Abstract
Panax ginseng is used to enhance stamina and relieve fatigue as well as physical stress. Ginsenoside, the effective component of ginseng, regulates cardiovascular function. This study was to examine the effect of ginsenosides Rb1 and Re on cardiac contractile function at the cellular level. Ventricular myocytes were isolated from adult rat hearts and were stimulated to contract at 0.5 Hz. Contractile properties analysed included: peak shortening (PS), time-to-90%PS (TPS), time-to-90% relengthening (TR90), and fluorescence intensity change (ΔFFI). Nitric oxide synthase (NOS) activity was determined by the 3H-arginine to 3H-citrulline conversion assay.
Both Rb1 and Re exhibited dose-dependent (1 – 1000 nM) inhibition in PS and ΔFFI, with maximal inhibitions between 20 – 25%. Concurrent application Rb1 and Re did not produce any additive inhibition on peak shortening amplitude (with a maximal inhibition of 24.9±6.1%), compared to Rb1 or Re alone. Pretreatment with the NOS inhibitor Nω-nitro-L-arginine methyl ester (L-NAME, 100 μM) abolished the effect of Rb1 and Re. Both Rb1 and Re significantly (P<0.05) stimulated NOS activity concentration-dependently.
This study demonstrated a direct depressant action of ginsenosides on cardiomyocyte contraction, which may be mediated in part through increased NO production.
Keywords: Ginsenoside Rb1 and Re, myocyte shortening, intracellular Ca2+ transients, nitric oxide
Introduction
Panax ginseng is widely used in traditional Chinese medicine to enhance stamina and capacity to cope with fatigue as well as physical stress. Mounting evidence has indicated therapeutic potential of Panax ginseng on the CNS (memory, learning and behaviour), neuroendocrine function, carbohydrate and lipid metabolism, immune function, and the cardiovascular system (Wang & Ren, 1988; Chen, 1996; Gillis, 1997). The mechanisms of action of ginseng remain largely unclear. Nevertheless, the therapeutic effects of ginseng have been attributed to its active ingredients, ginsenosides, sugar conjugates of dihydroxyl or trihydroxyl dammarane triterpenes. Ginsenosides are normally fractioned into two groups based on the types of aglycone, namely the panaxadiol group (e.g., Rb1 and Rc) and panaxatriol group (e.g., Rg1 and Re) (Figure 1). Ginseng extracts rich in ginsenosides have been found to facilitate learning and memory, delay the ageing process (Petkov, 1990), prevent neuronal loss under hypoxia (Seong & Kim, 1997), improve the muscle oxygen utilization and physical performance in human (Pieralisi et al., 1991). Ginseng or ginsenosides has been shown to slow down the deterioration of cardiac contraction (Toh, 1994), protect against myocardial ischaemia/reperfusion damage (Chu & Chen, 1990), free radicals production (Chen, 1996), and development of arrhythmias (Toh, 1994). These results indicated that ginseng was able to delay cardiac mitochondrial impairment and muscle contraction deterioration.
Figure 1.
Chemical structures of ginsenoside Rb1 and Re.
Although the cardiac performance under chronic ginseng treatment appears to be improved, data of acute administration of ginseng or ginsenosides on cardiovascular function has, however, been controversial (Gillis, 1997). Whereas some studies reported enhanced cardiac function (Toh, 1994), others claimed little or depressed cardiac function (Jin, 1996; Jin & Liu, 1994; Guan et al., 1996; Chen et al., 1994). Ginsenoside Re was shown to markedly depress the P wave in ECG with no effect on P – R, QRS, and Q – T intervals or R wave (Jin & Liu, 1994). Ginsenosides were shown to inhibit papillary muscle contractility (Chen et al., 1994). Ginseng and ginsenoside have been reported to regulate membrane Ca2+ channel activity (Zhang et al., 1994) and stimulate nitric oxide (NO) production (Chen, 1996; Chen et al., 1997; Gillis, 1997), both of which play crucial roles in cardiovascular function. However, no evidence for a direct effect of ginsenoside on ventricular contractile function has been reported at the cellular level.
