Abstract
The present work characterizes, from a pharmacological and biochemical point of view, adenosine receptors in the human malignant melanoma A375 cell line.
Adenosine receptors were detected by RT – PCR experiments. A1 receptors were characterized using [3H]-DPCPX binding with a KD of 1.9±0.2 nM and Bmax of 23±7 fmol mg−1 of protein. A2A receptors were studied with [3H]-SCH 58261 binding and revealed a KD of 5.1±0.2 nM and a Bmax of 220±7 fmol mg−1 of protein. A3 receptors were studied with the new A3 adenosine receptor antagonist [3H]-MRE 3008F20, the only A3 selective radioligand currently available. Saturation experiments revealed a single high affinity binding site with KD of 3.3±0.7 nM and Bmax of 291±50 fmol mg−1 of protein.
The pharmacological profile of radioligand binding on A375 cells was established using typical adenosine ligands which displayed a rank order of potency typical of the different adenosine receptor subtype.
Thermodynamic data indicated that radioligand binding to adenosine receptor subtypes in A375 cells was entropy- and enthalpy-driven.
In functional assays the high affinity A2A agonists HE-NECA, CGS 21680 and A2A – A2B agonist NECA were able to increase cyclic AMP accumulation in A375 cells whereas A3 agonists Cl-IB-MECA, IB-MECA and NECA were able to stimulate Ca2+ mobilization.
In conclusion, all these data indicate, for the first time, that adenosine receptors with a pharmacological and biochemical profile typical of the A1, A2A, A2B and A3 receptor subtype are present on A375 melanoma cell line.
Keywords: Adenosine receptors, A375 melanoma cell line, cyclic AMP, calcium levels, thermodynamic studies
Introduction
Adenosine acts as an autacoid via interaction with four types of G protein-coupled receptors, termed A1, A2A, A2B and A3. These receptors are widely distributed in human body regulating virtually the function of every organ and tissue (Fredholm et al., 1994). They differ by their affinity for the endogenous agonist as well as by their pharmacological specificity. A1, A2A and A3 receptors are activated by submicromolar concentrations of adenosine, whereas only the A2B receptor appears to be activated when adenosine levels raise into the micromolar range (Fredholm et al., 2001). In addition, adenosine receptors operate through distinct signalling mechanisms. The A1 and A3 subtypes control most, if not all, their cellular effectors via pertussis toxin-sensitive G proteins of the Gi and Go family; in contrast, both A2A and A2B subtypes couple to Gs and thereby stimulate cyclic AMP formation (Ralevic & Burnstock, 1998). In particular, stimulation of A3 receptors induces an intracellular signalling that actives phospholipase C and D and increases calcium concentrations (Olah & Stiles, 1995; Abbracchio et al., 1995). Adenosine has been linked to a variety of physiological processes, including mast-cell mediation of hypotension (Fozard et al., 1996; Guieu et al., 2001), induction of apoptosis (Yao et al., 1997; Schrier et al., 2001), inhibition of tumour necrosis factor α production from macrophage-like cell line (Elenkov et al., 2000), inhibition of platelet-activating factor-induced chemotaxis of human eosinophils (Walker et al., 1997), neural and cardiac protection (Liang & Jacobson, 1998; Safran et al., 2001) and depression of locomotor activity (Jacobson et al., 1993; Marston et al., 1998). Increased concentrations of adenosine have been detected in the absence of adenosine deaminase activity (ADA) (Blackburn et al., 1996) or under hypoxic conditions, e.g. large solid tumours (Blay et al., 1997). The mechanisms of adenosine effects on different tumoural tissues have not yet been carefully explored. Adenosine analogues are also used as pharmacological agents (Olah & Stiles, 1995). In particular adenosine, acting at specific A2A receptors, promotes wound healing in both normal animals and in animals with impaired wound healing (Montesinos et al., 1997). Furthermore, adenosine stimulates angiogenesis in the dermis leading to improvement in function and appearance of the epidermis. Adenosine may also increase collagen and glicosaminoglycan production from fibroblasts and it is readily taken up by the cells (Ethier et al., 1993; Ethier & Dobson, 1997). In view of these hallmarks, clarification of the cellular and biochemical mechanisms of adenosine may be important for the understanding of its possible role in the pathogenesis of tumours of the skin, as basal cell carcinoma, squamous cell carcinoma, melanoma. Malignant melanoma is a serious form of skin cancer. Unfortunately, the incidence of this disease appears to be increasing, such that currently about 1 in 100 persons in the United States can expect to develop this cancer in a lifetime (Ariza et al., 1999). Recent advances in the understanding of extracellular adenosine-mediated transmembrane signalling through adenosine receptors together with the availability of molecular tools to study adenosine receptors will allow more detailed and precise evaluation of the effects of extracellular adenosine under well controlled conditions. To our knowledge, a complete investigation on adenosine receptors in tumours of the skin is not available. Prompted by these observations we performed, for the first time, a pharmacological and biochemical characterization of adenosine receptors in a human malignant melanoma cell line, A375. Adenosine receptors messengers (mRNA) were first revealed by RT – PCR experiments and then characterized by radioligand binding assays by using the selective A1-, A2A-, A3-antagonists [3H]-DPCPX, [3H]-SCH 58261 and [3H]-MRE 3008F20, respectively. The lack of available selective radioligands has until now hindered characterization of the A3 receptors in any tissue. Thus, this newly developed antagonist radioligand represents a major contribution to the study of this receptor subtype (Varani et al., 2000a). Moreover, the abilities of typical A2A and A2B agonists to increase the cyclic AMP intracellular levels has been evaluated as well as the effect of high affinity A3 agonists, such as Cl-IB-MECA, IB-MECA and NECA, in the induction of Ca2+ release. Finally, with the aim of obtaining new insights into the forces driving the coupling of adenosine receptors in human melanoma with selective ligands, a thermodynamic analysis of [3H]-DPCPX, [3H]-SCH 58261 and [3H]-MRE 3008F20 binding has been performed and the enthalpic (ΔH°) and entropic (ΔS°) contribution to the standard free energy (ΔG°) of the binding equilibrium were determined.
Methods
Drugs
[3H]-DPCPX (specific activity 120 Ci mmol−1) was purchased from NEN Research Products (Boston, MA, U.S.A.). [3H]-SCH 58261 (specific activity 68 Ci mmol−1) was a kind gift of Dr E. Ongini, Schering-Plough Research Institute (Milan, Italy). CPA, MECA, CPT, 8PT, CGS 21680, CHA, NECA, R-PIA, S-PIA, C1-IB-MECA, IB-MECA, CGS 15943, DPCPX were obtained from Research Biochemical International (Natick, MA, U.S.A.). HE-NECA was a kind gift of Prof G. Cristalli, Dipartimento di Scienze Chimiche, University of Camerino, Italy. SCH 58261, MRE 3055F20, MRE 3062F20, MRE 3048F20, MRE 3046F20, MRE 3008F20 were synthesized by Prof P.G. Baraldi, Dipartimento di Scienze Farmaceutiche, University of Ferrara, Italy. MRE 3008F20 was successively labelled for us with 3H-isotope ([3H]-MRE 3008F20, specific activity 67 Ci mmol−1, as previously described by Baraldi et al., 2000) at Amersham International (Buckinghamshire, U.K.). All other reagents were of analytical grade and obtained from commercial sources.
Cell culture
A375 cells (obtained from ATCC) were grown adherently and maintained in Dulbecco's Modified Eagle's Medium (DMEM), containing 10% foetal calf serum, penicillin (100 u ml−1), streptomycin (100 μg ml−1), L-glutamine (2 mM) at 37°C in 5% CO2/95% air. Cells were split two or three times weekly at a ratio between 1 : 5 and 1 : 10.
RT – PCR
Total cytoplasmic RNA was extracted from 107 cells by the acid guanidinium thiocyanate phenol method. The human A1 adenosine receptor sequence was amplified with 5′ primer sequence (GGA TGC CAC CTT CTG CTT CAT) and 3′ primer sequence (CTC GAA CTC GCA CTT GAT CAC) giving a 394 bp product. The human A2A adenosine receptor sequence was amplified with 5′ primer sequence (GGG GTA CCA GTG GAG GGA GTG C) and 3′ primer sequence (AAG CCG CGG AGA AAG ATA AAG A) giving a 208 bp product. The human A2B adenosine receptor sequence was amplified with 5′ primer sequence (ACG GTA CCA CAA GAA ACA AAG AGG AC) and 3′ primer sequence (AAG CCG CGG AGC CTA CTA CTG ACA) giving a 153 bp product. The human A3 adenosine receptor sequence was amplified with 5′ primer sequence (ACG GTG AGG TAC CAC AGC TTG TG) and 3′ primer sequence (ATA CCG CGG GAT GGC AGA CC) giving a 156 bp product. RT – PCR was carried out by using Access RT – PCR System (Promega), in 50 μl at the following conditions: first strand cDNA synthesis at 48°C for 45 min and 94°C for 2 min. Second strand cDNA synthesis and PCR amplification: 94°C for 30 s, 59.5°C (for A1 and A3) or 58.6°C (for A2A) or 54°C (for A2B) for 30 s and 68°C for 2 min (35 – 40 cycles). For β-actin amplification primers were as described elsewhere (Walther et al., 1994). Oligonucleotides were synthesized by M-Medical Genenco-Life Science, Florence, Italy. Subsequently, 10 μl of PCR products were run on the 2% agarose gel and visualized under u.v. illumination after ethidium bromide staining.
