Abstract
In the nucleus accumbens (NAc) of rats, the involvement of P2X and P2Y receptors in the generation of astrogliosis in vivo, was investigated by local application of their respective ligands. The agonists used had selectivities for P2X1,3 (α,β-methylene adenosine 5′-triphosphate; α,β-meATP), P2Y1,12 (adenosine 5′-O-(2-thiodiphosphate; ADP-β-S) and P2Y2,4,6 receptors (uridine 5′-O-(3-thiotriphosphate; UTP-γ-S). Pyridoxalphosphate-6-azophenyl-2,4-disulphonic acid (PPADS) was used as a non-selective antagonist. The astroglial reaction was studied by means of immunocytochemical double-labelling with antibodies to glial fibrillary acidic protein (GFAP) and 5-bromo-2′-deoxyuridine (BrdU).
The agonist-induced changes in comparison to the artificial cerebrospinal fluid (aCSF)-treated control side reveal a strong mitogenic potency of ADP-β-S and α,β-meATP, whereas UTP-γ-S was ineffective. The P2 receptor antagonist PPADS decreased the injury-induced proliferation when given alone and in addition inhibited all agonist effects.
The observed morphogenic changes included hypertrophy of astrocytes, elongation of astrocytic processes and up-regulation of GFAP. A significant increase of both GFAP-immunoreactivity (IR) and GFA-protein content (by using Western blotting) was found after microinfusion of α,β-meATP or ADP-β-S. In contrast, UTP-γ-S failed to increase the GFAP-IR. The morphogenic effects were also inhibited by pre-treatment with PPADS.
A double immunofluorescence approach with confocal laser scanning microscopy showed the localisation of P2X3 and P2Y1 receptors on the GFAP-labelled astrocytes.
In conclusion, the data suggest that P2Y (P2Y1 or P2Y12) receptor subtypes are involved in the generation of astrogliosis in the NAc of rats, with a possible minor contribution of P2X receptor subtypes.
Keywords: Astrocytes, astrogliosis, proliferation, P2 receptor agonists, P2 receptor antagonists, immunocytochemistry
Introduction
ATP represents one of the endogenous factors released from cells following injury (Gordon, 1986; Queiroz et al., 1997). This extracellular signalling molecule acts either by stimulating the P2X receptor family of ligand-gated cation channels (which mediate fast responses) or the P2Y family of G protein-coupled receptors (which mediate slow metabotropic responses) (Abbracchio & Burnstock, 1994; Ralevic & Burnstock, 1998). Currently seven P2X and six P2Y receptor-subtypes of human origin are known (Burnstock, 1999; von kügelgen & Wetter, 2000; Hollopeter et al., 2001).
Astrocytes express a variety of cell surface receptors which render them capable of responding to extracellular stimuli (Kimelberg, 1995). Cultured astrocytes from, for example, the cortex or the striatum were described to possess P2X- and/or P2Y-receptors (Bruner & Murphy, 1993; Walz et al., 1994; Ho et al., 1995; King et al., 1996; Centemeri et al., 1997). A trophic role of ATP in development as well as in tissue injury and repair is suggested because of the presence of P2 receptors on astroglial cells. In fact, extracellular nucleotides have been shown to induce morphogenic and mitogenic effects in astrocytes in vitro (Neary & Norenberg, 1992; Abbracchio et al., 1994; Neary et al., 1996a). After infusion of ATP into the rat brain an increase of GFAP-staining was observed also in vivo (Hindley et al., 1994). Astrogliosis is characterized by cellular hypertrophy, increase in glial fibrillary acidic protein (GFAP) and in some cases by proliferation (Norenberg, 1994).
In a previous study, we have reported that tissue injury and microinfusion of 2-methylthioATP (2-MeSATP; mixed P2X/P2Y receptor agonist) into the nucleus accumbens (NAc) of rats under in vivo conditions induces astrogliosis which could be inhibited by the P2 receptor antagonists pyridoxal-phosphate-6-azophenyl-2,4-disulphonic acid (PPADS) and reactive blue 2 (Franke et al., 1999a). These results suggest the involvement of P2 receptors in the generation of astrogliosis in vivo and the possibility that more than one P2 receptor mediates the responses of astrocytes in the NAc of rats. Co-expression of various P2 receptor subtypes in different tissues have been described (Ho et al., 1995; King et al., 1996).
In order to investigate which subtypes of nucleotide receptors are involved in the astrogliosis in the NAc in vivo, the more selective P2 receptor agonists α,β-methylene adenosine 5′-triphosphate (α,β-meATP; P2X1,3), adenosine 5′-O-(2-thiodiphosphate) (ADP-β-S; P2Y1 or P2Y12) and uridine 5′-O-(3-thiotriphosphate) (UTP-γ-S; P2Y2,4,6) were used (Franke et al., 1999b; 2000). These agonists and the P2 receptor antagonist PPADS were infused into the NAc of rats. Immunocytochemical double labelling with antisera to GFAP (specific marker for fibrous astrocytes), 5-bromo-2′-deoxyuridine (BrdU; proliferation marker) and to the P2X3 and P2Y1 receptor subtypes were performed to study GFAP-immunoreactivity (GFAP-IR) and proliferative activity of astrocytes as well as P2X3 and P2Y1 receptor localization. Western blotting was used to quantify the GFA-protein content of the NAc.
Methods
Animals
Male Wistar rats (WistLei; 280 – 320 g) were housed under a 12 h light – 12 h dark cycle and were allowed access to lab feed and water ad libitum.
Operational procedure and microinfusion
All animal use procedures were approved by the committee of Animal Care and Use of the relevant local governmental body in accordance with the law of experimental animal protection. The rats were fixed under anaesthesia (90 mg kg−1 i.p. ketamine hydrochloride and 15 mg kg−1 i.p. xylazine hydrochloride) in a stereotaxic frame. After opening the skull, a stainless steel cannula (O.D. 0.25 mm) was inserted into the core of the NAc (1.7 mm rostral to the bregma, 1.5 mm lateral to the sagittal suture, 6.5 mm below the surface of the hemisphere). The cannula was connected with a microinfusion pump via a flexible FEP-tubing. The effects of the following agonists were studied: α,β-meATP, ADP-β-S and UTP-γ-S. The rats received BrdU (0.1 nmol), or a mixture of PPADS (0.03 nmol) and BrdU at first; 15 min after terminating injection a second application containing the respective agonist (0.1 nmol, each) or a mixture of PPADS (0.03 nmol) and the agonist (0.1 nmol, each) followed. Artificial cerebrospinal fluid (aCSF (mM): NaCl 126, KCl 2.5, NaH2PO4 1.2, MgCl2 1.3, CaCl2 2.4, pH 7.4), or test substances were injected in a volume of 1 μl at a rate of 12 μl h−1.
