Abstract
We have investigated the effect of U73122, a specific inhibitor of phospholipase C (PLC), on acetylcholine-activated K+ currents (IKACh) in mouse atrial myocytes.
In perforated patch clamp mode, IKACh was activated by 10 μM acetylcholine. When atrial myocytes were pretreated with U73122 or U73343, IKACh was inhibited dose-dependently (half-maximal inhibition at 0.12±0.0085 and 0.16±0.0176 μM, respectively). The current-voltage relationships for IKACh in the absence and in the presence of U73122 showed that the inhibition occurred uniformly from −120 to +40 mV, indicating a voltage-independent inhibition.
When U73122 was applied after IKACh reached steady-state, a gradual decrease in IKACh was observed. The time course of the current decrease was well fitted to a single exponential, and the rate constant was proportional to the concentration of U73122.
When KACh channels were directly activated by adding 1 mM GTPγS to the bath solution in inside-out patches, U73122 (1 μM) decreased the open probability significantly without change in mean open time. When KACh channels were activated independently of G-protein activation by 20 mM Na+, open probability was also inhibited by U73122.
Voltage-activated K+ currents and inward rectifying K+ currents were not affected by U73122.
These findings show that inhibition by U73122 and U73343 of KACh channels occurs at a level downstream of the action of Gβγ or Na+ on channel activation. The interference with phosphatidylinositol 4,5-bisphosphate (PIP2)-channel interaction can be suggested as a most plausible mechanism.
Keywords: U73122; U73343; acetylcholine-activated K+ current; phospholipase C; phospholipase C inhibitor; atrial myocytes; phosphatidylinositol 4,5-bisphosphate; patch-clamp
Introduction
U73122 has been shown to inhibit phospholipase C (PLC) at low micromolar concentrations (Smith et al., 1990; Bleasdale et al., 1990), and it is one of the most widely used PLC inhibitors. Very often the structurally related U73343 is used as negative control, since it does not inhibit PLC (Smith et al., 1990; Bleasdale et al., 1990). Recent studies, however, indicate that the action of U73122 and U73343 may not be specific as originally thought, since both agents also inhibit phospholipase D activity (Bosch et al., 1998), and since U73122 elicits intracellular calcium release (Muto et al., 1997). Furthermore, the non-selective actions of U73122 were also shown for a variety of receptor-mediated signal transductions, such as adenosine A1-receptor (Walker et al., 1998) and histamine H1-receptor (Hughes et al., 2000).
ACh-activated K+ currents (IKACh) are responsible for the inhibitory effect of cardiac function by ACh (Jan & Jan, 1997; Yamada et al., 1998). Activation of IKACh by ACh receptors (mAChR) is mediated via the pertussis toxin-sensitive G-protein. G-protein-ion channel coupling mechanisms have been widely investigated for IKACh and its molecular equivalent G-protein-gated inwardly rectifying K+ channels (GIRK), and it is now believed that the direct binding of G protein Gβγ subunits to the channel protein opens GIRK channels (Huang et al., 1995; Krapivinsky et al., 1995; Kunkel & Peralta, 1995; Inanobe et al., 1995). In addition to Gβγ subunits, GIRK channels can also be activated by Na+ ions through G-protein-independent pathway (Sui et al., 1996; 1998). Both Gβγ subunits and Na+ ion cause a stabilization of phosphatidylinositol 4,5-bisphosphate (PIP2)-channel interaction, which is absolutely required for channel opening (Huang et al., 1998).
In the present study, we investigated the effects of U73122 and U73343 on IKACh in mouse atrial myocytes and found that both inhibit IKACh independently of PLC inhibition. The inhibition was almost complete at concentrations similar to or below those used for PLC inhibition. The target for U73122 action was investigated using inside-out patch recording, showing that the inhibition by U73122 occurs even when the channels are activated by Na+ independently of G-protein activation. These results suggest that U73122 inhibits KACh channels possibly by the interference with PIP2-channel interaction.