Ginseng is currently sold in the U.S. as a food additive and thus need not meet specific safety and efficacy requirements of the Food and Drug Administration. With the growing use of ginseng, it is important to know the direct cardiac effect of ginseng. To address this question, we examined the effect of ginsenoside Rb1 and Re on cell shortening, intracellular Ca2+ and nitric oxide synthase (NOS) activity in ventricular myocytes isolated from adult rat hearts.
Methods
Isolation of ventricular myocytes
The experimental procedures were approved by the animal investigation committee of the University of North Dakota. Single ventricular myocytes were isolated from adult male Sprague-Dawley rats (200 – 225 g) as described previously (Ren, 2000). Briefly, hearts were rapidly removed and perfused (at 37°C) with oxygenated (5% CO2-95% O2) Krebs-Henseleit bicarbonate (KHB) buffer (mM): NaCl 118, KCl 4.7, CaCl2 1.25, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25, N-[2-hydro-ethyl]-piperazine-N′-[2-ethanesulfonic acid] (HEPES) 10, glucose 11.1, pH 7.4. Hearts were subsequently perfused with a nominally Ca2+-free KHB buffer for 2 – 3 min followed by a 20 min perfusion with Ca2+-free KHB containing 223 U ml−1 collagenase (Worthington Biochemical Corp., Freehold, NJ, U.S.A.) and 0.1 mg ml−1 hyaluronidase (Sigma Chemical, St. Louis, MO, U.S.A.). After perfusion, the left ventricle was removed, minced and further digested with trypsin (Sigma) before being filtered through a nylon mesh (300 μm) and collected by centrifugation. Cells were initially washed with Ca2+-free KHB buffer to remove remnant enzyme and extracellular Ca2+ was added incrementally back to 1.25 mM.
Myocyte shortening and relengthening
Mechanical properties of ventricular myocytes were assessed by a video-based edge-detection system (IonOptix Corporation, Milton, MA, U.S.A.). Coverslips with cells attached were placed in a chamber mounted on the stage of an inverted microscope and superfused (at 30°C) with a buffer containing (in mM): NaCl 131, KCl 4, CaCl2 1, MgCl2 1, glucose 10, HEPES 10, at pH 7.4. The cells were field stimulated at a frequency of 0.5 Hz. The myocyte being studied was displayed on the computer monitor using an IonOptix MyoCam camera, which rapidly scans the image area at 120 Hz such that the amplitude and velocity of shortening/relengthening is recorded with good fidelity. The soft-edge software (IonOptix) was used to capture changes in cell length during shortening and relengthening. Cell shortening and re-lengthening were assessed using the following indices: peak shortening (PS), time-to-90% peak shortening (TPS) and time-to-90% re-lengthening (TR90), maximal velocities of shortening (+dL/dt) and re-lengthening(−dL/dt) (Ren, 2000).
Intracellular fluorescence measurement
Myocytes was loaded with fura-2/AM (0.5 μM) for 10 min and fluorescence measurements were recorded with a dual-excitation fluorescence photomultiplier system (Ionoptix Corp., Milton, MA, U.S.A.) as described (Ren, 2000). Myocytes were plated on glass cover slips on an Olympus X-70 inverted microscope and imaged through a Fluor×40 oil objective. Cells were exposed to light emitted by a 75 W lamp and passed through either a 360 or a 380 nm filter (bandwidths were±15 nm), while being stimulated to contract at 0.5 Hz. Fluorescence emissions were detected between 480 – 520 nm after first illuminating cells at 360 nm for 0.5 s then at 380 nm for the duration of the recording protocol (333 Hz sampling rate). The 360 nm excitation scan was repeated at the end of the protocol and qualitative changes in intracellular Ca2+ concentration ([Ca2+]i) were inferred from the ratio of the fluorescence intensity at two wavelengths. The average fura-2 dye leakage throughout the recording duration (20 s) as referred from the two 360 nm emission intensities is averaged at ∼5%. Cells will be discarded if dye leakage is >10%. Myocyte shortening were also simultaneously recorded from fura-2 loaded cells but were used for qualitative comparisons only, in order to avoid potential effects on contraction from intracellular Ca2+ buffering by fura-2. An IonOptix software (IonWizard) was used for data analysis where 3 – 5 traces will be averaged for each cell recording.
