Abstract
The influence of bradykinin (BK) on blood glucose and plasma insulin levels was investigated in anaesthetized rats.
Blood glucose level was dose-dependently increased by intravenous infusion of BK. This effect of BK was enhanced by captopril, an inhibitor of angiotensin-converting enzyme (ACE). Des-Arg9-bradykinin (DABK), a kinin B1 receptor agonist, did not modify blood glucose levels while the effect of BK was inhibited by Hoe-140, a kinin B2 receptor antagonist. The effect of BK was reduced by the NO-synthase inhibitor, Nω-nitro-L-arginine methyl ester (L-NAME), and by the cyclo-oxygenase inhibitor, indomethacin.
The effect of BK was suppressed by the association of propranolol with phentolamine or phenoxybenzamine. It was also reduced by hexamethonium, a ganglion-blocking drug. In adrenalectomized rats, the infusion of BK slightly decreased blood glucose levels.
The hyperglycaemic effect of adrenaline was suppressed by propranolol associated with phentolamine or phenoxybenzamine, but it was not modified by L-NAME.
Infusion of BK did not modify plasma insulin levels. However, after phentolamine and propranolol, BK induced a transient 2 fold rise in plasma insulin levels. The release of insulin was dose-dependent and inhibited by Hoe-140.
We conclude that infusion of BK induces, via a stimulation of B2 receptors, the release of NO and of prostanoids. The latter agents activate through a reflex pathway the release of catecholamines from the adrenal medulla. This release increases blood glucose levels and reduces plasma insulin levels. After adrenoceptor inhibition, BK induces a secretion of insulin, via the stimulation of B2 receptors.
Keywords: bradykinin, insulin, adrenaline, blood glucose, blood pressure, nitric oxide, prostanoids, adrenals
Introduction
According to Yang & Hsu (1995); 1997), bradykinin (BK) stimulates insulin release from the rat pancreas perfused in situ, in a glucose-dependent manner. Similarly, BK stimulates insulin secretion from clonal beta cells through the activation of B2 receptors (Saito et al., 1996; Yang et al., 1997b). The administration of a glucose load induces a larger increase in glucose plasma level and a smaller release of insulin in kininogen-deficient rats than in normal rats (Damas et al., 1999). These observations suggest that bradykinin might modulate insulin release in vivo.
However, chronic subcutaneous injections of BK did not modify plasma glucose and insulin levels in normal rats while such administration reduced plasma insulin levels in obese rats without affecting plasma glucose levels (Henriksen et al., 1998). In hyperglycaemic diabetic rats, the subcutaneous injection of BK alone increased blood glucose, but BK administered together with insulin further reduced circulating glucose levels (Dietze & Wicklmayr, 1977; Wicklmayr et al., 1980). In man, the direct arterial infusion of BK into the forearm has been reported to increase skeletal muscle glucose uptake (Dietze & Wicklmayr, 1977). Moreover, it has been demonstrated that angiotensin-converting enzyme (ACE) inhibitors have a beneficial effect on insulin sensitivity in several models of insulin resistance (Tomiyama et al., 1994; Kohlman et al., 1995; Erlich & Rosenthal, 1998; Henriksen et al., 1999). Inhibition of ACE prevents not only the formation of angiotensin II, but also the degradation of bradykinin. Indeed, ACE is the main metabolizing enzyme for bradykinin (Bhoola et al., 1992) and evidence is accumulating of the involvement of bradykinin in this beneficial effect of ACE inhibitors (Tomiyama et al., 1994; Kohlman et al., 1995; Erlich & Rosenthal, 1998; Henriksen et al., 1999; Damas et al., 1999).
These contrary observations led us to further examine the effects of BK on blood glucose and plasma insulin levels in anaesthetized rats. In the present study, we have used intravenous administration of BK which induces a hypotensive response and releases prostanoids and NO (Bhoola et al., 1992). Moreover, BK is able to stimulate the adrenal medulla and to induce a catecholamine secretion (Lecomte et al., 1961; Feldberg & Lewis, 1964; Staszewska-Barczak & Vane, 1967). This release affects the vascular response to bradykinin (Lecomte et al., 1961; Gardiner et al., 1992; Bjornstad-Ostensen & Berg, 1994) but also could influence plasma glucose and insulin levels.
Methods
Experiments were conducted as approved by the Ethical Committee of the Faculty of Medicine of the University of Liège. In this study, we used 304 male Wistar rats (mean weight 320 g). The animals were maintained in our breeding farm on a standard rat chow with tap water ad libitum until the experiment.
Measurement of blood glucose and plasma insulin levels
The fed animals were separated into several groups containing 4 – 12 animals, according to their pretreatment before the infusion of saline, bradykinin or adrenaline. All the animals were anaesthetized with sodium pentobarbital (Nembutal) 50 mg kg−1 intraperitoneally. Fifteen minutes later, a catheter was inserted into a jugular vein for BK, adrenaline or saline administration, and a second short catheter was introduced into a carotid artery to obtain blood samples. The animals were tracheotomized. In some rats, the abdomen was opened and the adrenals removed before BK administration. In sham-operated rats, the abdomen was opened and the intestines displaced to allow the sight of both adrenals.
