Abstract
Macrophage Stimulating Protein (MSP), a serum factor related to Hepatocyte Growth Factor, was originally discovered to stimulate chemotaxis of murine resident peritoneal macrophages. MSP is the ligand for Ron, a member of the Met subfamily of tyrosine kinase receptors. The effects of MSP on human macrophages and the role played in human pathophysiology have long been elusive.
We show here that human recombinant MSP (hrMSP) evokes a dose-dependent superoxide anion production in human alveolar and peritoneal macrophages as well as in monocyte-derived macrophages, but not in circulating human monocytes. Consistently, the mature Ron protein is expressed by the MSP responsive cells but not by the unresponsive monocytes. The respiratory burst evoked by hrMSP is quantitatively higher than the one induced by N-formylmethionyl-leucyl-phenylalanine and similar to phorbol myristate acetate-evoked one.
To investigate the mechanisms involved in NADPH oxidase activation, leading to superoxide anion production, different signal transduction inhibitors were used. By using the non selective tyrosine kinase inhibitor genistein, the selective c-Src inhibitor PP1, the tyrosine phosphatase inhibitor sodium orthovanadate, the phosphatidylinositol 3-kinase inhibitor wortmannin, the p38 inhibitor SB203580, the MEK inhibitor PD098059, we demonstrate that hrMSP-evoked superoxide production is mediated by tyrosine kinase activity, requires the activation of Src but not of PI 3-kinase. We also show that MAP kinase and p38 signalling pathways are involved.
These results clearly indicate that hrMSP induces the respiratory burst in human macrophages but not in monocytes, suggesting for the MSP/Ron complex a role of activator as well as of possible marker for human mature macrophages.
Keywords: Macrophage Stimulating Protein (MSP), Ron, monocyte/macrophages, superoxide anion production, cellular activation, signal transduction, protein kinases/phosphatases
Introduction
Macrophage Stimulating Protein (MSP) is a 78 kDa heterodimeric protein composed by a disulfide-linked 53 kDa α chain and a 23 kDa β chain. It belongs to the kringle protein family, that includes plasminogen, prothrombin and Hepatocyte Growth Factor (HGF) (Yoshimura et al., 1993). MSP is synthesized in the liver and circulates in the blood in nanomolar concentration as a biologically inactive single-chain precursor, pro-MSP. Proteolytic cleavage of pro-MSP, yielding the dimeric mature MSP required for the activation of its receptor, occurs on the cell surface of murine peritoneal macrophages and in wound fluids by the pro-MSP convertase activity, a trypsin-like serine protease (Nanney et al., 1998; Wang et al., 1996c). The specific receptor of MSP is the transmembrane tyrosine kinase Ron, which belongs to the Met proto-oncogene family. Ron is a heterodimeric receptor composed of extracellular α (35 kDa) and a transmembrane β (150 kDa) chains (Gaudino et al., 1994; Wang et al., 1994b). MSP ligand stimulation of Ron-expressing cells rapidly induces the tyrosine kinase activity of the receptor, and Ron signalling occurs through association with multiple effectors [e.g., phospholipase C-γ, phosphatidylinositol 3-kinase (PI 3-kinase), focal adhesion kinase, c-Src, mitogen-activated protein kinase (MAP kinase), c-Jun amino terminal kinase (JNK)] as a consequence of the phosphorylation of a unique multifunctional docking site, conserved in all members of the Met family (Danilkovitch & Leonard, 1999; Danilkovitch-Miagkova et al., 2000; Iwama et al., 1996; Wang et al., 1996b). Since its discovery as a serum protein able to induce responsiveness to the chemoattractant C5a, and to enhance spreading, phagocytosis and chemotaxis in mouse resident peritoneal macrophages (Leonard & Skeel, 1979), the MSP spectrum of activity has widened and this protein is now regarded as a pleiotrophic factor. MSP stimulates human and murine bone marrow megakaryocytopoiesis, induces apoptosis in erythroid cell lines, induces proliferation and migration of murine keratinocytes and liver progenitor cells, increases ciliary motility of human nasal cilia, stimulates bone resorption by human osteoclasts, and promotes migration of non-small cell lung tumour cells (Banu et al., 1996; Kurihara et al., 1996; Medico et al., 1996; Sakamoto et al., 1997; Wang et al., 1996a; Willett et al., 1998). An increased expression of mRNA for MSP has been shown in experimental models of inflammation and liver regeneration in the rat (Bezerra et al., 1994), while a decreased hepatic MSP production has been suggested to impair Kupffer cell phagocytosis in patients with fulminant hepatic failure (Harrison et al., 1994).
MSP also inhibits lipopolysaccharide (LPS)- and/or cytokine-induced nitric oxide (NO) release and iNOS expression in murine peritoneal macrophages (Chen et al., 1998; Wang et al., 1994a), by negative modulation of co-stimulatory signals activating the transcription factor NF-κB (Liu et al., 1999).
The expression of the mouse Ron homologue Stk has been regarded as a marker of terminal differentiation of murine peritoneal macrophages, since this receptor is present in peritoneal macrophages, but not in alveolar macrophages, acute exudate macrophages or circulating monocytes (Iwama et al., 1995).
Knock-out mice lacking the Ron/Stk receptor showed enhanced susceptibility to LPS-induced septic shock, increased NO production as well as increased responses in tests of delayed-type hypersensitivity (Correll et al., 1997). Moreover, it has been demonstrated that mice carrying a targeted loss of MSP have a delayed macrophage activation, although migration of mononuclear phagocytes into the peritoneal cavity is unaltered as compared to wild-type animals (Bezerra et al., 1998).
In mouse peritoneal resident macrophages, as well as in peritoneal exudate macrophages, Ron becomes expressed and tyrosine phosphorylated upon MSP stimulation. Consequently, iNOS expression and NO release from mature peritoneal exudate macrophages are inhibited (Chen et al., 1998). Interestingly, endotoxin-induced NO production down-regulates Ron expression in murine macrophages (Wang et al., 2000).
As far as NO production is concerned, human monocyte/macrophages are at variance from the rodent ones. While murine macrophages rapidly produce large amounts of NO after challenge with classical stimuli such as IFN-γ, TNF-α or LPS, human monocytes and tissue macrophages usually do not. The actual amount of NO metabolites released by human monocyte/macrophages is extremely low when compared to that produced by rodent cells, although human monocytes and macrophages express the iNOS gene (Albina, 1995).
Recently, Ron immunoreactivity has been detected in dermal macrophages of normal human skin as well as in macrophages from burn wound skin (Nanney et al., 1998); however, the functions exerted by MSP on human monocyte/macrophages are still elusive.
This prompted us to investigate the effects of MSP on human mononuclear phagocytes of different origins: circulating monocytes, monocyte-derived macrophages, alveolar macrophages and peritoneal macrophages. In the inflammatory and immune processes, monocytes and macrophages operate phagocytosis, chemotaxis, antigen processing and presentation, secretion of enzymes and cytokines as well as oxy-radical production (Morrissette et al., 1999). Therefore, we used these cells to evaluate the ability of MSP to evoke superoxide anion (O2−) production, as compared to other stimuli such as N-formylmethionyl-leucyl-phenylalanine (FMLP) and phorbol 12-myristate 13-acetate (PMA). Moreover, by using different pharmacological inhibitors, we investigated the signal transduction pathways involved.
Methods
Human recombinant macrophage stimulating protein
Human recombinant MSP (hrMSP) was expressed in Sf9 insect cells, by the baculovirus expression system, using Bac-N-BlueTM AcMNPV DNA (Invitrogen). Conditioned medium was treated prior to use with 2% foetal calf serum (FCS) for 1 h at 37°C, in order to allow MSP processing to the mature two-chain form (Gaudino et al., 1994). The biological activity of hrMSP was tested by scatter assay and by Ron tyrosine phosphorylation on cells expressing the recombinant receptor, i.e., Madin-Darby Canine Kidney/Ron (MDCK/Ron) and NIH-3T3 RON fibroblasts, respectively. The titer of hrMSP was expressed as the highest dilution able to induce cell scatter in MDCK/Ron. The scatter assay was conducted with a standard procedure as previously described (Medico et al., 1996); the hrMSP used in this work had an average titer of 250 scatter units ml−1. Results were also validated and confirmed by using a commercially available source of MSP (R & D Systems); in this case, scatter activity was observed at 8 ng ml−1.
Expression of the Ron receptor
Ron expression in different cell populations was evaluated by Western immunoblot analysis. Confluent cell monolayers (5×106 macrophages or monocytes) were lysed with 750 μl buffer B (20 mM Tris base- pH 7.4, 1% v v−1 Triton X-100, 0.5 % w v−1 Na-deoxycholate, 0.1 % w v−1 SDS, 50 mM NaCl, 5 mM EDTA) supplemented with phenylmethanesulfonyl fluoride (PMSF; 1 mM), sodium orthovanadate (1 mM), aprotinin (10 μg ml−1), pepstatin (10 μg ml−1), leupeptin (50 μg ml−1). Cell lysates were cleared by centrifugation at 15,000×g for 15 min at 4°C. Equal amounts (800 μg) of total proteins from each cell line, determined using the BCA protein assay reagent kit, were immunoprecipitated with stirring for 2 h at 4°C with the anti-Ron specific antisera adsorbed to 40 μl of protein A-Sepharose 4B packed beads.