Nitric oxide assay
The activity of nitric oxide synthase (NOS) was evaluated by the 3H-arginine to 3H-citrulline conversion assay (Nickola et al., 2000). Briefly, plated ventricular myocytes were placed in HBSS medium (20 mM HEPES, 1% pen-strep, 0.1% BSA) for 20 min at 30°C before being replaced with HBSS containing 1 μCi ml−1 3H-arginine with Trasylol (0.2 KIU ml−1) and ginsenoside Rb1 or Re (Sigma, 1 – 1000 nM). The cells were then incubated for 15 min before the reaction was terminated with TRIS containing 5 mM cold arginine and 4 mM EDTA. The cells were lysed with 20 mM TRIS, sonicated, harvested and diluted with 1 : 1 (v v−1) H2O/Dowex-50 W (20 – 50, 8% cross-linked). The mixture of cells and resin Dowex-50 W was then passed through a polypropylene EconoColumn (BioRad Laboratories, Inc., Hercules, CA, U.S.A.). The effluent was collected and 3H-citrulline was counted with scintillation buffer.
Data analysis
Data are presented as mean±s.e.mean Statistical significance (P<0.05) was estimated by analysis of variance (ANOVA) followed up by a Dunnett's post hoc analysis.
Results
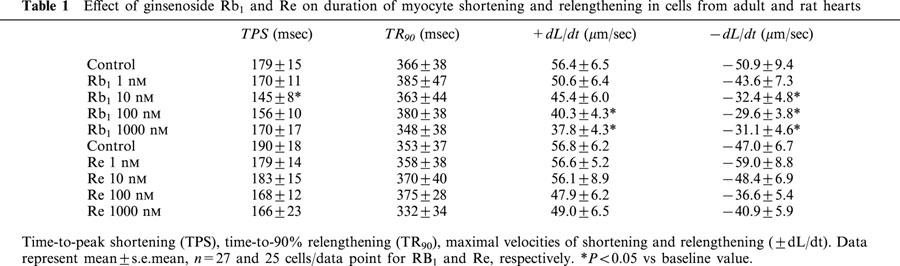
Effect of Rb1 and Re on myocyte shortening (PS)
The average cell length used in this study was 142.1±3.3 μm (n=114). Acute exposure of Rb1 and Re did not affect resting myocyte cell length over the range of concentrations tested. A representative trace depicting the effect of Rb1 and Re (100 nM) on myocyte shortening is shown in Figure 2A. At the end of a 15-min exposure to this concentration of Rb1 and Re, percentage myocyte shortening (PS) was decreased by 24.1 and 26.4%, respectively. Rb1 and Re exhibited little effect on duration of shortening (TPS) and relengthening (TR90) (Table 1). Rb1 and Re (1 – 1000 nM) elicited a concentration-dependent depression of PS, with a maximal inhibition of 25.6 and 25.7% respectively. The concentration at which Rb1 and Re displayed 50% of the maximal response (EC50) was 5.1±2.1 and 5.6±2.6 nM, respectively (P>0.05, Figure 2B). In a separate experiment, concurrent application of both Rb1 and Re elicited little additive inhibition on PS compared to Rb1 or Re alone. The inhibition elicited by concurrent administration of Rb1 and Re was 24.9±6.1 and 22.3±7.2%, for ginsenosides at 100 and 100 nM respectively (P<0.05 vs baseline, n=12 cells). The depressive effect of Rb1 and Re on cell shortening was maximal within 8 min of exposure and was reversible upon washout (data not shown). The inhibitory effect of Rb1 was associated with depressed maximal velocities of shortening or relengthening (±dL/dt) with little response on the duration of shortening (TPS) and relengthening (TR90) (with exception of TPS at 10 nM). Neither±dL/dt nor TPS/TR90 was affected by Re (Table 1).
