Abstract
This investigation reports the possible role of the endocannabinoid anandamide in modulating appetitive behaviour. Given that cannabinoids have been used clinically to stimulate appetite in HIV and cancer chemotherapy patients, there has been a renewed interest in the involvement of cannabinoids in appetite modulation. This is the first report on the administration of anandamide into the ventromedial hypothalamus. Pre-satiated rats received an intrahypothalamic injection of anandamide (50 ng 0.5 μl−1) followed by measurement of food intake at 3 h post injection. Administration of anandamide induced significant hyperphagia. Pretreatment with the selective CB1 cannabinoid antagonist SR 141716 (30 μg 0.5 μl−1), 30 min prior to anandamide injection resulted in an attenuation of the anandamide-induced hyperphagia (P<0.001). This study demonstrates that intrahypothalamic anandamide initiates appetite by stimulation of CB1 receptors, thus providing evidence on the involvement of hypothalamic endocannabinoids in appetite initiation.
Keywords: Anandamide, appetite, SR 141716
Introduction
Although research implicating the role of Anandamide (AEA), endogenous cannabinoid, in the stimulation and maintaining of appetite is not yet extensive (Williams & Kirkham, 1999; Hao et al., 2000; di marzo et al., 2001), AEA has been identified to play a definitive role in appetite regulation (Fride et al., 2001). However the precise role of AEA in appetitive processes remains to be established. At present, cannabinoid-induced feeding has been a phenomenon mainly supported by anecdotal accounts. The identification of endogenous ligands such as AEA (Devane et al., 1992) and the description of the first selective and potent cannabinoid CB1 receptor antagonist SR 141716 (Rinaldi-Carmona et al., 1994) have substantiated the involvement of endogenous cannabinoids in the regulation of appetite (Williams & Kirkham, 1999). In addition to this, several studies have reported that the blockade of central CB1 receptors by SR 141716 suppresses food intake in laboratory animals (Arnone et al., 1997; Colombo et al., 1998; Simiand et al., 1998).
Whilst this accumulated evidence supports the role of the brain cannabinoid system in the control of food intake, studies involving direct central administration of cannabinoids are relatively scarce, thus little is known about the role of endogenous cannabinoids in the central regulation of feeding. It is interesting to note that daily food intake is normally under the control of the hypothalamus (Torelli et al., 2000) and that cannabinoid receptors have been identified within several hypothalamic regions (Mailleux & Vanderhaeghen, 1992; Romero et al., 1998). Furthermore di marzo et al. (2001) recently reported increased endocannabinoid levels within the hypothalamus as a result of defective leptin signalling. In particular there appears to be a higher concentration of cannabinoid receptor mRNA within the ventromedial hypothalamus (VMH) in comparison to that in other nuclei within the hypothalamus (Mailleux & Vanderhaeghen, 1992). In light of this we investigated the effect of AEA administration into the VMH. Using the CB1 antagonist, SR 141716, we also investigated whether central AEA acts on CB1 receptors to modulate appetite.
Methods
Animals and housing
Male albino Glaxo-Wistar rats weighing between 250 – 350 g were used. Prior to experimentation rats were kept at 22°C with a 12 h light – dark cycle, lights on at 0515 h.
Following surgery (see below) rats were individually housed with free access to standard rat food (ARMTM pellets) and water.
The experimental protocol was approved by the Victorian College of Pharmacy, Monash University Animal Experimentation Ethics Committee and conforms to the guidelines set out by the National Health and Medical Research Council and all government regulations.
Surgery
Seven days prior to experimentation, rats were anaesthetized with a sodium methohexitone (18 mg kg−1 i.p.)/sodium amylobarbitone (30 mg kg−1 i.p.) mixture and placed in a stereotaxic frame. The skull was exposed and a hole (2 mm i.d.) was drilled above the VMH (co-ordinates Lateral −0.4 mm, Rostral −1.5 mm, from bregma). The co-ordinates were selected according to the stereotaxic atlas of Paxinos & Watson (1986). The animals were implanted with stainless steel 23-gauge guide cannulae, which were anchored using dental acrylic cement and two stainless steel screws. Each guide cannula was sealed by a stainless steel stylet. To prevent postoperative infection, the animals were injected with ticarcillin (25 mg kg−1 i.p.).
Central drug administration
Each treatment group consisted of 7 – 9 animals. The animals were gently restrained during microinjection. The stylet was removed and a stainless steel cannula (V −7.9 mm) connected by polyethylene tubing to a microinjection pump (Carnegie Medicin, Stockholm, Sweden) was inserted via the guide cannula. In order to examine the effect of AEA on the food intake of rats, three different concentrations were used: 25, 50 and 150 ng 0.5 μl−1 infused over 10 s.