Immunocomplexes were washed twice with lysis buffer and proteins from immunoprecipitates were solubilized in boiling Laemmli buffer (Tris-HCl 62.5 mM, pH 6.8, 10% v v−1 glycerol, 1% w v−1 SDS, 1% v v−1 β-mercaptoethanol, 0.01% v v−1 Bromophenol blue) for 5 min in reducing conditions. Denatured proteins were subsequently separated on 8% SDS – PAGE and transferred to nitro-cellulose filters (Hybond, Amersham, U.K.) in buffer C (Tris base 50 mM, pH 8.3, glycine 192 mM, 20% v v−1 methanol). The nitrocellulose filter was treated for 1 h with buffer D (Tris base 25 mM, pH 7.4, NaCl, 150 mM 0.1% v v−1 Tween 20, 5% w v−1 bovine serum albumin (BSA) fraction V and incubated overnight in the same buffer with the relevant primary antibody. After washing in buffer D, filters were incubated for 60 min with anti-Ron specific antiserum (Gaudino et al., 1994) or anti-phosphotyrosine antibody (U.B.I.) clone 4G10, diluted 1 : 5000 in buffer D and then washed again. Antibody-labelled proteins were visualized by the enhanced chemiluminescence system (ECL).
Isolation of human monocyte/macrophage populations
Human monocytes (HMs) were isolated from heparinized venous blood (30 – 40 ml) of healthy volunteers (aged 22 – 45) by standard techniques of dextran sedimentation, Ficoll – Paque gradient centrifugation (400×g, 30 min, room temperature) and recovered by thin suction at the interface. Cells were then washed twice with phosphate-buffered salt solution (PBS) and resuspended at 1 – 2×107/ml in RPMI 1640 medium supplemented with 5% FCS, 2 mM glutamine, 10 mM HEPES, 50 μg/ml streptomicin and 5 U/ml penicillin. Cell viability, as assessed by Trypan blue dye exclusion, was >98%. Purified HMs were obtained by adhesion and assessed with the pan-leukocyte monoclonal antibody HLE-1 (anti-CD45); the monoclonal antibody LEUm3 (anti-CD14), a marker for the LPS receptor, was also used to better define blood monocytes (Ziegler-Heitbrock, 2000). Briefly, 100 μl of cell suspension were plated in six-well tissue culture plates (35 mm diameter; Costar, U.K.) and allowed to adhere for 90 min at 37°C in a humidified atmosphere containing 5% CO2, as described (Brunelleschi et al., 1998). The non-adherent cells (mainly lymphocytes) were removed by three gentle washings with PBS.
Monocyte-derived macrophages (MDMs) were prepared from HMs (see above) cultured for 7 – 8 days in a CO2 incubator at 37°C in RPMI 1640 medium containing 10% FCS, glutamine, HEPES and antibiotics; medium was exchanged every 2 – 3 days. MDMs were defined as macrophage-like cells according to Gantner et al. (1997), by evaluating the decrease in the surface monocyte marker CD14. The great majority of HMs (92±5%) was CD14+, while a significantly lower percentage of MDMs (23±5%; P<0.01 vs HMs; n=5) was CD14+. Moreover, the absence of CD1a expression demonstrated that no differentiation towards dendritic cells occurred in our MDM preparations.
Alveolar macrophages (AMs) were isolated from bronchoalveolar lavage (BAL) as described (Brunelleschi et al., 1996). After informed consent was obtained from each subject (one healthy volunteer, one smoker, one patient with lung cancer, one with a larynx cancer, one patient with lung fibrosis and three patients with pulmonary sarcoidosis) and pretreatment with parenteral atropine sulphate (0.5 mg), airways were anaesthetized with 2% lidocaine. A fiberoptic bronchoscope was advanced and wedged into the middle lobe under direct visualization. Lavage was carried out with 200 ml of pre-warmed (37°C) sterile saline solution in ten 20-ml aliquots with immediate gentle vacuum (syringe) aspiration after each injection. The fluid so obtained was filtered through two layers of sterile surgical gauze and centrifuged (400×g, 30 min). The whole BAL pellet was washed twice in PBS, resuspended in RPMI 1640 medium (supplemented as above) and plated in tissue culture plates. After 2 hours at 37°C in humidified 5% CO2 atmosphere, non-adherent cells (mainly lymphocytes) were gently removed and AMs were used for the experiments. CD14 expression on AMs varied according to the disease (Brunelleschi et al., 1996), being 28±3% in the three patients with pulmonary sarcoidosis, 75% in the smoker subject and 35 – 50% in the others.
Peritoneal macrophages (PMs) were obtained from ascitic fluid (0.8 – 4 l) of cirrhotic patients and purified by adhesion. Briefly, ascitic fluid was centrifuged (400×g, 30 min); the pellet was resuspended in PBS, stratified on Ficoll – Paque gradient and centrifuged (400×g, 30 min). Cells were recovered by thin suction at the interface, washed twice in PBS, resuspended in RPMI 1640 medium and plated as above. CD14 expression on PMs was 32+6% (n=4).
Assay of superoxide anion (O2−) production
Adherent monocytes (HMs) or macrophages (MDMs, PMs, AMs) (0.6 – 1×106 cells/dish) were washed twice with PBS, incubated in sterile culture medium (RPMI 1640 without phenol red) and challenged with increasing concentrations of hrMSP or commercially available MSP for 30 min. The effects of hrMSP were compared with those evoked by known standard stimuli, e.g., the bacterial peptide N-Formylmethionyl-leucyl-phenylalanine (FMLP) and the direct protein kinase C activator phorbol 12-mirystate 13-acetate (PMA).
Superoxide anion (O2−) production was evaluated by the superoxide dismutase (SOD)-inhibitable cytochrome C reduction, the absorbance changes being recorded at 550 nm in a Beckman DU 650 spectrophotometer. O2− production was expressed as nmol cytochrome C reduced/106cells/30 min, using an extinction coefficient of 21.1 mM (Brunelleschi et al., 1996). To avoid interference with spectrophotometrical recordings of O2− production, adherent cells were incubated with RPMI 1640 without phenol red. FCS, at the concentration used to activate hrMSP, did not modify the respiratory burst (data not shown). O2− production was evaluated also in cells treated with control conditioned medium from non-infected Sf9; these values were subtracted from those obtained with hrMSP. Results so obtained were confirmed by using a commercial source of MSP. Experiments were performed in duplicate or triplicate; control values (e.g., basal O2− production in the absence of stimuli) were subtracted from all determinations.
Effects of signal transduction inhibitors on O2− production in human peritoneal macrophages
Reported selective inhibitors of signal transduction were used to evaluate the possible modulation of NADPH oxidase activation in human peritoneal macrophages. In these experiments, PMs were pretreated with the PI 3- kinase inhibitor wortmannin (10−10 – 10−7 M, 30 min preincubation according to Chen et al., 1998), the non-selective tyrosine kinase inhibitor genistein (10−6 – 10−4 M, 30 min preincubation according to Torres & Forman, 1999), the tyrosine phosphatase inhibitor sodium orthovanadate (10−5 – 10−4 M, 60 min preincubation), the p38 MAP kinase inhibitor SB 203580 (10−7 – 10−5 M, 60 min preincubation according to Rane et al., 1997), the MEK inhibitor PD 098059 (10−7 – 10−5 M, 60 min preincubation according to Downey et al., 1998) or the selective Src inhibitor PP1 (10−8 – 10−5 M, 30 min preincubation according to Erdreich-Epstein et al., 1999) and then evaluated for O2− production by hrMSP, PMA or FMLP as previously described. All signal transduction inhibitors (except for sodium orthovanadate) were dissolved in DMSO as a stock solution of 10 mM. Non-stimulated (basal) and stimulated PMs were added the same amount of DMSO (when required) as plates treated with the signal transduction inhibitors. DMSO 0.1% lowered of about 15 – 20% the responses evoked by FMLP, PMA and hrMSP (data not shown). None of the signal transduction inhibitors affected O2− production per se. Results are expressed as percentage of control, O2− production evoked by each stimulus (FMLP, PMA and hrMSP) in the absence of inhibitor being 100%.