After 10 min of equilibration, during which the metabolic changes induced by the anaesthesia and the surgical stress vanished (Rao, 1992), and after heparinization (500 U kg−1), a blood sample (20 – 30 μl) was withdrawn from the carotid artery to measure blood glucose. An infusion (0.25 ml min−1) of BK, adrenaline or saline was then administered for 10 min and the blood glucose level was measured at the end of the infusion and then every 15 min for 45 min. At the end of the period of observation, blood was withdrawn by cardiac puncture and citrate was added (3.8%; 1/9, v v−1). Plasma was separated by centrifugation at 2500×g for 10 min at 4°C, and kept at −20°C until assayed. Plasma insulin concentrations were measured by radioimmunoassay (Quabbe, 1969) using INS – RIA from Medgenix (Wevelgem, Belgium) with human insulin as standard. Blood glucose was measured on samples obtained from the carotid artery, using Accutrend glucose from Roche (Brussels, Belgium).
Drugs
Bradykinin acetate (BK), Des-Arg9-bradykinin (DABK), phentolamine hydrochloride, Nω-L-arginine methyl ester hydrochloride (L-NAME) were obtained from Sigma (Antwerpen, Belgium), propranolol hydrochloride from ICI (Macclesfield, U.K.), phenoxybenzamine hydrochloride from Euro-Biochem (Bierges, Belgium), hexamethonium chloride from Poviet (Amsterdam, The Netherlands), indomethacin from Merck Sharp and Dohme (Rahway, N.J., U.S.A.), heparin from Léo (Zaventem, Belgium), captopril from Squibb (Brussels, Belgium), L-adrenaline bitartrate from Calbiochem (Bierges, Belgium), and nembutal from Sanofi (Brussels, Belgium). Hoe-140 (D-arg(Hyp3,Thy5,D-Tic7,Oic8) bradykinin) was a kind gift of Hoechst AG (Frankfurt, Germany). All these drugs were dissolved or diluted in physiological saline except indomethacin which was dissolved in Tris HCl (0.15 M, pH 7.8) and phenoxybenzamine which was dissolved in 1,2-propanediol.
Statistical analysis
Results are given as mean±s.e.mean. Statistical significance was evaluated using an analysis of variance followed by Fisher's protected least significant difference or using Student's paired t-test as appropriate. Differences between means were considered significant when a 2-tailed value of P was less than 0.05.
Results
Changes in blood glucose levels induced by bradykinin
In a first series of experiments, BK was injected as an i.v. bolus, at doses ranging from 3 to 100 nmol kg−1 and the blood withdrawn 15 – 60 min after the injection. No significant modification of the plasma insulin concentration or of the blood glucose level was found (data not shown). In a second series of experiments, BK was infused intravenously for 10 min at doses ranging from 10 to 70 nmol kg−1 min−1. Under these conditions, BK at doses of 20 nmol kg−1 min−1 or higher, transiently increased blood glucose level. This effect was dose dependent. The increase in blood glucose was maximal at the end of the period of infusion and progressively decreased with time (Figure 1). Comparatively, the infusion of a similar volume of saline slightly reduced blood glucose from 8.43±0.72 to 7.66±0.40 mM (n=6).
Figure 1.
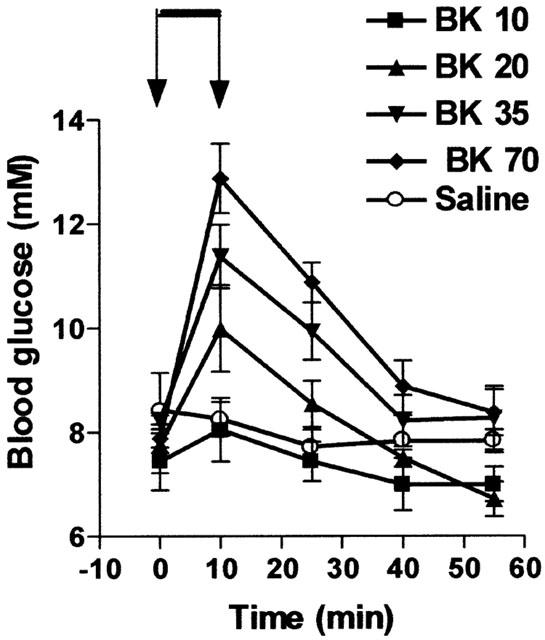
Influence of BK on blood glucose levels. BK was infused for 10 min from time 0 – 10 (indicated by the arrows) at four different doses (10, 20, 35 or 70 nmol kg−1 min−1). Results show the changes in blood glucose levels as a function of time: mean±s.e.mean of six values for each point.
As various effects of BK are increased by ACE inhibitors (Bhoola et al., 1992), we examined the influence of captopril, an ACE inhibitor (2 mg kg−1), on the hyperglycaemic action of BK using a dose of BK (10 nmol kg−1 min−1) that had previously been found not to induce hyperglycaemia. The acute administration of captopril alone did not modify blood glucose and after the administration of this drug, a saline infusion reduced the level of blood glucose from 7.66±0.45 mM to 6.94±0.24 mM (n=6; P<0.01). However, as shown Figure 2, the hyperglycaemic effect of BK was markedly increased in captopril treated animals.
Figure 2.