Figure 2.
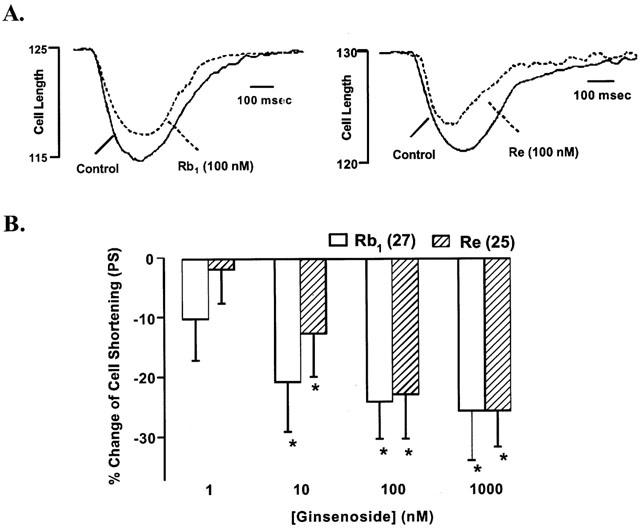
(A) Typical experiment showing the effect of ginsenoside Rb1 and Re on myocyte shortening in ventricular myocyte isolated from adult male rat hearts. Myocyte shortening and relengthening were recorded at 25°C before and 10 min after ginsenoside Rb1 and Re (100 nM) administration. (B) Concentration-dependent response to ginsenoside Rb1 and Re (1 – 1000 nM) on myocyte shortening. The basal PS is 5.22±0.58 and 5.26±0.71% of the cell length in Rb1 and Re group, respectively. Mean±s.e.mean, n=25 – 27/data group. *P<0.05 vs baseline value.
Table 1.
Effect of ginsenoside Rb1 and Re on duration of myocyte shortening and relengthening in cells from adult and rat hearts
Effect of Rb1 and Re on intracellular Ca2+ transients
To determine whether Rb1 and Re-induced inhibition of myocyte shortening was due to reduced availability of intracellular free Ca2+, [Ca2+]i in response to electrical stimuli in the presence of various concentrations of Rb1 and Re was examined. Representative traces of intracellular Ca2+ transients shown in Figure 3A depicts that 100 nM Rb1 and Re decreased ΔFFI by 25.9 and 24.1%, respectively. Both Rb1 and Re elicited concentration-dependent inhibitions of ΔFFI, with a maximal inhibition of 20.0 and 25.4%, respectively (Figure 3). The EC50 of the inhibitory response of Rb1 and Re on ΔFFI was 2.6±1.1 and 3.5±1.5 nM, respectively (P>0.05). The inhibitory response of ΔFFI suggests that a decrease in intracellular free Ca2+ is likely to be responsible for Rb1 and Re-induced depressive action on myocyte shortening. Neither the resting intracellular Ca2+ (360 : 380 ratio: 0.90±0.02, n=18) nor the fluorescence decay time (398±47 msec, n=18) was affected either ginsenoside (data not shown).
Figure 3.

Effect of ginseno side Rb1 and Re on intracellular Ca2+ transient changes (Δ[Ca2+]i) in ventricular myocytes. Top panel: typical experiment showing the effect of ginsenoside Rb1 and Re (100 nM) on intracellular Ca2+ transients. Solid and dashed traces show Ca2+ transients recorded from a fura-2 loaded myocyte before and 10 min after ginsenoside Rb1 and Re exposure; Lower panel: concentration-dependent response of ginsenoside Rb1 and Re (1 – 1000 nM) on intracellular Ca2+ transient changes (ΔFFI) in ventricular myocytes. Δ[Ca2+]i is expressed as the per cent change of respective baseline value. Mean±s.e.mean, n=17 – 18/data group, *P<0.05 vs baseline value.