Methods
Cell isolation
The isolation of single atrial myocytes from mice was performed as previously described (Cho et al., 2001) with minor modification. Mice were injected with pentobarbitone (6 mg mouse−1, intraperitoneal) and heart was quickly removed after deep anaesthesia was established. The heart was cannulated by 24G needle and then retrogradely perfused via the aorta on a Langendorff apparatus. During coronary perfusion all perfusates were maintained at 37°C and equilibrated with 100% O2. Initially the heart was perfused with normal Tyrode solution for 2 – 3 min to clear the blood. The heart was then perfused with Ca2+ free solution for 3 min. Finally the heart was perfused with enzyme solution for 12 min. Enzyme solution contains 0.14 mg ml−1 collagenase (Yakult) in Ca2+ free solution. After perfusion with enzyme solution, the atria were separated from the ventricles, chopped into small pieces. Single cells were dissociated in high-K+ and low-Cl− solution from these small pieces using blunt-tip glass pipette and stored in the same solution at 4°C until use.
Materials and solutions
Normal Tyrode solution contained (mM): NaCl 140, KCl 5.4, MgCl2 0.5, CaCl2 1.8, glucose 10, HEPES 5, titrated to pH 7.4 with NaOH. Ca2+ free solution contained (mM): NaCl 140, KCl 5.4, MgCl2 0.5, glucose 10, HEPES 5, titrated to pH 7.4 with NaOH. The high-K+ and low-Cl− solution contained (mM): KOH 70, KCl 40, L-glutamic acid 50, taurine 20, KH2PO4 20, MgCl2 3, glucose 10, HEPES 10, EGTA 0.5. The pipette solution for perforated patches contained (mM): KCl 140, HEPES 10, MgCl2 1, EGTA 5, titrated to pH 7.2 with KOH. For single-channel experiments, the bath solution contained (mM): KCl 140, EGTA 5, MgCl2 1, HEPES 5, glucose 5, pH 7.4 (with KOH). The pipettes solution contained (mM): KCl 140, CaCl2 1.8, MgCl2 1, HEPES 5, pH 7.4 (with KOH).
Acetylcholine (Sigma) was dissolved in deionized water to make a stock solution (10 mM) and stored at −20°C. On the day of experiments one aliquot was thawed and used. U73122 (Biomol) or U73343 (Biomol) was first dissolved in DMSO as a stock solution and then used at the final concentration in the solution. Final concentrations of DMSO did not exceed 0.1% and were without effect on IKACh. Free Mg2+ and ATP concentrations were estimated as described by Vivaudou et al. (1991). General chemical agents, including GTPγS and ATP, were purchased from Sigma. All experiments were conducted at 35±1°C. In the presence of ACh, 10 μM glibenclamide was applied to inhibit the ATP- sensitive K+ channel. To ensure the rapid solution change, the rate of superfusion was kept above 5 ml min−1. Considering that the volume of the bath was 100 μl, bath content could be replaced within 30 s when a new solution was applied.
Voltage-clamp recording and analysis
Whole cell currents were recorded from single isolated myocytes in a perforated patch configuration using nystatin (Sigma, 200 μg ml−1). Voltage clamp was performed using an Axopatch-1C amplifier (Axon instruments). The patch pipettes were pulled from borosilicate capillaries (Harvard apparatus) using a Narishige puller (PP-83, Japan). We used patch pipettes with a resistance of 2 – 3 MΩ when filled with the above pipette solutions. After the gigaseal was made, we usually waited 10 – 15 min until the series resistance decreased below 10 MΩ to allow the successful whole cell configuration. In most cells, this condition remained stable during the whole period of experiment. But, when the series resistance began to increase, we stopped recording and the data were discarded. The electrical signals were displayed during the experiments on an oscilloscope (Tektronix, TDS 210) and a chart recorder (Gould). Voltage clamp and data acquisitions were performed by a digital interface (Digidata 1200, Axon Instruments) coupled to an IBM compatible computer using pClamp software 5.7.1 (Axon instruments) at a sampling rate of 1 – 2 kHz, and filtered at 5 kHz.
Single channel activity was recorded in the inside-out patch configuration by using Axopatch 200A amplifier (Axon instruments). Fire-polished pipettes (5 – 6 MΩ) were used. Channel activity was monitored at −80 mV at a sampling rate of 5 kHz, and filtered at 1 kHz. Single channel records were analysed by using PCLAMP software. Parameters used for single-channel analysis include the total open probability, (NPo), and the mean open time. Results are displayed as averages over 5-s bins.
Statistics and presentation of data
The results in the text and in the figures are presented as means±s.e.mean (n=number of cells tested). Statistical analyses were performed using the Student's t-test. The difference between two groups was considered to be significant when P<0.01, and not significant when P>0.05.
