Abstract
Increased incidence of impotence is associated with some selective serotonin-reuptake-inhibitors (SSRIs), but the pathophysiological mechanism is unknown. Paroxetine and citalopram are extensively used SSRIs, but only paroxetine has been shown to inhibit nitric oxide synthase (NOS) activity. NO is a key mediator of penile erection. Thus, the aim of this study was to determine the effects of paroxetine and citalopram on erectile function and NO production, in a rat model. Application of cavernosal nerve electrical stimulation produced frequency-related intracavernosal pressure (ICP) increases, which were inhibited by the NOS inhibitor, NG-nitro-L-arginine (0.3 mg kg−1). Acute or chronic (2 weeks) paroxetine-treatment (10 mg kg−1) reduced ICP-responses, while citalopram did not. Paroxetine, but not citalopram, significantly reduced nitrite+nitrate plasma levels by 61.4% and inhibited penile neuronal NOS (nNOS) protein expression by 31.2% after chronic treatment. The results show that paroxetine inhibits erectile responses in rats. We propose that this effect is due to reduced NO production and nNOS expression.
Keywords: Selective serotonin reuptake inhibitor, erectile dysfunction, nitric oxide
Introduction
Selective serotonin reuptake inhibitors (SSRIs) are useful therapeutic agents for the management of depression and other psychiatric disorders. Treatment with these agents is associated with a high incidence of sexual dysfunction, reaching incidence rates above 50% in some studies (Zajecka et al., 1997). SSRI-induced sexual dysfunction affects men and women and includes orgasm delay, anorgasmia, delayed ejaculation and impotence (Labbate et al., 1998), which usually disappear when the treatment is interrupted (Rothschild, 1995). In men, the appearance of some degree of erectile dysfunction in more than 40% of patients after SSRI treatment, by an unknown mechanism, has been reported (Kennedy et al., 2000). SSRI antidepressants vary in their likelihood of producing these side effects (Arias et al., 2000). Paroxetine and citalopram are well known SSRIs extensively used in clinical practice (Nemeroff, 1993; Keller, 2000). Paroxetine has often been reported to induce sexual dysfunction (Zajecka et al., 1997; Labbate et al., 1998; Kennedy et al., 2000), while citalopram has been shown to affect sexual function to a lesser degree (Mendels et al., 1999).
Paroxetine has been reported to inhibit nitric oxide synthase (NOS) activity in vitro and in vivo (Finkel et al., 1996). Nitric oxide (NO) is a key mediator of penile smooth muscle relaxation and penile erection (Ignarro et al., 1990; Kim et al., 1991). Neuronal NOS (nNOS) in the nitrergic nerves and endothelial NOS (eNOS) in the endothelium of lacunar spaces and penile vessels are responsible for NO generation in the penis.
Thus, the aim of this work was to evaluate the effects of acute and chronic treatment with paroxetine and citalopram on erectile responses in male rats, on nitric oxide production in vivo and on nNOS and eNOS expression in penile tissue.
Methods
Male Sprague-Dawley rats were used for this study. For the chronic studies the animals were divided into three groups. The paroxetine and citalopram treated groups received a daily dose of 10 mg kg−1 of paroxetine or citalopram by means of two intraperitoneal injections per day of 5 mg kg−1 of the drugs dissolved in saline. The control group received two injections per day with the equivalent volume of saline.
Erectile responses to cavernosal nerve stimulation in anaesthetized rats
Male Sprague-Dawley rats were anaesthetized with urethane (1.25 g kg−1). The surgical procedure consisted of dissection and isolation of the right cavernous nerve through an abdominal midline incision and exposure of penile crura through a transverse perineal incision. Intracavernosal pressure (ICP) measurements were accomplished by insertion into the right crus of a 23-gauge needle connected to a disposable pressure transducer (Abbott, Sligo, Ireland) and a data acquisition system (ADInstruments, Castle Hill, Australia). Right carotid artery and left external jugular vein were catheterized for constant blood pressure measurement and saline or drug infusion, respectively. Electrical stimulation was applied by a delicate platinum bipolar hook electrode connected to a stimulator and current amplifier (Cibertec, Madrid, Spain). Parameters of electrical stimulation consisted of pulses with a duration of 1 ms and 1.5 mA of current intensity for 1 min. Frequency – response curves were performed by applying stimulation at 1 and 3 Hz with an interval of 3 min between both frequencies.
