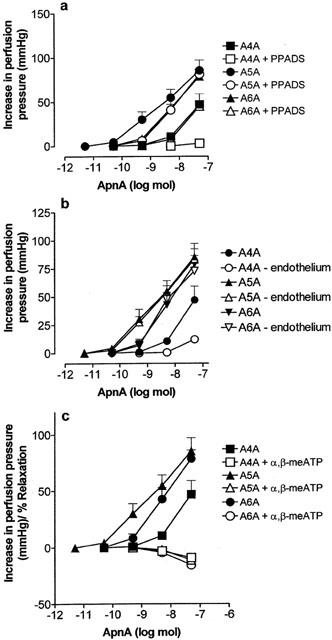
Dose response curves to ApnAs under control conditions (solid symbols) and (a) in the presence of pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS; 10 μM), (b) after endothelium removal, and (c) in the presence of α,β-methylene ATP (α,β-meATP; 10 μM), in the rat isolated perfused methoxamine-preconstricted mesenteric arterial bed. n=5 – 6. Data are shown as mean+s.e.mean.
Characterization of rapid relaxations to ApnAs and ApnGs (n=4 – 6) revealed in the presence of α,β-meATP
In order to characterize the rapid relaxations to ApnAs and ApnGs (n=4 – 6) revealed when contractions were blocked by P2X receptor desensitization with α,β-meATP (10 μM), these were investigated in the additional presence of suramin (100 μM), PPADS (10 μM), 8-PSPT (1 μM), or after endothelium removal. No clear pattern of effect of these treatments was observed. In the presence of α,β-meATP rapid relaxations to ApnAs were unaffected by 8-PSPT, but those to Ap4G and Ap6G were attenuated (n=6). With the exception of Ap4G, relaxations to ApnAs and ApnGs were unaffected by PPADS (n=6). Relaxations to Ap4A, Ap4G and Ap6G were attenuated by endothelium removal (n=6). Suramin attenuated relaxations to Ap4A, Ap4G and Ap6G, but appeared to prevent full desensitization of the P2X receptor, as Ap5A was still able to elicit vasoconstriction (18.8±4.7 mmHg, at 50 nmol; n=6).
Effect of PPADS on rapid and prolonged relaxations to ApnGs and ApnAs
PPADS (10 μM) significantly attenuated rapid relaxations to Ap3G (P<0.05) and Ap3A (P<0.001) (n=4 – 6), but had no effect on rapid relaxations to Ap2G and Ap2A (Figures 5 and 7a,b). PPADS virtually abolished prolonged relaxations to Ap4G, Ap6G and Ap4A, and significantly attenuated prolonged relaxations to Ap5G (P<0.001), Ap5A (P<0.001) and Ap6A (P<0.01) (n=4 – 6) (Figures 5 and 7c,d).
Figure 7.

Effect of pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS; 10 μM) on relaxation dose-response curves to ApnGs and ApnAs (n=2 – 6) in the rat isolated perfused methoxamine-preconstricted mesenteric arterial bed. Rapid relaxations to: (a) Ap2G and Ap3G (n=4 – 6); (b) Ap2A and Ap3A (n=4 – 6). Prolonged relaxations to: (c) Ap4G, Ap5G and Ap6G (n=6); (d) Ap4A, Ap5A and Ap6A (n=5 – 6). Data are shown as mean+s.e.mean.
Effect of α,β-meATP on rapid and prolonged relaxations to ApnGs and ApnAs
α,β-meATP (10 μM) had no significant effect on rapid relaxations to Ap2G, Ap3G and Ap2A, but rapid relaxations to Ap3A were slightly attenuated (n=4 – 6) (Figures 4, 5 and 8a,b). In contrast, α,β-meATP abolished prolonged relaxations to Ap4G, Ap5G, Ap6G, Ap4A, Ap5A and Ap6A (n=4 – 6) (Figures 4, 5 and 8c,d).
Figure 8.