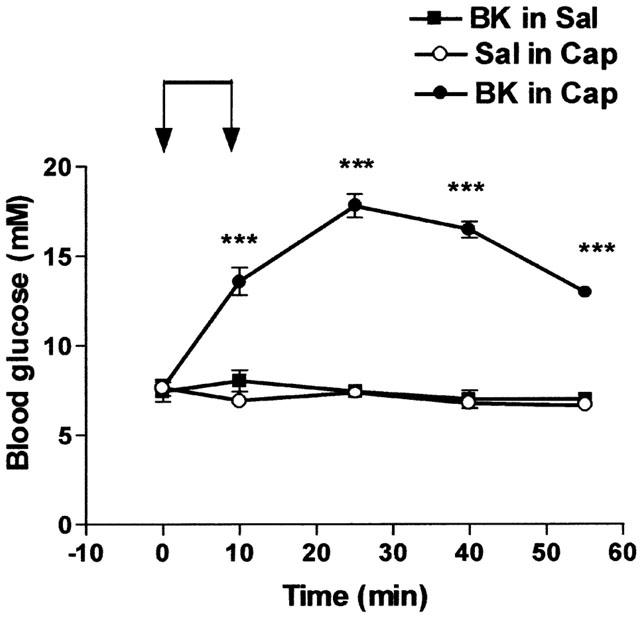
Influence of captopril on the hyperglycaemic effect of BK. Saline (Sal) or BK (10 nmol kg−1 min−1) was infused for 10 min from time 0 – 10 (indicated by the arrows) in rats pretreated with saline (Sal) or in captopril-treated (Cap, 2 mg kg−1) rats. Results show the changes in blood glucose levels as a function of time: mean±s.e.mean of six values for each point. ***P<0.001.
Des-arg9-bradykinin, which stimulates bradykinin B1-receptor (Marceau, 1995), infused for 10 min at a dose of 70 nmol kg−1 min−1 was without effect on glucose level in normal rats or in rats pretreated with captopril (Table 1). On the other hand, the hyperglycaemic effect of BK was suppressed by Hoe-140, a bradykinin B2-receptor antagonist (Wirth et al., 1991), administered at doses of 0.25 or 0.75 nmol kg−1 before the infusion of BK, while HOE-140 alone had no effect on basal blood glucose (Figure 3). The hyperglycaemic effect of BK thus depended on a stimulation of B2-receptors.
Table 1.
Influence of Des-Arg9-bradykinin (DABK) on blood glucose level
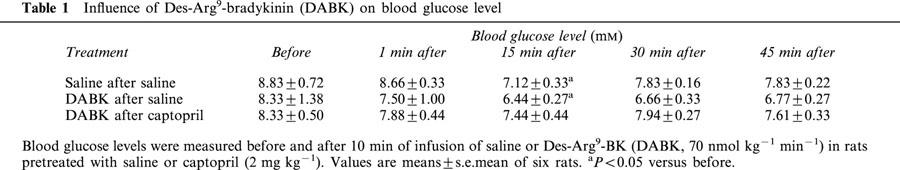
Figure 3.
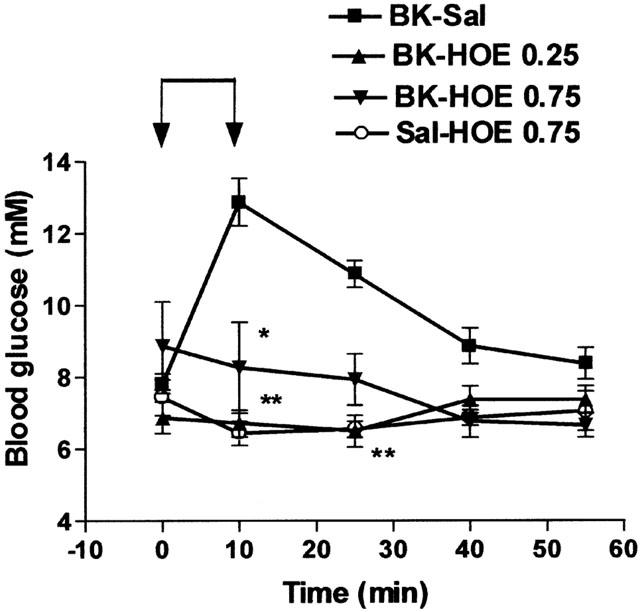
Influence of HOE-140 on the hyperglycaemic effect of BK. Saline (Sal) or BK (70 nmol kg−1 min−1) was infused for 10 min from time 0 – 10 (indicated by the arrows) in rats pretreated with saline (Sal) or HOE-140 (HOE, 0.25 or 0.75 nmol kg−1). Results show the changes in blood glucose levels as a function of time: mean±s.e.mean of six values for each point. *P<0.05, **P<0.01.
Several effects of BK depend on the release of nitric oxide or of prostanoids (Bhoola et al., 1992). In order to understand the mechanism of the hyperglycaemic effect of BK, the animals were treated by various agents before the infusion of the maximum dose of BK used earlier (70 nmol kg−1 min−1). The acute administration of these agents had no influence on the basal level of blood glucose and the infusion of saline in pretreated-animals slightly reduced this level. The influence of L-NAME, a NO-synthase inhibitor, or of indomethacin, a cyclo-oxygenase inhibitor, were thus evaluated. L-NAME (100 mg kg−1) was administered intravenously 10 min before BK. Indomethacin (3 mg kg−1) was administered intraperitoneally 60 min before BK. The increase in blood glucose level induced by BK was significantly reduced by 64% by L-NAME and by 42% by indomethacin (Figure 4).
Figure 4.