For evaluation of acute effects of the treatments on erectile responses, a control stimulation at 1 and 3 Hz was performed and, after an stabilization period, paroxetine or citalopram dissolved in 20% hydroxy-propyl-B-cyclodextrin (HPBCD) or the vehicle alone were intravenously administered. The stimulation was repeated at 60 min from the administration.
Determination of nitrite+nitrate plasma levels
Blood samples from rats treated with vehicle, paroxetine or citalopram were obtained and plasma was separated by centrifugation (1000×g). Plasma samples were passed through a 10,000 nominal molecular weight limit filter (Millipore, Bedford, MA, U.S.A.) by centrifugation (2500×g). Total NO derivatives (nitrites+nitrates) were determined in the filtrate by Griess reaction using a commercial kit from Cayman Chemical Co. (Ann Arbor, MI, U.S.A.). Once the reaction was completed, the absorbance at 540 nM was measured in a microplate reader (Vitek, France) to obtain nitrite plus nitrate concentrations.
eNOS and nNOS expression (Western blotting)
Frozen corpus cavernosum tissue from each rat was homogenized in lysis buffer (10 mM Tris pH 7.4, 1% SDS, 1 mM sodium orthovanadate, 2 mM PMSF and 12.5 μg ml−1 aprotinin). Equal amounts of protein from each sample (10 μg) were submitted to 8% SDS – PAGE and transferred to nitrocellulose membranes (Bio-Rad Laboratories, Madrid, Spain). eNOS and nNOS were identified using specific monoclonal antibodies (1 : 2500; Transduction Laboratories, Lexington, KY, U.S.A.) followed by incubation with horseradish peroxidase-conjugated goat anti-mouse secondary antibody (1 : 10000; Chemicon, Temecula, CA, U.S.A.), and visualized using ECL detection reagents (Amersham Pharmacia Biotech, Buckinghamshire, U.K.) and a film exposition system (Hyperfilm, Amersham Pharmacia Biotech). Bands were quantified using an image analyser software (NIH Image). Samples from control rats and from one of the treated groups were run in each gel.
Drugs and materials
Paroxetine and citalopram were provided by Forest Laboratories. Urethane, NG-nitro-L-arginine (L-NNA), hydroxy-propyl-B-cyclodextrin (HPBCD), sodium dodecyl-sulphate (SDS), sodium orthovanadate, phenyl-methyl-sulphonyl-fluoride (PMSF) and aprotinin were obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.).
Results
Cavernosal nerve electrical stimulation caused a frequency-dependent increase of intracavernosal pressure (ICP) in anaesthetized male rats. These erectile responses were dependent on NO production since they were dose-dependently inhibited by the treatment with the NO synthase inhibitor L-NNA at 0.3 mg kg−1 (Figure 1) and 3 mg kg−1 (data not shown). Acute administration of 10 mg kg−1 citalopram did not modify erectile responses, but the same dose of paroxetine produced a significant impairment of ICP increases to cavernosal nerve stimulation (Figure 1). Treatment with 10 mg kg−1 day−1 paroxetine or citalopram for 2 weeks did not modify basal mean arterial pressure (MAP) of anaesthetized rats (107.9±6.3, 99.0±5.3, and 99.7±5.4 mm Hg, for vehicle, paroxetine and citalopram treated rats, respectively). The erectile responses of the rats chronically treated with paroxetine were significantly reduced when compared with those of the control rats. The response to 1 Hz stimulus was reduced by 64.1%, and the 3 Hz response by 17.4%. Chronic treatment with citalopram did not significantly alter the erectile responses of the rats (Figure 2).
Figure 1.

Effects of acute intravenous administration of vehicle (20% HPBCD), 10 mg kg−1 paroxetine, 10 mg kg−1 citalopram and 0.3 mg kg−1 NG-nitro-L-arginine (L-NNA) on erectile responses in anaesthetized male rats. Data are expressed as the mean±s.e.mean of the area under the curve (AUC; mm Hg×s) of intracavernosal pressure (ICP) increase to cavernosal nerve stimulation normalized by mean arterial pressure (MAP) values. **P<0.01 vs frequency – response curve in vehicle treated group by a two-factors ANOVA test.
Figure 2.