Membrane preparation
For membrane preparation the culture medium was removed. The cells were washed with PBS and scraped off T75 flasks in ice-cold hypotonic buffer (5 mM Tris HCl, 2 mM EDTA, pH 7.4). The cell suspension was homogenized with Polytron and the homogenate was spun for 10 min at 1000×g. The supernatant was then centrifuged for 30 min at 100,000×g. The membrane pellet was resuspended in 50 mM Tris HCl buffer pH 7.4 for A1 adenosine receptors, 50 mM Tris HCl buffer pH 7.4, 10 mM MgCl2 for A2A adenosine receptors, 50 mM Tris HCl buffer pH 7.4, 10 mM MgCl2, 1 mM EDTA for A3 adenosine receptors and incubated with 3 IU ml−1 of Adenosine deaminase for 30 min at 37°C. Then the suspension was stored at −80°C. The protein concentration was determined according to a Bio-Rad method (Bradford, 1976) with bovine albumin as a standard reference.
Radioligand binding assays
Association kinetic studies of [3H]-DPCPX (2 nM), [3H]-SCH 58261 (4 nM) and [3H]-MRE 3008F20 (4 nM) were performed incubating membranes obtained by A375 cells at 25°C for A1 and at 4°C for A2A and A3, respectively. For the measurement of the association rate, the reaction was terminated at different times (from 5 to 200 min) by rapid filtration under vacuum, followed by washing with 5 ml ice-cold buffer four times. For dissociation studies, membranes were pre-incubated with [3H]-DPCPX (2 nM), [3H]-SCH 58261 (4 nM) and [3H]-MRE 3008F20 (4 nM) at 25°C for A1 and at 4°C for A2A and A3, respectively, for 150 min. Specific binding was then measured at 5 – 120 min after the addition of 1 μM DPCPX, SCH 58261 and MRE 3008F20 for A1, A2A and A3, respectively. Saturation binding experiments of [3H]-DPCPX, [3H]-SCH 58261 and [3H]-MRE 3008F20 to A375 cells were performed incubating membranes (100 μg of protein/assay) at 25°C for A1 and at 4°C for A2A and A3, respectively, for 150 min. Competition experiments of 2 nM [3H]-DPCPX, 4 nM [3H]-SCH 58261 and 4 nM [3H]-MRE 3008F20 were performed in duplicate in a final volume of 100 μl in test tubes containing 50 mM Tris HCl buffer (10 mM MgCl2 for A2A, 10 mM MgCl2 and 1 mM EDTA for A3), pH 7.4 and 100 μl of membranes and at least 12 – 14 different concentrations of typical adenosine receptor agonists and antagonists. Non-specific binding was defined as the binding in the presence of 1 μM DPCPX, SCH 58261 and MRE 3008F20 for A1, A2A and A3, respectively and, at the KD value for each radioligand, was about 30% of total binding. Bound and free radioactivity were separated by filtering the assay mixture through Whatman GF/B glass-fiber filters using a Micro-Mate 196 cell harvester (Packard Instrument Company). The filter bound radioactivity was counted on a Top Count Microplate Scintillation Counter (efficiency 57%) with Micro-Scint 20.
Measurement of cyclic AMP levels
A375 cells (5×106 cells/assay) were suspended in 0.5 ml of incubation mixture (mM): NaCl 150, KCl 2.7, NaH2PO4 0.37, MgSO4 1, CaCl2 1, glucose 5, HEPES 1, MgCl2 10, pH 7.4 at 37°C, containing 0.5 mM 4-(3-butoxy-4-methoxybenzyl)-2-imidazolidinone (Ro 20-1724) as phosphodiesterase inhibitor, 2.0 IU ml−1 adenosine deaminase and preincubated for 10 min in a shaking bath at 37°C. Then typical adenosine agonists plus forskolin (10 μM for A1 – A3, 1 μM for A2A – A2B) were added to the mixture and incubated for a further 5 min. The reaction was terminated by the addition of cold 6% trichloroacetic acid (TCA). The TCA suspension was centrifuged at 2000×g for 10 min at 4°C and the supernatant was extracted four times with water-saturated diethyl ether. The final aqueous solution was tested for cyclic AMP levels by a competition protein binding assay carried out essentially according to Varani et al. (2000b). Samples of cyclic AMP standards (0 – 10 pmol) were added to each test tube containing Trizma base 0.1 M; aminophylline 8.0 mM; 2 mercaptoethanol 6.0 mM; pH 7.4 and [3H]-cyclic AMP in a total volume of 0.5 ml. The binding protein, previously prepared from bovine adrenal glands, was added to the samples previously incubated at 4°C for 150 min. After the addition of charcoal the samples were centrifuged at 2000×g for 10 min. The clear supernatant (0.2 ml) was mixed with 4 ml of Atomlight and counted in a LS-1800 Beckman scintillation counter.
Calcium mobilization assays
Changes in [Ca2+] were measured with the fluorescent indicator fura 2-AM, according to Varani et al., 2000b. Briefly, cells were loaded for 15 min with 4 μM fura 2-AM in HBS (mM): HEPES pH 7.4 20, NaCl 140, KCl 4, K2HPO4 1, MgCl2 1, CaCl2 1, D-glucose 10 and 0.1% BSA, buffer at 37°C. The cells were centrifuged at 1000×g for 10 min to remove extracellular dye and were resuspended in HBS buffer at 2×106 cells ml−1. Fluorescence was monitored with a LS50, Perkin-Elmer, Norwalk, CT spectrofluorimeter in cuvettes thermostatically controlled at 37°C and continuously stirred.
Thermodynamic analysis
For a generic binding equilibrium L+R=LR (L=ligand, R=receptor), the affinity constant KA is directly related to the standard free energy ΔG° (ΔG°=−RTlnKA) which can be separated in its enthalpic and entropic contributions according to the Gibbs equation: ΔG°=ΔH°−TΔS°. ΔG° is calculated as −RTlnKA at 25°C, while the determination of the other thermodynamic parameters (ΔH° and ΔS°) is performed by KA measurements at different temperatures (KA=1 KD−1 where KD is the dissociation constant at equilibrium). Two general cases can be distinguished: (1) ΔCp° (the difference in standard specific heats at constant pressure of the equilibrium) is nearly zero. In this case, the equation (δln Ka)/δ (1 T−1)=−ΔH° R−1 gives a linear van't Hoff plot ln KA versus (1/T) and standard enthalpy can be calculated from its slope, −ΔH° R−1, while standard entropy is calculated as ΔS°=(ΔH°−ΔG°)T−1 with T=298.15 K and R=8.314 J mol−1 K−1; (2) ΔCp° is different from zero. The plot ΔG° versus T is often parabolic and other mathematical methods (Gilli et al., 1994) for calculating the thermodynamic parameters of the equilibrium are available. Saturation experiments of [3H]-DPCPX, [3H]-SCH 58261 and [3H]-MRE 3008F20 binding to the human A1, A2A and A3, respectively, were carried out at 4, 10, 20 and 25°C, in a thermostatic bath assuring a temperature of±0.1°C, using concentrations ranging from 0.5 – 30 nM. In the present case, the van't Hoff plot can be considered as being essentially linear and the first method was applied.
Data analysis and statistics
For saturation binding experiments, determination of receptor affinity (KD) and receptor density (BMAX) was performed using the non linear least-squares curve fitting program LIGAND (Munson & Rodbard, 1980). LIGAND was also used to determine inhibitory binding constant (KI) values from the competition binding experiments. EC50 and KI values were expressed as geometric means with 95% confidence intervals (CI). All other data are expressed as arithmetic mean±s.e.mean. Unless otherwise stated, data are represented by three separate experiments, done in triplicate.
Results
RT – PCR
Total cDNA obtained from the human malignant melanoma A375 cell line was amplified with specific primers for β-actin and the obtained 654 bp product was run in agarose gel and is shown in Figure 1, lane 6. To exclude the presence of residual DNA contamination, RNA was directly amplified with β-actin primers and, as reported in lane 7, we did not find the amplified product confirming the absence of DNA contamination. So we amplified reverse-transcribed RNAs of A1 (lane 2), A2A (lane 3), A2B (lane 4) and A3 (lane 5) adenosine receptors. Detectable amounts of message for all four adenosine receptor subtypes were found.
Figure 1.