Effect of Rb1 and Re on myocyte shortening in the presence of NOS inhibitor L-NAME
Rb1 and Re has been shown to increase nitric oxide (NO) accumulation, an important regulator in the cardiovascular system (Chen, 1996; Chen et al., 1997; Gillis, 1997). Constitutive NOS (cNOS) and inducible NOS (iNOS) are both present in cardiac myocytes (Kelly et al., 1996; Kanai et al., 1997). To examine the potential mechanism of action for ginsenosides, the effect of Rb1 and Re on myocyte shortening was re-examined in the presence of the NOS inhibitor, L-NAME (100 μM). This concentration of L-NAME has been shown to effectively inhibit the NOS activity (Brady et al., 1992; Nickola et al., 2000) and itself alone had no effect on cell shortening over 30 min (Nickola et al., 2000). As shown in Figure 4A, both Rb1 and Re-induced decrease in PS was completely abolished by L-NAME. These data suggest that Rb1 and Re may exert its inhibition on cardiac contraction, at least in part, through NO production.
Figure 4.
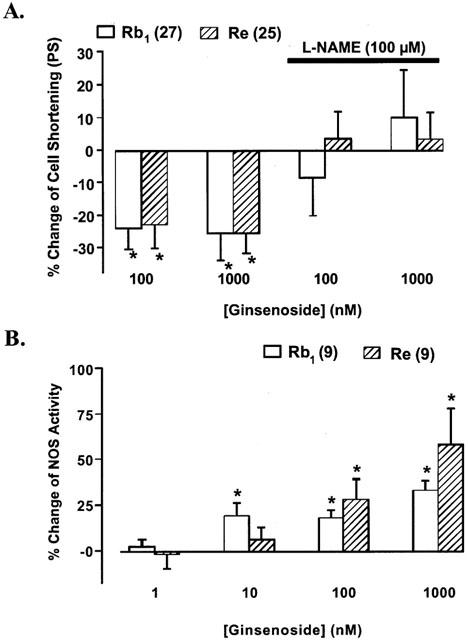
(A) Effect of NOS inhibitor L-NAME (100 μM) on ginsenoside Rb1 and Re-induced action on cardiac myocyte shortening. Bar graphs exhibiting the response of ginsenoside Rb1 and Re in the absence of L-NAME are redrawn from data shown in Figure 2. n=25 – 27/group for cells not pretreated with L-NAME and 25 per group for cells pretreated with L-NAME. (B) Concentration-dependent response of ginsenoside Rb1 and Re (1 – 1000 nM) on NOS activity (arginine – citrulline conversion) in myocytes from adult rat hearts. All the c.p.m. (3H-citrulline) counts were normalized to respective control (without Rb1 or Re) to reduce inter-assay variation such as total radioactivity loaded. n=9. Mean±s.e.mean, *P<0.05 vs baseline value.
Effect of Rb1 and Re on NOS activity
To further ensure the potential involvement of NO in Rb1 and Re-induced cardiac contractile action, the effect of Rb1 and Re on NOS activity was measured directly. Data presented in Figure 4B indicate that Rb1 and Re elicited a concentration-dependent increase in NOS activity in isolated ventricular myocytes.
Discussion
Our study demonstrated that ginsenoside Rb1 and Re depressed ventricular myocyte shortening and intracellular Ca2+ transients in isolated cardiac myocytes. The ginsenosides-evoked cardiac depressive response was inhibited by L-NAME, suggesting a role of NO production in ginsenosides-induced response. The 3H-arginine to 3H-citrulline conversion assay revealed that both ginsenosides directly stimulated NOS activity. These observations suggested that ginsenosides Rb1 and Re depress cardiac myocyte contraction consistent with the observations at the multicellular level when ginsenoside was applied acutely (Chen et al., 1994).