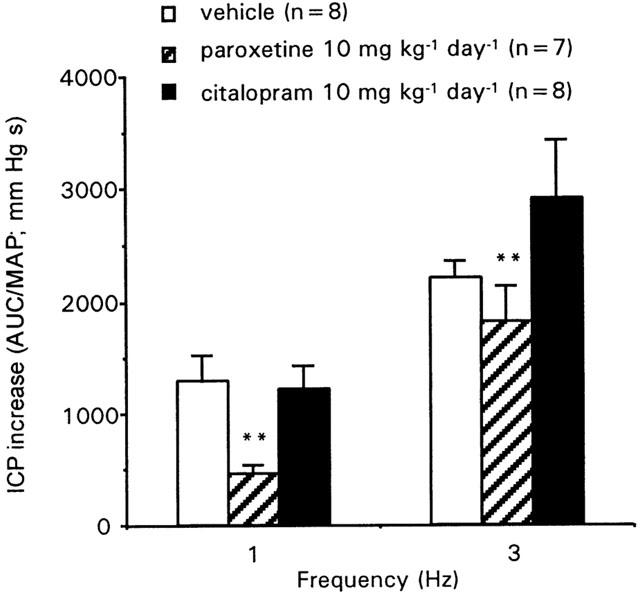
Erectile responses elicited by cavernosal nerve electrical stimulation in anaesthetized rats chronically treated with vehicle (saline), paroxetine or citalopram for 2 weeks. Data are expressed as the mean±s.e.mean of the area under the curve (AUC; mm Hg×s) of intracavernosal pressure (ICP) increase to cavernosal nerve stimulation normalized by mean arterial pressure (MAP) values. **P<0.01 vs frequency – response curve in vehicle treated group by a two-factors ANOVA test.
Paroxetine-treated rats showed a 61% reduction (P<0.05) in nitrite+nitrate plasma levels when compared to vehicle treated rats, while these levels were not significantly affected by citalopram treatment (Figure 3). Both eNOS and nNOS were detected in all rat penises, but while the expression of eNOS was not affected by any treatment, paroxetine, but not citalopram, produced a significant reduction (31.2%, P<0.01) of the amount of nNOS protein in rat penile tissue (Figure 4).
Figure 3.
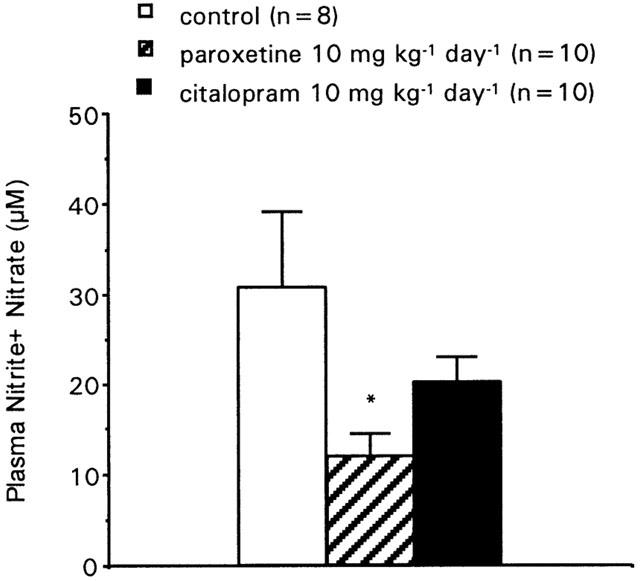
Plasma nitrite plus nitrate levels in rats chronically treated with vehicle (saline), paroxetine or citalopram for 2 weeks. Data are expressed as the mean±s.e.mean of nitrite+nitrate plasma concentrations (in μM). *P<0.05 vs vehicle treated group by one-way ANOVA followed by Student – Newman – Keuls post-hoc test.
Figure 4.
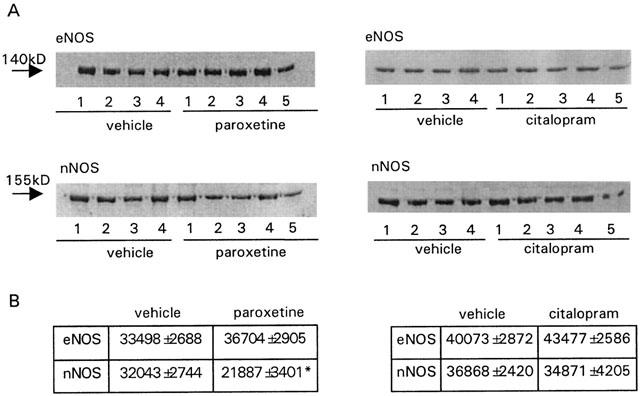
Effects of paroxetine and citalopram (10 mg kg−1 day−1 for 2 weeks) on endothelial (A) and neuronal (B) nitric oxide synthase (NOS) expression in rat penile tissue. Data are expressed as the mean±s.e.mean of the density of bands (in pixels). n indicates the number of rats used for determinations. **P<0.01 vs vehicle treated rats by one-way ANOVA followed by Student – Newman – Keuls post-hoc test.