Results
U73122 inhibits KACh channels
Figure 1 shows the IKACh recorded from a single isolated atrial myocyte using the nystatin-perforated patch clamp technique. When the membrane potential was held at −40 mV, application of 10 μM ACh induced a rapid activation of outward IKACh followed by a decrease in currents due to desensitization. Desensitization was fully recovered after 4 min washout, and the amplitude of IKACh at second exposure was equivalent to that obtained at the first exposure (data not shown), as was previously reported (Cho et al., 2001). However, when cells were pretreated with U73122 (0.2 μM) for 3 min before the second application of ACh, the peak and steady-state IKACh were significantly inhibited (Figure 1a). This inhibition was sustained, and the IKACh was not recovered by re-exposure to ACh after washout of U73122. When the concentration of U73122 was increased to 1 μM, IKACh was almost abolished, indicating that inhibitory effect of U73122 on IKACh is concentration-dependent (Figure 1b). Dose – response relationships for the inhibition of IKACh by the pretreatment of U73122 for 3 min are shown in Figure 1c. The data were fitted with the Hill equation, showing that the concentration for the half-maximal inhibition (IC50) was 0.12±0.0085 μM and a Hill coefficient was 2.33±0.31 (n=8). This concentration is only about one tenth of the concentration required for the half-maximal inhibition of PLC (IC50) which was reported to be 1.0 – 2.0 μM (Bleasdale et al., 1990; Smith et al., 1990).
Figure 1.

Effect of U73122 on IKACh. Chart recordings of the whole-cell current at a holding potential of −40 mV. The dotted line indicates zero current level. (a) The applications of 10 μM ACh and 0.2 μM U73122 are indicated by the horizontal bar above the current trace. The vertical deflections of current trace are the responses to voltage ramps. (b) An experiment with the same protocol as described in (a) with 1 μM U73122. (c) Dose-response relationship for U73122. The amplitude of peak IKACh in the absence of U73122 was regarded as control. The per cent inhibition was plotted against U73122 concentration.
Since it is not certain that the effect of U73122 reached steady-state after 3 min, we investigated the kinetics of inhibition by adding U73122 after IKACh reached quasi-steady-state (Figure 2a). At 0.2 μM, IKACh decreased very slowly, and the inhibition was far from steady-state after 3 min. But the inhibition continued, and almost complete inhibition was obtained after 10 min (Figure 2b). The rate of the current decrease became much faster at higher concentrations of U73122. When the time course of the current decrease was fitted to single exponential functions, time constants (τ) at each concentration (0.2, 1, 10) of U73122 were 214.06±7.29, 48.04±0.01, 8.67±0.40 s, respectively. From these values, association rate constant was calculated to be 0.01±0.0002 μM−1s−1 (n=5, Figure 2c).
Figure 2.

The kinetics of U73122 inhibition. (a) Currents were recorded at a holding potential of −40 mV and IKACh was elicited by application of 10 μM ACh. Various concentrations of U73122 were added after IKACh reached quasi-steady-state. (b) The amplitude of IKACh were normalized by the value of the first exposure to U73122. The time constants (τ) were obtained from a single exponential fitting to the decaying traces of IKACh. (c) The reciprocal of time constants was plotted against U73122 concentration. The solid line represents the least-squares fit of the data to the relation 1/τ=k+1[D]+k−1. Data were expressed as means±s.e.mean.
To determine the influence of the membrane potential on the inhibition of U73122 on IKACh, the current – voltage (I – V) relationships were obtained from the current response induced by voltage ramps between +60 and −120 mV (at a speed of 0.6 V s−1) from the holding potential of −40 mV. The ramps were applied before ACh application (a), at peak activation of IKACh (b), and at peak activation of IKACh in the presence of U73122 after pretreatment for 3 min (c), as indicated in Figure 1a. Net IKACh was obtained by subtracting control (a) from the current response in the presence of ACh (b,c), and corresponding I – V curves were plotted in Figure 3a. Apart from the decrease in conductance in the presence of U73122, no significant change in the shape of I – V curves was noticed. The per cent inhibition of IKACh by U73122 at −120, −40, and +40 mV were 65.7±12.9, 71.9±8.7, and 70.8±8.1%, respectively (n=3, Figure 3b). They were not significantly different (P>0.05), indicating that the inhibition of IKACh by U73122 is voltage-independent.
Figure 3.