After a postinjection period of 4 days the rats were transcardially perfused under thiopental sodium-anaesthesia with paraformaldehyde (2%) in sodium acetate buffer (pH 6.5) followed by paraformaldehyde (2%)/glutaraldehyde (0.1%) in sodium borate buffer (pH 8.5). Serial coronal sections (50 μm thick) from the NAc were obtained by using a vibratome (TSE, Bad Homburg, Germany) and collected as free-floating slices in 0.1 M Tris (pH 7.6).
Immunocytochemistry
The GFAP-staining procedure was performed as previously described by Franke (1995). GFAP was characterized with rabbit anti-cow GFAP antiserum (1 : 600; DAKO, Glostrup, Denmark) and biotinylated protein A (1 : 400; Calbiochem, La Jolla, CA, U.S.A.). For the detection of the astroglial marker the streptavidin/biotin technique (1 : 125; StreptABComplex; DAKO) and 3,3′-diaminobenzidine tetrahydrochloride (DAB; 0.05%; Sigma) were used.
Mitotic astrocytes were identified by immunostaining of the incorporated BrdU. After DNA denaturation (2 N HCl) and neutralization (borate buffer; 0.15 M; pH 8.5) the slices were incubated with a mouse monoclonal antibody against BrdU (Clone Bu20a; 1 : 75; DAKO) followed by incubation with horse biotinylated anti-mouse immunoglobulins (1 : 100; Vector Labs., Burlingame, CA, U.S.A.) and with ABC Elite Kit (1 : 50; Vectastain; Vector Labs.). Peroxidase activity was visualized with DAB (0.07%) containing nickel ammonium sulphate (1%) plus cobalt chloride (1%) (DAB-Ni/Co) and hydrogen peroxide, which renders a black reaction product. After mounting on slide glasses all stained sections were dehydrated in a series of graded ethanol, processed through n-butylacetate and covered with entellan (Merck, Darmstadt, Germany).
Single GFAP-staining was used for characterizing morphogenic changes (hypertrophy, elongation and changes in GFAP-IR). For GFAP-/BrdU-double staining experiments to characterize mitogenic changes, the slices were first processed for anti-GFAP-labelling followed by BrdU-immunolabelling. The two reaction products could be distinguished by their different colours (GFAP: brown; BrdU: dark-blue to violet) and by their specific intracellular location (GFAP, in the cytoplasm and processes; BrdU, in the nuclei).
Immunofluorescence
After washing with Tris-buffered saline (TBS, 0.05 M; pH 7.6) and blocking with normal goat serum (NGS) in TBS the slices (coronal sections from the NAc; 50 μm thick) were incubated in an antibody mixture of mouse anti-GFAP (1 : 1000; Sigma) and of rabbit anti-P2X3 receptor antibody (1 : 1000, GlaxoWellcome, Cambridge, U.K.) or of rabbit anti-P2Y1 receptor antibody (1 : 1500, SmithKline Beecham Pharmaceuticals, U.K.) with 0.1% Triton X-100 in 1% NGS in TBS for 48 h at 4°C. The secondary antibodies employed for the simultaneous localization of the two primary antibodies were Cy2-conjugated goat anti-mouse IgG (1 : 500; Jackson Immuno Research, Baltimore, U.S.A.) and Cy3-conjugated goat anti-rabbit IgG (1 : 800; Jackson Immuno Research), respectively. The sections were washed three times for 5 min each in 1% NGS in TBS and then incubated for 2 h in a solution containing a mixture of the secondary antibodies with 1% NGS in TBS. After intensive washing and mounting on slide glasses all stained sections were dehydrated in a series of graded ethanol, processed through n-butylacetate and covered with entellan (Merck, Darmstadt, Germany). Control experiments were carried out without primary antibody or by pre-adsorption of the antibody with the immunizing peptides.
Confocal microscopy
The double-immunofluorescence was investigated by a scanning confocal microscope (LSM 510, Zeiss, Oberkochen, Germany) equipped with an argon laser emitting at 488 nm and a helium/neon laser emitting at 543 nm. The two reaction products were distinguished by their different fluorescence: GFAP by the green Cy2-immunofluorescence and the P2X3 or the P2Y1 receptors by the red Cy3-immunofluorescence.
GFAP-Western blotting
Sample preparation
Immediately after excision, brain tissue samples were rapidly frozen and stored at −70°C. The frozen tissue was homogenized in phosphate buffer (pH 7.4; 0.06 M potassium phosphate, 1 mM EDTA). Protein concentrations were measured according to the method of Bradford (1976).
Immunoblotting and GFAP-quantification
2.5 μl (containing 0.25 μg protein) of the control and test sample preparation (as duplicates) were separated electrophoretically on 12% resolving polyacrylamide mini-gels using a Mini Protean II electrophoresis unit (BIO-RAD Laboratories GmbH, Germany) and then quantitatively transferred to nitrocellulose sheets (0.45 μm). After incubation for 1 h in TRIS-buffered saline containing 5% membrane blocking reagent, the membranes were exposed to primary anti-GFAP antibody (1 : 4000; DAKO) for 1 h. Subsequently, the sheets were incubated with biotinylated anti-rabbit antibody (1 : 1333; Amersham Pharmacia Biotech., U.K.) for 1 h followed by incubation of the blots with diluted streptavidin-horseradish-peroxidase (1 : 2000; Amersham) for 20 min. Enhanced chemiluminescence (ECL)-reagents and ECL-hyperfilm (Amersham) were used for detection.
Quantification and statistical analysis
Proliferating cells were identified according to morphological criteria (Franke et al., 1999a). All GFAP-positive cells and all GFAP-/BrdU-double stained cells were counted under a light microscope (Axioskop; Zeiss, Oberkochen, Germany) with a 20× objective within a square (0.5×0.5 mm) in identical areas of the NAc for each experimental situation (Figure 1). The cells were counted in the section containing the centre of the needle tract. Each value represents five replications for each condition. The results were expressed as a percentage of cells in the same region of the aCSF-treated side. For determination of statistically significant differences between the ipsi- and contralateral region, the Mann-Whitney-test was used. The individual groups were compared with one-way ANOVA using the Bonferroni-test.
Figure 1.

Horizontal section of the rat brain including the nucleus accumbens (NAc). The schematic localization of the needle tracts and the areas in which the cells were counted (1: core 1; 2: core 2; 3: ventral shell; 4: piriform cortex; according to Franke et al., 1999a) are shown.