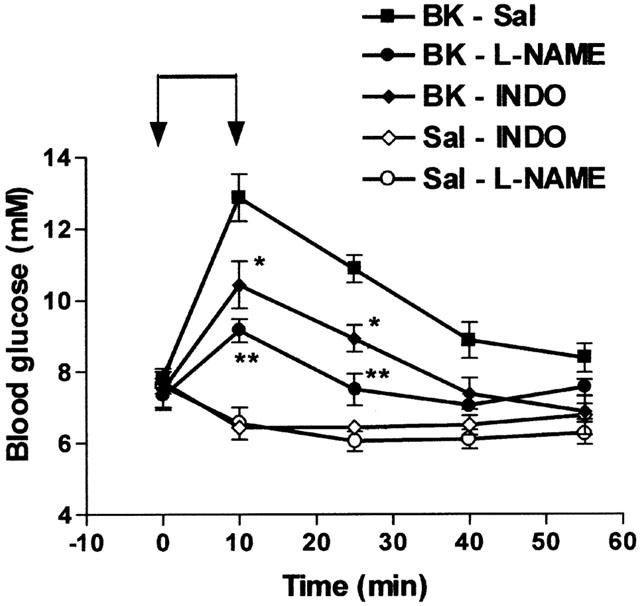
Influence of L-NAME or of indomethacin on the hyperglycaemic effect of BK. Saline (Sal) or BK (70 nmol kg−1min−1) was infused for 10 min from time 0 – 10 (indicated by the arrows) in rats previously treated with saline (Sal) indomethacin (3 mg kg−1) or L-NAME (100 mg kg−1). Results show the changes in blood glucose levels as a function of time: mean±s.e.mean of six values for each point. *P<0.05, **P<0.01.
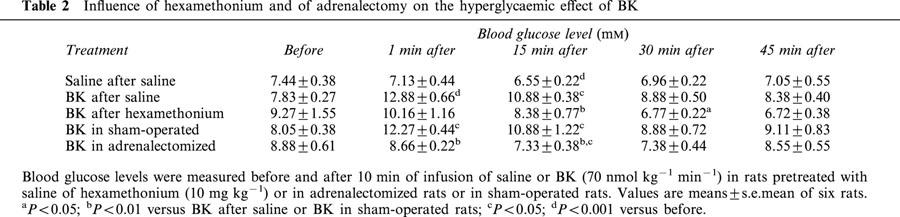
Similarly, the hyperglycaemic effect of BK was reduced by propranolol (2 mg kg−1 i.v.), a beta-adrenoceptor antagonist, and suppressed by the combination of propranolol with phentolamine (2 mg kg−1 i.v., Figure 5) or with phenoxybenzamine (10 mg kg−1 i.v., Figure 6), two alpha-adrenoceptor antagonists. The effect of BK was also suppressed by hexamethonium, a ganglion-blocking agent (10 mg kg−1 i.v.) (Table 2). On the other hand, adrenalectomy completely prevented the hyperglycaemic effect of BK. Indeed, BK slightly but significantly reduced blood glucose levels in adrenalectomized rats (Table 2).
Figure 5.
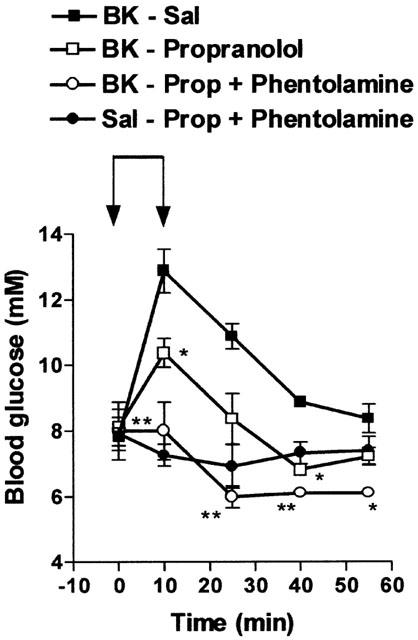
Influence of propranolol and phentolamine on the hyperglycaemic effect of BK. Saline (Sal) or BK (70 nmol kg−1 min−1) was infused for 10 min from time 0 – 10 (indicated by the arrows) in rats previously treated with saline (Sal), propranolol (2 mg kg−1) or propranolol (prop) plus phentolamine (2 mg kg−1). Results show the changes in blood glucose levels as a function of time: mean±s.e.mean of six values for each point. *P<0.05, **P<0.01.
Figure 6.
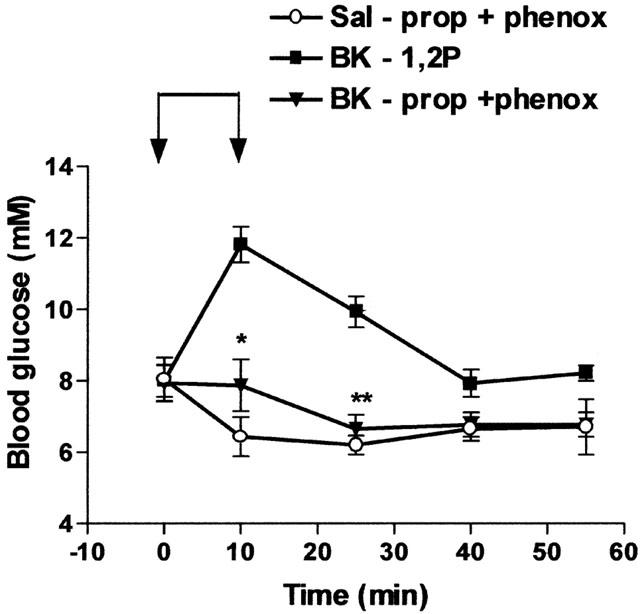
Influence of propranolol and phenoxybenzamine on the hyperglycaemic effect of BK. Saline (Sal) or BK (70 nmol kg−1 min−1) was infused for 10 min from time 0 – 10 (indicated by the arrows) in rats previously treated with 1,2-propanediol (1,2P) or propranolol (prop, 2 mg kg−1) plus phenoxybenzamine (phenox, 10 mg kg−1). Results show the changes in blood glucose levels as a function of time: mean±s.e.mean of six values for each point. *P<0.05, **P<0.01.