AEA (25, 50 or 150 ng 0.5 μl−1 in 10 s), SR 141716 (30 μg 0.5 μl−1 in 10 s) or vehicle (saline) was microinjected into the VMH. The inner cannula remained in place for 3 min post microinjection. After resealing the guide cannula with a stylet the animals were returned to their individual home cage. Rats had unlimited access to water and a pre-weighed quantity of food. In some animals the microinjection of AEA was preceded by 30 min with central administration of SR 141716.
Experiments commenced at 1000 h. Food pellets were weighed and their weight was recorded before pretreatment (−30 min) and immediately prior to central administration (0 min) of AEA, SR 141716 or vehicle and at 180 min post microinjection.
At the end of the experiment, the rats were deeply anaesthetized with pentobarbitone (>100 mg kg−1 i.p.). Each rat was microinjected with dye into the VMH and its brain was rapidly removed, frozen and coronal sections were cut. An observer, unaware of the results obtained, determined the position of the injection site. Only results obtained from rats with correctly positioned guide cannula are presented.
Drugs
The following drugs were used: Anandamide, arachidonylethanolamide (Tocris), and SR 141716, (N-piperidino -5-(4-chlorphenyl)-1-(2,4-dichlorophenyl)-4-methyl-3-pyrazole-carboxamide) (Sanofi Recherche).
For intrahypothalamic injection AEA in a soya/water (4 : 1) emulsion (poloxamer as emulsifier) was dissolved in 0.9% w v−1 saline to give the required concentration.
For intrahypothalamic administration, SR 141716 (30 mg) was transferred to a glass vial and suspended in Tween-80 by vortex and then made up to volume with 0.9% w v−1 saline, resulting in a final concentration of 60 μg μl−1 with 4% Tween-80.
Statistical analysis
Groups of drug-treated rats were compared with the respective vehicle treated group using one-way analysis of variance (ANOVA). If the ANOVA revealed statistically significant differences (P<0.05), post-hoc Dunn Multiple Comparison test was used to determine which treatment groups were different. When data was not normally distributed or did not exhibit equal variance, an ANOVA on ranks was performed and when a significant difference (P<0.05) was determined amongst groups, Dunn's test was used as a multiple comparison to control.
Results
Rats normally consume the major proportion of their daily food intake during the period of darkness (8 – 10 g 100 g−1 body weight) (Taylor & Jamshidi, 1999). As illustrated in Figure 1, intrahypothalamic administration of vehicle had no effect on the appetite of such pre-satiated rats. At 25 ng (data not shown) and 150 ng AEA had no effect on food intake (Figure 1). When rats were microinjected with 50 ng AEA into the VMH, there was a significant increase in food intake compared to saline treated animals (P<0.001). This was despite animals previously consuming their total daily food intake. SR 141716 (30 μg) had no effect on appetite compared to that of vehicle-treated animals. When SR 141716 was injected 30 min prior to 50 ng AEA, it inhibited the AEA-induced spontaneous food intake.
Figure 1.
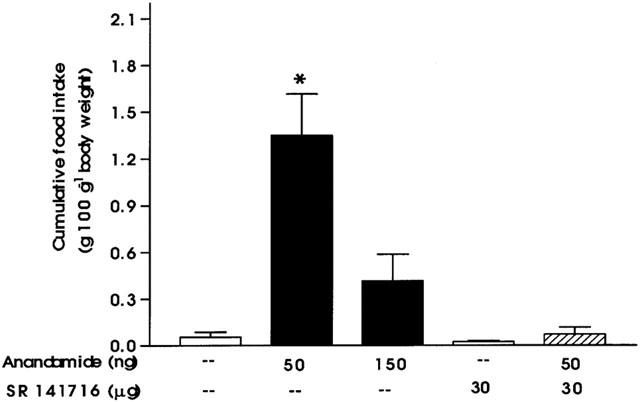
Effect of injection into the ventromedial hypothalamus of AEA 50 ng (n=8), 150 ng (n=8), saline (n=9), SR 141716 30 μg (n=9), and SR 141716 injected 30 min prior to AEA 50 ng (n=7) intrahypothalamically on food intake in pre-satiated rats (*P<0.001, when compared with vehicle). Results are expressed as mean+s.e.mean.
Histological analysis of injection cannulae placements using dye allowed for the exclusion of ‘off-target' cannulae from the study. Only rats with ‘on-target' cannulae (within the area outlined in Figure 2) were included in the study. It should be noted that when the cannula was not within the target area there was no increase in food intake upon the administration of AEA.
Figure 2.