Effect of the membrane potential on the inhibition of U73122 on IKACh. (a) The I – V curves for net IKACh at peak in the absence (b-a) and in the presence of U73122 (c-a) were from the data in Figure 1a. (b) The bar graph of the extent of inhibition measured at −120, −40, and +40 mV (n=3). Note that they were not significantly different (P>0.05).
To test the possibility that the inhibition of IKACh by U73122 is caused by PLC inhibition, we examined the effect of U73343, which is structurally related to U73122 but lacks PLC inhibitory activity. As shown in Figure 4a, U73343 inhibited IKACh. Effect of U73343 was completely reversed after 10 min washout, whereas the effect of U73122 was hardly reversed. Dose – response relationships for the inhibition of IKACh by the pretreatment of U73343 for 3 min are shown in Figure 4b. The data were fitted with the Hill equation, showing that the concentration for the half-maximal inhibition (IC50) was 0.16±0.0176 μM and a Hill coefficient was 1.33±0.18 (n=5). Above result shows that both U73122 and U73343 inhibit IKACh irrespective of PLC inhibition.
Figure 4.

Effect of U73343 on IKACh. Chart recordings of the whole-cell current at a holding potential of −40 mV. The dotted line indicates zero current level. (a) The applications of 10 μM ACh and 0.1 μM U73343 are indicated by the horizontal bars above the current trace. (b) Dose-response relationship for U73343. The amplitude of peak IKACh in the absence of U73343 was regarded as control. The per cent inhibition was plotted against U73343 concentration.
U73122 inhibits KACh channels activated by GTPγS or Na+ in inside-out patches
In order to investigate the level at which U73122 is acting for inhibiting IKACh, we examined whether U73122 inhibited KACh channels in excised patches. To test the possibility that the inhibition produced by U73122 occurred through alteration in the coupling between G protein and the M2 muscarinic receptor, M2 muscarinic receptor was by-passed and KACh channels were directly activated by adding 1 mM GTPγS to the bath solution. Figure 5a shows a representative trace of single-channel activity recorded after perfusing GTPγS from an inside-out patch of atrial membrane held at −80 mV. Usually, two or three channels were included in one patch, but the amplitude of a single channel opening was well resolved. When the I – V relationship for single channel currents was obtained at various potentials, it showed an inward rectification with a mean slope conductance of 42.4±0.7 pS (n=4) in an inward direction (data not shown). Mean open time under control condition was 0.77±0.04 ms (n=4) at a patch membrane potential of −80 mV. These are characteristic features of KACh channel in heart cells (Sakmann et al., 1983; Soejima & Noma, 1984; Logothetis et al., 1987; Kurachi et al., 1990). When 1 μM U73122 was applied to the bath solution, the KACh channel activity was markedly decreased, while the single channel amplitude was not changed. The open probability, NPo was decreased from 0.048±0.01 to 0.003±0.001 (n=4, P<0.01, Figure 5b) by 1 μM U73122. Mean open time was not significantly affected by 1 μM U73122 (0.72±0.17 ms, n=4, P>0.05). This result suggests that the inhibition by U73122 of KACh channels occurs at the level downstream of G-protein activation. U73122 may possibly act either on coupling between G-protein and KACh channel or on PIP2-channel interaction. To resolve these two possibilities, KACh channel were activated by Na+ ions in the absence of agonist and internal GTP.
Figure 5.
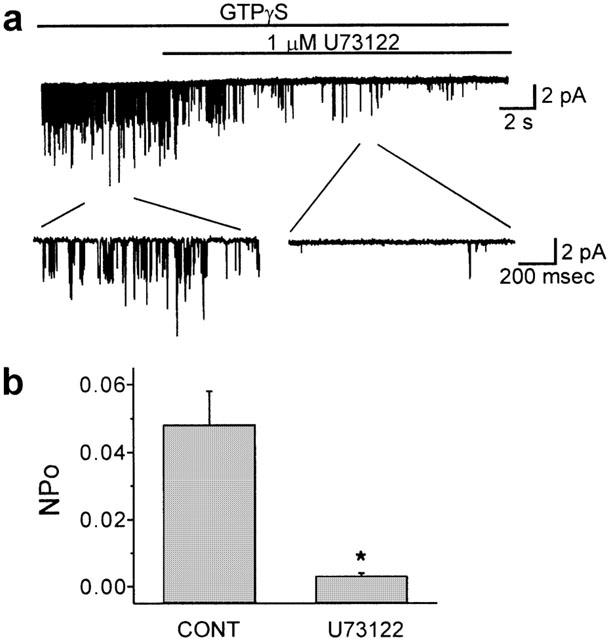
U73122 inhibits KACh channels activated by GTPγS. (a) KACh channels in inside-out patch were activated by bath perfusion of 1 mM GTPγS at a holding potential of −80 mV. (b) Changes in open probability (NPo) after U73122. Values are means±s.e.mean of four cells. *P<0.01 vs control.