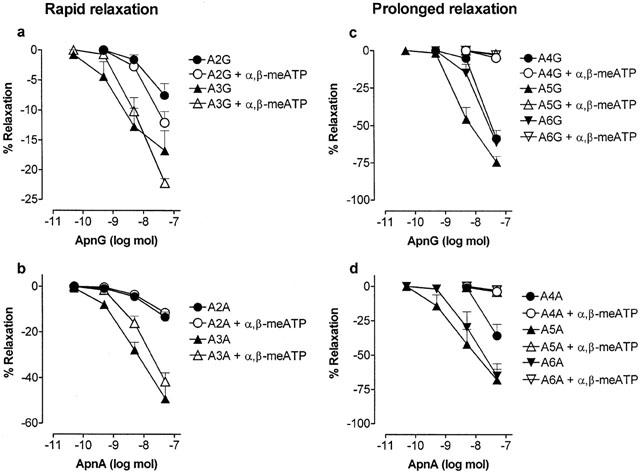
Effect of α,β-methylene ATP (α,β-meATP; 10 μM) on relaxation dose-response curves to ApnGs and ApnAs (n=2 – 6) in the rat isolated perfused methoxamine-preconstricted mesenteric arterial bed. Rapid relaxations to: (a) Ap2G and Ap3G (n=4 – 6); (b) Ap2A and Ap3A (n=4 – 6). Prolonged relaxations to: (c) Ap4G, Ap5G and Ap6G (n=6); (d) Ap4A, Ap5A and Ap6A (n=5 – 6). Data are shown as mean+s.e.mean.
Effect of endothelium removal on rapid and prolonged relaxations to ApnGs and ApnAs
Endothelial denudation attenuated rapid relaxations to Ap3G and Ap3A (P<0.001), but had no effect on rapid relaxations to Ap2G and Ap2A (n=6) (Figures 4 and 9a,b). Endothelium removal had no significant effect on prolonged relaxations to Ap4G, Ap5G and Ap6G. Prolonged relaxations to Ap4A were blocked and those to Ap5A (P<0.01) and Ap6A (P<0.05) were augmented (n=6) (Figures 4 and 9c,d).
Figure 9.
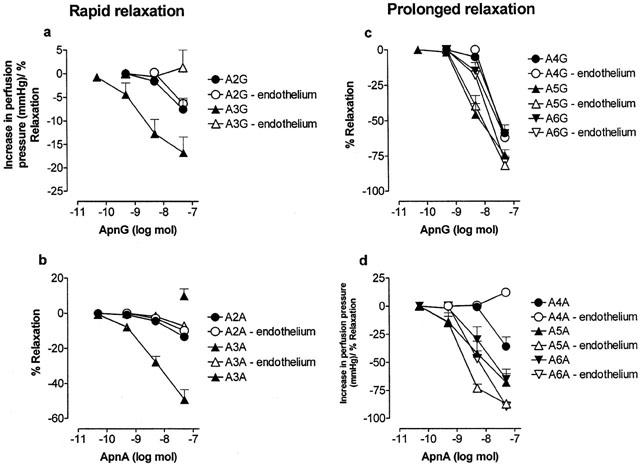
Effect of endothelium removal on relaxation dose-response curves to ApnGs and ApnAs (n=2 – 6) in the rat isolated perfused methoxamine-preconstricted mesenteric arterial bed. Rapid relaxations to: (a) Ap2G and Ap3G (n=6); (b) Ap2A and Ap3A (n=6). Prolonged relaxations to: (c) Ap4G, Ap5G and Ap6G (n=6); (d) Ap4A, Ap5A and Ap6A (n=5 – 6). Data are shown as mean+s.e.mean. In the absence of endothelium the attenuated rapid relaxation response to Ap3A was followed by a small constriction (isolated symbol in panel b).
Effect of GpnGs in the rat isolated mesenteric arterial bed
The GpnGs were inactive or elicited very weak vasorelaxation, with the exception of Gp2G, which was a vasoconstrictor (n=4 – 6) (Figure 10a). The effect of Gp2G was blocked by PPADS (10 μM; n=4) and α,β-meATP (10 μM; n=6), but was not significantly affected by removal of the endothelium (n=6) (Figure 10b,c). In the presence of α,β-meATP a small vasorelaxation to Gp2G was observed (Figure 10b).