The content of GFA-protein was detected by enhanced chemiluminescence. The intensity of the staining was quantified using a ScanJet 4c scanner (Hewlett Packard) and the Sigma Gel software from Jandel Scientific. Staining of the control side preparations was taken as 100%.
Materials
Drugs used were: adenosine 5′-O-(2-thiodiphosphate (ADP-β-S), 5-bromo-2′-deoxyuridine (BrdU), 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma, Deisenhofen, Germany), α,β-methylene adenosine 5′-triphosphate (α,β-meATP; RBI; Natick, MA, U.S.A.), uridine 5′-O-(3-thiotriphosphate) (UTP-γ-S; Inspire Pharmaceuticals Inc., Durham, NC, U.S.A.), pyridoxalphosphate-6-azophenyl-2,4-disulphonic acid (PPADS; Biotrend, Köln, Germany), ketamine hydrochloride (Parke-Davis; Berlin, Germany) and xylazine hydrochloride (Bayer, Leverkusen, Germany).
Results
Insertion of the injection cannula and infusion of aCSF caused astrogliosis characterized by an increase in the number of single-stained (GFAP) and double stained (GFAP/BrdU) cells, by up-regulation of GFAP-IR, as well as by hypertrophy of astrocytes after a postinjection time of 4 days. Microinfusion of the P2 receptor agonists and antagonists modified the extent of astrogliosis.
Cells that had synthesized DNA in the presence of BrdU were immunocytochemically identified with antibodies to BrdU. Stained nuclei appeared as black or blue-purple precipitates. Reactive astrocytes (single- and double-stained) were found near the needle tract and in adjacent subfields, up to the cortical regions on both sides. Examples of single- and double-stained cells are illustrated in Figure 2.
Figure 2.
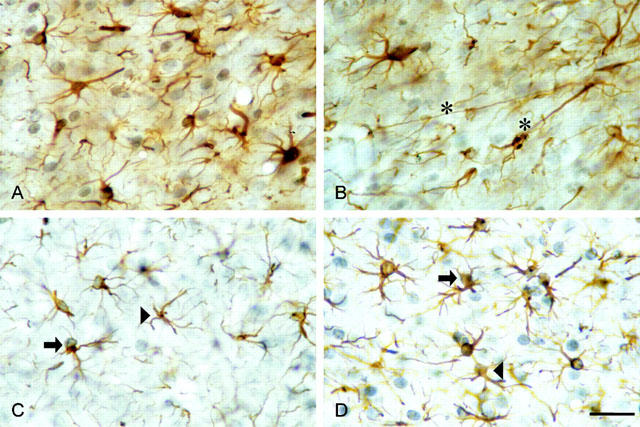
Glial fibrillary acidic protein (GFAP)-/bromodeoxyuridine (BrdU)-double stained cells in the NAc of the rat (GFAP: brown, cytoplasm and processes; BrdU: dark-blue to violet, nuclei). (A,B) Effects of α,β-meATP on astrocytes in the NAc of rats after a postinjection time of 4 days. Asterisks mark examples of intensive elongation of astrocytic processes. (C,D) Single- (arrowhead) and double-stained cells (arrow) 4 days after ADP-β-S-application. (C) Artificial cerebrospinal fluid (aCSF)-treated control side. (D) ADP-β-S-treated side (scale bar: 20 μm).
Mitogenic effects
The effects of all agonists were evaluated 4 days after microinfusion into the NAc. The mitogenic effect of ADP-β-S (0.1 nmol) was the most powerful of all investigated agonists. Not only the number of GFAP-positive cells but also the number of GFAP-/BrdU-double stained cells (Figure 3A) increased in the studied regions, when compared to the aCSF-treated control side. PPADS (0.03 nmol) alone decreased the number of GFAP- and GFAP-/BrdU-double stained cells (see Franke et al., 1999a). In addition, PPADS (0.03 nmol) counteracted the effect of ADP-β-S (0.1 nmol; Figure 3A). It is noteworthy that an unequivocal antagonistic interaction between PPADS and ADP-β-S was observed only when cells were double stained for GFAP and BrdU. The microinfusion of α,β-meATP increased the number of GFAP-positive cells and GFAP-/BrdU double-stained cells (Figure 3B) in most of the investigated areas, when compared to the aCSF-treated control side. PPADS (0.03 nmol) partially counteracted the mitogenic effects of α,β-meATP (0.1 nmol; see e.g. the core 2 and cortex regions for the GFAP-/BrdU-double stained cells). This antagonism, however, was by far not as marked as in the case of ADP-β-S (compare Figure 3A,B).
Figure 3.
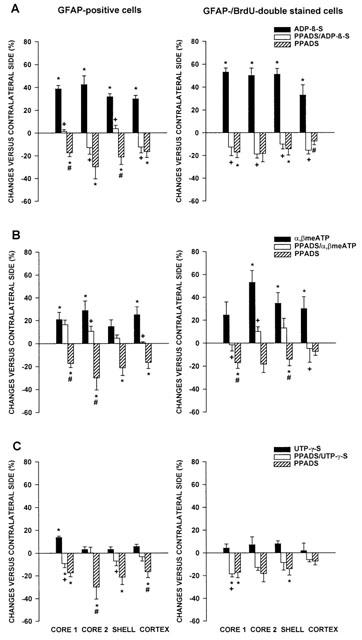
Effects of ADP-β-S, PPADS plus ADP-β-S and PPADS alone (A), α,β-meATP, PPADS plus α,β-meATP and PPADS alone (B), and UTP-γ-S, PPADS plus UTP-γ-S and PPADS alone (C) on the number of GFAP-positive cells and the number of GFAP-/BrdU-double stained cells in the NAc of rats after a postinjection time of 4 days. Rats received at first an ipsilateral injection of aCSF, followed by agonist (0.1 nmol), or PPADS alone (0.03 nmol) or at first PPADS (0.03 nmol), followed by a mixture of PPADS (0.03 nmol) and agonist (0.1 nmol). The contralateral NAc received two injections of aCSF as a control. The values are expressed as a percentage of controls and represent the mean±s.e.m. of six animals per group (*P<0.05, versus aCSF group; +P<0.05, agonist versus PPADS/agonist group; #P<0.05, PPADS versus PPADS/agonist group).