Ginsenosides are deglycosylated by intestinal bacteria to active forms such as 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol (M1) after oral administration. The pharmacokinetics of ginsenosides suggests that the concentration range used in our in vitro study is consistent with that of plasma ginsenoside level after oral administration (Wang et al., 1999). In our study, both Rb1 and Re exerted inhibition on electrically-stimulated intracellular Ca2+ rise, consistent with a previous report (Guan et al., 1996), indicating that the ginsenosides depress myocyte contraction through inhibition of intracellular Ca2+ rise. The similarity in inhibition efficacy (such as maximal inhibition and EC50) between Rb1 and Re as well as the fact that concurrent administration of the two ginsenosides did not produce any additive inhibitory response indicate that Rb1 and Re may share similar machinery in their inhibitor response. Although the mechanism underneath the inhibitory response of ginsenosides is still not clear, the ability of ginsenosides to shorten the plateau of action potential indicates potential involvement of Ca2+ channel blockade (Jin & Liu, 1994). This was confirmed by single channel recording that ginsenoside decreased Ca2+ channel open time and open probability in isolated ventricular myocytes (Zhang et al., 1994; Zhong et al., 1995).
In this study, L-NAME abolished the depressant effect of both Rb1 and Re. L-NAME inhibits all forms of NOS, but inhibition of iNOS requires extensive hydrolysis and may not be achieved during the short incubation time utilized in these in vitro experiments. Thus, we expect greater selectivity towards the constitutive form of NOS present in cardiomyocytes (Kanai et al., 1997). The involvement of NO was further supported by the fact that both Rb1 and Re stimulate NOS activity in these cardiac myocytes. Recent studies have suggested that the antioxidant, organ-protective and cardiovascular actions of ginseng or ginsenosides are due to their ability to enhance NO synthesis in multiple cell types (Chen, 1996; Gillis, 1997), although reports regarding cardiac myocytes have not been seen. Cardiac myocytes constitutively express the endothelial isoform of NOS (eNOS) and ambient NO levels are known to regulate cardiac contractile function (Kelly et al., 1996; Kanai et al., 1997). Our previous study indicated that eNOS activity can be modified within 15 – 60 min which associated with contractile function change (Nickola et al., 2000). The role of iNOS induction, which usually takes a few hours to occur (Shah, 2000), in the pathogenesis of heart disease is unclear, but may account for the contractile dysfunction due to cytokines. NO has been reported to elicit a biphasic effect on cardiac contraction including potentiation at lower concentrations and attenuation at higher concentrations (Mery et al., 1993; Xu et al., 1998). This is paradoxically similar to the biphasic effect of ginsenoside on cardiac contraction, including a positive response with lower dose and a negative action with higher dose (Chen et al., 1994). The lack of biphasic response in our study is not clear but may be related to the absence of other ginsenosides that may oppose Rb1 and Re. Furthermore, both Rb1 and Re failed to alter NOS activity at a concentration (10 nM) where mechanical response was seen, suggesting potential involvement of other mechanisms (although technical factors such as NOS assay sensitivity should not be precluded).
In conclusion, our study demonstrates, for the first time, a direct cardiac depressive response of ginsenoside Rb1 and Re at the ventricular myocyte level, possibly through a NO-mediated mechanism. The cardiac depression associated the ginseng-induced NO-mediated vasodilatation (Kang et al., 1995; Chen, 1996) should favour a reduced after-load for the heart and benefit cardiac pump function. These results implicate the clinical value of ginseng in cardiovascular disease in patients with hypertension and heart failure (Jin, 1996; Sung et al., 2000). The exact nature of cardiac contractile effect of Panax ginseng is still far from clear. Future studies should focus on the cardiac excitation-contraction coupling and membrane ion channels with Rb1, Re and other purified ginsenosides. These approaches will be essential to lighten the cellular effect and pharmacological profiles of the ever-increasingly used Panax ginseng
Acknowledgments
This work was supported in part by grants from American Heart Association-Northland Affiliate (9960204Z), American Diabetes Association and University New Faculty Award. GIS was funded through North Dakota Experimental Program to Stimulate Competitive Research (EPSCoR).