It has been shown previously that Na+ ions can stimulate KACh channel activity in an ATP-dependent manner in the absence of G protein signaling, by stabilizing PIP2-channel interaction (Sui et al., 1996; 1998). In this respect, Na+ ion is acting similarly to Gβγ subunit, However, the binding site for Na+ in the channel protein was shown to be different from that for Gβγ (Logothetis & Zhang, 1999; Ho & Murrell-Lagnado, 1999a, 1999b; Zhang et al., 1999). We postulated that if U73122 interferes Gβγ activation of KACh channels, U73122 would not suppress Na+-induced activation of KACh channels. As shown in Figure 6a, application of 20 mM Na+ with 5 mM Mg-ATP to inside-out patches activated KACh channels. This channel showed an inward rectification with a single channel conductance of 42.9±1.2 pS (n=5) and its mean open time at −80 mV was 0.75±0.15 ms (n=5). These characteristics were similar to those reported for the KACh channel (Sakmann et al., 1983; Soejima & Noma, 1984; Logothetis et al., 1987; Kurachi et al., 1990) and not different from those for the GTPγS-induced channel (Figure 5). Thereafter, 1 μM U73122 was included in the bath solution, and the KACh channel activity decreased in the continued presence of MgATP/Na+. The open probability, NPo was decreased from 0.117±0.015 to 0.007±0.002 (n=5, P<0.01, Figure 6b) by 1 μM U73122. However, the mean open time was not significantly affected by U73122 (0.72±0.17 ms, n=5, P>0.05). This result suggests that U73122 inhibits KACh channels by interfering with activation steps common to both Gβγ subunits and Na+ ion, and PIP2-channel interaction appears to be the most plausible target for the action of U73122.
Figure 6.

U73122 inhibits KACh channels activated by Na+. (a) KACh channels in inside-out patch were activated by bath perfusion of 20 mM Na+ with 5 mM MgATP at a holding potential of −80 mV. (b) changes in open probability (NPo) after U73122. Values are means±s.e.mean of five cells. *P<0.01 vs control.
Effect of U73122 on other K+ channels
From above experiments, the possibility of direct blockade of KACh channels by U73122 is not completely excluded. To test this possibility, we tested the effect of U73122 on different types of ion channels. Considering that most K+ channel blockers are not specific to a certain type of K+ channel but block other K+ channels, we tested the effects of U73122 on voltage-activated K+ currents and inward rectifying K+ (IRK) currents. Na+ currents were inactivated by the prepulse to −40 mV for 10 ms, and Ca2+ currents were blocked with the 1 μM nicardipine. As shown in Figure 7a,b, U73122 at 1 μM hardly affected the transient outward currents and delayed rectifier K+ currents elicited by depolarizing pulse. IRK currents activated by hyperpolarizing pulse were not affected, either. I – V curves were shown in Figure 7c. This result implies that the effect of U73122 on ionic currents of atrial myocytes was specific to KACh channels.
Figure 7.

Effects of 1 μM U73122 on voltage dependent K+ currents recorded using the standard whole-cell clamp technique. Superimposed current traces induced by hyperpolarizing and depolarizing pulses from a holding potential of −80 mV before (a) and after (b) 1 μM U73122. Current – voltage relationship for the peak current (circles) and the current at the end of 300 ms test pulse (squares) in the absence (closed symbols) and in the presence (open symbols) of U73122 (c).
Discussion
The results of the present study can be summarized as follows: (1) U73122 and its inactive counterpart U73343 inhibit acetylcholine-activated K+ currents (IKACh) in mouse atrial myocytes at concentrations commonly used in experiments investigating the role of PLC. (2) Inhibition of KACh channels by U73122 also occurred in inside-out patches when channels were activated directly by adding 1 mM GTPγS or 20 mM Na+ with 5 mM Mg-ATP to the bath solution. (3) Inward rectifying K+ (IRK) currents and voltage-activated K+ currents were not affected by U73122.