After microinfusion of UTP-γ-S (0.1 nmol), the number of GFAP-positive cells significantly increased in the immediate vicinity of the application site only (core 1; Figure 3C). There was no further measurable increase observed either in the number of GFAP-stained or GFAP-/BrdU-double stained cells (Figure 3C). PPADS (0.03 nmol) interfered with the effect of UTP-γ-S (0.1 nmol) in the region core 1 (GFAP-positive cells).
Finally, the number of GFAP-/BrdU-double stained cells was averaged in the four investigated areas of the NAc after microinjection of ADP-β-S, α,β-meATP and UTP-γ-S (0.1 nmol each) and compared to the aCSF-treated contralateral side (Figure 4; data for the mitogenic effects of 2-MeSATP were taken from a previous study; Franke et al., 1999a). The most marked increase in the number of GFAP-/BrdU-double stained cells was caused by ADP-β-S, followed by 2-MeSATP and α,β-meATP. UTP-γ-S had almost no mitogenic activity.
Figure 4.
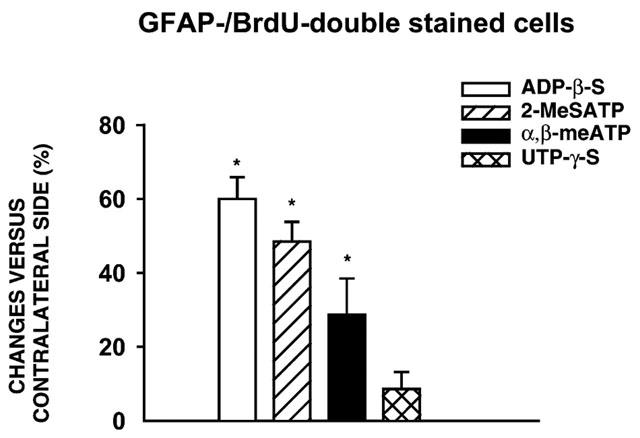
Comparison of the sum of GFAP-/BrdU-double stained cells in various areas of the NAc 4 days after microinjection of P2 receptor agonists. Similar results with 2-MeSATP are included from a previous publication (Franke et al., 1999a). Values are expressed as a percentage of controls and represent the mean±s.e.m. of six animals per group (*P<0.05, versus aCSF group). The differences in the effects of ADP-β-S, 2-MeSATP and α,β-meATP are statistically significant (P<0.05).
Morphogenic effects
Microinfusion of the P2 receptor agonists resulted in hypertrophy and up-regulation of the GFAP-IR in the cytoplasm and processes of astrocytes after a postinjection period of 4 days (Figure 2A,B,D) in comparison to the aCSF-treated control side (Figure 2C). The elongation of astrocytic processes was particularly pronounced after α,β-meATP application (Figure 2B).
In order to verify that the increase of GFAP-IR after α,β-meATP (0.1 nmol)- or ADP-β-S (0.1 nmol)-microinfusion results from an increase in GFA-protein content and not only from hypertrophy of the cells in combination with increased antigenicity (Franke, 1995), the GFA-protein content was measured using immunoblotting. A quantitative evaluation of these data indicated that ADP-β-S and α,β-meATP increased the GFA-protein content by 28±8% (Figure 5A) and by 46±5% (data not shown). This effect could be blocked by pre-treatment with PPADS (0.03 nmol; see Figure 5A for ADP-β-S). Figure 5B illustrates the increase in GFA-protein content after ADP-β-S-microinfusion in comparison to the aCSF-treated control side.
Figure 5.
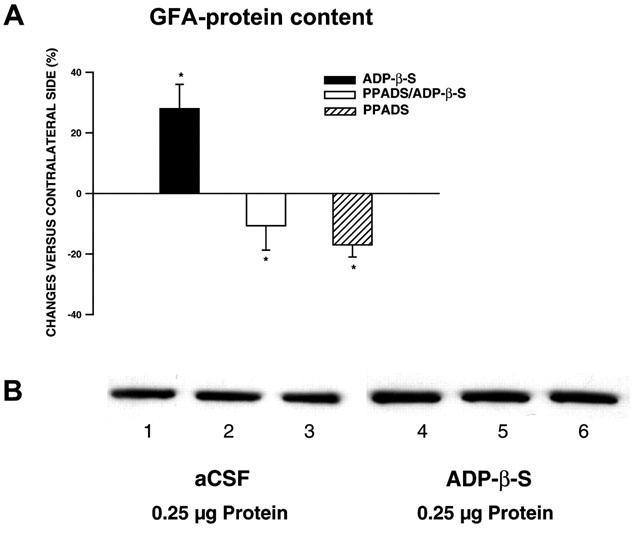
GFA-protein was detected by immunoblotting followed by measurement of chemiluminescence on nitrocellulose filter. The protein content was determined after ADP-β-S microinfusion in comparison with the aCSF-treated control side following a postinjection time of 4 days. (A) Quantification of the ADP-β-S-induced GFA-protein content in comparison with that induced by ADP-β-S plus PPADS or PPADS alone. The values are expressed as a percentage of controls and represent the mean±s.e.m. of three animals per group (*P<0.05, versus aCSF-treated side). (B) Immunoblot analysis of preparations of the aCSF- (lanes 1 – 3) and ADP-β-S-treated sides (lanes 4 – 6) using GFAP antibodies. Each lane contains 0.25 μg protein.
Immunocytochemical demonstration of P2X and P2Y receptors
Double labelling of astrocytes with antibodies against GFAP and the P2X3 as well as P2Y1 receptor-subtypes, proved the localization of these receptors on activated astrocytes in the NAc of rats. Figure 6 shows examples of the localization of P2X3 (A – F) and P2Y1 (G – L) receptors on GFAP-positive astrocytes. The strong immunoreactivity for these receptors was found after microinfusion of the specific agonists α,β-meATP (P2X3 receptors; Figure 6D – F) and ADP-β-S (P2Y1 receptors; Figure 6J – L) on GFAP-positive astrocytes. The situation after microinfusion of aCSF alone is shown in Figure 6A – C and G – I. P2X3 and P2Y1 receptors were localized both on GFAP-positive astroglial processes and on the cell somata.
Figure 6.

Confocal images of double immunofluorescence for GFAP (A,D,G,J; Cy2-green fluorescence) and P2 receptor-subtypes (B,E,H,K; Cy3-red immunofluorescence) to characterize the receptor-localization on astrocytes in the NAc of rats. ACSF (A – C, G – I), α,β-meATP (D – F) or ADP-β-S (J – L) were microinfused 4 days before preparation. Double labelling for GFAP and the P2X3 receptor subtype is shown in C,F and for the P2Y1 receptor-subtype in I,L (scale bar: 10 μm (A – L). The expression of P2X3 receptors in GFAP-immunoreactive astrocytes after aCSF microinfusion has been demonstrated earlier (Franke et al., 2001).