Drugs and analytical reagents
Dextran T-500, Ficoll – Paque and protein A-Sepharose 4B packed beads were obtained from Pharmacia (Uppsala, Sweden). Monoclonal antibodies HLE-1 (anti-CD45) and Leu-M3 (anti-CD14) were from Becton Dickinson (San Jose, CA, U.S.A.). The anti-phosphotyrosine antibody clone 4G10 was from U.B.I. (Lake Placid, NY, U.S.A.). PBS, RPMI 1640 (with or without phenol red), FCS. BSA, glutamine, HEPES, streptomicin, penicillin, FMLP, SOD, cytochrome C, Tris base, Na-deoxycholate, NaCl, EDTA, aprotinin, pepstatin, leupeptin, Bromophenol blue, glycine, glycerol, methanol and Tween 20 were obtained from Sigma (Milwaukee, WI, U.S.A.). Triton X-100 and β-mercaptoethanol were from Fluka (Buchs, Switzerland); PMSF was from Promega (Madison, WI, U.S.A.). SDS and DMSO were from Merck (Darmstadt, Germany). Commercial MSP was from R&D Systems (Minneapolis, MN, U.S.A.). PMA, wortmannin, genistein, sodium orthovanadate, PD098059 (2′-amino-3′-methoxyflavone), PP1 (4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]-pyrimidine) and SB203580, ([4 - (4 - fluorophenyl) -2 (4 - methylsulfinylphenyl)5-(4-pyridyl)-1H-imidazole]) were purchased from Alexis Biochemicals (Vinci, FI, Italy). BCA Protein Assay Reagent Kit was from Pierce (Rockford, IL, U.S.A.). Nitro-cellulose filters (Hybond) and the enhanced chemiluminescence system (ECL) were from Amersham (Buckinghamshire, U.K.). Tissue-culture plates were purchased from Costar Ltd (Buckinghamshire, U.K.)
Data and statistical analysis
Data are expressed as means±s.e.mean of ‘n' independent determinations. Concentration-response curves for hrMSP were constructed and logarithmically transformed. EC50 values were interpolated from curves of best-fit. When required, statistical evaluation was performed by Student's t-test.
Results
Human recombinant MSP displays full biological activity
To assess the biological activity of hrMSP produced in insect cells, preliminary tests were done in MDCK and in NIH-3T3 cells, transfected with human recombinant Ron and stabilized in culture after marker selection (Ron/MDCK and NIH-3T3 RON). These transfectants constitutively express the Ron receptor, which can be stimulated by the MSP ligand, so representing optimal tools to measure biochemical and biological activities of hrMSP. A typical feature of the Met subfamily receptors is the capability to induce dissociation and motility of epithelial monolayers, known as ‘scatter' effect. The scatter activity induced by the recombinant MSP used in this work was evaluated in Ron/MDCK. As reported in Figure 1A, hrMSP was able to induce scatter of Ron/MDCK cells; the maximal dilution of the conditioned medium of insect cells expressing MSP, at which the scatter effect was detectable, resulted 1 : 250. This value was indicated as one ‘Scatter Unit'; consequently the conditioned medium typically contained 250 Scatter Units ml−1. The scatter activity evoked by hrMSP 10 Scatter Units ml−1 is similar to the one evoked by 80 ng ml−1 commercial MSP (Figure 1A).
Figure 1.
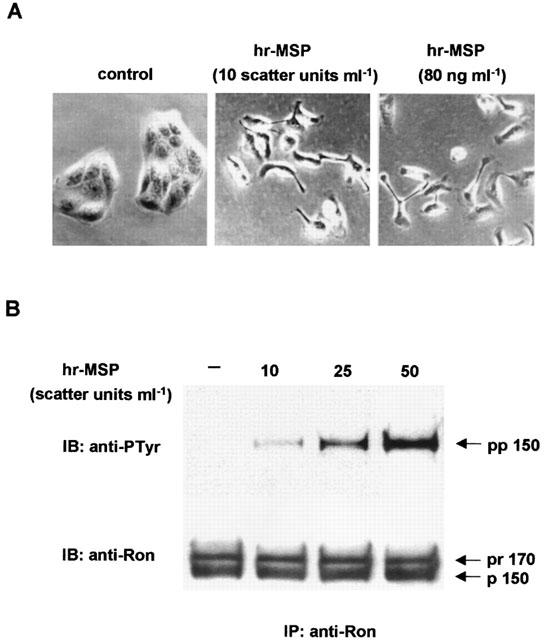
Human recombinant MSP displays full biological activity. (A) Scatter activity induced by hrMSP or commercial MSP in Ron/MDCK cells. Left panel: control mock stimulated cells grown in a characteristic round colony with epithelial morphology; middle panel: cells after stimulation with hrMSP (10 Scatter Units ml−1) dissociated and displayed fibroblastoid appearance; right panel: cells after stimulation with commercial MSP 80 ng ml−1. (B) Immunoprecipitation with anti-Ron antibodies (IP:anti-Ron), followed by immunoblotting of lysates from NIH-3T3 RON cells stimulated with hrMSP (10 – 50 Scatter Units ml−1). The filter has been probed either with anti-protein (anti-Ron; IB:anti-Ron) and with anti-phosphotyrosine (anti-PTyr; IB:anti-Ptyr) antibodies. The recombinant MSP induced the tyrosine phosphorylation of the 150 kDa β-chain (pp 150) of the Ron receptor in a dose-dependent manner; pr 170 is the single-chain precursor of Ron.
The primary effect of ligand stimulation of tyrosine kinase receptors such as Ron, is the auto-transphosphorylation of the receptor itself, that can be evaluated using anti-phosphotyrosine antibodies. As shown in Figure 1B, Ron tyrosine phosphorylation induced by recombinant MSP was checked in NIH-3T3 RON cells, where hrMSP dose-dependently (10 – 50 Scatter Units ml−1) induced tyrosine phosphorylation of 150 kDa β-chain of the receptor, as evaluated by immunoprecipitation and immunoblotting with anti-phosphotyrosine (pp150) and anti-Ron antisera (p150) (Figure 1B). By using IB: anti-Ron, we demonstrated that an equal amount of protein is present in all the lanes. These results, which were also validated by using commercial MSP (data not shown), demonstrated that the hrMSP displayed full biological activities and was suitable for further investigation on human cells.
Human macrophages of different origin express Ron
Murine Stk/Ron receptor has been found expressed in resident peritoneal macrophages (Iwama et al., 1995). In order to investigate the human counterpart, the expression of the Ron receptor has been investigated in human MDMs, as well as in AMs, PMs and HMs (Figure 2). The anti-Ron antiserum used here (Gaudino et al., 1994) requires to enrich the concentration of extracted Ron protein by immunoprecipitation before immunoblotting, due to the high non-specific background observed in direct Western blotting.
Figure 2.
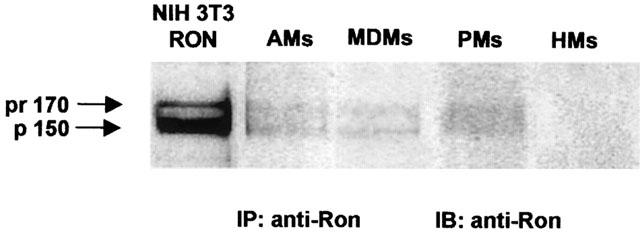
Representative Western blots showing Ron expression in human macrophages of different origin (AMs: alveolar macrophages, MDMs: monocyte-derived macrophages, PMs: peritoneal macrophages), but not in human monocytes (HMs). Immunoprecipitation with anti-Ron antibodies (IP: anti-Ron) followed by immunoblotting with the same antibodies (IB: anti-Ron) from lysates of the indicated cells. The mature 150 kDa Ron β-chain protein (p150) and the single chain precursor of 170 kDa (pr170) are detectable in control NIH-3T3 RON cells as well as in AMs, MDMs and PMs. No Ron protein expression can be detected in HMs. Representative of three different experiments.
Immunoblotting analysis on immunoprecipitates made with anti-Ron antiserum, demonstrated that AMs, PMs and MDMs, but not HMs, express the Ron protein, although at lower level as compared to the positive control, NIH-3T3 RON cells (Figure 2). The anti-Ron antiserum recognizes both the mature 150 kDa Ron β-chain protein (p150) and the single-chain precursor of 170 kDa (pr170; biologically inactive).
hrMSP evokes O2− production in macrophages of different origin
Human recombinant MSP had no effects on adherent monocytes (HMs) collected from peripheral blood of healthy donors, while in the range 1 – 600 ng ml−1 (that is, 0.125 – 75 Scatter Units ml−1) it dose-dependently evoked O2− production from monocyte-derived macrophages (MDMs), peritoneal macrophages (PMs) obtained from ascitic fluid of four cirrhotic patients and alveolar macrophages (AMs) collected from eight different patients (Figure 3). Maximal O2− production was not significantly different among the three human macrophage populations evaluated, reaching about 40 nmol cytochrome C reduced /106 cells (Figure 1 and Table 1). The EC50 values for hrMSP were 110, 187 and 224 ng ml−1 in MDMs, PMs and AMs, respectively.
Figure 3.