The concentration of U73122 for inhibiting IKACh was much lower than that for inhibiting PLC. ED50 for inhibition of IKACh when U73122 was pretreated for 3 min was 0.12 μM (Figure 1), while ED50 for inhibiting PLC was 1 – 2 μM (Bleasdale et al., 1990; Smith et al., 1990). If pretreatment period is increased, complete inhibition of IKACh can be obtained at concentration one tenth of ED50 for PLC inhibition. Furthermore, it was confirmed in the present study that the effect of U73122 is specific to IKACh, without effects on other K+ channels (Figure 7), Na+ channels (data not shown) and Ca2+ channels (data not shown). For the purpose of KACh channel inhibitor, a better choice may be U73343 which has no action on PLC. ED50 of U73343 for inhibition of IKACh is similar to U73122 (0.16±0.0176 μM), and inhibitory effect is reversible.
The selective inhibition of KACh channels by U73122 and U73343 is notable, since no pharmacological tools are available to distinguish KACh channels from IRK channels. Inwardly rectifying K+ channels are inhibited by cationic channels blockers (e.g., Cs+, tetraethylammonium+, and Ba2+), but these ions discriminate poorly among the different types of inwardly rectifying K+ channels (Hille, 1992). A limited number of peptide toxins inhibit inwardly rectifying K+ channels, yet none has been isolated that selectively inhibits KACh channels (Lu & MacKinnon, 1997; Imredy et al., 1998; Jin & Lu, 1998).
Mechanism for inhibition of KACh channels by U73122
We have shown in our previous study that the activation and desensitization of IKACh are not affected by PLC inhibition by neomycin (Cho et al., 2001). In the present study, we show that IKACh is inhibited by U73122 which was originally used as a PLC inhibitor, but the effect is not attributable to PLC inhibition. It is further supported by the result showing that U73343 also exerts the same effect on IKACh (Figure 4). Such nonspecific actions of U73122 have already been reported by many authors, such as Bosch et al. (1998), Cenni & Picard (1999), Muto et al. (1997), Walker et al. (1998) and Hughes et al. (2000). From the results of the present study and a recent report by Meyer et al. (2001), the inhibition of IKACh must be added to the growing list of nonspecific effects of U73122. As a mechanism of nonspecific action of U73122, the interference with G-protein signalling of several neurotransmitter receptors was reported (Walker et al., 1998; Hughes et al., 2000). In the present study, however, we exclude this mechanism by showing that U73122 inhibits KACh channels that is activated by GTPγS or by Na+, by-passing the G-protein-coupled receptor. These findings suggest that U73122 interferes with an activation mechanism that is common to both Gβγ and Na+ ion. According to the current understandings about the molecular mechanism of KACh channel activation, both GTPγS and Na+ activate KACh channels by stabilizing the interaction of channels with PIP2. We therefore propose that U73122 and U73343 antagonize interaction of PIP2 with KACh channels, causing the inhibition of the current. This model is suggested to be a mechanism of bupivacaine inhibition of GIRK channel (Zhou et al., 2001).
As a mechanism of IKACh inhibition by U73122, we could not entirely exclude the possibility of direct channel blockade, but only present indirect evidence. In single channel recordings, U73122 did not alter the amplitude or mean open time of single channel currents, implying that it does not act as an open channel blocker. U73122 did not show voltage-dependence or use-dependence which is often found for the K+ channel blockers. No cross reactivity with other K+ channels may also regarded as indirect evidence.
In conclusion, U73122, which has often been used in signal transduction pathway research as a PLC inhibitor, is also able to inhibit KACh channels in atrial myocytes of the mice, possibly by interfering with PIP2-channel interaction.
Acknowledgments
This study was supported by BK21 Human Life Sciences and National R & D Project from Ministry of Science & Technology.