Discussion
Following trauma or ischemia, large amounts of nucleotides and nucleosides (ATP, GTP, UTP, adenosine) enter the extracellular space. They may play a key role in initiating brain repair mechanisms including astrogliosis. The glial scar is thought to be a limiting factor in CNS regeneration following cellular damage (Bovolenta et al., 1992; Abbracchio et al., 1999). Application of ATP itself or of its structural analogues to cultures of astrocytes mimicked the changes which occur during gliotic processes (Neary, 1996; Abbracchio et al., 1999). Microinfusion of 2-MeSATP into the NAc of rats stimulated the gliosis following CNS injury and led to an increase in GFAP expression and proliferation; this effect was prevented by the P2 receptor antagonist PPADS (Franke et al., 1999a). When given alone, PPADS depressed the astrogliosis caused by vehicle injection, suggesting the involvement of endogenous ATP in this process (Franke et al., 1999a).
Since ATP may activate several types of P2 receptors, it is possible that more than one receptor-subtype mediates the astrocytic response. The present study shows that agonists with preference for certain P2 receptor-subtypes, such as α,β-meATP and ADP-β-S (but not UTP-γ-S), also induce morphogenic and mitogenic changes of astrocytes, although with different potencies. The P2 receptor antagonist PPADS reduced the agonist effects. The relatively hydrolysis-resistant ATP analogue α,β-meATP is supposed to act on P2X1 and P2X3 homomeric or P2X4/6 heteromeric receptor-types without stimulating P2Y receptors (Ralevic & Burnstock, 1998). In contrast, the non-hydrolysable analogues ADP-β-S (P2Y1 or P2Y12) and UTP-γ-S (P2Y2/4/6) appear to activate certain subtypes of P2Y, but not P2X receptors. UTP-γ-S is equipotent with uridine 5′-triphosphate itself (UTP; Lazarowski et al., 1996).
Significant mitogenic effects, the appearance of hypertrophic astrocytes and the presence of elongated and thickened astrocytic processes as well as an increase in the GFAP-IR and GFA-protein content after microinfusion of α,β-meATP suggest the involvement of P2X receptors in the astrogliotic process observed. The proliferative changes evoked by α,β-meATP, however, were less pronounced in comparison to the infusion of the mixed P2X/P2Y receptor agonist 2-MeSATP. Hence, the mitogenic effects of 2-MeSATP may be due to stimulation of both P2X and P2Y receptors at astrocytes. In fact, the P2Y1 (or P2Y12; Hollopeter et al., 2001) receptor agonist ADP-β-S had a more marked effect than 2-MeSATP in up-regulating the number of GFAP-positive and GFAP-/BrdU-positive cells and in the induction of phenotypic changes (see Figure 4). Because the used agonist does not allow a discrimination between the P2Y1 and P2Y12 receptor subtypes, the participation of P2Y12 receptors cannot be excluded. The P2Y2,4,6 receptor agonist UTP-γ-S appeared to be inactive, suggesting no involvement of these receptor types. Our results support the hypothesis that P2Y receptor stimulation may activate intracellular pathways inducing cell differentiation and proliferation (e.g. Gallagher & Salter, 1999) more potently than P2X receptor stimulation.
Astrocytic swelling is often associated with increased GFAP-IR, probably as a consequence of increased synthesis of GFAP, unmasking of GFAP-subunits and/or enhanced exposure of epitopes on GFAP caused by dissociation and dispersion of glial filaments within the astrocytic cytoplasm and processes (for references, see Franke, 1995). However, the present results (Figure 6) show that the increase in GFA-protein is a genuine part of the described astrogliosis induced by both – the P2X and P2Y agonists.
The described in vivo astrocytic changes are in agreement with previous reports using in vitro systems. Exposure of primary cultures of striatal astroglial cells to α,β-meATP results in marked stellation of GFAP-positive cells, in elongation of astrocytic processes, stimulation of DNA synthesis, and increase in astroglial cell number (Abbracchio et al., 1994; Neary et al., 1994; Brambilla et al., 2000). Similar effects on astrocytic elongation were observed with ATP and other P2 receptor agonists (α,β-meATP, ADP-β-S, 2-MeSATP and, to a lesser extent, with UTP) (Abbracchio et al., 1995; Bolego et al., 1997). Astrocytes from some CNS areas were activated by 2-MeSATP as well as UTP, indicating a co-expression of P2Y and P2U (renamed P2Y2) receptors (dorsal spinal cord, Ho et al., 1995; cerebral cortex, King et al., 1996). In contrast, cultured hippocampal astrocytes responded to ATP, ADP and 2-MeSATP with the elevation of intracellular calcium, while UTP was inactive, excluding the involvement of UTP-binding sites coupled to calcium (Ca2+) signalling (Bernstein et al., 1998). Reverse transcriptase-polymerase chain reaction (RT – PCR) studies with primer pairs for cloned rat P2Y receptors showed that rat cortical astrocytes express P2Y1 as well as P2Y2 and P2Y4 receptor subtypes. Transcripts for P2Y6 receptors were not detected (Lenz et al., 2000).
The present results suggest the expression of both P2Y and P2X receptors on astrocytes in the NAc of rats. Autoradiography using [3H]α,β-meATP revealed that there are binding sites in the rat NAc for this P2X agonist (Bo & Burnstock, 1994). Immunostaining with P2Y1 receptor-antibodies demonstrated the presence of this receptor-type in the NAc of the mouse as well (Cousens et al., 2000). The present immunofluorescence study used antibodies against P2X3 and P2Y1 receptors and showed their co-expression with GFAP on astrocytes after α,β-meATP- and ADP-β-S-microinfusion, respectively.
PPADS is an antagonist at certain subtypes of P2X and P2Y receptors (Ralevic & Burnstock, 1998). In the present study, the mitogenic and morphogenic effects of P2 receptor agonists were blocked by PPADS. The marked elongation of astrocytic processes by α,β-meATP in primary cultures of astrocytes from rat striatum or cortex as well as in human astrocytoma cells could be also reversed by PPADS (Brambilla et al., 2000). Finally, PPADS, when given alone, decreased the gliotic process, probably by antagonizing the effect of nucleotides released as a consequence of tissue injury (Franke et al., 1999a). The marginal effect of PPADS on UTP-γ-S suggests that the agonist acts at PPADS-insensitive P2Y2 receptors (see von kügelgen & Wetter, 2000).