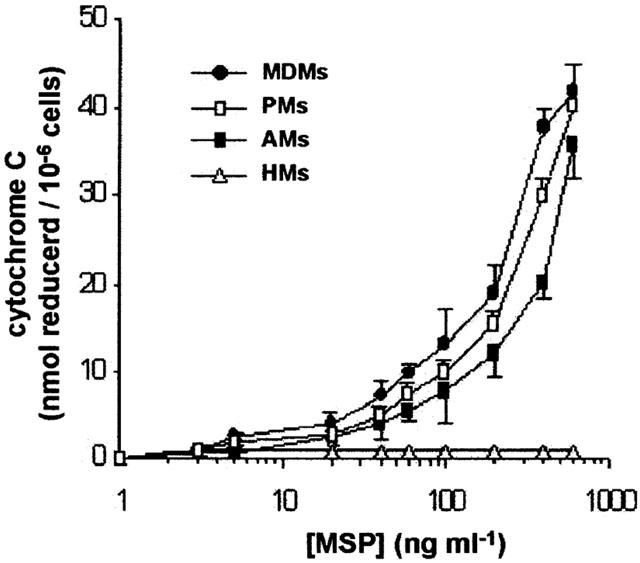
Human recombinant MSP evokes O2− production in human macrophages of different origin, but not in human monocytes. Cells (0.6 – 1×106/well) were challenged with hrMSP for 30 min. Means±s.e.mean of 4 – 8 experiments.
Table 1.
O2− production induced by hrMSP, PMA and FMLP in monocyte/macrophages
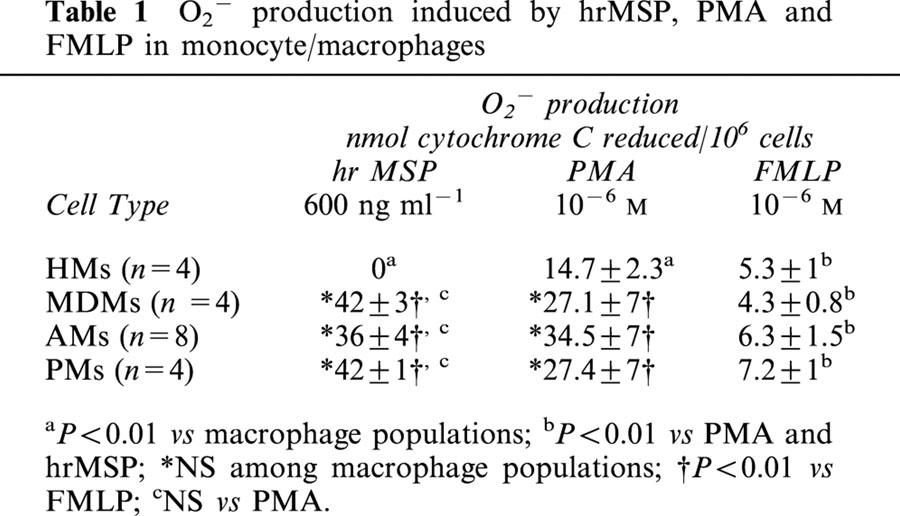
Human macrophages of different origin, as well as HMs, undergo a respiratory burst, when challenged in vitro with the bacterial peptide FMLP or the protein kinase C activator, PMA: hrMSP, uneffective in HMs, was more potent than FMLP in evoking O2− production in MDMs, PMs and AMs (P<0.01; Table 1), and evoked a respiratory burst quantitatively similar to the PMA-induced one in the three macrophage populations evaluated (Table 1).
By evaluating individual O2− production in AMs obtained from eight different patients, a more pronounced effect of hrMSP was observed in the smoker patient and the three with sarcoidosis as compared to the control volunteer and patients with cancer, suggesting a possible role for MSP in interstitial lung pathologies (data not shown): however, the limited number of patients evaluated did not allow any definitive conclusion.
Signal transduction and O2− production in PMs
The intracellular signals transduced by the MSP/Ron activated complex, involved in NADPH-oxidase activation, are far from being elucidated. In order to get insight into this mechanism, the effects of different signal transduction inhibitors on the respiratory burst induced by hrMSP were evaluated in human PMs and compared with those elicited by the classical PMA and FMLP stimuli. In these experiments, FMLP and PMA were used at 10−6 M and produced 4.5±0.6 and 28.6±4 nmol cytochrome C reduced/106 cells, respectively (n=5), while hrMSP was used at 400 ng ml−1 and produced 36±4 nmol cytochrome C reduced /106 cells (n=5).
The tyrosine kinase inhibitor genistein blocks the increase in tyrosine phosphorylation and the morphological changes induced by MSP in IL-3-dependent Ba/F3 cells expressing Ron (Mera et al., 1999), inhibits FMLP-evoked O2− production but has little effect on the respiratory burst induced by PMA in human neutrophils (Tan et al., 1998). In the range 1 – 100 μM, genistein inhibited O2− production evoked by hrMSP or FMLP in human PMs, but had an inconsistent effect on PMA-evoked one: maximal inhibition amounted to 30±3% vs hrMSP and 21±4% (n=5) vs FMLP, IC50 values being 6.3 μM and 9 μM, respectively. These effects were confirmed by the enhancement of the respiratory burst evoked by hrMSP and FMLP, when the tyrosine phosphatase inhibitor sodium orthovanadate was present (Figure 4). PI 3-kinase, a member of the phosphatidylinositol kinase family, has been frequently associated to MSP/Ron signalling (Chen et al., 1998, Danilkovitch & Leonard, 1999; Wang et al., 1996b), whereas FMLP is well characterized as one of the most effective and rapid activators of PI 3-kinase (Stephens et al., 1991). The PI 3-kinase specific inhibitor wortmannin reduces MSP-evoked epithelial cell migration (Wang et al., 1996b), prevents MSP-induced inhibition of NO production in macrophage cell lines (Chen et al., 1998) and also potently inhibits the phosphorylation of p47phox and O2− release in human neutrophils stimulated by FMLP, but not by PMA (Ding et al., 1995). In our experiments, wortmannin did not affect hrMSP- or PMA-evoked O2− production, while in the concentration range 0.1 – 100 nM, it inhibited FMLP-evoked respiratory burst in human PMs (Figure 5). Maximal inhibition (68±6%; n=5) was achieved at 100 nM; the IC50 value was 4.07 nM.
Figure 4.
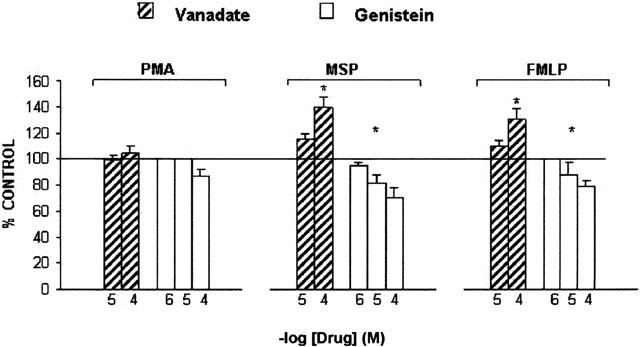
Effects of genistein or vanadate on O2− production evoked by hrMSP, FMLP or PMA in PMs. PMs were pretreated with the tyrosine kinase inhibitor genistein for 30 min or the tyrosine phosphatase inhibitor vanadate for 60 min and then challenged with fixed concentrations of hrMSP (400 ng ml−1), FMLP (10−6 M) or PMA (10−6 M). O2− production evoked by single stimulus was taken as 100%; results are expressed as per cent of single stimulus-induced respiratory burst. Means±s.e.mean of five experiments. *P<0.05 vs PMA; hrMSP vs FMLP is not significant (NS).
Figure 5.
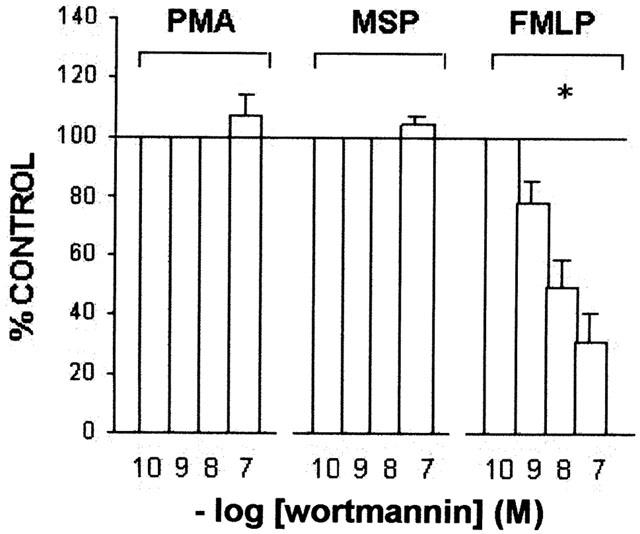
Effects of wortmannin on O2− production evoked by hrMSP, FMLP or PMA in PMs. PMs were pretreated with the PI 3-kinase inhibitor wortmannin for 30 min and then challenged with fixed concentrations of hrMSP (400 ng ml−1), FMLP (10−6 M) or PMA (10−6 M). O2− production evoked by single stimulus was taken as 100%; results are expressed as per cent of single stimulus-induced respiratory burst. Means±s.e.mean of five experiments. *P<0.05 vs PMA and hrMSP; PMA vs hrMSP is not significant (NS).