Figure 10.
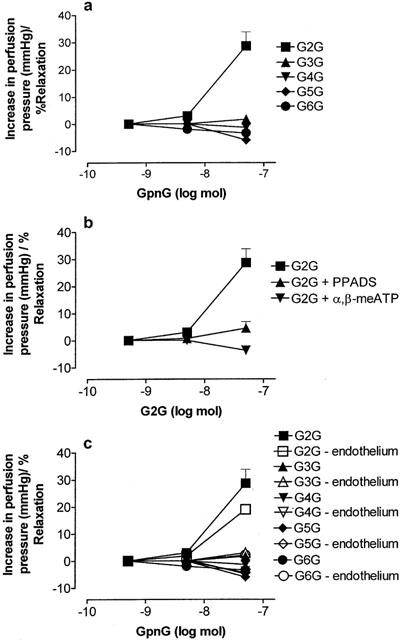
Dose-response curves to GpnG (n=2 – 6) in the rat isolated perfused methoxamine-preconstricted mesenteric arterial bed. (a) Control conditions; (b) responses to GpnG in the presence of pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS; 10 μM) and α,β-methylene ATP (α,β-meATP; 10 μM); (c) responses to GpnGs with and without endothelium removal (n=4 – 6). Data are shown as mean+s.e.mean. Where error bars do not appear, these fall within the symbol.
Discussion
This study has shown that for ApnGs as well as for ApnAs, the length of the polyphosphate chain is a crucial determinant of vasomotor activity via P2 receptors in the rat isolated mesenteric arterial bed. Hence, short chain ApnGs and ApnAs (n=2 – 3) are vasorelaxants (via endothelial P2Y1-like receptors when n=3), whilst longer chain ApnGs and ApnAs (n=4 – 6) are vasoconstrictors, via smooth muscle P2X1-like receptors. In addition, this study shows that contractile ApnAs and ApnGs (n=4 – 6) can additionally mediate vasorelaxation; prolonged vasorelaxation following vasoconstriction, and rapid vasorelaxation after blockade of P2X1 receptor-mediated vasoconstriction. Regarding the purine moiety, one adenine is crucial and sufficient for vasoactivity as GpnGs were largely inactive, and ApnAs and ApnGs approximately equipotent.
Ap4A, Ap5A and Ap6A were vasoconstrictors, with Ap4A being the least potent, as reported previously in the rat isolated mesenteric arterial bed (Ralevic et al., 1995). The pD30 values were not significantly different from those we reported previously for ApnAs, indicating consistency between the commercially available (Ralevic et al., 1995) and synthesized compounds (present study) and reproducibility of the assay. A similar potency order of ApnAs, as activators of P2X1-like receptor inward currents and mediators of vasoconstriction, has recently been reported in rat mesenteric artery rings and acutely dissociated smooth muscle cells (Lewis et al., 2000). In the present study, Ap4G, Ap5G and Ap6G elicited vasoconstriction with a similar potency, and order of potency, as the corresponding ApnAs. Similar activities of contractile ApnAs and their corresponding ApnGs have also been reported in the rat isolated perfused kidney (van der giet et al., 1997; Schlüter et al., 1998). In contrast, Lewis et al. (2000) found that ApnGs were significantly less potent than the corresponding ApnAs at mediating P2X responses in rat mesenteric arteries and dissociated smooth muscle cells. The reason for this difference is not entirely clear. However, in the present study dinucleotides were applied luminally as bolus doses, and there was a small but significant decrease in the contractile action of Ap5G and Ap6G after removing the endothelium, which was not observed for the corresponding ApnAs, which may indicate a partial degradation of the ApnGs by endothelial ectohydrolases. Hence, in the presence of the endothelium ATP may be produced, which masks the lower efficacy of Ap5G and Ap6G at the P2X1 receptor.