Discussion
The existence of adverse events affecting sexual function in patients undergoing treatment with SSRIs is a common clinical observation (Zajecka et al., 1997; Kennedy et al., 2000). The mechanism underlying increased incidence of sexual dysfunction in these patients is not well understood. The known mechanism of action of these drugs involves the inhibition of serotonin reuptake by neurons, increasing the levels of this neurotransmitter in the synapse. While high levels of dopamine are related to promoting sexual function, high levels of serotonin, in general, are thought to inhibit sexual behaviour (Hull et al., 1999). In relation to this fact, the increased levels of serotonin in some regions of the central nervous system could be the reason for the development of sexual dysfunction associated with SSRIs. Nevertheless, although all SSRIs enhance serotonin levels in the brain not all of them produce the same effects on sexual function. Indeed, an increase of incidence of erectile dysfunction in patients treated with paroxetine has often been reported, while a lesser effect of citalopram on sexual function has also been published (Mendels et al., 1999). Furthermore, the activation of some serotonin receptor subtypes is known to inhibit (5-HT1A), but others stimulate (5-HT2C), penile erection (Millan et al., 1997).
Relaxation of trabecular smooth muscle is needed to achieve and maintain penile erection (Sáenz de Tejada et al., 1991). Nitric oxide is a key mediator of penile smooth muscle relaxation (Ignarro et al., 1990; Kim et al., 1991), which is released by nitrergic nerves within the trabecular and penile arterial tissues as well as the endothelia that line the lacunar spaces and the intima of penile arteries (Kim et al., 1991). Nitric oxide synthase-like immunoreactivity has been identified in nerves and endothelia in corpus cavernosum tissue (Burnett et al., 1993) and the activity of this enzyme has been characterized in corpus cavernosum tissue homogenates (Kim et al., 1991). Inhibitors of NOS inhibit penile erection elicited by the stimulation of the pelvic nerves (Holmquist et al., 1991). Indeed, in our model, the intravenous administration of a NOS inhibitor caused a dose-related reduction of erectile responses. The inhibition of erectile responses by chronic and acutely administered paroxetine suggests that this molecule also inhibits NOS activity as previously demonstrated in hamster brain and depressed patients (Finkel et al., 1996). The inhibition of NOS activity would explain the reduced erectile response to cavernosal nerve stimulation. The interference of paroxetine with the catalytic activity of NOS could be related to the reported ability of this agent to inhibit, potently, the activity of cytochrome P450 isozymes (Preskorn, 1993), which are structurally related to NOS. In contrast, citalopram is known to have no or negligible activity towards cytochrome P450 isozymes (Greenblatt et al., 1998).
However, the production of NO is also regulated at the level of NOS protein expression. Paroxetine, at similar doses to those used in other chronic studies (Hajos-Korcsok et al., 2000) showed NOS inhibitory activity in chronic experiments with reduction of nNOS protein expression and NO production, as demonstrated in the reduced nitrate+nitrite plasma levels. Further research is needed to determine the mechanism responsible for the unexpected effect of paroxetine on nNOS expression. It can be speculated that the lack of adequate erectile responses could produce down-regulation of nNOS, since the opposite effect has been observed in rats after chronic pharmacological induction of erections (Escrig et al., 1999). The accuracy of the observed reduction with paroxetine of nNOS expression is supported by the use of monoclonal antibodies against eNOS and nNOS, which excludes the possibility of undesirable cross-reactions seen with some polyclonal antibodies (Lin et al., 1998). Since the NO released from nitrergic nerves in the penis plays a key role in trabecular smooth muscle relaxation and penile erection, reduced expression of nNOS in this tissue could be determinant for reduced NO production and the appearance of diminished erectile responses in the rats treated with paroxetine. Knockout mice lacking the gene encoding for nNOS have normal erectile function, likely preserved by overexpression of eNOS as a compensatory mechanism (Burnett et al., 1996). In our model, the reduced expression of nNOS in rat penile tissue did not lead to enhanced eNOS expression. This difference is probably due to the fact that we administered the pharmacological treatment to sexually mature animals while knockout mice have nNOS deficiency during development, facilitating regulatory processes. Thus, in addition to the possible inhibitory effect of paroxetine on NOS activity, the reduction of nNOS expression without concomitant eNOS overexpression could account for the effects of this compound on penile erection. The lack of effect on erectile responses with acute or chronic administration of citalopram is consistent with the lack of significant effects of this molecule on NOS expression, NO production and the reported lesser sexual dysfunction in patients (Mendels et al., 1999).