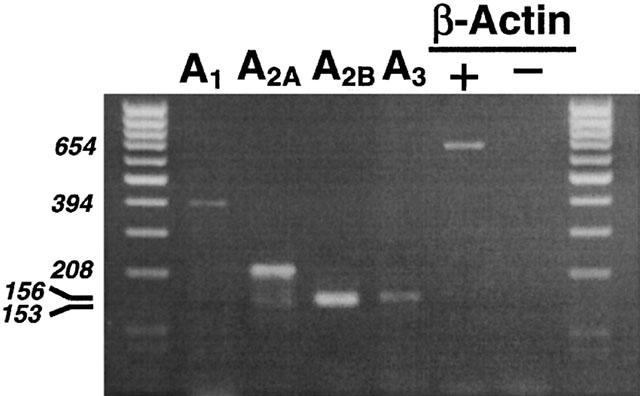
Expression of the adenosine receptors and β-actin transcripts in A375 cells. The PCR product in lane 2 is 394 bp long according to the human A1 receptor sequence, that in lane 3 is 208 bp long according to the human A2A receptor sequence, that in lane 4 is 153 bp long according to the human A2B receptor sequence and that in lane 5 is 156 bp long according to the human A3 receptor sequence. Lane 6 is 654 bp long according to the human β-actin sequence. Lanes 1 and 8 are molecular weight markers. Total RNA was extracted as described in Methods and used for RT – PCR (lanes 2 – 6) or directly used for PCR to detect β-actin DNA contaminant sequences (lane 7). Ten μl of PCR product were loaded in each lane. RT – PCR products obtained from A375 cells and separated on a 2% agarose gel.
Kinetic studies
Association studies indicated that equilibrium was reached after approximately 120, 90 and 150 min for [3H]-DPCPX, [3H]-SCH 58261 and [3H]-MRE 3008F20, respectively, and was stable for at least 5 h (Figure 2A – C). [3H]-DPCPX, [3H]-SCH 58261 and [3H]-MRE 3008F20 binding was rapidly reversed by the addition of 1 μM DPCPX, SCH 58261 and MRE 3008F20, respectively, as shown in Figure 2D – F. A two-site fit of association and dissociation curves was not significantly better than a one-site fit (P<0.05). The rate constants were kobs=0.0193±0.0005, 0.0253±0.0008, 0.0220±0.0004 min−1 and k−1=0.0136±0.0007, 0.0161± 0.0004, 0.0145±0.0005 min−1 from a t1/2=36, 31 and 49 min, for A1, A2A and A3, respectively. With these values, kinetic dissociation constants (KD) of 4.8, 7.0 and 7.7 nM, for A1, A2A and A3, respectively, were calculated.
Figure 2.
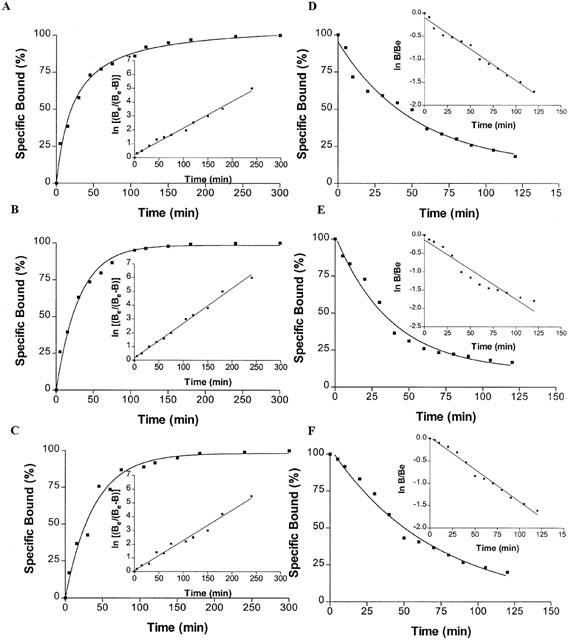
(A – C) Kinetics of [3H]-DPCPX, [3H]-SCH 58261 and [3H]-MRE 3008F20 binding to human A1, A2A and A3 adenosine receptors in A375 cells with association curves representative of a single experiment, which was replicated three times with similar results. The inset shows the data transformed as a semi-log plot, where Be is the specific binding measured at equilibrium and B is the specific binding at time t. (D – F) Kinetics of [3H]-DPCPX, [3H]-SCH 58261 and [3H]-MRE 3008F20 binding to human A1, A2A and A3 adenosine receptors in A375 cells with dissociation curves representative of a single experiment, which was replicated three times with similar results. The inset shows the data transformed as a semi-log plot, where B is the specific binding at time t and Be is the specific binding measured at equilibrium.
Saturation studies
The expression of adenosine receptors in A375 cells was determined performing saturation binding experiments with [3H]-DPCPX, [3H]-SCH 58261 and [3H]-MRE 3008F20 to label A1, A2A and A3 receptors, respectively. A single saturable binding site was detected for all adenosine receptors. Saturation of [3H]-DPCPX shows a KD value of 1.9±0.2 nM and Bmax of 23±7 fmol mg−1 of protein (Figure 3A). [3H]-SCH 58261 exhibits high affinity for A2A receptors with a KD value of 5.1±0.2 nM and Bmax of 220±7 fmol mg−1 of protein (Figure 3B). Figure 3C reports a saturation curve of [3H]-MRE 3008F20 to the adenosine A3 receptor with a KD value of 3.3±0.7 nM and Bmax value of 291±50 fmol mg−1 protein.
Figure 3.
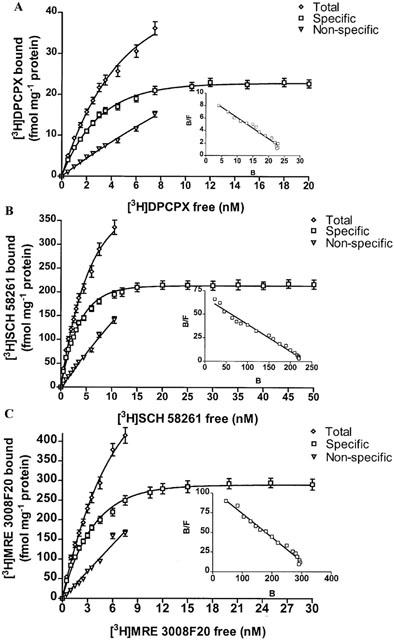
Saturation of [3H]-DPCPX, [3H]-SCH 58261 and [3H]-MRE 3008F20 binding to A1, A2A and A3 adenosine receptors in A375 cells. Experiments were performed as described in Methods. Values are the means and vertical lines s.e. of the mean of four separate experiments performed in triplicate. In the inset the Scatchard plot of the same data is shown.
Competition studies
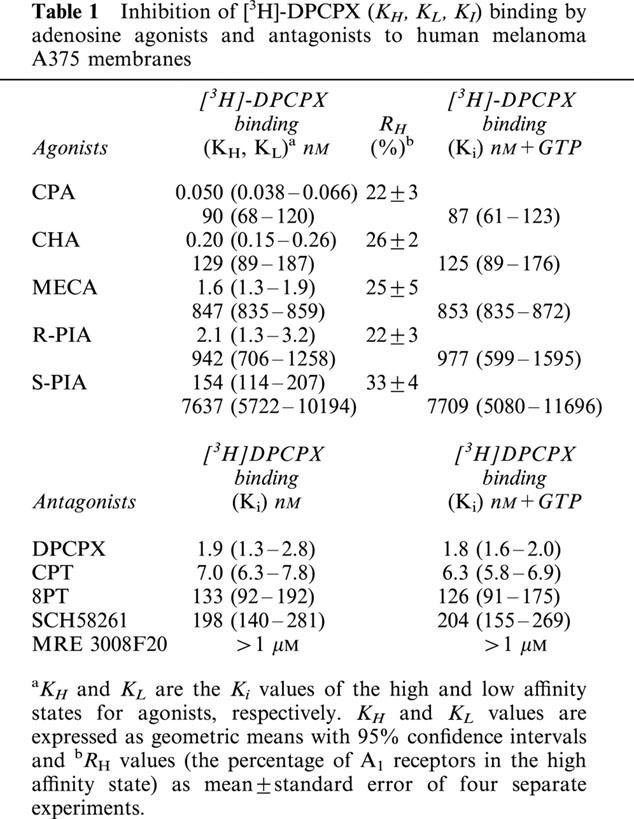
Competition curves for [3H]-DPCPX binding of various agonists of adenosine receptors were best fitted assuming a two-state model corresponding to a high and low affinity state of the A1 adenosine receptor (Figure 4A), a phenomenon expected for a G-protein-coupled receptor. Affinities are reported in Table 1 as KI, KH and KL values, and the percentage of receptors in the high affinity state (RH) is also shown. To test whether the high affinity state of the A1 receptor was linked to a guanine nucleotide regulatory protein, the effect of GTP on the affinity states was examined. The presence of 100 μM GTP shifted the competition curves with agonists from a biphasic to a monophasic shape (LIGAND, P<0.01), with a K1 close to the low affinity sites, as shown in Table 1. In contrast the above treatment did not change the shape of the competition curves with antagonists. The rank order of potency of adenosine agonists to compete with [3H]-DPCPX binding to A375 membranes was: CPA>CHA>MECA>R-PIA>S-PIA. Competition of [3H]-DPCPX was stereoselective, with R-PIA being approximately 73 times more active than its stereoisomer, S-PIA. The order of potency of the receptor antagonists to compete with [3H]-DPCPX was: DPCPX>CPT>8PT>SCH 58261>MRE 3008F20. The selective A1 receptor antagonist DPCPX displayed high affinity value, being the most potent antagonist compound.