ApnAs and ApnGs are degraded by asymmetrical and symmetrical hydrolases and phosphorylases to yield ATP, ADP, AMP, adenosine, GTP, GDP, GMP and guanosine. However, the very much higher contractile activity of the dinucleotides compared with their metabolites (e.g. Ap5A, Ap6A and Ap4A are 126 fold, 63 fold and 32 fold more potent, respectively than ATP at mediating contractions in the rat mesenteric arterial bed (Ralevic et al., 1995)) indicates that it is unlikely that their actions are mediated principally by mononucleotide breakdown products. Similar conclusions were drawn by Schlüter et al. (1998), who showed that all potential degradation products of the ApnGs were considerably less active than the diguanosine polyphosphates at mediating vasoconstriction in the rat isolated perfused kidney. Further support for a direct action of ApnAs and ApnGs was provided with the finding that recovery of these compounds in the effluent of the perfused kidney was similar to that of the nonhydrolyzable compound α,β-meATP, and that their half lives when incubated with smooth muscle cells in culture were of the order of 1 h (Schlüter et al., 1998). Moreover, there is direct evidence for an action of ApnAs (n=4 – 6) at recombinant P2X1 receptors (Wildman et al., 1999) and for ApnAs and ApnGs (n=4 – 6) in evoking contraction and P2X receptor currents (Lewis et al., 2000). However, Lewis et al. (2000) did reveal a mismatch between the activities of longer chain length polyphosphates at mediating contraction in mesenteric arteries and inward currents in dissociated smooth muscle cells, the latter carried out under concentration clamp conditions where agonist breakdown is minimized, which indicates that the activity of certain dinucleotides at P2X receptors may be overestimated due to metabolic breakdown.
Contractions mediated by ApnAs and ApnGs were blocked by PPADS, a P2 receptor antagonist (Lambrecht et al., 1992; Ziganshin et al., 1994; Windscheif et al., 1994) and by α,β-meATP, indicating actions at P2X1 receptors, the principal isoform of P2X receptor in rat mesenteric arteries (Lewis & Evans, 2000). Contractions were largely unaffected by endothelium removal, consistent with actions mediated via receptors on the smooth muscle. Lewis et al. (2000) also found that responses to ApnAs and ApnGs were mediated via P2X1 receptors in rat mesenteric artery dissociated smooth muscle cells. In contrast, a suramin- and PPADS-resistant component of vasoconstriction to Ap4A and Ap6A in rat kidney may be mediated independently of P2X receptors (van der giet et al., 1997).
A large and prolonged vasorelaxation following vasoconstriction by the longer chain purines (n=4 – 6) was observed for both ApnAs and ApnGs. Prolonged vasorelaxation following P2X receptor-mediated vasoconstriction has previously been shown for ATP in the rat isolated mesenteric arterial bed, and is endothelium-independent and blocked by PPADS and by α,β-meATP desensitization (Stanford & Mitchell, 1998; Ralevic, 2001). The present study shows that prolonged vasorelaxations mediated by ApnAs and ApnGs (n=4 – 6) are also endothelium-independent and blocked by α,β-meATP and PPADS. Interestingly, the rank order of potency of the ApnAs and ApnGs in eliciting prolonged vasorelaxation was similar to that of the contractile potency order of the compounds, suggesting a possible involvement of P2X receptor activation in initiation of the response. PPADS-sensitive, endothelium-independent prolonged relaxations following vasoconstriction to ApnAs have also recently been described in rat isolated mesenteric resistance arteries (Steinmetz et al., 2000).
Interestingly, in the presence of α,β-meATP rapid vasorelaxations to ApnAs and ApnGs (n=4 – 6) were observed, indicating that actions of these compounds at contractile P2X1-like receptors normally opposes rapid vasorelaxation. Similarly, in the rat raised-tone isolated perfused kidney, suramin block of Ap4A-mediated vasoconstriction revealed vasorelaxation (van der giet et al., 1997). The present study is the first full report of vasorelaxant actions of Ap4G, Ap5G and Ap6G, masked under normal conditions by the contractile actions of these compounds. Rapid relaxations preceding vasoconstriction to ApnAs have recently been reported in rat isolated preconstricted mesenteric resistance arteries (Steinmetz et al., 2000). Dual and opposing actions of dinucleotides were also reported by Busse et al. (1988) who described an Ap4A-mediated endothelium-dependent vasorelaxation, which was reversed to a pronounced contraction after endothelium removal in rabbit mesenteric arteries. Collectively, these data indicate that contractile ApnAs and ApnGs can have complex effects at multiple purine receptors in the vasculature.