The potent and selective Src-inhibitor PP1 has been shown to inhibit O2− production evoked by immune complexes in bone marrow-derived human macrophages (Erdreich-Epstein et al., 1999) as well as adhesion-dependent NADPH oxidase activation in human eosinophils (Lynch et al., 1999). In our hands, PP1 dose-dependently (10 nM – 10 μM) inhibited the respiratory burst evoked by hrMSP and PMA, IC50 value being 0.4 μM for both stimuli, while less active against FMLP (IC50=1.85 μM) (Figure 6).
Figure 6.
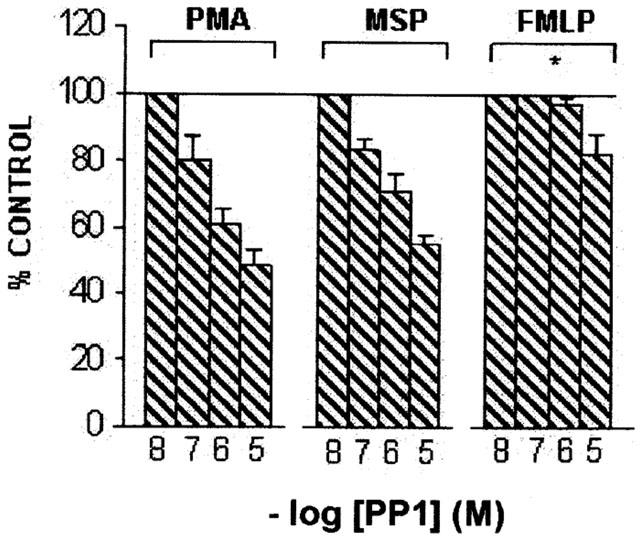
Effects of PP1 on O2− production evoked by hrMSP, FMLP or PMA in PMs. PMs were pretreated with the selective Src inhibitor PP1 for 30 min and then challenged with fixed concentrations of hrMSP (400 ng ml−1), FMLP (10−6 M) or PMA (10−6 M). O2− production evoked by single stimulus was taken as 100%; results are expressed as per cent of single stimulus-induced respiratory burst. Means±s.e.mean of five experiments. *P<0.05 vs PMA and hrMSP; PMA vs hrMSP is not significant (NS).
SB203580 is an ATP binding site inhibitor of the α and β isoforms of p38, a member of the MAP kinase family, which is required for FMLP stimulation of the neutrophil respiratory burst (Rane et al., 1997). This drug has been shown to prevent PMA- and FMLP-induced O2− production in neutrophils (Lal et al., 1999) as well as NADPH oxidase activity in eosinophils (Lynch et al., 1999). In our experiments, SB203580 dose-dependently (0.1 – 10 μM) inhibited PMA-, hrMSP- and FMLP- evoked respiratory burst (Figure 7), IC50 values being 0.37, 1 and 0.87 μM.
Figure 7.
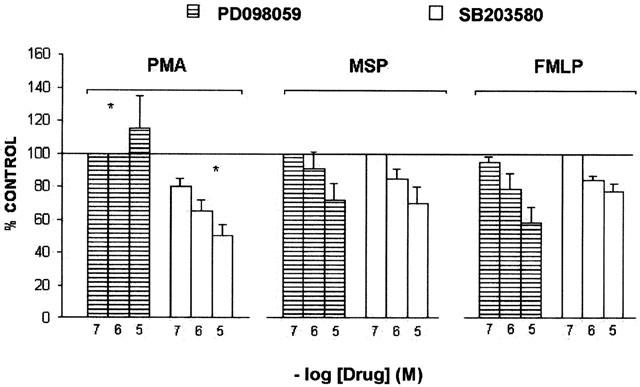
Effects of SB203580 or PD098059 on O2− production evoked by hrMSP, FMLP or PMA in PMs. PMs were pretreated with the p38 MAP kinase inhibitor SB203580 or the MEK inhibitor PD098059 for 60 min and then challenged with fixed concentrations of hrMSP (400 ng ml−1), FMLP (10−6 M) or PMA (10−6 M). O2− production evoked by single stimulus was taken as 100%; results are expressed as per cent of single stimulus-induced respiratory burst. Means±s.e.mean of five experiments. *P<0.05 vs FMLP and hrMSP; FMLP vs hrMSP is not significant (NS).
PD098059 is a MEK (MAP kinase kinase) inhibitor and, as a consequence, inhibits also tyrosine phosphorylation and activation of ERK-1 and ERK-2, the two isoforms mainly expressed in neutrophils (Downey et al., 1998). It has been reported to interfere with FMLP- and zymosan-induced respiratory burst in the same cells (Downey et al., 1998). However, others have demonstrated that it inhibits chemotaxis but not O2− production in FMLP-stimulated neutrophils (Kuroki & O'flaherty, 1997). In this work, PD0908059 was used at 0.1 – 10 μM and inhibited hrMSP- and FMLP-evoked respiratory burst, IC50 values being 1.56 μM vs hrMSP and 1 μM vs FMLP. However, it had no inhibitory effects on the O2− production induced by PMA, which, at the highest PD0908059 concentration evaluated, resulted slightly but non-significantly increased over PMA alone (Figure 7).
Discussion
The results presented here indicate that human macrophages of different origin (alveolar, peritoneal and in vitro-differentiated macrophages) express the tyrosine kinase receptor Ron, whereas in human circulating monocytes no Ron protein was detected. These results also confirm and further extend previous observations in murine macrophages (Iwama et al., 1995) as well as in the monocytic THP-1 cell line, which expressed Ron only when differentiated to macrophages by IFN-γ plus TNF-α treatment (Chen et al., 1996). Interestingly, Ron expression has been observed in human dermal macrophages from both normal and burn wound skin (Nanney et al., 1998).
Human Ron mRNA is expressed in the lung (Gaudino et al., 1994) and the protein has been detected at the apical surface of the bronchiolar ciliated epithelium, but not in goblet cells (Sakamoto et al., 1997). Moreover, MSP content ranged from 1.3 to 5.8 ng/ml in BAL fluids obtained from four healthy non-smokers, but the absolute concentration of MSP in the bronchoalveolar space, where AMs are located, was possibly higher as suggested by Sakamoto et al. (1997). It is attractive to speculate that MSP, via circulation (Wang et al., 1996c), can be supplied to the bronchoalveolar spaces, where it binds and activates Ron on AMs to induce the respiratory burst, as suggested by our results. The stimulatory effect of hrMSP on AMs was comparable to the one evoked by the tumour promoter PMA and higher than the chemotactic peptide FMLP-induced one. Similar findings have been reported in human nasal cilia, where MSP ability to increase ciliary motility is similar to that of LTC4 and other inflammatory mediators (Sakamoto et al., 1997). Moreover, by evaluating MSP levels in induced sputum, Takano et al. (2000) found a significant increase in patients with bronchiectasis as compared with normal controls. They also reported that half of the MSP in sputum is present as mature MSP, that is the biologically active alpha/beta chain heterodimer (Takano et al., 2000). Furthermore, the elevated O2− production measured after challenge with hrMSP in AMs isolated from patients with sarcoidosis might suggest a peculiar involvement of MSP in interstitial lung diseases.
We also demonstrate that the tyrosine kinase receptor Ron is expressed in MDMs and PMs isolated from ascitic fluid of cirrhotic patients, and that hrMSP induces a dose-dependent respiratory burst. In this case, too, hrMSP induced a higher amount of O2−, as compared to FMLP, strongly implying this factor as a potent regulator of macrophage activity.
The Ron receptor is functional in the macrophage lineage cells but not in circulating monocytes, since hrMSP activates only human macrophages of different origin to produce superoxide anion. This is a novel activity proposed for MSP, not described so far, that suggests a role of MSP in host defense and control of infection.
The source of reactive oxygen intermediates is the NADPH oxidase, a multi-component enzyme complex which transfers a single electron from NADPH to oxygen, resulting in the production of O2−. The O2− so generated is in turn the key element for the production of hydrogen peroxide, hydroxyl radical, singlet oxygen and long-living oxidants.
NADPH oxidase, which comprises both cytosolic and membrane components, is inactive until the cell is stimulated by phagocytosis or various inflammatory mediators. Activation is a multi-step process, not completely elucidated, which comprises partial activation of p47phox, translocation of p47phox, p67phox and p40phox to the membrane, final phosphorylation of p47phox, coupled to the acquisition of the catalytic activity of the enzyme (Babior, 1999).