Figure 4.
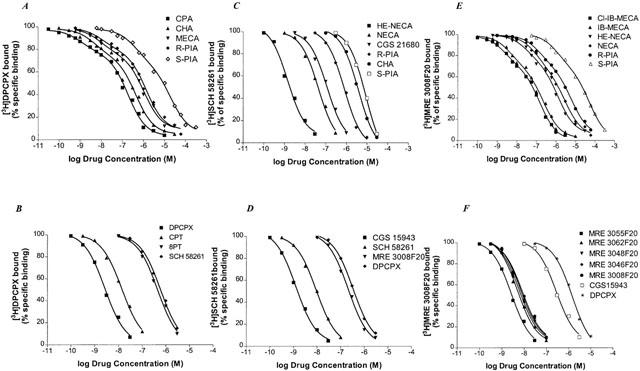
Competition curves of specific [3H]-DPCPX, [3H]-SCH 58261 and [3H]-MRE 3008F20 binding to human A1, A2A and A3 adenosine receptors in A375 cells by adenosine agonists (A,C,E) and antagonists (B,D,F). Curves are representative of a single experiment from a series of four independent experiments. Competition experiments were performed as described in Methods.
Table 1.
Inhibition of [3H]-DPCPX (KH, KL, KI) binding by adenosine agonists and antagonists to human melanoma A375 membranes
KI values for a series of adenosine receptor agonists and antagonists obtained in the competition of [3H]-SCH 58261 binding are summarized in Table 2. Figure 4C,D shows the competition curves of adenosine agonists and antagonists in human malignant melanoma A375 membranes, respectively. The rank order of potency of adenosine agonists to compete with [3H]-SCH 58261 binding to A375 membranes was: HE-NECA>NECA>CGS 21680>R-PIA>CHA>S-PIA. The order of potency of the receptor antagonists to compete with [3H]-SCH 58261 was: CGS 15943>SCH 58261>MRE 3008F20>DPCPX. HE-NECA and NECA were the most potent agonists, their affinity being in the low nanomolar range, while the selective A1 receptor antagonist DPCPX displayed the worst affinity value. CGS 15943 and SCH 58261 were the most potent antagonist compounds. Competition of [3H]-SCH 58261 was stereoselective, with R-PIA being approximately 14 times more active than its stereoisomer, S-PIA. Hill coefficients of all compounds were not significantly different from unity (data not shown).
Table 2.
Inhibition of [3H]-SCH 58261 binding (KI) by adenosine agonists and antagonists to human melanoma A375 membranes
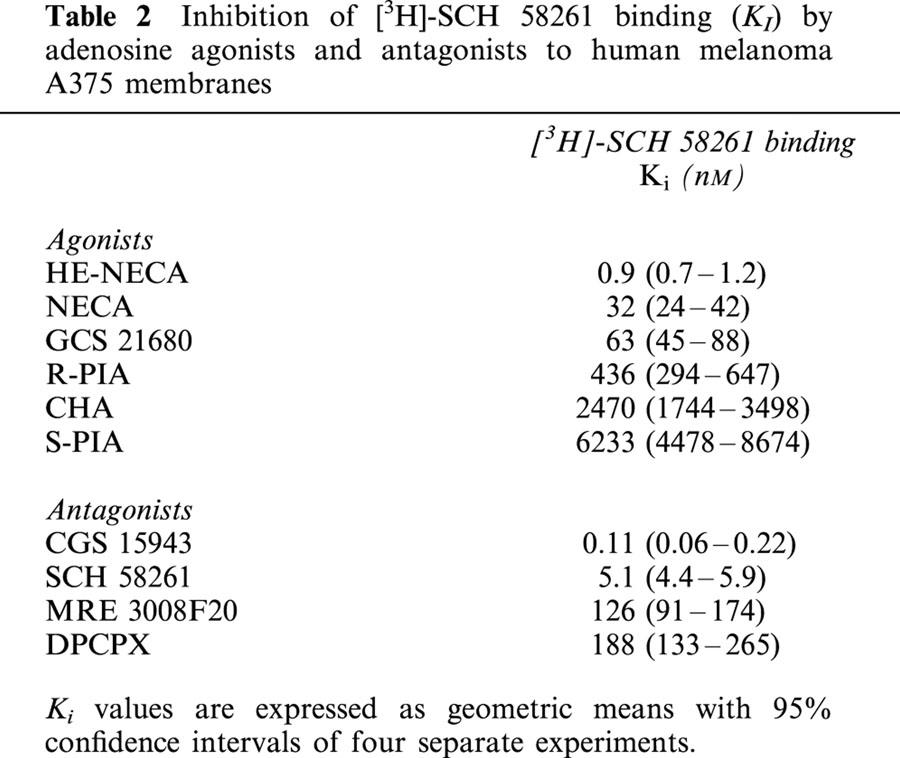
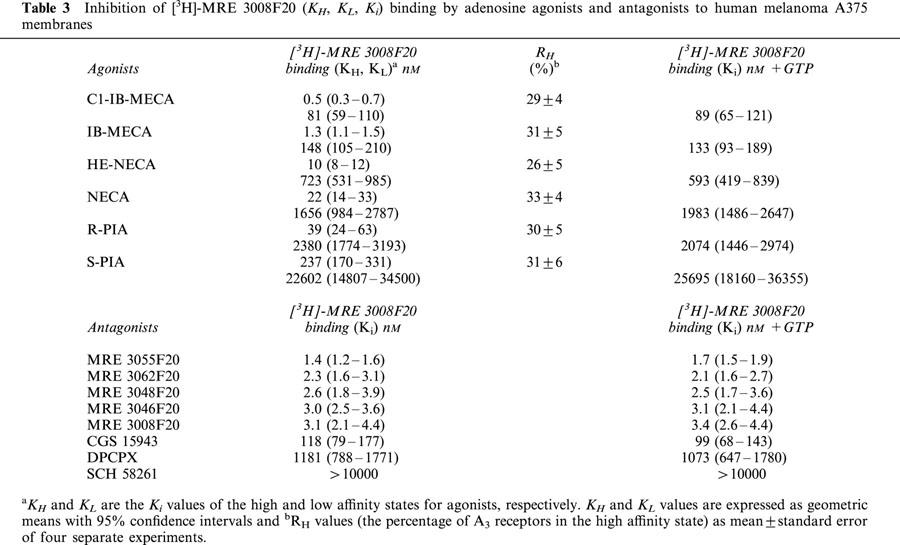
Table 3 shows the comparison of affinities, expressed as KI, KH and KL values, of selected adenosine receptor agonists and antagonists to human A3 receptor expressed in A375 cells using [3H]-MRE 3008F20, and the percentage of receptors in the high affinity state (RH) is also shown. Only one affinity state could be detected with a slope factor near unity in the presence of GTP. The order of potency in [3H]-MRE 3008F20 inhibition assays for adenosine receptor agonists resulted to be: Cl-IB-MECA>IB-MECA>HE-NECA>NECA>R-PIA>S-PIA. R-PIA was approximately six times more active (KH=40 nM; KL=2400 nM) than its stereoisomer, S-PIA (KH=240 nM; KL=23000 nM) showing the stereoselectivity of PIA binding. Antagonist competition curves for [3H]-MRE 3008F20 binding gave the following rank order of potency: MRE 3055F20>MRE 3062F20>MRE 3048F20>MRE 3046F20>MRE 3008F20>CGS 15943>DPCPX each of which exhibited Hill slopes near to unity, either in the absence or in the presence of GTP (Figure 4F, Table 3). SCH 58261 showed a K1 value>10 μM.
Table 3.
Inhibition of [3H]-MRE 3008F20 (KH, KL, Ki) binding by adenosine agonists and antagonists to human melanoma A375 membranes
Assay of cyclic AMP levels
Functional coupling of the A2A adenosine receptors in A375 cells to adenylyl cyclase was investigated by measuring cyclic AMP production in response to HE-NECA, NECA and CGS 21680 as A2A receptor agonists. All these agonists were able to increase cyclic AMP levels displaying an order of potency similar to that observed in binding affinities for the adenosine A2A receptor. HE-NECA appeared to be the most potent compound (EC50=28 nM (95% CI: 23 – 34; n=4) followed by NECA and CGS 21680 (EC50=131 nM (95% CI: 90 – 190, n=4) and 246 nM (95% CI:190 – 320; n=4), respectively) (Figure 5). The selective A2A antagonist SCH 58261 (100 nM) totally inhibited the rise in cyclic AMP levels induced by HE-NECA and NECA (100 nM).
Figure 5.