Experiments carried out in order to characterize the receptor mediating rapid relaxations to contractile ApnAs and ApnGs (n=4 – 6) after P2X receptor blockade produced mixed results. In the presence of α,β-meATP rapid relaxations to ApnAs were not blocked by 8-PSPT, suggesting no involvement of an adenosine P1 receptor, but neither were they all blocked by PPADS or endothelium removal, appearing to rule out an involvement of P2Y1 and P2Y2 receptors. Consistent with this is the report that Ap4A, Ap5A and Ap6A are weak or inactive at recombinant P2Y1 receptors (Pintor et al., 1996). In contrast, rapid relaxations to ApnGs were variously blocked by endothelium removal, PPADS and 8-PSPT, suggesting possible actions at both P1 and P2 receptors. Although suramin was able to block responses to both ApnAs and ApnGs these data may have to be viewed with caution as Ap5A was still able to elicit vasoconstriction, suggesting that there was no longer full desensitization of P2X receptors. Whether the relaxations are due to direct actions of the compounds, or due to vasorelaxant fragments generated by ectoenzymatic hydrolysis, remains to be determined.
Vasorelaxations to Ap2A and Ap3A were observed and, as reported previously, Ap3A was more potent than Ap2A (Ralevic et al., 1995). In the present study we additionally show for the first time that the shorter phosphate chain ApnGs, Ap2G and Ap3G, are vasorelaxants in the rat isolated mesenteric arterial bed. Both were less potent than the corresponding ApnA, and Ap3G was more potent than Ap2G. Interestingly, responses to both Ap3A and Ap3G were endothelium-dependent, and were blocked by PPADS, but those to Ap2A and Ap2G were independent of the endothelium and were unaffected by PPADS. It seems likely, therefore, that relaxations to Ap3A and Ap3G are mediated by PPADS-sensitive P2Y1 receptors and not by PPADS-insensitive P2Y2 receptors coexpressed on the endothelium (Ralevic & Burnstock, 1996). In line with this suggestion, Ap3A, but not Ap2A, is an agonist at recombinant P2Y1 receptors (Pintor et al., 1996). In contrast, in the rat isolated perfused kidney vasorelaxant responses to Ap3A and Ap3G are mediated via A2 receptors, and these compounds also elicit vasoconstriction via A1 receptors (van der giet et al., 1997). In the present study, relaxation by Ap2A and Ap2G may be mediated via adenosine A2 (A2B subtype) receptors on the smooth muscle of rat mesenteric arteries (Rubino et al., 1995). Thus, the length of the polyphosphate chain can determine not only the selectivity and potency of these purine compounds at different subtypes of P2 receptors, but also their selectivity for P2 versus P1 receptors.
The GpnGs were largely inactive, except for Gp2G (which elicited α,β-meATP- and PPADS-sensitive P2X1-like receptor-mediated vasoconstriction), indicating that expression of an adenine moiety is crucial for vasoactivity of dipurine polyphosphates. Moreover, a single adenine was sufficient for full activity of the molecule, as potency was generally not greater with two adenines (ApnAs) compared to one adenine (ApnGs). GpnGs have also been shown to be inactive as modulators of vascular tone in the rat isolated perfused kidney, but are potent modulators of growth in vascular smooth muscle cells (Schlüter et al., 1998).
ApnAs, ApnGs and GpnGs are found in a number of different cell types, including hepatocytes, adrenal medullary chromaffin and myocardial granules, erythrocytes and platelets (Rapaport & Zamenick, 1976; Flodgaard & Klenow, 1982; Lüthje & Ogilvie, 1983; Rodriguez-Del-castillo et al., 1988; Schlüter et al., 1994; Luo et al., 1999a,1999b). Platelets and erythrocytes may be particularly significant sources of these compounds relevant to the regulation of vascular tone. Endothelial cell damage is a trigger for platelet aggregation, which may lead to the release of ApnAs, ApnGs and GpnGs to vasoactive concentrations extracellularly, and vasoconstriction. However, evidence of a prolonged relaxation that follows contraction to ApnAs and ApnGs (n=4 – 6) indicates that the vasospasmic action of the purines may be self-limiting.