Table 2.
Influence of hexamethonium and of adrenalectomy on the hyperglycaemic effect of BK
Changes in blood glucose levels induced by adrenaline
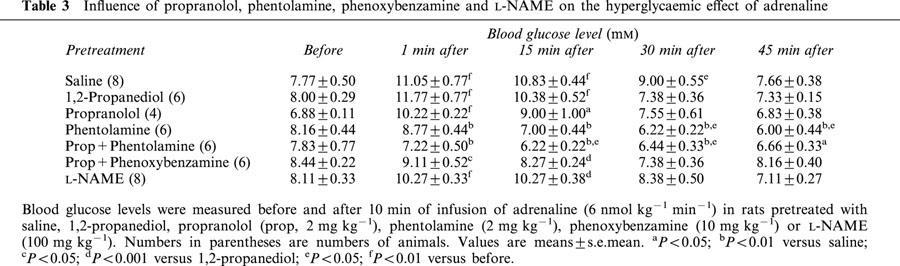
As the effect of BK on blood glucose was suppressed by adrenoceptor antagonists, the influence of these drugs on the hyperglycaemic effect of adrenaline was evaluated. Adrenaline was infused for 10 min at a dose of 6 nmol kg−1 min−1 and blood glucose levels were measured before and during 45 min after this infusion. Results are shown Table 3. At this dose, adrenaline increased blood glucose levels during a period of 30 min following its intravenous infusion. Phentolamine (2 mg kg−1), injected alone before adrenaline, had a strong inhibitory influence on the peak increase in glucose induced by adrenaline. Propranolol (2 mg kg−1) administered alone accelerated the return to the baseline. Phentolamine and propranolol in association suppressed the hyperglycaemic effect of adrenaline. In the same way, the effect of adrenaline was inhibited by phenoxybenzamine (10 mg kg−1) and propranolol in association. However, the hyperglycaemic effect of adrenaline was not significantly modified by L-NAME (100 mg kg−1) (Table 3).
Table 3.
Influence of propranolol, phentolamine, phenoxybenzamine and L-NAME on the hyperglycaemic effect of adrenaline
Release of insulin by bradykinin
Assay of insulin was initially performed on plasma samples withdrawn at the end of the period of observation: 45 min after bradykinin infusion. No significant changes in plasma insulin levels were observed in the different groups of animals treated by the various doses of BK with or without the different pharmacological agents (results not shown). Thus, in another series of experiments, rats were infused with bradykinin or saline as previously described but the blood was withdrawn just at the end of the infusion. In these animals, blood glucose levels were also measured before and after the infusion. Results are shown Table 4.
Table 4.
Changes in plasma insulin levels induced by BK
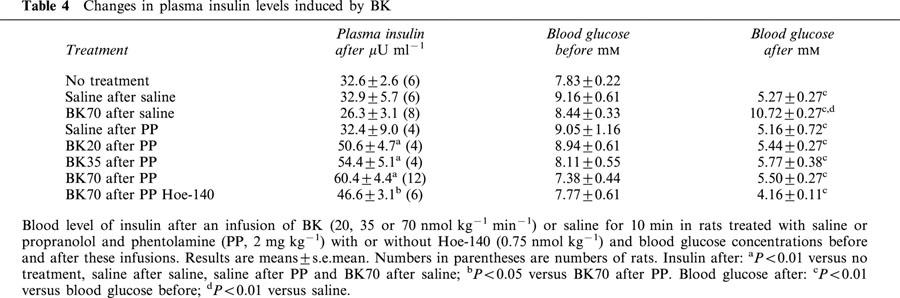
Infusion of saline, in normal rats or in rats treated with phentolamine and propranolol, induced a decrease in blood glucose levels as previously reported while plasma insulin levels were, at the end of this infusion, similar to the level observed after anesthesia. In normal rats, BK induced an increase in blood glucose as previously described but did not significantly modify insulin levels. However, in rats treated with propranolol and phentolamine (2 mg kg−1), plasma insulin levels were significantly increased by BK while blood glucose levels were decreased (Table 4). The increase in plasma insulin levels induced by BK in animals pretreated with propranolol and phentolamine was dose-dependent; furthermore, it was inhibited by Hoe-140 (0.75 nmol kg−1) (Table 4).
Discussion
Bolus intravenous injection of BK did not modify blood glucose levels while intravenous infusion of BK induced a dose-dependent hyperglycaemia. The hyperglycaemic effect of BK was suppressed by adrenalectomy. The rapid development of this hyperglycaemia suggests that adrenal steroids are not involved in this effect as the influence of glucocorticoids on plasma level of glucose takes a long time to become apparent (Stojanovska et al., 1990). The effect of BK was inhibited by the pretreatment of the animals with adrenoceptor antagonists, mainly by alpha-adrenoceptor antagonists. It thus depends on a release of catecholamines from the adrenal medulla.
The stimulation of the adrenal medulla by BK has been already described in the 1960s (Lecomte et al., 1961; Feldberg & Lewis, 1964; Staszewska-Barczak & Vane, 1967). This stimulation explains the secondary pressor response to intravenous injection of BK (Lecomte et al., 1964; Lang & Pearson, 1968) and participates in the haemodynamic changes induced by this peptide (Gardiner et al., 1992). To obtain an increase in blood glucose, our results show that the stimulation of the adrenal medulla by BK must be protracted while the cardiovascular repercussions of this stimulation are elicited by a bolus injection of BK (Lecomte et al., 1964). In other words, the amount of catecholamines necessary to induce an increase in blood glucose seems to be higher than the amount eliciting cardiovascular modifications. Our results probably explain why subcutaneous injection of BK had no effect on plasma glucose concentrations in normal rats (Henriksen et al., 1998).