NO has been demonstrated to inhibit the oxidase by preventing its assembly during activation (Fujii et al., 1997) and it is tempting to speculate that hrMSP- evoked O2− production in our cells might result, as least in part, from its ability to inhibit NO release, as demonstrated in murine macrophages (Chen et al., 1998; Liu et al., 1999).
The tyrosine kinase activity of Src-like protein kinases, as well as PI 3-kinase and MAP kinases, have also been associated to activation of the NADPH oxidase (Downey et al., 1998; Erdreich-Epstein et al., 1999; Lal et al., 1999; Rane et al., 1997). The majority of these studies have been performed using human neutrophils, while few of them were conducted on monocyte/macrophages. In rat AMs, different MAP kinases have been shown to be responsible for NADPH oxidase activation in response to zymosan-activated serum (Torres & Forman, 1999); in another macrophage population, the human bone marrow-derived macrophages, tyrosine kinases of the Src family have been demonstrated to play a key role in O2− production evoked by immune complexes (Erdreich-Epstein et al., 1999).
The mechanism of hrMSP-induced O2− production in human macrophages is still to be defined. However, PI 3-kinase does not seem to play a key role in the process, although this enzyme has been implicated in MSP/Ron signal transduction in other cell types. Our data show that wortmannin had no significant inhibitory effect on hrMSP-evoked O2− production, while markedly inhibited FMLP-evoked (but not PMA-evoked) respiratory burst. These observations are consistent with the implication of PI 3-kinase in chemotaxis and in NADPH oxidase activation of human eosinophils elicited by agonists acting through G protein-coupled receptors (Elsner et al., 1996). Moreover, also in the respiratory burst of human neutrophils, wortmannin has been repetitively reported to inhibit FMLP- but not PMA- induced responses (Kodama et al., 1999; Tudan et al., 1999).
The involvement of tyrosine kinases in NADPH oxidase activation in PMs was evaluated by using selective and non-selective inhibitors. The non-selective tyrosine kinase inhibitor genistein inhibited hrMSP- and FMLP-evoked O2− production in human PMs, while inactive against PMA, so confirming previous data in human neutrophils (Tan et al., 1998). The increased O2− production that we observed in the presence of the tyrosine phosphatase inhibitor sodium vanadate further confirmed the role of genistein in the respiratory burst. Sodium vanadate enhanced by 40% hrMSP- and FMLP-evoked respiratory burst, but did not affect PMA-evoked one. It has to be taken into account that the role of this phosphatase inhibitor in PMA-stimulated macrophages is controversial, being either inhibitory (Conde et al., 1995) or stimulatory (Green & Phillips, 1994).
PP1, a selective inhibitor of the Src-family tyrosine kinase activity, dose-dependently inhibited hrMSP- and PMA-evoked O2− production, while less active versus FMLP. This finding is partially at variance with previous observations in bone marrow-derived human macrophages (Erdreich-Epstein et al., 1999), showing a large inhibitory effect (about 80% with PP1 10 μM) in immune complexes-induced respiratory burst but no effect on PMA-evoked one. We have no definitive explanation for this fact, but differences in the macrophage cell type might be envisaged, since, as reported by the authors (Erdreich-Epstein et al., 1999), 99.5% of bone marrow-derived human macrophages (recovered from the screens used to filter bone marrow after harvest, grown and differentiated in M-CSF) were CD14 positive. Recent investigations in epithelial cells have shown that Ron activation is integrin-dependent and that adhesion of RON-expressing epithelial cells to extracellular matrix requires the tyrosine kinase activity of c-Src and Ron itself as well. Actually, collagen-induced Ron phosphorylation becomes reduced in cells transfected with a dominant-negative kinase-inactive Src construct, suggesting that Ron is phosphorylated by activated Src (Danilkovitch-Miagkova et al., 2000). The fact that Src can phosphorylate Ron in epithelial cells when stimulated by collagen, but not by MSP, indicates that the pattern of Ron tyrosine phosphorylation induced by extracellular matrix is distinct from that induced by MSP. Moreover, MSP addition to collagen-adherent cells resulted in an increased Ron phosphorylation and kinase activity as compared to MSP or collagen alone (Danilkovitch-Miagkova et al., 2000). Whether the known Src inhibitor PP1 affects MSP-evoked respiratory burst either through the direct inhibition of Src or indirectly, requires further investigations. However, in our opinion, our observations on macrophages further strengthen the role of Src in MSP/Ron signalling.
The dual specificity kinase MEK (MAP kinase kinase) activates ERKs (Extracellular-regulated protein kinases) and p38, which are members of the MAP kinase family. MEK inhibition by PD098059 prevents O2− production in zymosan-stimulated neutrophils (Downey et al., 1998), while conflicting results have been reported for FMLP (Downey et al., 1998; Kuroki & O'flaherty, 1997). On the other hand, inhibition of p38 by SB203580 prevents NADPH oxidase activation by FMLP and by PMA (Lal et al., 1999).
Our results indicate that PD 098059 inhibited hrMSP- and FMLP- evoked respiratory burst, while a slight increase was observed at 10 μM versus PMA. On its turn, the p38 inhibitor SB 203580 displayed a clear-cut inhibitory effect on all evaluated stimuli, i.e. hrMSP, FMLP and PMA, in accordance with results obtained in human neutrophils (Lal et al., 1999).
These results are in agreement with the observation that in Ron-transfected MDCK cells, MSP activates Ras by activation and translocation of the nucleotide exchange factor SOS (Li et al., 1995) and confirm that ERKs and p38 are primary targets of the pathway induced by Ron, via the Ras/MAP kinase pathway. It is well known that the activity of NADPH oxidase can be regulated also by Rac, a small GTP binding protein of the Ras family (Babior, 1999; Irani & Goldschmidt-Clermont, 1998), that has been shown activated by several tyrosine kinase receptors (Babior, 1999; Boehm et al., 1999). A possibility therefore exists that the pattern of activation of NADPH oxidase by MSP could also rely on Rac.
However, the occasional discrepancies observed in some experiments on signal transduction inhibitors are due to the pathways elicited by the different stimuli, given that FMLP acts through a G-protein-coupled receptor, unlike MSP and PMA. Another explanation relates to possible structural differences in the mechanism of NADPH oxidase activation between macrophages and other phagocytes (e.g., neutrophils, where most experiments have been conducted).
The role of reactive oxygen species has recently been re-evaluated and growing evidence points to their physiological function as signalling molecules in the regulation of intracellular pathways; protein tyrosine kinases, protein tyrosine phosphatases, phospholipase A2 and the transcription factor NF-κB were identified as potential targets of hydrogen peroxide (Finkel, 1998). Reactive oxygen species produced by the NADPH oxidase could regulate the dynamic equilibrium between the opposing effects of the tyrosine kinases and tyrosine phosphatases (Finkel, 1998). Therefore, one can speculate that the O2− produced by MSP in human macrophages of different origin might also act as a second messenger for signal transduction.
Acknowledgments
We wish to thank Dr Massimo Sartori, Ospedale Maggiore della Carità, Novara (Italy) and Dr Antonio Di Stefano, Fondazione ‘Salvatore Maugeri', Veruno (Italy) for kindly providing ascitic fluids and alveolar macrophages, respectively. We thank Dr Flavio Mignone for skilled technical assistance. We also are indebted to Prof Guido Monga, School of Medicine, University of Piemonte Orientale ‘A. Avogadro', Novara (Italy) for helpful advice and discussion. This work was supported by CNR grant 9701213 PF49, Progetto Finalizzato “Biotecnologie”, by AIRC and by MURST PRIN 2000. A preliminary account of this work was presented at the VI Joint Meeting of British Pharmacological Society and Società Italiana di Farmacologia, Birmingham, 18 – 21 December 2000 (Br. J. Pharmacol. 133, 38P, 2001).