In conclusion, we report here that both acute and chronic treatments with the SSRI paroxetine cause erectile dysfunction in a rat model. We propose a rationale for the inhibitory effect of paroxetine on erectile function, involving both an inhibition of NOS activity and a reduced expression of nNOS in penile tissue. These factors lead to decreased NO production and hence, impaired penile smooth muscle relaxation and erection. These effects seem to be specific for paroxetine, since another SSRI, citalopram, does not share any of the effects exerted by paroxetine on erectile function or NO production.
Acknowledgments
We want to acknowledge Jose L. Llergo, Elena Cercas and Argentina Fernández for their excellent technical assistance.
Abbreviations
- eNOS
endothelial nitric oxide synthase
- HPBCD
hydroxy-propyl-B-cyclodextrin
- ICP
intracavernosal pressure
- nNOS
neuronal nitric oxide synthase
- SSRIs
selective serotonin reuptake inhibitors
References
- ARIAS F. , PADIN J.J. , RIVAS M.T., SANCHEZ A. Sexual dysfunctions induced by serotonin reuptake inhibitors. Atención Primaria. 2000;26:389–394. doi: 10.1016/S0212-6567(00)78688-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNETT A.L., NELSON R.J., CALVIN D.C., LIU J.X., DEMAS G.E., KLEIN S.L., KRIEGSFELD L.J., DAWSON V.L., DAWSON T.M., SNYDER S.H. Nitric oxide-dependent penile erection in mice lacking neuronal nitric oxide synthase. Mol. Med. 1996;2:288–296. [PMC free article] [PubMed] [Google Scholar]
- BURNETT A.L., TILLMAN S.L., CHANG T.S., EPSTEIN J.L., LOWENSTEIN C.J., BREDT D.S., SNYDER S.H., WALSH P.C. Immunohistochemical localization of nitric oxide synthase in the autonomic innervation of the human penis. J. Urol. 1993;150:73–76. doi: 10.1016/s0022-5347(17)35401-0. [DOI] [PubMed] [Google Scholar]
- ESCRIG A., MARIN R., MAS M. Repeated PGE1 treatment enhances nitric oxide and erection responses to nerve stimulation in the rat penis by upregulating constitutive NOS isoforms. J. Urol. 1999;162:2205–2210. doi: 10.1016/S0022-5347(05)68160-8. [DOI] [PubMed] [Google Scholar]
- FINKEL M.S., LAGHRISSI-THODE F., POLLOCK B.G., RONG J. Paroxetine is a novel nitric oxide synthase inhibitor. Psychopharmacol. Bull. 1996;32:653–658. [PubMed] [Google Scholar]
- GREENBLATT D.J., VON MOLTKE L.L., HARMATZ J.S., SHADER R.I. Drug interactions with newer antidepressants: role of human cytochrome P450. J. Clin. Psychiatry. 1998;59 Suppl 15:19–27. [PubMed] [Google Scholar]
- HAJOS-KORCSOK E., MCTAVISH S.F., SHARP T. Effect of a selective 5-hydroxytryptamine reuptake inhibitor on brain extracellular noradrenaline: microdialysis studies using paroxetine. Eur. J. Pharmacol. 2000;407:101–107. doi: 10.1016/s0014-2999(00)00723-8. [DOI] [PubMed] [Google Scholar]
- HOLMQUIST F., STIEF C.G., JONAS U., ANDERSSON K.E. Effects of the nitric oxide synthase inhibitor NG-nitro-L-arginine on the erectile response to cavernous nerve stimulation in the rabbit. Acta Physiol. Scand. 1991;143:299–304. doi: 10.1111/j.1748-1716.1991.tb09236.x. [DOI] [PubMed] [Google Scholar]
- HULL E.M., LORRAIN D.S., DU J., MATUSZEWICH L., LUMLEY L.A., PUTNAM S.K., MOSES J. Hormone-neurotransmitter interactions in the control of sexual behavior. Behav. Brain Res. 1999;105:105–116. doi: 10.1016/s0166-4328(99)00086-8. [DOI] [PubMed] [Google Scholar]