Abbreviations
- GIRK
G-protein-gated inwardly rectifying K+
- IKACh
acetylcholine-activated K+ currents
- PIP2
phosphatidylinositol 4,5-biphosphate
- PLC
phospholipase C
References
- BLEASDALE J.E., THAKUR N.R., GREMBAN R.S., BUNDY G.L., FITZPATRICK F.A., SMITH R.J., BUNTING S. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J. Pharmacol. Exp. Ther. 1990;255:756–768. [PubMed] [Google Scholar]
- BOSCH R.R., PATEL A.M., VAN EMST-DE VRIES S.E., SMEETS R.L., DEPONT J.J., WILLEMS P.H. U73122 and U73343 inhibit receptor-mediated phospholipase D activation downstream of phospholipase C in CHO cells. Eur. J. Pharmacol. 1998;346:345–351. doi: 10.1016/s0014-2999(98)00070-3. [DOI] [PubMed] [Google Scholar]
- CENNI B., PICARD D. Two compounds commonly used for phospholipase C inhibition activate the nuclear estrogen receptors. Biochem. Biophys. Res. Commun. 1999;261:340–344. doi: 10.1006/bbrc.1999.1017. [DOI] [PubMed] [Google Scholar]
- CHO H., NAM G.B., LEE S.H., EARM Y.E., HO W.K. Phosphatidylinositol 4,5-Bisphosphate is acting as a signal molecule in alpha 1-adrenergic pathway via the modulation of acetylcholine-activated K+ channels in mouse atrial myocytes. J. Biol. Chem. 2001;276:159–164. doi: 10.1074/jbc.M004826200. [DOI] [PubMed] [Google Scholar]
- HILLE B. Ionic channels of excitable membranes. Sinauer: Sunderland, MA; 1992. [Google Scholar]
- HO I.H., MURRELL-LAGNADO R.D. Molecular mechanism for sodium-dependent activation of G protein-gated K+ channels. J. Physiol. 1999a;520:645–651. doi: 10.1111/j.1469-7793.1999.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HO I.H., MURRELL-LAGNADO R.D. Molecular determinants for sodium-dependent activation of G protein-gated K+ channels. J. Biol. Chem. 1999b;274:8639–8648. doi: 10.1074/jbc.274.13.8639. [DOI] [PubMed] [Google Scholar]
- HUANG C.L., SLESINGER P.A., CASEY P.J., JAN Y.N., JAN L.Y. Evidence that direct binding of to the GIRK1 protein-gated inwardly rectifying K+ channel is important for channel activation. Neuron. 1995;15:1133–1143. doi: 10.1016/0896-6273(95)90101-9. [DOI] [PubMed] [Google Scholar]
- HUANG C.-L., FENG S., HILGEMANN D.W. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- HUGHES S.A., GIBSON W.J., YOUNG J.M. The interaction of U-73122 with the histamine H1 receptor: implications for the use of U-73122 in defining H1 receptor-coupled signalling pathways. Naunyn. Schmiedebergs. Arch. Pharmacol. 2000;362:555–558. doi: 10.1007/s002100000326. [DOI] [PubMed] [Google Scholar]
- IMREDY J.P., CHEN C., MACKINNON R. A snake toxin inhibitor of inward rectifier potassium channel ROMK1. Biochemistry. 1998;37:14867–14874. doi: 10.1021/bi980929k. [DOI] [PubMed] [Google Scholar]
- INANOBE A., MORISHIGE K.I., TAKAHASHI N., ITO H., YAMADA M., TAKUMI T., NISHINA H., TAKAHASHI K., KANAHO Y., KATADA T., KURACHI Y. G directly binds to the carboxyl terminus of the G protein-gated muscarinic K+ channel, GIRK1. Biochem. Biophys. Res. Commun. 1995;212:1022–1028. doi: 10.1006/bbrc.1995.2072. [DOI] [PubMed] [Google Scholar]
- JAN L.Y., JAN Y.N. Voltage-gated and inwardly rectifying potassium channels. J. Physiol. (Lond.) 1997;505:267–282. doi: 10.1111/j.1469-7793.1997.267bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIN W., LU Z. A novel high-affinity inhibitor for inward-rectifier K+ channels. Biochemistry. 1998;37:13291–13299. doi: 10.1021/bi981178p. [DOI] [PubMed] [Google Scholar]
- KRAPIVINSKY G., KRAPIVINSKY L., WICKMAN K., CLAPHAM D.E. G binds directly to the G protein-gated K channel, IKACh. J. Biol. Chem. 1995;270:29059–29062. doi: 10.1074/jbc.270.49.29059. [DOI] [PubMed] [Google Scholar]
- KUNKEL M.T., PERALTA E.G. Identification of domains conferring protein regulation on inward rectifier potassium channels. Cell. 1995;83:443–449. doi: 10.1016/0092-8674(95)90122-1. [DOI] [PubMed] [Google Scholar]