Previous studies have shown that BK can influence catecholamine release from the adrenal medulla in vivo by direct and indirect nervous mechanisms (Terragno & Terragno, 1979). Though the secondary catecholamine-mediated hypertension induced by bolus injection of BK was not modified by hexamethonium (Damas, 1972), this ganglion-blocking agent largely reduced the hyperglycaemic effect of BK. This result indicates that infusion of BK mainly stimulates the adrenal medulla through a reflex pathway. The excitation of this reflex pathway depends on the stimulation of B2 receptors by BK, as the hyperglycaemic effect of BK was suppressed by Hoe-140, a B2 receptor antagonist (Wirth et al., 1991), and as Des-arg9-BK, a B1 receptor agonist (Marceau, 1995), was without influence on blood glucose concentration. It should be added that though the infusion of BK mainly activates catecholamine secretion from the adrenal medulla through an indirect mechanism, BK can also directly stimulate catecholamine release by the stimulation of B2 receptors (Dendorfer et al., 1996). As the hyperglycaemic effect of BK but not that of adrenaline was inhibited by L-NAME, NO would be involved in the excitation of the indirect mechanism causing the release of catecholamines.
Several mechanisms could explain the indirect stimulation of the adrenal medulla by BK (Clark, 1979). This stimulation might be baroreflex-mediated. In several conditions and vascular tissues, BK is able to release nitric oxide and prostanoids which amplify or mediate the vascular effects of the peptide (Bhoola et al., 1992). Indeed, it has been observed that indomethacin and L-NAME reduce the extent of the hypotension induced by BK (Damas & Deby, 1974; Damas, 1977; Nasjletti & Malik, 1979; Rees et al., 1990; Gardiner et al., 1990; O'shaughnessy et al., 1992; Bjornstad-Ostensen & Berg, 1994). Similarly, indomethacin and L-NAME reduce the hyperglycaemic effect of BK. Other mechanisms could be simultaneously involved in the indirect stimulation of the sympathetic system by BK such as the excitation of paravascular pain receptors by this peptide (Clark, 1979; Bachelard et al., 1992). This stimulation is largely reduced by cyclo-oxygenase inhibitors (Ferreira et al., 1973; Juan, 1978).
BK infused into anaesthetized rats increased blood glucose levels without significantly affecting plasma insulin levels. However, when the adrenoceptors were inhibited by propranolol and phentolamine, BK induced a small decrease in blood glucose levels associated with an increase in plasma insulin. The stimulation of insulin release by BK was thus inhibited in normal rats by the effects of catecholamines. In the presence of adrenoceptor antagonists, the release of insulin was dose-dependent and blocked by Hoe-140. This releasing effect of BK thus involves an excitation of B2 receptors, as it has been observed in vitro (Yang & Hsu, 1995; Saito et al., 1996; Yang et al., 1997a). Direct addition of BK into the perfusate of rat pancreas transiently induced a 3 fold increase in the release of insulin for a few minutes followed by a sustained smaller increase in insulin secretion (Yang et al., 1997a; Yang & Hsu, 1997). Similarly, in our experiments performed in vivo, infusion of BK transiently induced a 2 fold increase in plasma insulin levels. The effects of BK on rat pancreas seem to be complex: BK has been reported to enhance insulin and glucagon secretion but to inhibit somatostatin release when directly added into the perfusate of the isolated rat pancreas (Yang et al., 1997a; Abu-Basha et al., 2000). However, the release of insulin appears to predominate and to be more sustained (Yang et al., 1997a). Indeed, in vivo, blood glucose levels decreased after BK infusion in adrenalectomized rats.
In conclusion, BK stimulates insulin release in vivo when the effects of catecholamines are inhibited. The significance of this effect of BK will be the subject of other studies.
Acknowledgments
We thank Dr K.J. Wirth from Hoechst-Marion-Roussel for the kind supply of Hoe-140 and Mrs A. Rombaux and Mr V. Bourdon for their excellent technical help.