- IGNARRO L.J., BUSH P.A., BUGA G.M., WOOD K.S., FUKUTO J.M., RAJFER J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem. Biophys. Res. Commun. 1990;170:843–850. doi: 10.1016/0006-291x(90)92168-y. [DOI] [PubMed] [Google Scholar]
- KELLER M.B. Citalopram therapy for depression: a review of 10 years of European experience and data from U.S. clinical trials. J. Clin. Psychiatry. 2000;61:896–908. [PubMed] [Google Scholar]
- KENNEDY S.H., EISFELD B.S., DICKENS S.E., BACCHIOCHI J.R., BAGBY R.M. Antidepressant-induced sexual dysfunction during treatment with moclobemide, paroxetine, sertraline, and venlafaxine. J. Clin. Psychiatry. 2000;61:276–281. doi: 10.4088/jcp.v61n0406. [DOI] [PubMed] [Google Scholar]
- KIM N., AZADZOI K.M., GOLDSTEIN I., SÁENZ DE TEJADA I. A Nitric Oxide-like factor mediates nonadrenergic-noncholinergic neurogenic relaxation of penile corpus cavernosum smooth muscle. J. Clin. Invest. 1991;88:112–118. doi: 10.1172/JCI115266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LABBATE L.A., GRIMES J., HINES A., OLESHANSKY M.A., ARANA G.W. Sexual dysfunction induced by serotonin reuptake antidepressants. J. Sex. Marital Ther. 1998;24:3–12. doi: 10.1080/00926239808414663. [DOI] [PubMed] [Google Scholar]
- LIN C.-S., LAU A., BAKIRCIOGLU E., TU R., WU F., WEEK S., NUNES L., LUE T.F. Analysis of neuronal nitric oxide synthase isoform expression and identification of human nNOS-μ. Biochem. Biophys. Res. Commun. 1998;253:388–394. doi: 10.1006/bbrc.1998.9658. [DOI] [PubMed] [Google Scholar]
- MENDELS J., KIEV A., FABRE L.F. Double-blind comparison of citalopram and placebo in depressed outpatients with melancholia. Depress. Anxiety. 1999;9:54–60. [PubMed] [Google Scholar]
- MILLAN M.J., PEGLION J.L., LAVIELLE G., PERRIN-MONNEYRON S. 5-HT2C receptors mediate penile erections in rats: actions of novel and selective agonists and antagonists. Eur. J. Pharmacol. 1997;325:9–12. doi: 10.1016/s0014-2999(97)89962-1. [DOI] [PubMed] [Google Scholar]
- NEMEROFF C.B. Paroxetine: an overview of the efficacy and safety of a new selective serotonin reuptake inhibitor in the treatment of depression. J. Clin. Psychopharmacol. 1993;13 Suppl 2:10S–17S. [PubMed] [Google Scholar]
- PRESKORN S.H. Recent pharmacologic advances in antidepressant therapy for the elderly. Am. J. Med. 1993;94:2S–12S. [PubMed] [Google Scholar]
- ROTHSCHILD A.J. Selective serotonin reuptake inhibitor-induced sexual dysfunction: efficacy of a drug holiday. Am. J. Psychiatry. 1995;152:1514–1516. doi: 10.1176/ajp.152.10.1514. [DOI] [PubMed] [Google Scholar]
- SÁENZ DE TEJADA I., MOROUKIAN P., TESSIER J., KIM J.J., GOLDSTEIN I., FROHRIB D. Trabecular smooth muscle modulates the capacitor function of the penis. Studies on a rabbit model. Am. J. Physiol. 1991;260:H1590–H1595. doi: 10.1152/ajpheart.1991.260.5.H1590. [DOI] [PubMed] [Google Scholar]
- ZAJECKA J., MITCHELL S., FAWCETT J. Treatment-emergent changes in sexual function with selective serotonin reuptake inhibitors as measured with the Rush Sexual Inventory. Psychopharmacol. Bull. 1997;33:755–760. [PubMed] [Google Scholar]