Abbreviations
- ±dL/dt
maximal velocities of shortening/relengthening
- FFI
fura-2 fluorescence intensity, τ, fluorescence decay time
- NOS
nitric oxide synthase
- PS
peak shortening
- TPS
time-to-PS
- TR90
time-to-90% relengthening
References
- BRADY A.J., POOLE-WILSON P.A., HARDING S.E., WARREN J.B. Nitric oxide production within cardiac myocytes reduces their contractility in endotoxemia. Am. J. Physiol. Heart Circ. Physiol. 1992;263:H1963–H1966. doi: 10.1152/ajpheart.1992.263.6.H1963. [DOI] [PubMed] [Google Scholar]
- CHEN X. Cardiovascular protection by ginsenosides and their nitric oxide releasing action. Clin. Exp. Pharmacol. Physiol. 1996;23:728–732. doi: 10.1111/j.1440-1681.1996.tb01767.x. [DOI] [PubMed] [Google Scholar]
- CHEN X., SALWINSKI S., LEE T.J-F. Extracts of ginko biloba and ginsenosides exert cerebral vasorelaxation via a nitric oxide pathway. Clin. Exp. Pharmacol. Physiol. 1997;24:958–959. doi: 10.1111/j.1440-1681.1997.tb02727.x. [DOI] [PubMed] [Google Scholar]
- CHEN X., YANG S., CHEN L., MA X., CHEN Y., WANG L., SHU C. The effect of PQS and its monomer ginsenoside on heart. J. Trad. Chin. Med. 1994;19:617–621. [PubMed] [Google Scholar]
- CHU G.X., CHEN X. Anti-lipid peroxidation and protection of ginsenosides against ischemia and reperfusion injuries in rats. Acta. Pharmacol. Sin. 1990;11:119–123. [PubMed] [Google Scholar]
- GILLIS C.N. Panax ginseng pharmacology: A nitric oxide link. Biochem. Pharmacol. 1997;54:1–8. doi: 10.1016/s0006-2952(97)00193-7. [DOI] [PubMed] [Google Scholar]
- GUAN Y.Y., MIAO L.Y., SUN J.J. Effects of Rb1 from the Panax notoginseng saponins on arrhythmia and Ca2+ movement in rat heart cells. Drug Dev. Res. 1996;39:179–183. [Google Scholar]
- JIN Z.Q. The action of ginsenoside Re on inotropy and chronotropy of isolated atria prepared from guinea pigs. Planta. Medica. 1996;62:314–316. doi: 10.1055/s-2006-957891. [DOI] [PubMed] [Google Scholar]
- JIN Z.Q., LIU C.M. Effect of Ginsenoside Re of the Electrophysiological Activity of the Heart. Planta. Medica. 1994;60:192–193. [PubMed] [Google Scholar]
- KANAI A.J., MESAROS S., FINKEL M.S., ODDIS C.V., BIRDER L.A., MALINSKI T. β-Adrenergic regulation of constitutive nitric oxide synthase in cardiac myocytes. Am. J. Physiol. 1997;273:C1371–C1377. doi: 10.1152/ajpcell.1997.273.4.C1371. [DOI] [PubMed] [Google Scholar]
- KANG S.Y., SCHINI-KERTH V.B., KIM N.D. Ginsenosides of the protopanaxatriol group cause endothelium-dependent relaxation in rat aorta. Life Sci. 1995;56:1577–1586. doi: 10.1016/0024-3205(95)00124-o. [DOI] [PubMed] [Google Scholar]
- KELLY R.A., BALLIGAND J.L., SMITH T.W. Nitric oxide and cardiac function. Circ. Res. 1996;79:363–380. doi: 10.1161/01.res.79.3.363. [DOI] [PubMed] [Google Scholar]
- MERY P.F., PAVOINE C., BELHASSEN L., PECKER F., FISCHEISTER R. Nitric oxide regulates cardiac Ca current: involvement of cGMP-inhibited and cGMP-stimulated phosphodiesterases through guanyly cyclase activation. J. Biol. Chem. 1993;268:26286–26295. [PubMed] [Google Scholar]