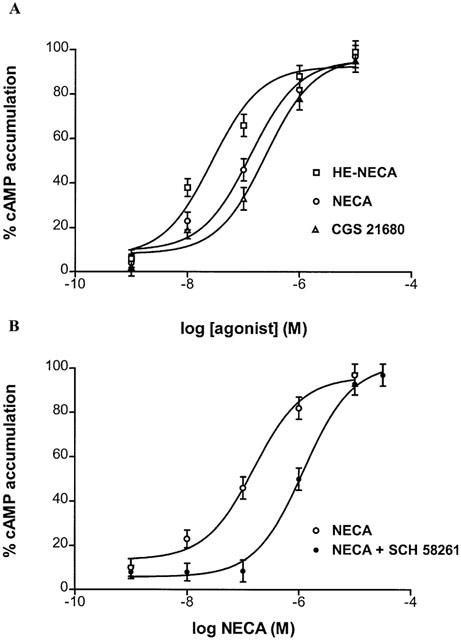
Stimulation curves of cyclic AMP levels by HE-NECA, NECA and CGS 21680 in A375 cells (A). Effect of increasing concentrations of NECA on cyclic AMP accumulation in A375 cells in the absence and in the presence of 100 nM A2A selective antagonist SCH 58261 (B). Data (mean of three experiments±s.e.mean) are given in percentages.
Functional coupling of the A2B adenosine receptors in A375 cells to adenylyl cyclase was also investigated. We repeated the concentration-response curve of NECA-induced cyclic AMP accumulation in the presence of SCH 58261 100 nM, a concentration that produced maximal blockade of A2A receptors while leaving A2B receptors intact. The concentration-response curve of NECA+SCH 58261 100 nM (Figure 5B) was a typical sigmoidal curve with EC50=1206 nM (95% CI: 879 – 1655, n=4) consistent with the presence on surface membranes of functional A2B receptors.
The A1 receptor agonist, CHA, and Cl-IB-MECA and IB-MECA, potent and selective A3 agonists, in the range 0.1 – 10.000 nM, failed to significantly decrease cyclic AMP levels following stimulation with 10 μM forskolin, in the absence and in the presence of SCH 58261 (100 nM).
Calcium mobilization assays
The A3 receptor agonist, Cl-IB-MECA, was able to stimulate Ca2+ release in A375 cells (Figure 6A) producing a rapid rise followed by a sustained increase in [Ca2+]i. Chelation of extracellular Ca2+ with EGTA (500 μM) did not influence the initial rise in [Ca2+]i but abolished a second phase of elevations in [Ca2+]i, suggesting that the first rapid increase was due to Ca2+ release from intracellular Ca2+ pools, while the second phase of elevation appeared to result from an influx of extracellular Ca2+. A375 cells responded to Cl-IB-MECA in a concentration dependent way with a calculated EC50 of 24 μM (95% CI: 19 – 32; n=4) (Figure 6B). IB-MECA and NECA were also able to produce increases in [Ca2+]i levels but in a higher range of concentrations, according to their order of potency in binding experiments performed in A375 cells. To verify the adenosine receptor subtype mediating the Cl-IB-MECA-induced calcium response, the effect of pre-incubation for 10 min with different antagonists of A1 (DPCPX), A2A (SCH 58261), A2A/A2B (ZM 241385) or A3 (MRE 3008F20) adenosine receptors on Cl-IB-MECA-(10 μM)-stimulated calcium influx was evaluated. DPCPX, SCH 58261 and ZM 241385 were used at 0.1 μM because at this concentration they selectively block A1, A2A and A2A/A2B receptors, respectively. These antagonists did not prevent Cl-IB-MECA mediated calcium influx, whereas the A3 antagonist MRE 3008F20 at the same concentration (0.1 μM) reduced calcium levels by 30±3%. Furthermore, 1 μM MRE 3008F20 completely abolished Cl-IB-MECA induced calcium influx (data not shown).
Figure 6.
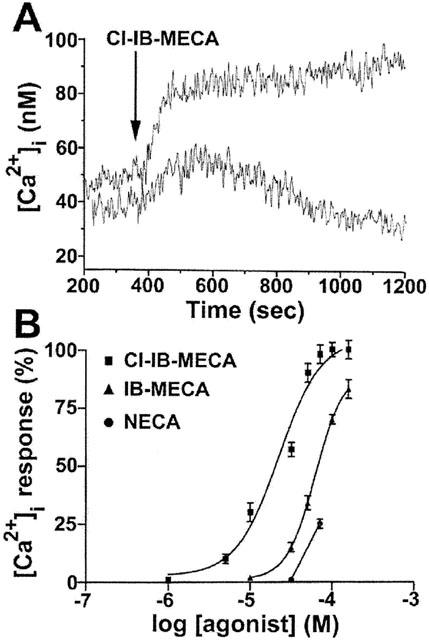
Effect of Cl-IB-MECA (30 μM) on intracellular calcium mobilization. Lower trace was obtained in calcium free medium and in the presence of 0.5 mM EGTA (A). Concentration dependent stimulation of Ca2+ mobilization by Cl-IB-MECA, IB-MECA and NECA in A375 cells (B). Data (mean of three experiments±s.e.mean) are given in percentages.
Thermodynamic analysis
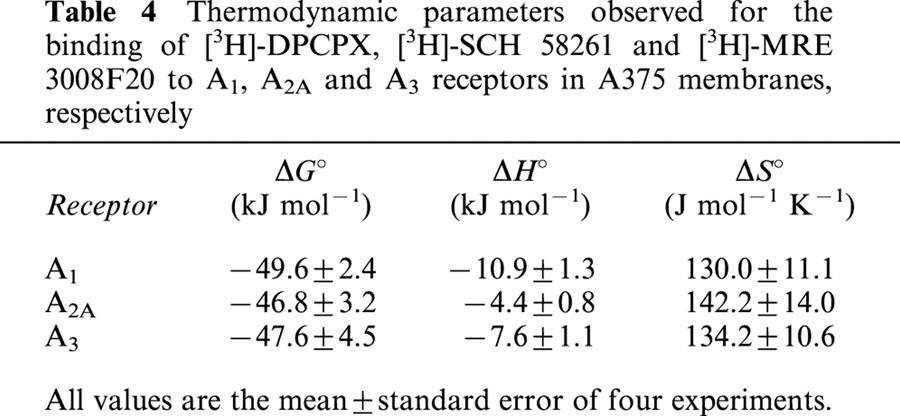
KD and Bmax derived from the saturation experiments of [3H]-DPCPX, [3H]-SCH 58261 and [3H]-MRE 3008F20 binding to A1, A2A and A3 adenosine receptors performed at the four temperatures selected were found within the following range: KD=1.3 – 1.9, 5.1 – 5.9 and 3.3 – 4.3 nM and Bmax=23 – 25, 220 – 240 and 291 – 310 fmol mg−1 protein, for A1, A2A and A3 receptors, respectively. While the dissociation constant (KD) changed with temperature, Bmax values obtained from [3H]-DPCPX, [3H]-SCH 58261 and [3H]-MRE 3008F20 saturation experiments appeared to be independent of it. Figure 7A – C shows the van't Hoff plot lnKA versus 1/T of the [3H]-DPCPX, [3H]-SCH 58261 and [3H]-MRE 3008F20 binding to the A1, A2A and A3 adenosine receptors, respectively, and the final equilibrium thermodynamic parameters (expressed as mean values±standard error of three independent determinations) are expressed in Table 4. The linearity of the plot was statistically significant (ΔCp°, equilibrium heat capacity change, ≈0) and its slope (−ΔH° R−1) was positive for all receptors, a property which has been found to be typical for antagonist binding to adenosine receptors (Borea et al., 1996).
Figure 7.
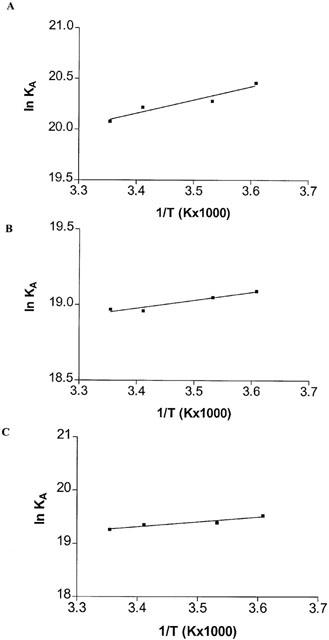
Van't Hoff plot showing the effect of temperature on the equilibrium binding association constant, KA=1/KD of [3H]-DPCPX, [3H]-SCH 58261 and [3H]-MRE 3008F20. The plot is essentially linear in the temperature range investigated 4 – 25°C.
Table 4.
Thermodynamic parameters observed for the binding of [3H]-DPCPX, [3H]-SCH 58261 and [3H]-MRE 3008F20 to A1, A2A and A3 receptors in A375 membranes, respectively
Discussion
The goal of the present study was the pharmacological and biochemical characterization of adenosine receptors in the human malignant melanoma A375 cell line.