P2X and P2Y receptors may cause astrocytic activation by utilizing distinct and independent transduction pathways. P2X-receptors are ATP-gated ion channels and act as mediators of fast excitatory neurotransmission in the CNS. These receptors allow the rapid non-selective passage of cations across the cell membrane, resulting in an increase of intracellular calcium concentration ([Ca2+]i) and in a depolarization of the cell membrane (Ralevic & Burnstock, 1998). The P2X receptor-selective agonist α,β-meATP was relatively ineffective in evoking inward currents in astrocyte mRNA-injected oocytes and in stimulating mitogen-activated protein kinase (MAPK) in cultured astrocytes, suggesting that P2X receptors are only weakly expressed in astrocytes (King et al., 1996). α,β-meATP used at concentrations which induced reactive astrogliosis in striatal glial cell cultures did not elicit any significant increase of [Ca2+]i (Centemeri et al., 1997). Although α,β-meATP stimulates only cloned P2X receptor subtypes, and none of the cloned P2Y receptor subtypes, the existence of a not yet cloned P2Y receptor sensitive to α,β-meATP cannot be excluded (Windscheif et al., 1995). It has been concluded that the gliotic response to α,β-meATP is mediated by an ‘atypical' G-protein-coupled P2Y receptor rather than by a P2X receptor (Abbracchio et al., 1999). Further studies of Abbracchio and co-workers suggest that this ‘gliotic' P2Y receptor is linked to the activation of phospholipase A2 via a pertussis toxin-sensitive mechanism, accompanied by significantly increased expression of cyclooxygenase (COX)-2 (Bolego et al., 1997; Abbracchio et al., 1999; Brambilla et al., 1999; 2000).
The P2Y receptors couple to phosphatidylinositol-specific phospholipase C (PI-PLC), which stimulate the inositol phosphate formation (IP3) and calcium mobilization (Pearce et al., 1989; Kastritsis et al., 1992; Salter & Hicks, 1994; Fam et al., 2000). They couple also to the extracellular signal regulated protein kinase/mitogen-activated protein kinase (ERK/MAPK) cascade which is crucial for cellular proliferation and differentiation (Neary & Zhu, 1994; Neary et al., 1999a, 1999b; Lenz et al., 2000). Coupling to adenylate cyclase by some P2Y receptors has also been described (Webb et al., 1996). Activation of protein kinases by, for example, α,β-meATP leads to induction of immediate early response genes (e.g. c-fos), which may regulate late response genes mediating long-term phenotypic changes, such as GFAP-induction (Abbracchio et al., 1996; Bolego et al., 1997). The GFAP-gene contains a binding site for activator protein (AP-1) in its promotor-sequence complexes which would be highly consistent with the increased expression of this protein following exposure of astroglial cells to purine analogues (Neary & Norenberg, 1992; Abbracchio et al., 1995; Neary et al., 1996b; Bolego et al., 1997). A rapid and transient up-regulation of c-fos messenger RNA was observed in cultured astrocytes after treatment with ADP-β-S (Priller et al., 1998).
In conclusion, ATP and its structural analogues, but not UTP-γ-S, caused astrogliosis in the NAc of rats. Although P2X receptors are also expressed at astrocytes and the P2X receptor agonist α,β-meATP had a considerable activity, P2Y receptors may be primarily involved in this effect.
Acknowledgments
The authors thank Dr W. Pendergast (Inspire Pharmaceuticals Inc., Durham, North Carolina, U.S.A.) for the supply of UTP-γ-S, Dr E.J. Kidd (GlaxoWellcome, Cambridge, U.K. for the supply of the P2X3 receptor antibody and Dr D. Moore (Department of Neurobiology, The Babraham Institute, Cambridge and SmithKline Beecham Pharmaceuticals, U.K.) for the P2Y1 receptor antibody. The authors are grateful to J. Fichtler, M. Henschke and A. Rast for technical assistance. This study was supported by the Deutsche Forschungsgemeinschaft (IL 20/9-1) and by the ‘Bundesministerium für Bildung, Forschung und Technologie (BMB+F)' and Interdisciplinary Center for Clinical Research at the University of Leipzig (01KS9504, Project Z10).
Abbreviations
- aCSF
artificial cerebrospinal fluid
- ADP-β-S
adenosine 5′-O-(2-thiodiphosphate)
- BrdU
5-bromo-2′-deoxyuridine
- [Ca2+]i
intracellular calcium concentration
- DAB
3,3′-diaminobenzidine
- ERK
extracellular signal regulated protein kinase
- GFAP
glial fibrillary acidic protein
- GFAP-IR
GFAP-immunoreactivity
- MAPK
mitogen-activated protein kinase
- α,β-meATP
α,β-methylene adenosine 5′-triphosphate
- NAc
nucleus accumbens
- Pl-PLC
phosphatidylinositol-specific phospholipase C
- PPADS
pyridoxalphosphate-6-azophenyl-2,4-disulphonic acid
- UTP-γ-S
uridine 5′-O-(3-thiotriphosphate)
References
- ABBRACCHIO M.P., BURNSTOCK G. Purinoceptors: are there families of P2X and P2Y purinoceptors. Pharmac. Ther. 1994;64:445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- ABBRACCHIO M.P., BRAMBILLA R., CERUTI S., CATTABENI F. Signalling mechanisms involved in P2Y receptor-mediated reactive astrogliosis. Progr. Brain Res. 1999;120:333–342. doi: 10.1016/s0079-6123(08)63567-0. [DOI] [PubMed] [Google Scholar]
- ABBRACCHIO M.P., CERUTI S., BOLEGO C., PUGLISI L., BURNSTOCK G., CATTABENI F.Trophic roles of P2 purinoceptors in central nervous system astroglial cells P2 Purinoceptors: Localisation, Function and Transduction Mechanisms 1996Chichester: John Wiley & Sons; 142–147.ed. Chadwick, D.J. & Goode, J.A., Ciba Foundation Symposium 198 [DOI] [PubMed] [Google Scholar]