Abbreviations
- AMs
alveolar macrophages
- BAL
bronchoalveolar lavage
- ERK
extracellular signal-regulated kinase
- FMLP
N-formylmethionyl-leucyl-phenylalanine
- HMs
human monocytes
- hrMSP
human recombinant macrophage stimulating protein
- iNOS
inducible NO synthase
- LPS
lipopolysaccharide
- MAP
mitogen-activated protein
- MDMs
monocyte-derived macrophages
- MEK
MAP kinase kinase
- MSP
macrophage stimulating protein
- NO
nitric oxide
- O2−
superoxide anion
- PD 098059
[2′-amino-3′-methoxyflavone]
- PI 3-kinase
phosphatidylinositol 3-kinase
- PMA
phorbol 12-mirystate 13-acetate
- PMs
peritoneal macrophages
- PP1
4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]-pyrimidine
- SB203580
[4-(4-fluorophenyl)-2(4-methylsulfinylphenyl)5-(4-pyridyl)-1H-imidazole]
References
- ALBINA J.E. On the expression of nitric oxide synthase by human macrophages. Why no NO. J. Leukoc. Biol. 1995;58:643–649. doi: 10.1002/jlb.58.6.643. [DOI] [PubMed] [Google Scholar]
- BABIOR B.M. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- BANU N., PRICE D.J., LONDON R., DENG B., MARK M., GODOWSKI P.J., AVRAHAM H. Modulation of megakaryocytopoiesis by human macrophage-stimulating protein, the ligand for the RON receptor. J. Immunol. 1996;156:2933–2940. [PubMed] [Google Scholar]
- BEZERRA J.A., CARRICK T.L., DEGEN J.L., WITTE D., DEGEN S.J.F. Biological effects of targeted inactivation of hepatocyte growth factor-like protein in mice. J. Clin. Invest. 1998;101:1175–1183. doi: 10.1172/JCI1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEZERRA J.A., LANEY D.W., JR, DEGEN S.J. Increased expression of mRNA for hepatocyte growth factor-like protein during liver regeneration and inflammation. Biochem. Biophys. Res. Commun. 1994;203:666–673. doi: 10.1006/bbrc.1994.2234. [DOI] [PubMed] [Google Scholar]
- BOEHM J.E., CHAIKA O.V., LEWIS R.E. Rac-dependent anti-apoptotic signaling by the insulin receptor cytoplasmic domain. J. Biol. Chem. 1999;274:28632–28636. doi: 10.1074/jbc.274.40.28632. [DOI] [PubMed] [Google Scholar]
- BRUNELLESCHI S., BORDIN G., COLANGELO D., VIANO I. Tachykinin receptors on human monocytes: their involvement in rheumatoid arthritis. Neuropeptides. 1998;32:215–223. doi: 10.1016/s0143-4179(98)90040-3. [DOI] [PubMed] [Google Scholar]
- BRUNELLESCHI S., GUIDOTTO S., VIANO I., FANTOZZI R., POZZI E., GHIO P., ALBERA C. Tachykinin activation of human alveolar macrophages in tobacco smoke and sarcoidosis: a phenotypical and functional study. Neuropeptides. 1996;30:456–464. doi: 10.1016/s0143-4179(96)90010-4. [DOI] [PubMed] [Google Scholar]
- CHEN Y.Q., FISHER J.H., WANG M.H. Activation of the RON receptor tyrosine kinase inhibits inducible nitric oxide synthase (iNOS) expression by murine peritoneal exudate macrophages: phosphatidylinositol-3 kinase is required for RON-mediated inhibition of iNOS expression. J. Immunol. 1998;161:4950–4959. [PubMed] [Google Scholar]
- CHEN Q., DEFRANCES M.C., ZARNEGAR R. Induction of met proto-oncogene (hepatocyte growth factor receptor) expression during human monocyte-macrophage differentiation. Cell Growth Differ. 1996;7:821–832. [PubMed] [Google Scholar]
- CONDE M., CHIARA M.D., PINTADO E., SOBRINO F. Modulation of phorbol ester-induced respiratory burst by vanadate, genistein and phenylarsine oxide in mouse macrophages. Free Radic. Biol. Med. 1995;18:343–348. doi: 10.1016/0891-5849(94)00126-5. [DOI] [PubMed] [Google Scholar]
- CORRELL P.H., IWAMA A., TONDAT S., MAYRHOFER G., SUDA T., BERNSTEIN A. Deregulated inflammatory response in mice lacking the STK/RON receptor tyrosine kinase. Genes Funct. 1997;1:69–83. doi: 10.1046/j.1365-4624.1997.00009.x. [DOI] [PubMed] [Google Scholar]
- DANILKOVITCH A., LEONARD E.J. Kinases involved in MSP/RON signaling. J. Leukoc. Biol. 1999;65:345–348. doi: 10.1002/jlb.65.3.345. [DOI] [PubMed] [Google Scholar]
- DANILKOVITCH-MIAGKOVA A., ANGELONI D., SKEEL A., DONLEY S., LERMAN M., LEONARD E.J. Integrin-mediated RON growth factor receptor phosphorylation requires tyrosine kinase activity of both the receptor and c-Src. J. Biol. Chem. 2000;275:14783–14786. doi: 10.1074/jbc.C000028200. [DOI] [PubMed] [Google Scholar]
- DING J., VLAHOS C.I., LIU R., BROWN R.F., BADWEY J.A. Antagonists of phosphatidylinositol 3-kinase block activation of several novel protein kinases in neutrophils. J. Biol. Chem. 1995;270:11684–11691. doi: 10.1074/jbc.270.19.11684. [DOI] [PubMed] [Google Scholar]
- DOWNEY G.P., BUTLER J.R., TAPPER H., FIALKOW L., SALTIEL A.R., RUBIN B.B., GRINSTEIN S. Importance of MEK in neutrophil microbicidal responsiveness. J. Immunol. 1998;160:434–443. [PubMed] [Google Scholar]
- ELSNER J., HOCHSTETTER R., KIMMIG D., KAPP A. Human eotaxin represents a potent activator of the respiratory burst of human eosinophils. Eur. J. Immunol. 1996;26:1919–1925. doi: 10.1002/eji.1830260837. [DOI] [PubMed] [Google Scholar]
- ERDREICH-EPSTEIN A., LIU M., KANT A.M., IZADI K.D., NOLTA J.A., DURDEN D.L. Cbl functions downstream of Src kinases in FcγRI signaling in primary human macrophages. J. Leukoc. Biol. 1999;65:523–534. doi: 10.1002/jlb.65.4.523. [DOI] [PubMed] [Google Scholar]
- FINKEL T. Oxygen radicals and signaling. Curr. Opin. Cell. Biol. 1998;10:248–253. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- FUJII H., ICHIMORI K., HOSHIAI K., NAKAZAWA K. Nitric oxide inactivates NADPH oxidase in pig neutrophils by inhibiting its assembling process. J. Biol. Chem. 1997;272:32773–32788. doi: 10.1074/jbc.272.52.32773. [DOI] [PubMed] [Google Scholar]
- GANTNER F., KUPFERSCHMIDT R., SCHUDT C., WENDEL A., HATZELMANN A. In vitro differentiation of human monocytes to macrophages: change of PDE profile and its relationship to suppression of tumor necrosis factor-α release by PDE inhibitors. Br. J. Pharmacol. 1997;121:221–231. doi: 10.1038/sj.bjp.0701124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAUDINO G., FOLLENZI A., NALDINI L., COLLESI C., SANTORO M., GALLO K.A., GODOWSKI P.J., COMOGLIO P.M. Ron is a heterodimeric tyrosine kinase receptor activated by the HGF homologue MSP. EMBO J. 1994;13:3524–3532. doi: 10.1002/j.1460-2075.1994.tb06659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN S.P., PHILLIPS W.A. Activation of the macrophage respiratory burst by phorbol myristate acetate: evidence for both tyrosine-kinase-dependent and -independent pathways. Biochim. Biophys. Acta. 1994;1222:241–248. doi: 10.1016/0167-4889(94)90175-9. [DOI] [PubMed] [Google Scholar]
- HARRISON P., DEGEN S.J., WILLIAMS R., FARZANEH F. Hepatic expression of hepatocyte-growth factor-like/macrophage-stimulating protein mRNA in hepatic fulminant failure. Lancet. 1994;344:27–29. doi: 10.1016/s0140-6736(94)91050-2. [DOI] [PubMed] [Google Scholar]
- IRANI K., GOLDSCHMIDT-CLERMONT P.J. Ras, superoxide and signal transduction. Biochem. Pharmacol. 1998;55:1339–1346. doi: 10.1016/s0006-2952(97)00616-3. [DOI] [PubMed] [Google Scholar]
- IWAMA A., WANG M.H., YAMAGUCHI N., OHNO N., OKANO K., SUDO T., TAKEYA M., GERVAIS F., MORISSETTE C., LEONARD E.J., SUDA T. Terminal differentiation of murine resident peritoneal macrophages is characterized by expression of the STK protein tyrosine kinase, a receptor for macrophage-stimulating protein. Blood. 1995;86:3394–3403. [PubMed] [Google Scholar]
- IWAMA A., YAMAGUCHI N., SUDA T. STK/RON receptor tyrosine kinase mediates both apoptotic and growth signals via the multifunctional docking site conserved among the HGF receptor family. EMBO J. 1996;15:5866–5875. [PMC free article] [PubMed] [Google Scholar]