Abbreviations
- ACE
angiotensin-converting enzyme
- BK
bradykinin
- Cap
captopril
- DABK
Des-Arg9-bradykinin
- Indo
indomethacine
- L-NAME
Nω-nitro-L-arginine methyl ester
- 1,2P
1,2-propanediol
- Phenox
phenoxybenzamine
- PP
propranolol and phentolamine
- prop
propranolol
- Sal
saline
References
- ABU-BASHA E.A., MAKOWSKI J.P., YIBCHOK-ANUN S., HSU W.H. Stimulatory effect of bradykinin (BK) on glucagon secretion from the perfused rat pancreas: involvement of BK2 receptors. Metabolism. 2000;49:1370–1373. doi: 10.1053/meta.2000.9511. [DOI] [PubMed] [Google Scholar]
- BACHELARD H., GARDINER S.M., KEMP P.A., BENNETT T. Involvement of capsaicin-sensitive neurones in the haemodynamic effects of exogenous vasoactive peptides: studies in conscious, adult Long Evans rats treated neonatally with capsaicin. Br. J. Pharmacol. 1992;105:202–210. doi: 10.1111/j.1476-5381.1992.tb14235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHOOLA K.D., FIGUEROA C.D., WORTHY K. Bioregulation of kinins: kallikreins, kininogens and kininases. Pharmacol. Rev. 1992;44:1–80. [PubMed] [Google Scholar]
- BJORNSTAD-OSTENSEN A., BERG T. The role of nitric oxide, adrenergic activation and kinin-degradation in blood pressure homeostasis following an acute kinin-induced hypotension. Br. J. Pharmacol. 1994;113:1567–1573. doi: 10.1111/j.1476-5381.1994.tb17175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK W.G.Kinins and the peripheral and central nervous systems Handbook of Experimental Pharmacology Bradykinin, Kallidin and Kallikrein 1979Berlin: Springer-Verlag; 311–356.Vol 25 suppled. Erdös E. pp [Google Scholar]
- DAMAS J. Rôle des ions calcium lors de la stimulation par la bradykinine de la médullo-surrénale du rat. Arch. Int. Physiol. Biochim. 1972;80:511–520. doi: 10.3109/13813457209075246. [DOI] [PubMed] [Google Scholar]
- DAMAS J., DEBY C. Libération de prostaglandines par la bradykinine chez le Rat. C. R. Soc. Biol. 1974;168:375–378. [PubMed] [Google Scholar]
- DAMAS J. Inhibition du pouvoir prostaglandino-libérateur de la bradykinine chez le rat. C. R. Soc. Biol. 1977;171:685–689. [PubMed] [Google Scholar]
- DAMAS J., BOURDON V., LEFEBVRE P.J. Insulin sensitivity, clearance and release in kininogen-deficient rats. Exp. Physiol. 1999;84:549–557. doi: 10.1111/j.1469-445x.1999.01812.x. [DOI] [PubMed] [Google Scholar]
- DENDORFER A., HÄUSER W., FALIAS D., DOMINIAK P. Bradykinin increases catecholamine release via B2 receptors. Pflügers Arch. 1996;432 Suppl.:R99–R106. [PubMed] [Google Scholar]
- DIETZE G., WICKLMAYR M. Evidence for a participation of the kallikrein-kinin system in the regulation of muscle metabolism during muscular work. FEBS Lett. 1977;74:205–208. doi: 10.1016/0014-5793(77)80847-8. [DOI] [PubMed] [Google Scholar]
- ERLICH Y., ROSENTHAL T. Contribution of bradykinin to the beneficial effects of ramipril in the fructose-fed rat. J. Cardiovasc. Pharmacol. 1998;31:581–584. doi: 10.1097/00005344-199804000-00017. [DOI] [PubMed] [Google Scholar]
- FELDBERG W., LEWIS G.P. The action of peptides on the adrenal medulla. Release of adrenaline by bradykinin and angiotensin. J. Physiol. 1964;171:98–108. doi: 10.1113/jphysiol.1964.sp007364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERREIRA S.H, MONCADA S., VANE J.R. Prostaglandins and the mechanism of analgesia produced by aspirin-like drugs. Br. J. Pharmacol. 1973;49:86–97. doi: 10.1111/j.1476-5381.1973.tb08270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., COMPTON A.M., KEMP P.A., BENNETT T. Regional and cardiac haemodynamic responses to glyceryl trinitrate, acetylcholine, bradykinin and endothelin-1 in conscious rats: effects of NG-nitro-L-arginine methyl ester. Br. J. Pharmacol. 1990;101:632–639. doi: 10.1111/j.1476-5381.1990.tb14132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., KEMP P.A., BENNETT T., BOSE C., FOULKES R., HUGHES B. Involvement of β2-adrenoceptors in the regional haemodynamic responses to bradykinin in conscious rats. Br. J. Pharmacol. 1992;105:839–848. doi: 10.1111/j.1476-5381.1992.tb09066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENRIKSEN E.J., JACOB S., FOGT D.L., DIETZE G.J. Effect of chronic bradykinin administration on insulin action in an animal model of insulin resistance. Am. J. Physiol. 1998;275:R40–R45. doi: 10.1152/ajpregu.1998.275.1.R40. [DOI] [PubMed] [Google Scholar]
- HENRIKSEN E.J., JACOB S., KINNICK T.R., YOUNGBLOOD E.B., SCHMIT M.B., DIETZE G.J. ACE inhibition and glucose transport in insulin-resistant muscle: role of bradykinin and nitric oxide. Am. J. Physiol. 1999;277:R332–R336. doi: 10.1152/ajpregu.1999.277.1.R332. [DOI] [PubMed] [Google Scholar]
- JUAN H. Prostaglandins as modulators of pain. Gen. Pharmacol. 1978;9:403–409. doi: 10.1016/0306-3623(78)90025-3. [DOI] [PubMed] [Google Scholar]