In conclusion, we have shown that for ApnGs as well as for ApnAs, the length of the phosphate chain has a pivotal role in determining activity at P2 receptors in the rat isolated mesenteric arterial bed. Hence, when the phosphate chain is short (n=2 – 3) the compounds are vasodilators, whilst when the chain is long (n=4 – 6) the compounds are vasoconstrictors via P2X1-like receptors. Moreover, we have shown for the first time that contractile ApnAs and ApnGs (n=4 – 6) can mediate vasorelaxation; prolonged vasorelaxation following vasoconstriction, and rapid relaxation after block of P2X1 receptor-mediated constriction. Regarding the purine moiety, one adenine is crucial and sufficient for vasoactivity as GpnGs are largely inactive, and ApnA and ApnGs approximately equipotent.
Acknowledgments
We are grateful to the Royal Society for financial support.
Abbreviations
- ApnAs
diadenosine polyphosphates
- ApnGs
adenosine polyphospho guanosines
- GpnGs
guanosine polyphospho guanosines
- α,β-me ATP
α,β-methylene ATP
- PPADS
pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid
References
- BUSSE R., OGILVIE A., POHL U. Vasomotor activity of diadenosine triphosphate and adenosine tetraphosphate in isolated arteries. Am. J. Physiol. 1988;254:H828–H832. doi: 10.1152/ajpheart.1988.254.5.H828. [DOI] [PubMed] [Google Scholar]
- FLODGAARD H., KLENOW H. Abundant amounts of the diadenosine 5′,5′′′-P1,P4-tetraphosphate are present and releasable, but metabolically inactive in human platelets. Biochem. J. 1982;208:737–742. doi: 10.1042/bj2080737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYLE C.H.V. Pharmacological activity of adenine dinucleotides in the periphery: possible receptor classes and transmitter function. Gen. Pharmacol. 1990;21:827–831. doi: 10.1016/0306-3623(90)90440-w. [DOI] [PubMed] [Google Scholar]
- HOYLE C.H.V., HILDERMAN R.H., PINTOR J.J., SCHLÜTER H., KING B.F. Diadenosine polyphosphates as extracellular signaling molecules. Drug Dev. Res. 2001;52:260–273. [Google Scholar]
- JANKOWSKI J., HAGEMANN J., TEPEL M., VAN DER GIET M., STEPHAN N., HENNING L., GOUNI-BERTHOLD I., SACHINIDIS A., ZIDEK W., SCHLÜTER H. Dinucleotides as growth promoting extracellular mediators: presence of dinucleoside diphosphates Ap2A, Ap2G and Gp2G in releasable granules of platelets. J. Biol. Chem. 2001;276:8904–8909. doi: 10.1074/jbc.M009527200. [DOI] [PubMed] [Google Scholar]
- JANKOWSKI J., TEPEL M., VAN DER GIET M., TENTE I.M., HENNING L., JUNKER R., ZIDEK W., SCHLÜTER H. Identification and characterization of P(1), P(7)-di(adenosine-5′)-heptaphosphate from human platelets. J. Biol. Chem. 1999;274:23926–23931. doi: 10.1074/jbc.274.34.23926. [DOI] [PubMed] [Google Scholar]
- LAMBRECHT G., FRIEBE T., GRIMM U., WINDSCHEIF U., BUNGARDT E., HILDEBRANDT C., BÄUMERT H.G., SPATZ-KUMBEL G., MUTSCHLER E. PPADS, a novel functionally selective antagonist of P2 purinoceptor-mediated responses. Eur. J. Pharmacol. 1992;217:217–219. doi: 10.1016/0014-2999(92)90877-7. [DOI] [PubMed] [Google Scholar]