- KURACHI Y., ITO H., SUGIMOTO T. Positive cooperativity in activation of the cardiac muscarinic K+ channel by intracellular GTP. Pflugers Arch. 1990;416:216–218. doi: 10.1007/BF00370247. [DOI] [PubMed] [Google Scholar]
- LOGOTHETIS D.E., KURACHI Y., GALPER J., NEER E.J., CLAPHAM D.E. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987;325:321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- LOGOTHETIS D.E., ZHANG H. Gating of G protein-sensitive inwardly rectifying K+ channels through phosphatidylinositol 4,5-bisphosphate. J. Physiol. 1999;520:630. doi: 10.1111/j.1469-7793.1999.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LU Z., MACKINNON R. Purification, characterization, and synthesis of an inward-rectifier K+ channel inhibitor from scorpion venom. Biochemistry. 1997;36:6936–6940. doi: 10.1021/bi9702849. [DOI] [PubMed] [Google Scholar]
- MEYER T., WELLNER-KIENITZ M.C., BIEWALD A., BENDER K., EICKEL A., POTT L. Depletion of phosphatidylinositol 4,5-biphosphate by activation of phospholipase C-coupled receptors causes slow inhibition but not desensitization of G-protein-gated inward rectifier K+ current in artrial myocytes. J. Biol. Chem. 2001;276:5650–5658. doi: 10.1074/jbc.M009179200. [DOI] [PubMed] [Google Scholar]
- MUTO Y., NAGAO T., URUSHIDANI T. The putative phospholipase C inhibitor U73122 and its negative control, U73343, elicit unexpected effects on the rabbit parietal cell. J. Pharmacol. Exp. Ther. 1997;282:1379–1388. [PubMed] [Google Scholar]
- SAKMANN B., NOMA A., TRAUTWEIN W. Acetylcholine activation of single muscarinic K+ channels in isolated pacemaker cells of the mammalian heart. Nature. 1983;303:250–253. doi: 10.1038/303250a0. [DOI] [PubMed] [Google Scholar]
- SOEJIMA M., NOMA A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflugers Arch. 1984;400:424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- SUI J.L., CHAN K.W., LOGOTHETIS D.E. Na+ activation of the muscarinic K+ channel by a G-protein-independent mechanism. J. Gen. Physiol. 1996;108:381–391. doi: 10.1085/jgp.108.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUI J.L., PETIT-JACQUES J., LOGOTHETIS D.E. Activation of the atrial KACh channel by the betagamma subunits of G proteins or intracellular Na+ ions depends on the presence of phosphatidylinositol phosphates. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1307–1312. doi: 10.1073/pnas.95.3.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH R.J., SAM L.M., JUSTEN J.M., BUNDY G.L., BALA G.A., BLEASDALE J.E. Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J. Pharmacol. Exp. Ther. 1990;253:688–697. [PubMed] [Google Scholar]
- WALKER E.M., BISPHAM J.R., HILL S.J. Nonselective effects of the putative phospholipase C inhibitor, U73122, on adenosine A1 receptor-mediated signal transduction events in Chinese hamster ovary cells. Biochem. Pharmacol. 1998;56:1455–1462. doi: 10.1016/s0006-2952(98)00256-1. [DOI] [PubMed] [Google Scholar]
- VIVAUDOU M.B., ARNOULT C., VILLAZ M. Skeletal muscle ATP-sensitive K+ channels recorded from sarcolemmal blebs of split fibers: ATP inhibition is reduced by magnesium and ADP. J. Membr. Biol. 1991;122:165–175. doi: 10.1007/BF01872639. [DOI] [PubMed] [Google Scholar]
- YAMADA M., INANOBE A., KURACHI Y.G. Protein regulation of potassium ion channels. Pharmacol. Rev. 1998;50:723–757. [PubMed] [Google Scholar]
- ZHANG H., HE C., YAN X., MIRSHAHI T., LOGOTHETIS D.E. Activation of inwardly rectifying K+ channels by distinct PtdIns(4,5)P2 interactions. Nat. Cell Biol. 1999;1:183–188. doi: 10.1038/11103. [DOI] [PubMed] [Google Scholar]
- ZHOU W., ARRABIT C., CHOE S., SLESINGER P.A. Mechanism underlying bupivacaine inhibition of G protein-gated inwardly rectifying K+ channels. Proc. Natl. Acad. Sci. U.S.A. 2001;98:6482–6487. doi: 10.1073/pnas.111447798. [DOI] [PMC free article] [PubMed] [Google Scholar]