- KOHLMAN O., JR, NEVES F.A.R., GINOZA M., TAVARES A., CEZARETTI M.L., ZANELLA M.T., RIBEIRO A.B., GAVRAS I., GAVRAS H. Role of bradykinin in insulin sensitivity and blood pressure regulation during hyperinsulinemia. Hypertension. 1995;25:1003–1007. doi: 10.1161/01.hyp.25.5.1003. [DOI] [PubMed] [Google Scholar]
- LANG W.J., PEARSON L. Studies on the pressor responses produced by bradykinin and kallidin. Br. J. Pharmacol. 1968;32:330–338. doi: 10.1111/j.1476-5381.1968.tb00976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LECOMTE J., TROQUET J., CESSION-FOSSION A. Sur la nature de l'hypertension artérielle provoquée par la bradykinine et la kallidine. Arch. Int. Pharmacodyn. Ther. 1964;147:518–524. [Google Scholar]
- LECOMTE J., TROQUET J., DRESSE A. Stimulation médullo-surrénalienne par la bradykinine. Arch. Int. Physiol. Biochim. 1961;69:89–91. doi: 10.3109/13813456109092781. [DOI] [PubMed] [Google Scholar]
- MARCEAU F. Kinin B1 receptors: a review. Immunopharmacology. 1995;30:1–26. doi: 10.1016/0162-3109(95)00011-h. [DOI] [PubMed] [Google Scholar]
- NASJLETTI A., MALIK K.U. Relationships between the kallikrein-kinin and prostaglandin systems. Life Sci. 1979;25:99–109. doi: 10.1016/0024-3205(79)90380-1. [DOI] [PubMed] [Google Scholar]
- O'SHAUGHNESSY K.M., NEWMAN C.M., WARREN J.B. Inhibition in the rat of nitric oxide synthesis in vivo does not attenuate the hypotensive action of acetylcholine, ATP or bradykinin. Exp. Physiol. 1992;77:285–292. doi: 10.1113/expphysiol.1992.sp003588. [DOI] [PubMed] [Google Scholar]
- QUABBE H.J. Modification der radioimmunologischen Insulinbestimmung nach Hales und Randle. Diabetologia. 1969;5:101–107. doi: 10.1007/BF01212004. [DOI] [PubMed] [Google Scholar]
- RAO R.H. Changes in insulin sensitivity from stress during repetitive sampling in anaesthetized rats. Am. J. Physiol. 1992;262:R1033–R1039. doi: 10.1152/ajpregu.1992.262.6.R1033. [DOI] [PubMed] [Google Scholar]
- REES D.D., PALMER R.M., SCHULZ R., HODSON H.F., MONCADA S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br. J. Pharmacol. 1990;101:746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO Y., KATO M., KUBOHARA Y., KOBAYASHI I., TATEMOTO K. Bradykinin increases intracellular free Ca2+ concentration and promotes insulin secretion in the clonal β-cell line, HIT-T15. Biochem. Biophys. Res. Commun. 1996;221:577–580. doi: 10.1006/bbrc.1996.0638. [DOI] [PubMed] [Google Scholar]
- STASZEWSKA-BARCZAK J., VANE J.R. The release of catecholamines from the adrenal-medulla by peptides. Br. J. Pharmacol. 1967;30:655–667. doi: 10.1111/j.1476-5381.1967.tb02172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOJANOVSKA L., ROSELLA G., PROIETTO J. Evolution of dexamethasone-induced insulin resistance in rats. Am. J. Physiol. 1990;258:E748–E756. doi: 10.1152/ajpendo.1990.258.5.E748. [DOI] [PubMed] [Google Scholar]
- TERRAGNO N.A., TERRAGNO A.Release of vasoactive substances by kinins Handbook of experimental Pharmacology Bradykinin, Kallidin and Kallikrein 1979Berlin: Springer-Verlag; 401–426.Vol 25 suppl.ed. Erdös E. pp [Google Scholar]
- TOMIYAMA H., KUSHIRO T., ABETA H., TAKAHASHI A., FURUKAWA L., ASAGAMI T., HINO T., SAITO F., OTSUKA Y., KURUMATANI H., KOBAYASHI F., KANMATSUSE K., KAJIWARA N. Kinins contribute to the improvement of insulin sensitivity during treatment with angiotensin converting enzyme inhibitor. Hypertension. 1994;23:450–455. doi: 10.1161/01.hyp.23.4.450. [DOI] [PubMed] [Google Scholar]
- WICKLMAYR M., DIETZE G., GÜNTHER B., SCHIFMANN R., BÖTTGER I., GEIGER R., FRITZ H., MEHNERT H. The kallikrein-kinin system and muscle metabolism–Clinical aspects. Agents Actions. 1980;10:339–343. doi: 10.1007/BF01971436. [DOI] [PubMed] [Google Scholar]
- WIRTH K.J., HOCK F.J., ALBUS U., LINZ W., ALPERMANN H.G., ANAGNOSTOPOULOS H., HENKE S., BREIPOHL G., KONIG W., KNOLLE J., SCHOLKENS B.A. HOE 140 is a new potent and long acting bradykinin-antagonist. Br. J. Pharmacol. 1991;102:774–777. doi: 10.1111/j.1476-5381.1991.tb12249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG C., HSU W.H. Stimulatory effect of bradykinin on insulin release from the perfused rat pancreas. Am. J. Physiol. 1995;268:E1027–E1030. doi: 10.1152/ajpendo.1995.268.5.E1027. [DOI] [PubMed] [Google Scholar]
- YANG C., HSU W.H. Glucose-dependency of bradykinin-induced insulin secretion from the perfused rat pancreas. Regul. Pept. 1997;71:23–28. doi: 10.1016/s0167-0115(97)01011-2. [DOI] [PubMed] [Google Scholar]
- YANG C., CHAO J., HSU W.H. The effect of bradykinin on secretion of insulin, glucagon, and somatostatin from the perfused rat pancreas. Metabolism. 1997a;46:1113–1115. doi: 10.1016/s0026-0495(97)90201-8. [DOI] [PubMed] [Google Scholar]
- YANG C., LEE B., CHEN T.H., HSU W.H. Mechanisms of bradykinin-induced insulin secretion in clonal beta cell line RINm5F. J. Pharmacol. Exp. Ther. 1997b;282:1247–1252. [PubMed] [Google Scholar]