- KODAMA T., HAZEKI K., HAZEKI O., OKADA T., UI M. Enhancement of chemotactic peptide-induced activation of phosphoinositide 3-kinase by granulocyte-macrophage colony-stimulating factor and its relation to the cytokine-mediated priming of neutrophil superoxide anion production. Biochem. J. 1999;337:201–209. [PMC free article] [PubMed] [Google Scholar]
- KURIHARA N., IWAMA A., TATSUMI J., IKEDA K., SUDA T. Macrophage-stimulating protein activates STK receptor tyrosine kinase on osteoclasts and facilitates bone resorption by osteoclast-like cells. Blood. 1996;87:3704–3710. [PubMed] [Google Scholar]
- KUROKI M., O'FLAHERTY J.T. Differential effects of a mitogen-activated protein kinase kinase inhibitor on human neutrophil responses to chemotactic factors. Biochem. Biophys. Res. Commun. 1997;232:474–477. doi: 10.1006/bbrc.1997.6296. [DOI] [PubMed] [Google Scholar]
- LAL A.S., CLIFTON A.D., ROUSE J., SEGAL A.W., COHEN P. Activation of the neutrophil NADPH oxidase is inhibited by SB 203580, a specific inhibitor of SAPK2/p38. Biochem. Biophys. Res. Commun. 1999;259:465–470. doi: 10.1006/bbrc.1999.0759. [DOI] [PubMed] [Google Scholar]
- LEONARD E.J., SKEEL A.H. Enhancement of spreading, phagocytosis and chemotaxis by macrophage stimulating protein (MSP) Adv. Exp. Med. Biol. 1979;121B:181–194. doi: 10.1007/978-1-4684-8914-9_16. [DOI] [PubMed] [Google Scholar]
- LI B.Q., WANG M.H., KUNG H.F., RONSIN C., BREATNACH R., LEONARD E.J., KAMATA T. Macrophage-stimulating protein activates Ras by both activation and translocation of the SOS nucleotide exchange factor. Biochem. Biophys. Res. Comm. 1995;216:110–118. doi: 10.1006/bbrc.1995.2598. [DOI] [PubMed] [Google Scholar]
- LIU Q.P., FRUIT K., WARD J., CORRELL P.H. Negative regulation of macrophage activation in response to IFN-gamma and lipopolysaccharide by the STK/RON receptor tyrosine kinase. J. Immunol. 1999;163:6606–6613. [PubMed] [Google Scholar]
- LYNCH O.T., GIEMBYCZ M.A., BARNES P.J., HELLEWELL P.G., LINDSAY M.A. “Outside-in” signalling mechanisms underlying CD11b/CD18-mediated NADPH oxidase activation in human adherent blood eosinophils. Br. J. Pharmacol. 1999;128:1149–1158. doi: 10.1038/sj.bjp.0702892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEDICO E., MONGIOVI A.M., HUFF J., JELINEK M.A., FOLLENZI A., GAUDINO G., PARSONS J.T., COMOGLIO P.M. The tyrosine kinase receptors Ron and Sea control “scattering” and morphogenesis of liver progenitor cells in vitro. Mol. Biol. Cell. 1996;7:495–504. doi: 10.1091/mbc.7.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERA A., SUGA M., ANDO M., SUDA T., YAMAGUCHI N. Induction of cell shape changes through activation of the interleukin-3 common beta chain receptor by the RON receptor-type tyrosine kinase. J. Biol. Chem. 1999;274:15766–15774. doi: 10.1074/jbc.274.22.15766. [DOI] [PubMed] [Google Scholar]
- MORRISSETTE N., GOLD E., ADEREM A. The macrophage: a cell for all seasons. Trends Cell Biol. 1999;9:199–201. doi: 10.1016/s0962-8924(99)01540-8. [DOI] [PubMed] [Google Scholar]
- NANNEY L.B., SKEEL A., LUAN J., POLIS S., RICHMOND A., WANG M.H., LEONARD E.J. Proteolytic cleavage and activation of pro-macrophage-stimulating protein and upregulation of its receptor in tissue injury. J. Invest. Dermatol. 1998;111:573–581. doi: 10.1046/j.1523-1747.1998.00332.x. [DOI] [PubMed] [Google Scholar]
- RANE M.J., CARRITHERS S.L., ARTHUR J.M., KLEIN J.B., MCLEISH K.R. Formyl peptide receptors are coupled to multiple mitogen-activated protein kinase cascades by distinct signal transduction pathways. J. Immunol. 1997;159:5070–5078. [PubMed] [Google Scholar]
- SAKAMOTO O., IWAMA A., AMITANI R., TAKEHARA T., YAMAGUCHI N., YAMAMOTO T., MASUYAMA K., YAMANAKA T., ANDO M., SUDA T. Role of macrophage-stimulating protein and its receptor, RON tyrosine kinase, in ciliary motility. J. Clin. Invest. 1997;99:701–709. doi: 10.1172/JCI119214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEPHENS L.R., HUGHES K.T., IRVINE R.F. Pathway of phosphatidylinositol (3,4,5)- trisphosphate synthesis in activated neutrophils. Nature. 1991;351:33–39. doi: 10.1038/351033a0. [DOI] [PubMed] [Google Scholar]
- TAKANO Y., SAKAMOTO O., SUGA M., SUDA T., ANDO M. Elevated levels of macrophage stimulating protein in induced sputum of patients with bronchiectasis. Respir. Med. 2000;94:784–790. doi: 10.1053/rmed.2000.0822. [DOI] [PubMed] [Google Scholar]
- TAN A.S., AHMED N., BERRIDGE M.V. Acute regulation of glucose transport after activation of human peripheral blood neutrophils by phorbol myristate acetate, fMLP and granulocyte-macrophage colony-stimulating factor. Blood. 1998;91:649–655. [PubMed] [Google Scholar]
- TORRES M., FORMAN H.J. Activation of several MAP kinases upon stimulation of rat alveolar macrophages: role of the NADPH oxidase. Arch. Biochem. Biophys. 1999;366:231–239. doi: 10.1006/abbi.1999.1225. [DOI] [PubMed] [Google Scholar]
- TUDAN C., JACKSON J.K., PELECH S.L., ATTARDO G., BURT H. Selective inhibition of protein kinase C, mitogen-activated protein kinase, and neutrophil activation in response to calcium pyrophosphate dihydrate crystals, formyl-methionyl-leucyl-phenylalanine, and phorbol esters by O-(chloroaceyl-carbamoyl) fumagillol (AGM-1470; TNP-470) Biochem. Pharmacol. 1999;58:1869–1880. doi: 10.1016/s0006-2952(99)00287-7. [DOI] [PubMed] [Google Scholar]
- WANG M.H., COX G.W., YOSHIMURA T., SHEFFLER L.A., SKEEL A., LEONARD E.J. Macrophage-stimulating protein inhibits induction of nitric oxide production by endotoxin- or cytokine-stimulated mouse macrophages. J. Biol. Chem. 1994a;269:14027–14031. [PubMed] [Google Scholar]
- WANG M.H., DLUGOSZ A.A., SUN Y., SKEEL A., LEONARD E.J. Macrophage-stimulating protein induces proliferation and migration of murine keratinocytes. Exp. Cell Res. 1996a;226:39–46. doi: 10.1006/excr.1996.0200. [DOI] [PubMed] [Google Scholar]
- WANG M.H., FUNG H.L., CHEN Y.Q. Regulation of the RON receptor tyrosine kinase expression in macrophages: blocking the RON gene transcription by endotoxin-induced nitric oxide. J. Immunol. 2000;164:3815–3821. doi: 10.4049/jimmunol.164.7.3815. [DOI] [PubMed] [Google Scholar]
- WANG M.H., MONTERO-JULIAN F.A., DAUNY I., LEONARD E.J. Requirement of phosphatidylinositol-3 kinase for epithelial cell migration activated by human macrophage stimulating protein. Oncogene. 1996b;13:2167–2175. [PubMed] [Google Scholar]
- WANG M.H., RONSIN C., GESNEL M.C., COUPEY L., SKEEL A., LEONARD E.J., BREATHNACH R. Identification of the ron gene product as the receptor for the human macrophage stimulating protein. Science. 1994b;266:117–119. doi: 10.1126/science.7939629. [DOI] [PubMed] [Google Scholar]
- WANG M.H., SKEEL A., LEONARD E.J. Proteolytic cleavage and activation of pro-macrophage-stimulating protein by resident peritoneal macrophage membrane proteases. J. Clin. Invest. 1996c;97:720–727. doi: 10.1172/JCI118470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLETT C.G., WANG M.H., EMANUEL R.L., GRAHAM S.A., SMITH D.I., SHRIDHAR V., SUGARBAKER D.J., SUNDAY M.E. Macrophage-stimulating protein and its receptor in non-small-cell lung tumors: induction of receptor tyrosine phosphorylation and cell migration. Am. J. Respir. Cell. Mol. Biol. 1998;18:489–496. doi: 10.1165/ajrcmb.18.4.2978. [DOI] [PubMed] [Google Scholar]
- YOSHIMURA T., YUHKI N., WANG M.H., SKEEL A., LEONARD E.J. Cloning, sequencing and expression of human macrophage stimulating protein (MSP, MST1) confirms MSP as a member of the family of kringle protein, and locates the MSP gene on chromosome 3. J. Biol. Chem. 1993;268:15416–15468. [PubMed] [Google Scholar]
- ZIEGLER-HEITBROCK H.W.L. Definition of human blood monocytes. J. Leukoc. Biol. 2000;67:603–606. doi: 10.1002/jlb.67.5.603. [DOI] [PubMed] [Google Scholar]