- ABBRACCHIO M.P., CERUTI S., LANGFELDER R., CATTABENI F., SAFFREY M.J., BURNSTOCK G. Effects of ATP analogues and basic fibroblast growth factor on astroglial cell differentiation in primary cultures of rat striatum. Int. J. Dev. Neurosci. 1995;13:685–693. doi: 10.1016/0736-5748(95)00064-x. [DOI] [PubMed] [Google Scholar]
- ABBRACCHIO M.P., SAFFREY M.J., HÖPKER V., BURNSTOCK G. Modulation of astroglial cell proliferation by analogues of adenosine and ATP in primary cultures of rat striatum. Neuroscience. 1994;59:67–76. doi: 10.1016/0306-4522(94)90099-x. [DOI] [PubMed] [Google Scholar]
- BERNSTEIN M., BEHNISCH T., BALSCHUN D., REYMANN K.G., REISER G. Pharmacological characterization of metabotrophic and purinergic receptors linked to Ca2+ signalling in hippocampal astrocytes. Neuropharmacol. 1998;37:169–178. doi: 10.1016/s0028-3908(98)00012-4. [DOI] [PubMed] [Google Scholar]
- BRAMBILLA R., BURNSTOCK G., BONAZZI A., CERUTI S., CATTABENI F., ABBRACCHIO M.P. Cyclo-oxygenase-2 mediates P2Y receptor-induced reactive astrogliosis. Br. J. Pharmacol. 1999;126:563–567. doi: 10.1038/sj.bjp.0702333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAMBILLA R., CERUTI S., MALORNI W., CATTABENI F., ABBRACCHIO M.P. A novel gliotic P2 receptor mediating cyclooxygenase-2 induction in rat and human astrocytes. J. Auton. Nerv. Syst. 2000;81:3–9. doi: 10.1016/s0165-1838(00)00152-1. [DOI] [PubMed] [Google Scholar]
- BO X., BURNSTOCK G. Distribution of [3H]α,β-methylene ATP binding sites in rat brain and spinal cord. NeuroReport. 1994;5:1601–1604. doi: 10.1097/00001756-199408150-00015. [DOI] [PubMed] [Google Scholar]
- BOLEGO C., CERUTI S., BRAMBILLA R., PUGLISI L., CATTABENI F., BURNSTOCK G., ABBRACCHIO M.P. Characterization of signalling pathways involved in ATP and basic fibroblast growth factor-induced astrogliosis. Br. J. Pharmacol. 1997;121:1692–1699. doi: 10.1038/sj.bjp.0701294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVOLENTA P., WANDOSELL F., NIETO-SAMPEDRO M. CNS glial scar tissue: a source of molecules which inhibit central neurite outgrowth. Progr. Brain Res. 1992;94:367–379. doi: 10.1016/s0079-6123(08)61765-3. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BRUNER G., MURPHY S. Purinergic P2Y receptors on astrocytes are directly coupled to phospholipase A2. Glia. 1993;7:219–224. doi: 10.1002/glia.440070305. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. Current status of purinergic signalling in the nervous system. Progr. Brain Res. 1999;120:3–10. doi: 10.1016/s0079-6123(08)63541-4. [DOI] [PubMed] [Google Scholar]
- CENTEMERI C., BOLEGO C., ABBRACCHIO M.P., CATTABENI F., PUGLISI L., BURNSTOCK G., NICOSIA S. Characterization of the Ca2+ responses evoked by ATP and other nucleotides in mammalian brain astrocytes. Br. J. Pharmacol. 1997;121:1700–1706. doi: 10.1038/sj.bjp.0701293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COUSENS D., DISNEY G., WATSON M., FERRAGUTI F., XUEREB J., HURLE M., PEDRICK M., PATEL K., BARNES A., MARSHALL F.P2Y receptor distribution in human and mouse tissue Drug. Dev. Res. 200050108238 [Google Scholar]
- FAM S.R., GALLAGHER C.J., SALTER M.W. P2Y1 purinoceptor-mediated Ca2+ signaling and Ca2+ wave propagation in dorsal spinal cord astrocytes. J. Neurosci. 2000;20:2800–2808. doi: 10.1523/JNEUROSCI.20-08-02800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKE H. Influence of chronic alcohol treatment on the GFAP-immunoreactivity in astrocytes of the hippocampus in rats. Acta histochem. 1995;97:263–271. doi: 10.1016/S0065-1281(11)80187-X. [DOI] [PubMed] [Google Scholar]
- FRANKE H., KRÜGEL U., ILLES P. P2 Receptor-mediated proliferative effects on astrocytes in vivo. Glia. 1999a;28:190–200. [PubMed] [Google Scholar]
- FRANKE H., KRÜGEL U., ILLES P. P2Y and P2X Receptor-mediated mitogenic effects on astrocytes in vivo. Br. J. Pharmacol. 1999b;128 Suppl. P:153. [PubMed] [Google Scholar]
- FRANKE H., KRÜGEL U., SCHMIDT R., PREIß R., ILLES P.P2Y Receptor-mediated astrogliosis in vivo Drug. Dev. Res. 20005091172 [Google Scholar]
- FRANKE H., GROSCHE J., SCHÄDLICH H., KRÜGEL U., ALLGAIER C., ILLES P.P2X receptor expression on astrocytes in the nucleus accumbens of rats Neuroscience 2001(in press) [DOI] [PubMed]
- GALLAGHER C.J., SALTER M.W. Nucleotide receptor signalling in spinal cord astrocytes: Findings and functional implications. Progr. Brain Res. 1999;120:311–322. doi: 10.1016/s0079-6123(08)63565-7. [DOI] [PubMed] [Google Scholar]
- GORDON L. Extracellular ATP: effects, sources and fate. Biochem. J. 1986;233:309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HINDLEY S., HERMAN M.A.R., RATHBONE M.P. Stimulation of reactive astrogliosis in vivo by extracellular adenosine diphosphate or an adenosine A2 receptor agonist. J. Neurosci. Res. 1994;38:399–406. doi: 10.1002/jnr.490380405. [DOI] [PubMed] [Google Scholar]
- HOLLOPETER G., JANTZEN H.-M., VINCENT D., LI G., ENGLAND L., RAMAKRISHNAN V., YANG R.-B., NURDEN A., JULIUS D., CONLEY P.B. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- HO C., HICKS J., SALTER MW. A novel P2-purinoceptor expressed by a subpopulation of astrocytes from the dorsal spinal cord of the rat. Br. J. Pharmacol. 1995;116:2909–2918. doi: 10.1111/j.1476-5381.1995.tb15944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASTRITSIS C.H.C., SALM A.K., MCCARTHY K. Stimulation of the P2Y purinergic receptor on type 1 astroglia results in inositol phosphate formation and calcium mobilization. J. Neurochem. 1992;58:1277–1284. doi: 10.1111/j.1471-4159.1992.tb11339.x. [DOI] [PubMed] [Google Scholar]