- LEWIS C.J., EVANS R.J. Lack of run-down of smooth muscle P2X receptor currents recorded with the amphotericin permeabilized patch technique, physiological and pharmacological characterization of the properties of mesenteric artery P2X receptor ion channels. Br. J. Pharmacol. 2000;131:1659–1666. doi: 10.1038/sj.bjp.0703744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS C.J., GITTERMAN D.P., SCHLÜTER H., EVANS R.J. Effects of diadenosine polyphosphates (ApnAs) and adenosine polyphospho guanosines (ApnGs) on rat mesenteric artery P2X receptor ion channels. Br. J. Pharmacol. 2000;129:124–130. doi: 10.1038/sj.bjp.0702993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUO J., JANKOWSKI J., KNOBLOCH M., VAN DER GIET M., GARDANIS K., RUSS T., VAHLENSIECK U., NEUMANN J., SCHMITZ W., TEPEL M., DENG M.C., ZIDEK W., SCHLÜTER H. Identification and characterization of diadenosine 5′5′′′-P1,P2-diphosphate and diadenosine 5′,5′′′-P1,P3-triphosphate in human myocardial tissue. Faseb J. 1999a;13:695–705. doi: 10.1096/fasebj.13.6.695. [DOI] [PubMed] [Google Scholar]
- LUO J., JANKOWSKI J., TEPEL M., VAN DER GIET M., ZIDEK W., SCHLÜTER H. Identification of diadenosine hexaphosphates in human erythrocytes. Hypertension. 1999b;34:872–875. doi: 10.1161/01.hyp.34.4.872. [DOI] [PubMed] [Google Scholar]
- LÜTHJE J., OGILVIE A. The presence of diadenosine 5′,5′′′-P1,P3-triphosphate (Ap3A) in human platelets. Biochem. Biophys. Res. Commun. 1983;115:253–260. doi: 10.1016/0006-291x(83)90997-x. [DOI] [PubMed] [Google Scholar]
- PINTOR J., KING B.F., MIRAS-PORTUGAL M.T., BURNSTOCK G. Selectivity and activity of adenine dinucleotides at recombinant P2X2 and P2Y1 purinoceptors. Br. J. Pharmacol. 1996;119:1006–1012. doi: 10.1111/j.1476-5381.1996.tb15771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINTOR J., MIRAS-PORTUGAL M.T. P2 purinergic receptors for diadenosine polyphosphates in the nervous system. Gen. Pharmacol. 1995;26:229–235. doi: 10.1016/0306-3623(94)00182-m. [DOI] [PubMed] [Google Scholar]
- RALEVIC V. Mechanism of prolonged vasorelaxation to ATP in the rat isolated mesenteric arterial bed. Br. J. Pharmacol. 2001;132:685–692. doi: 10.1038/sj.bjp.0703868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Actions mediated by P2-purinoceptor subtypes in the isolated perfused mesenteric bed of the rat. Br. J. Pharmacol. 1988;95:637–645. doi: 10.1111/j.1476-5381.1988.tb11686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Discrimination by PPADS between endothelial P2Y- and P2U-purinoceptors in the rat isolated mesenteric arterial bed. Br. J. Pharmacol. 1996;118:428–434. doi: 10.1111/j.1476-5381.1996.tb15420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- RALEVIC V., HOYLE C.H.V., BURNSTOCK G. Pivotal role of phosphate chain length in vasoconstrictor versus vasodilator actions of adenine dinucleotides in rat mesenteric arteries. J. Physiol. 1995;483:703–713. doi: 10.1113/jphysiol.1995.sp020615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPAPORT E., ZAMENICK P.C. Presence of diadenosine 5′,5′′′-P1,P4-tetraphosphate (Ap4A) in mammalian cells in levels varying widely with proliferative activity of the tissue: a possible positive pleiotypic activator. Proc. Natl. Acad. Sci. U.S.A. 1976;73:3984–3988. doi: 10.1073/pnas.73.11.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODRIGUEZ-DEL-CASTILLO A., TORRES M., DELICADO F.G., MIRAS-PORTUGAL M.T. Subcellular distribution studies of diadenosine polyphosphates–Ap4A and Ap5A – in bovine adrenal medulla: presence in chromaffin granules. J. Neurochem. 1988;51:1696–1703. doi: 10.1111/j.1471-4159.1988.tb01147.x. [DOI] [PubMed] [Google Scholar]