We have firstly analysed the expression of the human adenosine receptors by checking the production of the RNA of all the adenosine receptors known. By RT – PCR we have detected the expression of the transcript of A1, A2A, A2B and A3 receptors. Furthermore, we have found strong differences on RNA expression being A1 and A3 mRNAs less expressed than A2A and A2B mRNAs. It should be noted that this result is not predictive of the presence of adenosine receptor in the membrane surface because posttranscriptional events can modulate the amount of protein. Thus, in the present study, we have characterized, for the first time, A1, A2A and A3 adenosine receptors on A375 membranes by using the selective radioligands [3H]-DPCPX, [3H]-SCH 58261 and [3H]-MRE 3008F20.
It is difficult to estimate the number of A2B receptors expressed in A375 cells because selective ligands for ligand-binding studies are not available. Recently, the new xanthine derivative antagonist MRS 1754 was characterized as a selective antagonist tritiated radioligand for A2B receptors (Ji et al., 2001). Unfortunately, this radioligand is not commercially available. In this light, a pharmacological characterization of this adenosine receptor subtype has not been performed.
To investigate the kinetic behaviour, the binding was carried out under pseudo-first order conditions. Analysis of association and dissociation kinetic parameters produced equilibrium constants for A1, A2A and A3 which are in good agreement with those obtained from the equilibrium saturation binding assays.
In saturation experiments the antagonist [3H]-DPCPX labelled a single saturable binding site with a good affinity but with a low receptor density (Bmax=23±7 fmol mg−1 of protein) while the radiolabelled [3H]-SCH 58261 identified a large number (Bmax=220±7 fmol mg−1 of protein) of high-affinity binding sites in A375 membranes. At the same time, [3H]-MRE 3008F20 labelled a single class of recognition sites with binding capacity (Bmax) of 291±50 fmol mg−1 protein. Interestingly, despite the ability to transcribe A2A and A3 adenosine receptor genes at different levels, A375 cells present on the membrane surface similar amounts of receptor protein. Thus, we have performed a more detailed pharmacological and biochemical characterization for A1, A2A and A3 receptor subtypes with the aim of increasing the evidences in support of the conclusion that the [3H]-DPCPX, [3H]-SCH58261 and [3H]-MRE 3008F20 binding sites in melanoma membranes are A1, A2A and A3 receptors. This goal comes from the results of competition binding assays and functional studies. As illustrated in Figure 4 and summarized in Tables 1, 2, 3, the agonist and antagonist affinities for [3H]-DPCPX, [3H]-SCH58261 and [3H]-MRE 3008F20 binding sites are consistent with the identification of these sites as A1, A2A and A3 (Gessi et al., 2000; Varani et al., 2000a).
Competition of [3H]-DPCPX by increasing concentrations of CPA, CHA, MECA, R-PIA and S-PIA (Table 1 and Figure 4A) revealed two binding sites for these agonists. The addition of GTP (100 μM) and subsequent uncoupling of receptors from G-proteins converted the curves of agonists from biphasic to monophasic. The similarity between Ki values determined in the presence of GTP and KL values obtained in the absence of GTP indicated a guanine nucleotide-mediated shift of the high affinity binding sites to a low affinity form, a finding in agreement with that reported for human A3 receptors transfected in CHO cells (Varani et al., 2000a). On the contrary, competition binding curves with antagonists were monophasic and did not change by the addition of GTP, indicating that antagonists bind equally well to both receptor states, whereas agonists discriminate between them.
HE-NECA, NECA and CGS 21680, A2A agonists, were significantly more potent than the A1 agonists R-PIA and CHA to compete with [3H]-SCH 58261 for binding sites. Also, unlabelled SCH 58261 was significantly more potent than the A1 antagonist DPCPX. Coupling of the A2A receptor to its G protein is tight (Palmer & Stiles, 1995; Varani et al., 1997). Hence, we did not perform competition experiments of agonists with [3H]-SCH 58261 binding in the presence of GTP because there is only slow dissociation of agonists from the receptor. The results of radioligand binding studies were consistent with the results of functional assays. We evaluated EC50 values obtained for the stimulation by HE-NECA, NECA and CGS 21680 of cyclic AMP levels. Interestingly, in the adenylyl cyclase assay, the compounds examined exhibited a rank order of potency very close to that observed in binding experiments. The block obtained in the presence of the selective A2A antagonist SCH 58261 in the increase of cyclic AMP levels induced by HE-NECA and NECA (100 nM) suggested that the stimulatory effect was essentially A2A-mediated.
To verify the expression of functional A2B receptors, we used the approach previously described by Feoktistov & Biaggioni, 1998. The non-selective agonist NECA can be used in conjunction with highly selective antagonists of other adenosine receptor subtype to selectively stimulate A2B receptors in cell systems where different adenosine receptors are coexpressed. Thus, the selective A2A-antagonist SCH 58261 can be useful in the discrimination of A2B function in A375 cells also coexpressing A2A receptors. Figure 5B shows an unequivocally A2B-mediated cyclic AMP response. These data indicate that the human melanoma A375 cells express an A2B-adenosine receptor coupled positively to adenylyl cyclase.
Agonists competition of [3H]-MRE 3008F20 yielded both a high and low-affinity state of the A3 receptor (Figure 5A). After uncoupling the A3 adenosine receptor from G proteins by GTP 100 μM treatment, binding of A3 agonists was to a single low-affinity state, identical to the binding of antagonists (Table 3), including a series of new substituted pyrazolo triazolo pyrimidines (Baraldi et al., 1999; Baraldi & Borea, 2000).
We found a lack of effectiveness of CHA, A1 agonist, and Cl-IB-MECA and IB-MECA, A3 agonists, to significantly decrease cyclic AMP levels following stimulation with forskolin. It may be suggested that the effect on the stimulation of cyclic AMP levels by A2A receptors prevailed over the A1- and A3-mediated inhibitory actions, suggesting a stronger potency of A2A receptors in signal transduction over the A1 and A3 receptors. Furthermore, in our experience, we have observed that the co-expression of receptors coupled with Gs proteins and receptors coupled with Gi proteins can mask adenylyl cyclase inhibitor activity of Gi coupled receptors. Our observations agree according to the signalling consequences obtained in doubly Gs/Gi-coupled chimaeric A1/A2A adenosine receptors in HEK-293 cells (Tucker et al., 2000). Furthermore, it is note worthy that it could well be that an adenylyl cyclase inhibition could not be detected because the selectivity that is found in binding is often not found in functional assays and, thus, stimulation and inhibition of the cyclase may occur at very similar concentrations. Thus, in order to exclude the A2A interference on A1-A3 signalling, we tested A1 and A3 agonists also in the presence of SCH 58261 aiming to block A2A receptors, but we did not find any effect in adenylyl cyclase inhibition. This could be explained recognizing that the magnitude of the cyclic AMP response depends not only on the number of receptors but also on the amount of available G protein and the activity of the effector enzyme (Arslan et al., 1999). In addition, it may be suggested that A1 and A3 receptors in A375 cells are not efficiently coupled to Gi proteins. In particular, A1 undetectable effects on adenylyl cyclase activity may be the consequence of its low expression on cell surface.
In calcium mobilization studies, the A3 agonist Cl-IB-MECA was able to increase [Ca2+]i (Figure 6A). EC50 value for this agonist was largely different if compared with its affinity determined in binding studies, according to that obtained in other cell systems (Abbracchio et al., 1995; Brambilla et al., 2000; Jacobson, 1998, Kohno et al., 1996a,1996b; Varani et al., 2000a; Gessi et al., 2001). This discrepancy, previously noted, seems to be a common characteristic of the A3 adenosine receptor but the reasons are not clearly known. We hypothesize that in a dynamic and complex cell system, A3 receptor may interact, after agonist binding, with different types of G proteins that differ in their ability to couple to different regulatory signalling systems, as reported for A2A receptors (Fredholm et al., 2000). Pre-treatment of A375 cells with the A3 antagonist MRE 3008F20 potently reduced the rise in intracellular calcium induced by a submaximal dose of Cl-IB-MECA. This block, not obtained with A1-A2A-A2B receptor antagonists pre-treatment, suggested that the adenosine receptor subtype involved in the calcium response could be the A3.
Finally, thermodynamic studies were performed and the enthalpic (ΔH°) and entropic (ΔS°) contributions to the standard free energy (ΔG°) of the binding equilibrium were determined. The linearity of the van't Hoff plot (Figure 7) indicates that ΔCp° values for the drug interaction are nearly zero, which means that ΔH° and ΔS° values are not significantly affected by temperature variations at least over the temperature range investigated (Borea et al., 1996). It is notable that the linearity of van't Hoff plots in a limited range of temperatures (4 – 25°C) appears to be a common feature of practically all membrane receptor ligands so far studied from a thermodynamic point of view (Gilli et al., 1994). Thermodynamic data obtained from the van't Hoff plot indicate that [3H]-DPCPX, [3H]-SCH 58261 and [3H]-MRE 3008F20 binding to human A1, A2A and A3 receptors is enthalpy- and entropy-driven (Table 4). This binding behaviour has previously been found to be typical of adenosine A1, A2A and A3 antagonists (Borea et al., 1996; Varani et al., 2000a). This is remarkable since the similarity in thermodynamic parameters may be the key for understanding the difficulty of obtaining selective adenosine receptor ligands. Although much progress has been made in recent years to synthesize potent and selective adenosine receptor ligands, today the result is that compounds exhibiting high affinity to only one subtype are an exception (Klotz, 2000).