- KIMELBERG H.K. Receptors on astrocytes – what possible functions. Neurochem. Int. 1995;26:27–40. doi: 10.1016/0197-0186(94)00118-e. [DOI] [PubMed] [Google Scholar]
- KING B.F., NEARY J.T., ZHU Q., WANG S., NORENBERG M.D., BURNSTOCK G. P2 purinoceptors in rat cortical astrocytes: expression, calcium-imaging and signalling studies. Neuroscience. 1996;74:1187–1196. doi: 10.1016/0306-4522(96)00209-6. [DOI] [PubMed] [Google Scholar]
- LAZAROWSKI E.R., WATT W.C., STUTTS M.J., BROWN H.A., BOUCHER R.C., HARDEN T.K. Enzymatic synthesis of UTPγS, a potent hydrolysis resistant agonist of P2U-purinoceptors. Br. J. Pharmacol. 1996;117:203–209. doi: 10.1111/j.1476-5381.1996.tb15175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENZ G., GOOTFRIED C., LUO Z., AVRUCH J., RODNIGHT R., NIE W.-J., KANG Y., NEARY J.T. P2Y purinoceptor subtypes recruit different Mek activators in astrocytes. Br. J. Pharmacol. 2000;129:927–936. doi: 10.1038/sj.bjp.0703138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEARY J.T.Trophic actions of extracellular ATP on astrocytes, synergistic interactions with fibroblast growth factors and underlying signal transduction mechanisms P2 Purinoceptors: Localisation, Function and Transduction Mechanisms 1996Chichester: John Wiley & Sons; 130–139.ed. Chadwick, D.J. & Goode, J.A., Ciba Foundation Symposium 198 [DOI] [PubMed] [Google Scholar]
- NEARY J.T., NORENBERG M.D. Signalling by extracellular ATP: physiological and pathological considerations in neuronal-astrocytic interactions. Progr. Brain Res. 1992;94:145–151. doi: 10.1016/s0079-6123(08)61746-x. [DOI] [PubMed] [Google Scholar]
- NEARY J.T., ZHU Q. Signaling by ATP receptors in astrocytes. Neuroreport. 1994;5:1617–1620. doi: 10.1097/00001756-199408150-00019. [DOI] [PubMed] [Google Scholar]
- NEARY J.T., BAKER L., JORGENSEN S.L., NORENBERG M.D. Extracellular ATP induces stellation and increases glial fibrillary acidic protein content and DNA synthesis in primary astrocyte cultures. Acta Neuropathol. 1994;87:8–13. doi: 10.1007/BF00386249. [DOI] [PubMed] [Google Scholar]
- NEARY J.T., KANG Y., BU Y., AKONG K., PETERS C.M. Mitogenic signaling by ATP/P2Y purinergic receptors in astrocytes: Involvement of a calcium-independent protein kinase C, extracellular signal-regulated protein kinase pathway distinct from the phosphatidyinositol-specific phospholipase C/calcium pathway. J. Neurosci. 1999b;19:4211–4220. doi: 10.1523/JNEUROSCI.19-11-04211.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEARY J.T., MCCARTHY M., CORNELL-BELL A., KANG Y. Trophic signaling pathways activated by purinergic receptors in rat and human astroglia. Progr. Brain Res. 1999a;120:323–332. doi: 10.1016/s0079-6123(08)63566-9. [DOI] [PubMed] [Google Scholar]
- NEARY J.T., RATHBONE M.P., CATTABENI F., ABBRACCHIO M.P., BURNSTOCK G. Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci. 1996a;19:13–18. doi: 10.1016/0166-2236(96)81861-3. [DOI] [PubMed] [Google Scholar]
- NEARY J.T., ZHU Q., KANG Y., DASH P.K. Extracellular ATP induces formation of AP-1 complexes in astrocytes via P2 purinoceptors. Neuroreport. 1996b;7:2893–2896. doi: 10.1097/00001756-199611250-00017. [DOI] [PubMed] [Google Scholar]
- NORENBERG M.D. Astrocyte responses to CNS injury. J. Neuropathol. Exp. Neurol. 1994;53:213–220. doi: 10.1097/00005072-199405000-00001. [DOI] [PubMed] [Google Scholar]
- PEARCE B., MURPHY S., JEREMY J., MORROW C., DANDONA P. ATP-evoked Ca2+ release from astrocytes: P2-purinergic receptors linked to phosphoinositide hydrolysis. J. Neurochem. 1989;52:971–977. doi: 10.1111/j.1471-4159.1989.tb02549.x. [DOI] [PubMed] [Google Scholar]
- PRILLER J., REDDINGTON M., HAAS C.A., KREUTZBERG G.W. Stimulation of P2Y-purinoceptors on astrocytes results in immediate early gene expression and potentiation of neuropeptide action. Neuroscience. 1998;85:521–525. doi: 10.1016/s0306-4522(97)00653-2. [DOI] [PubMed] [Google Scholar]
- QUEIROZ G., GEBICKE-HAERTER P.J., SCHOBERT A., STARKE K., VON KÜGELGEN I. Release of ATP from cultured rat astrocytes elicited by glutamate receptor activation. Neuroscience. 1997;78:1203–1208. doi: 10.1016/s0306-4522(96)00637-9. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptor for purines and pyrimidines. Pharmacological. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- SALTER M.W., HICKS J.L. ATP-evoked increase in intracellular calcium in neurons and glia from the dorsal spinal cord. J. Neurosci. 1994;14:1563–1575. doi: 10.1523/JNEUROSCI.14-03-01563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VON KÜGELGEN I., WETTER A. Molecular pharmacology of P2Y-receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 2000;362:310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- WALZ W., GIMPL G., OHLEMEYER C., KETTENMANN H. Extracellular ATP induced currents in astrocytes: involvement of a cation channel. J. Neurosci. 1994;38:12–18. doi: 10.1002/jnr.490380104. [DOI] [PubMed] [Google Scholar]
- WEBB T.E., FEOLDE E., VIGNE P., NEARY J.T., RUNBERG A., BARNARD E.A. The P2Y purinoceptor in rat brain microvascular endothelial cells couples to inhibition of adenylate cyclase. Br. J. Pharmacol. 1996;119:1385–1392. doi: 10.1111/j.1476-5381.1996.tb16050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINDSCHEIF U., PFAFF O., ZIGANSHIN A.U., HOYLE C.H.V., BÄUMERT H.G., MUTSCHLER E., BURNSTOCK G., LAMBRECHT G. Inhibitory action of PPADS on relaxant responses to adenine nucleotides or electrical field stimulation in guinea-pig taenia coli and rat duodenum. Br. J. Pharmacol. 1995;115:1509–1517. doi: 10.1111/j.1476-5381.1995.tb16644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