- RUBINO A., RALEVIC V., BURNSTOCK G. Contribution of P1 (A2b subtype) and P2-purinoceptors to the control of vascular tone in the rat isolated mesenteric arterial bed. Br. J. Pharmacol. 1995;115:648–652. doi: 10.1111/j.1476-5381.1995.tb14981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLÜTER H., GROSS I., BACHMANN J., KAUFMANN R., VAN DER GEIT M., TEPEL M., NOFER J., ASSMANN G., KARAS M., JANKOWSKI J., ZIDEK W. Adenosine(5′) oligophospho-(5′) guanosines and guanosine (5′) oligophospho-(5′) guanosines in human platelets. J. Clin. Invest. 1998;101:682–688. doi: 10.1172/JCI119882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLÜTER H., JANKOWSKI J., VAN DER GIET M., TEPEL M., ZIDEK W. Purification and identification of dinucleoside polyphosphates in cells and tissues. Drug Dev. Res. 2000;50:40. [Google Scholar]
- SCHLÜTER H., OFFERS E., BRUGGEMANN G., VAN DER GIET M., TEPEL M., NORDHOFF E., KARAS M., SPIEKER C., WITZEL H., ZIDEK W. Diadenosine phosphates and the control of blood pressure. Nature. 1994;367:186–188. doi: 10.1038/367186a0. [DOI] [PubMed] [Google Scholar]
- STANFORD S.J., MITCHELL J.A. ATP-induced vasodilatation in the rat isolated mesenteric bed exhibits two apparent phases. Br. J. Pharmacol. 1998;125:94P. [Google Scholar]
- STEINMETZ M., SCHLATTER E., BOUDIER H.A.J.S., RAHN K.H., DE MEY J.G.R. Diadenosine polyphosphates cause contraction and relaxation in isolated rat resistance arteries. J. Pharmacol. Exp. Ther. 2000;294:1175–1181. [PubMed] [Google Scholar]
- SUMIYOSHI R., NISHIMURA J., KAWASAKI J., KOBAYASHI S., TAKAHASHI S., KANAIDE H. Diadenosine polyphosphates directly relax porcine coronary arterial smooth muscle. J. Pharmacol. Exp. Ther. 1997;283:548–556. [PubMed] [Google Scholar]
- VAN DER GIET M., KHATTAB M., BÖRGEL J., SCHLÜTER H., ZIDEK W. Differential effects of diadenosine phosphates on purinoceptors in the rat isolated perfused kidney. Br. J. Pharmacol. 1997;120:1453–1460. doi: 10.1038/sj.bjp.0701074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILDMAN S.S., BROWN S.G., KING B.F., BURNSTOCK G. Selectivity of diadenosine polyphosphates for rat P2X receptor subunits. Eur. J. Pharmacol. 1999;367:119–123. doi: 10.1016/s0014-2999(98)00976-5. [DOI] [PubMed] [Google Scholar]
- WINDSCHEIF U., RALEVIC V., BÄUMERT H.G., MUTSCHLER E., LAMBRECHT G., BURNSTOCK G. Vasoconstrictor and vasodilator responses to various agonists in the rat perfused mesenteric arterial bed: selective inhibition by PPADS of contractions mediated via P2X-purinoceptors. Br. J. Pharmacol. 1994;113:1015–1021. doi: 10.1111/j.1476-5381.1994.tb17094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIGANSHIN A.U., HOYLE C.H.V., LAMBRECHT G., MUTSCHLER E., BÄUMERT H.G., BURNSTOCK G. Selective antagonism by PPADS at P2X-purinoceptors in rabbit isolated blood vessels. Br. J. Pharmacol. 1994;111:923–929. doi: 10.1111/j.1476-5381.1994.tb14827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