In conclusion all these data indicate, for the first time, that adenosine receptors present on A375 melanoma cell line have a pharmacological and biochemical profile typical of the A1, A2A, A2B and A3 receptor subtype.
Recently, it has been reported that human tumoural Jurkat cell line expresses high level of A3 receptors (Gessi et al., 2001) in agreement with our results in human melanoma cell line. Furthermore, it has been reported that A3 adenosine receptors increase cell survival of rat leukaemia mast cells by triggering an antiapoptotic signalling (Gao et al., 2001). In our opinion, the presence of adenosine receptors in a malignant cell type, as melanoma, suggests a potential role for adenosine in modulating tumoural processes (Ohana et al., 2001). Given this, we propose the full characterization of adenosine receptors in tumour and normal tissue with the aim to elucidate if A3 or a combination of different adenosine receptors could be associated to the development and promotion of malignant phenotype.
Abbreviations
- CGS 15943
5-amino-9-chloro-2-(furyl)1,2,4-triazolo[1,5-c] quinazoline
- CGS 21680
(2-[p-(2-carboxyethyl)-phenethyl-amino]-5′-N-ethyl-carboxamidoadenosine)
- CHA (N6-cyclohexyladenosine)
ClIBMECA N6 (3iodobenzyl) 2chloroadenosine 5′- N-methyluron-amide
- CPA
N6-cyclopentyladenosine, CPT, 8-cyclopentyltheophylline
- DPCPX
1,3-dipropyl-8-cyclopentyl-xanthine
- [3H]-DPCPX
1,3dipropyl8cyclopentylxanthine
- HENECA
(2-hexynyl-5′Nethylcarboxamidoadenosine)
- [3H]-MRE 3008F20
[3H]5-N-(4-methoxyphenylcarbamoyl)amino-8-propyl-2-(2-furyl) pyrazolo [4,3e]1,2,4triazolo [1,5c] pyrimidine
- [3H]-SCH58261
5amino7 (2phenylethyl) 2 (2furyl) pyrazolo [4,3e] 1,2,4triazolo[1,5c] pyrimidine
- IBMECA
N6 (3iodobenzyl)-adenosine-5′-N-methyluronamide
- MECA
5′-(N-methyl)carboxamidoadenosine
- MRE3008F20
5N(4methoxyphenyl-carbamoyl)amino-8-propyl-2(2furyl)-pyrazolo[4,3e]1,2,4-triazolo[1,5-c] pyrimidine
- MRE 3046F20
5-N-(4-metylphenyl-carbamoyl)amino-8-metyl-2(2furyl)-pyrazolo-[4,3e]-1,2,4-triazolo[1,5-c]pyrimidine
- MRE 3048F20
5-N-(4phenylcarbamoyl)amino-8-ethyl-2(2furyl)-pyrazolo-[4,3e]-1,2,4-triazolo[1,5-c]pyrimidine
- MRE 3055F20
5-N-(4phenylcarbamoyl)amino 8 propyl 2 (2furyl) pyrazolo[4,3e]1,2,4triazolo[1,5c]pyrimidine
- MRE 3062F20
5-N-(4-phenyl-carbamoyl)amino-8-butyl-2(2furyl)-pyrazolo-[4,3e]-1,2,4 triazolo[1,5-c]pyrimidine
- NECA
5′-N(ethyl)carboxamidoadenosine
- 8-PT
8phenyltheophylline
- R-PIA
R(−)-N6(2-phenyl-isopropyl)-adenosine
- SCH58261
7-(2-phenylethyl)-2-(2furyl) pyrazolo [4,3e]1,2,4triazolo [1,5c]pyrimidine
- S-PIA
S(−)N6(2phenylisopropyl)adenosine
References
- ABBRACCHIO M.P., BRAMBILLA R., CERUTI S., KIM H.O., VON LUBITZ D.K.J.E., JACOBSON K.A., CATTABENI F. G Protein-dependent activation of phospholipase C by adenosine A3 receptors in rat brain. Mol. Pharmacol. 1995;48:1038–1045. [PubMed] [Google Scholar]
- ARIZA M.E., BROOME-POWELL M., LAHTI J.M., KIDD V.J., NELSON M.A. Fas-induced apoptosis in human malignant melanoma cell lines is associated with the activation of the p34cdc2-related PITSLRE protein kinases. J. Biol. Chem. 1999;274:28505–28513. doi: 10.1074/jbc.274.40.28505. [DOI] [PubMed] [Google Scholar]
- ARSLAN G., KULL B., FREDHOLM B.B. Signaling via A2A adenosine receptor in four PC12 cell clones. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:28–32. doi: 10.1007/pl00005319. [DOI] [PubMed] [Google Scholar]
- BARALDI P.G., CACCIARI B., ROMAGNOLI R., SPALLUTO G., KLOTZ K-N., LEUNG E., VARANI K., GESSI S., MERIGHI S., BOREA P.A. Pyrazolo[4,3-e]1,2,4-triazolo[1,5-c]-pyrimidine derivatives as highly potent and selective human A3 adenosine receptor antagonists. A possible template for adenosine receptor subtypes. J. Med. Chem. 1999;42:4473–4478. doi: 10.1021/jm991114s. [DOI] [PubMed] [Google Scholar]
- BARALDI P.G., CACCIARI B., ROMAGNOLI R., VARANI K., MERIGHI S., GESSI S., BOREA P.A., LEUNG E., HICKEY S.L., SPALLUTO G. Synthesis and preliminary biological evaluation of [3H]-MRE 3008-F20: the first high affinity radioligand antagonist for the human A3 adenosine receptors. Bioorg. Med. Chem. Lett. 2000;10:209–211. doi: 10.1016/s0960-894x(99)00674-5. [DOI] [PubMed] [Google Scholar]
- BARALDI P.G., BOREA P.A. New potent and selective human adenosine A3 receptor antagonists. Trends Pharmacol. Sci. 2000;21:456–459. doi: 10.1016/s0165-6147(00)01581-9. [DOI] [PubMed] [Google Scholar]
- BLACKBURN M.R., DATTA S.K., WAKAMIYA M., VARTABEDIAN B.S., KELLEMS R.E. Metabolic and immunologic consequences of limited adenosine deaminase expression in mice. J. Biol. Chem. 1996;271:15203–15210. doi: 10.1074/jbc.271.25.15203. [DOI] [PubMed] [Google Scholar]
- BLAY J., WHITE T.D., HOSKIN D.W. The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res. 1997;57:2602–2605. [PubMed] [Google Scholar]
- BOREA P.A., DALPIAZ A., VARANI K., GESSI S., GILLI G. Binding thermodynamics at A1 and A2A adenosine receptors. Life Sci. 1996;59:1373–1388. doi: 10.1016/0024-3205(96)00311-6. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye-binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BRAMBILLA R., CATTABENI F., CERUTI S., BARBIERI D., FRANCESCHI C., KIM Y.C., JACOBSON K.A., KLOTZ K.N., LOHSE M.J., ABBRACCHIO M.P. Activation of the A3 adenosine receptor affects cell cycle progression and cell growth. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:225–234. doi: 10.1007/s002109900186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELENKOV I.J., CHROUSOS G.P., WILDER R.L. Neuroendocrine regulation of IL-12 and TNF-alpha/IL-10 balance. Clinical implications. Ann. N.Y. Acad. Sci. 2000;917:94–105. doi: 10.1111/j.1749-6632.2000.tb05374.x. [DOI] [PubMed] [Google Scholar]
- ETHIER M.F., CHANDER V., DOBSON J.G. Adenosine stimulates proliferation of human endothelial cells in culture. Am. J. Physiol. 1993;265:H131–H138. doi: 10.1152/ajpheart.1993.265.1.H131. [DOI] [PubMed] [Google Scholar]
- ETHIER M.F., DOBSON J.G. Adenosine stimulation of DNA synthesis in human endothelial cells. Am. J. Physiol. 1997;272:H1470–H1479. doi: 10.1152/ajpheart.1997.272.3.H1470. [DOI] [PubMed] [Google Scholar]
- FEOKTISTOV I., BIAGGIONI I. Pharmacological characterization of adenosine A2B receptors: studies in human mast cells co-expressing A2A and A2B adenosine receptor subtypes. Biochem. Pharmacol. 1998;55:627–633. doi: 10.1016/s0006-2952(97)00512-1. [DOI] [PubMed] [Google Scholar]
- FOZARD J.R., PFANNKUCHE H.J., SCHUURMAN H.J. Mast cell degranulation following adenosine A3 receptor activation in rats. Eur. J. Pharmacol. 1996;298:293–297. doi: 10.1016/0014-2999(95)00822-5. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B., ABBRACCHIO M.P., BURNSTOCK G., DALY J.W., HARDEN K., JACOBSON K.A., LEFF P., WILLIAMS M. VI: Nomenclature and classification of purinoceptors. Physiol. Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- FREDHOLM B.B., ARSLAN G., HALLDNER L., KULL B., SCHULTE G., WASSERMAN W. Structure and function of adenosine receptors and their genes. Naunyn Schmiedebergs Arch. Pharmacol. 2000;362:364–374. doi: 10.1007/s002100000313. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B., IRENIUS E., KULL B., SCHULTE G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem. Pharmacol. 2001;61:443–448. doi: 10.1016/s0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- GAO Z., LI B.S., DAY Y.J., LINDEN J. A3 adenosine receptor activation triggers phosphorylation of protein kinase B and protects rat basophilic leukemia 2H3 mast cells from apoptosis. Mol. Pharmacol. 2001;59:76–82. doi: 10.1124/mol.59.1.76. [DOI] [PubMed] [Google Scholar]