- NICKOLA M.W., WOLD L.E., COLLIGAN P.B., WANG G.J., SAMSON W.K., REN J. Leptin attenuates cardiac contraction in adult rat ventricular myocytes: Role of nitric oxide. Hypertension. 2000;36:501–505. doi: 10.1161/01.hyp.36.4.501. [DOI] [PubMed] [Google Scholar]
- PETKOV V.Ginseng as a remedy regulating aging process in brain (Experiments on rats) Recent advances in ginseng studies 1990Hirokawa Publishing Company, Tokyo; 83–98.ed: Shibata S, Ohtsuka Y, Saito H. pp [Google Scholar]
- PIERALISI G., RIPARI P., VECCHIET L. Effect of a standardized ginseng extract combined with dimethylaminoethanol bitartrate, vitamins, minerals, and trace elements on physical performance during exercise. Clin. Therapeutics. 1991;13:373–382. [PubMed] [Google Scholar]
- REN J. Attenuated cardiac contractile responsiveness to insulin-like growth factor I in ventricular myocytes from biobreeding spontaneous diabetic rats. Cardiovasc. Res. 2000;46:162–171. doi: 10.1016/s0008-6363(00)00011-0. [DOI] [PubMed] [Google Scholar]
- SEONG Y.H., KIM H.S. Inhibitory effects of ginseng total saponins on hypoxia-induced dysfunction and injuries of cultured astrocytes. Arch. Pharm. Res. 1997;2:103–109. doi: 10.1007/BF02973995. [DOI] [PubMed] [Google Scholar]
- SHAH A.M. Inducible nitric oxide synthase and cardiovascular disease. Cardiovasc. Res. 2000;45:145–148. doi: 10.1016/s0008-6363(99)00316-8. [DOI] [PubMed] [Google Scholar]
- SUNG J., HAN K.H., ZO J.H., PARK H.J., KIM C.H., OH B.H. Effects of red ginseng upon vascular endothelial function in patients with essential hypertension. Am. J. Chin. Med. 2000;28:205–216. doi: 10.1142/S0192415X00000258. [DOI] [PubMed] [Google Scholar]
- TOH H.T. Improved isolated heart contractility and mitochondrial oxidation after chronic treatment with Panax ginseng in rats. Am. J. Chinese Med. 1994;22:275–284. doi: 10.1142/S0192415X94000334. [DOI] [PubMed] [Google Scholar]
- WANG H., ZOU H., KONG L., ZHANG Y., PANG H., SU C., LIU G., HUI M., FU L. Determination of ginsenoside Rg3 in plasma by solid-phase extraction and high-performance liquid chromatography for pharmacokinetic study. J. Chromatogr. B. Biomed. Sci. Appl. 1999;731:403–409. doi: 10.1016/s0378-4347(99)00238-8. [DOI] [PubMed] [Google Scholar]
- WANG Z.G., REN J.Pharmacology and toxicology of traditional Chinese medicines: a historical perspective Current Problems in Nutrition, Pharmacology and Toxicology 1988John Libbey & Company Limited Press, London; 44–49.Ed: McLean A & Wahlqvist ML pp [Google Scholar]
- XU L., EU J.P., MEISSNER G., STAMLER J.S. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-s-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- ZHANG W.J., ZHONG G.G., JIANG Y., WANG X.M., WANG Z.F. Single channel analysis on calcium channel blockade action of panaxadiol and panaxatriol saponins on cultured rat ventricular myocytes. Acta. Pharmacol. Sin. 1994;2:173–176. [PubMed] [Google Scholar]
- ZHONG G.G., SUN C.W., LI Y.Y., QI H., ZHAO C.Y., JIANG Y., WANG X.M., YANG S.J., LI H. Calcium channel blockade and anti-free-radical actions of panaxadiol saponins Rb1, Rb2, Rb3, Rc and Rd. Acta. Pharmacol. Sin. 1995;16:255–260. [PubMed] [Google Scholar]