- GESSI S., VARANI K., MERIGHI S., ONGINI E., BOREA P.A. A2A adenosine receptors in human peripheral blood cells. Br. J. Pharmacol. 2000;129:2–11. doi: 10.1038/sj.bjp.0703045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GESSI S., VARANI K., MERIGHI S., MORELLI A., FERRARI D., LEUNG E., BARALDI P.A., SPALLUTO G., BOREA P.A. Pharmacological and biochemical characterization of A3 adenosine receptors in Jurkat T cells. Br. J. Pharmacol. 2001;134:116–126. doi: 10.1038/sj.bjp.0704254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLI P., FERRETTI V., GILLI G., BOREA P.A. Enthalpy-entropy compensation in drug-receptor binding. J. Phys. Chem. 1994;98:1515–1518. [Google Scholar]
- GUIEU R., BRUNET P., SAMPOL J., BECHIS G., FENOUILLET E., MEGE J.L., CAPO C., VITTE J., IBRAHIM Z., CARREGA L., LERDA D., ROCHAT H., BERLAND Y., DUSSOL B. Adenosine and hemodialysis in humans. J. Investig. Med. 2001;49:56–67. doi: 10.2310/6650.2001.34091. [DOI] [PubMed] [Google Scholar]
- JACOBSON K.A., NIKODIJEVIC O., SHI D., GALLO-RODRIGUEZ C., OLAH M.E., STILES G.L., DALY J.W. A role for central A3 adenosine receptors. Mediation of behavioural depressant effects. FEBS Lett. 1993;336:57–60. doi: 10.1016/0014-5793(93)81608-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBSON K.A. Adenosine A3 receptors: novel ligands and paradoxical effects. Trends Pharmacol. Sci. 1998;19:184–191. doi: 10.1016/s0165-6147(98)01203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JI X., KIM Y.C., AHERN D.G., LINDEN J., JACOBSON K.A. [3H]MRS 1754, a selective antagonist radioligand for A2B adenosine receptors. Biochem. Pharmacol. 2001;61:657–663. doi: 10.1016/s0006-2952(01)00531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLOTZ K.N. Adenosine receptors and their ligands. Naunyn. Schmiedebergs. Arch. Pharmacol. 2000;362:382–391. doi: 10.1007/s002100000315. [DOI] [PubMed] [Google Scholar]
- KOHNO Y., SEI Y., KOSHIBA M., KIM H.O., JACOBSON K.A. Induction of apoptosis in HL-60 human promyelocytic leukemia cells by adenosine A3 receptor agonists. Biochem. Biophys. Res. Commun. 1996A;219:904–910. doi: 10.1006/bbrc.1996.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOHNO Y., JI X., MAWHORTER S.D., KOSHIBA M., JACOBSON K.A. Activation of A3 adenosine receptors on human eosinophils elevates intracellular calcium. Blood. 1996B;88:3569–3574. [PMC free article] [PubMed] [Google Scholar]
- LIANG B.T., JACOBSON K.A. A physiological role of the adenosine A3 receptor: sustained cardioprotection. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6995–6999. doi: 10.1073/pnas.95.12.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSTON H.M., FINLAYSON K., MAEMOTO T., OLVERMAN H.J., AKAHANE A., SHARKEY J., BUTCHER S.P. Pharmacological characterization of a simple behavioral response mediated selectively by central adenosine A1 receptors, using in vivo and in vitro techniques. J. Pharmacol. Exp. Ther. 1998;285:1023–1030. [PubMed] [Google Scholar]
- MONTESINOS M.C., GADANGI P., LONGAKER M., SUNG J., LEVINE J., NILSEN D., REIBMAN J., LI M., JIANG C.K., HIRSCHHORN R., RECHT P.A., OSTAD E., LEVIN R.I., CRONSTEIN B.N. Wound healing is accelerated by agonist of adenosine A2 (Gαs–linked) receptors. J.Exp.Med. 1997;186:1615–1620. doi: 10.1084/jem.186.9.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNSON P.J., RODBARD D. Ligand: a versatile computerized approach for the characterization of ligand binding systems. Anal. Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- OHANA G., BAR-YEHUDA S., BARER F., FISHMAN P. Differential effect of adenosine on tumor and normal cell growth: focus on the A3 adenosine receptor. J. Cell. Physiol. 2001;186:19–23. doi: 10.1002/1097-4652(200101)186:1<19::AID-JCP1011>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- OLAH M.E., STILES G.L. Adenosine receptor subtypes: characterization and therapeutic regulation. Annu. Rev. Pharmacol. Toxicol. 1995;35:581–606. doi: 10.1146/annurev.pa.35.040195.003053. [DOI] [PubMed] [Google Scholar]
- PALMER T.M., STILES G.L. Adenosine receptors. Neuropharmacology. 1995;34:683–694. doi: 10.1016/0028-3908(95)00044-7. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- SAFRAN N., SHNEYVAYS V., BALAS N., JACOBSON K.A., NAWRATH H., SHAINBERG A. Cardioprotective effects of adenosine A1 and A3 receptor activation during hypoxia in isolated rat cardiac myocytes. Mol. Cell. Biochem. 2001;217:143–152. doi: 10.1023/a:1007209321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHRIER S.M., VAN TILBURG E.W., VAN DER MEULEN H., IJZERMAN A.P., MULDER G.J., NAGELKERKE J.F. Extracellular adenosine-induced apoptosis in mouse neuroblastoma cells: studies on involvement of adenosine receptors and adenosine uptake. Biochem. Pharmacol. 2001;61:417–425. doi: 10.1016/s0006-2952(00)00573-6. [DOI] [PubMed] [Google Scholar]
- TUCKER A.L., JIA L.G., HOLETON D., TAYLOR A.J., LINDEN J. Dominance of Gs in doubly Gs/Gi-coupled chimaeric A1/A2A adenosine receptors in HEK-293 cells. Biochem. J. 2000;352:203–210. [PMC free article] [PubMed] [Google Scholar]
- VARANI K., GESSI S., DALPIAZ A., ONGINI E., BOREA P.A. Characterization of A2A adenosine receptors in human lymphocyte membranes by [3H]-SCH 58261 binding. Br. J. Pharmacol. 1997;122:386–392. doi: 10.1038/sj.bjp.0701378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARANI K., MERIGHI S., GESSI S., KLOTZ K.N., LEUNG E., BARALDI P.G., CACCIARI B., ROMAGNOLI R., SPALLUTO G., BOREA P.A. [3H]MRE 3008F20: a novel antagonist radioligand for the pharmacological and biochemical characterization of human A3 adenosine receptors. Mol. Pharmacol. 2000a;57:968–975. [PubMed] [Google Scholar]
- VARANI K., PORTALUPPI F., GESSI S., MERIGHI S., ONGINI E., BELARDINELLI L., BOREA P.A. Dose and time effects of caffeine intake on human platelet adenosine A2A receptors: functional and biochemical aspects. Circulation. 2000b;102:285–289. doi: 10.1161/01.cir.102.3.285. [DOI] [PubMed] [Google Scholar]
- WALKER B.A., JACOBSON M.A., KNIGHT D.A., SALVATORE C.A., WEIR T., ZHOU D., BAI T.R. Adenosine A3 receptor expression and function in eosinophils. Am. J. Resp. Cell. Mol. Biol. 1997;16:531–537. doi: 10.1165/ajrcmb.16.5.9160835. [DOI] [PubMed] [Google Scholar]
- WALTHER W., STEIN U., EDER C. RNA analysis using miniprep RNA in reverse transcription PCR. Biotechniques. 1994;17:674–675. [PubMed] [Google Scholar]
- YAO Y., SEI Y., ABBRACCHIO M.P., JIANG J.L., KIM Y.C., JACOBSON K. Adenosine A3 receptor agonists protect HL60 and U-937 cells from apoptosis induced by A3 antagonists. Biochem. Biophys. Res. Comm. 1997;232:317–322. doi: 10.1006/bbrc.1997.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]