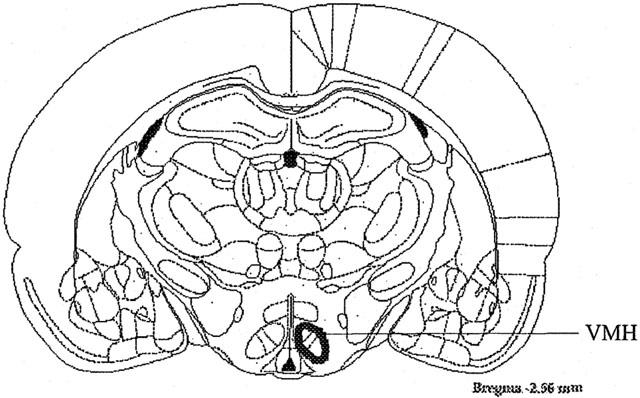
Schematic diagram of coronal brain section outlining the area within the hypothalamus in which the cannulae were placed to observe the effects of AEA and SR 141716 on appetite (Paxinos & Watson, 1986).
Discussion
Our results support the concept that brain cannabinoid systems are involved in appetitive behaviour. To our knowledge this study is the first to show that intrahypothalamic injection of AEA is effective in altering appetite, and that this effect is specific to the VMH. Administration of 25 and 150 ng AEA into the VMH had no effect on appetite. However 50 ng AEA caused a significant increase in food intake, generating an ‘inverted U' shape dose response effect to AEA. The hyperphagia produced by 50 ng AEA was significantly attenuated by the selective CB1 receptor antagonist SR 141716. This is in agreement with the results obtained by Williams & Kirkham (1999) who showed that cannabinoid-induced hyperphagia is mediated by central CB1 receptors. However, most studies conducted to date have used the peripheral route of drug injection to examine the effects of AEA and SR 141716 (Williams & Kirkham, 1999; Hao et al., 2000) on feeding, making this the first study to identify the central location of AEA induced feeding within the hypothalamus. Despite their differences in feeding paradigms and the injection routes of AEA, both previous studies also showed that AEA caused a stimulation of appetite. Contrary to these recent observations Crawley et al. (1993) did not observe any effect on food consumption of animals that had started eating during the experimental phase of injecting AEA peripherally. It should be noted that both the experimental circumstances and the peripheral dose of AEA in the Crawley et al. (1993) study were different to that of our study and the other two studies (Williams & Kirkham, 1999; Hao et al., 2000). In the current study and previous studies (Hao et al., 2000) animals were fed prior to injection of either intrahypothalamic or peripheral AEA. Thus, it can be hypothesized that AEA-induced feeding may predominantly depend on the satiety level of the animals. Perhaps it can also be postulated that endocannabinoid processes are (depending on the satiety level of animals) involved in the actual initiation of food intake, where exogenous AEA may act to facilitate the endogenous system.
Stimulation of appetite and feeding behaviour have been linked to reward and rewarding behaviour (Berridge, 1996). In addition to this, stimulation of rewarding processes and more specifically food intake, have been associated with the release of dopamine (Mark et al., 1994; Bassareo & Di Chiara, 1997). Given that cannabinoid ingestion has been linked to rewarding processes similar to those of other drugs of abuse (Gardner et al., 1988), it may be speculated from our results that the appetite stimulating action of AEA is part of the stimulation of reward or rewarding behaviour. Furthermore since it has been shown that cannabinoids can induce dopamine release (Navarro et al., 1993; Malone & Taylor, 1999), it can be postulated that AEA-induced feeding leads to rewarding behaviour and dopamine release. However the functional interaction between AEA-induced feeding and dopamine release requires further investigation.
Selective reduction of both standard rat food and sucrose intake by SR 141716 has been reported (Arnone et al., 1997; Colombo et al., 1998; Simiand et al., 1998). However in all reported cases the animals were injected during their feeding time irrespective of restricted (Arnone et al., 1997) and free (Colombo et al., 1998; Simiand et al., 1998) food access. In the current study, given the observation that vehicle-treated pre-satiated rats do not eat, it is not surprising that no effect was observed when SR 141716 was administered intrahypothalamically. Similarly, in the study where Williams & Kirkham (1999) peripherally injected pre-satiated rats with SR 141716 alone, SR 141716 had no effect on appetite. Therefore, from this and the observation that SR 141716 inhibited AEA induced feeding it can be postulated that in the VMH AEA acts on CB1 receptors to elicit food intake and that endocannabinoids play a role in the initiation of appetite. We propose that the endogenous cannabinoid AEA plays an activational role in ingestive behaviour. It is possible that this modulation however, may be overridden by the action of opposing systems, such as dopaminergic and/or serotonergic, upon alteration of satiety level.
In conclusion, this is the first demonstration that central cannabinoids play a role in the initiation of appetite by activation of CB1 receptors located in the VMH.
Acknowledgments
We would like to thank the Sanofi Recherche for the generous gift of SR 141716.
Abbreviations
- AEA
anandamide
- CB1
cannabinoid1
- VMH
ventromedial hypothalamus
References
- ARNONE M., MARUANI J., CHAPERON F., THIEBOT M.H., PONCELET M., SOUBRIE P., LE FUR G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacol. (Berl.) 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- BASSAREO V., DI CHIARA G. Differential influence of associative and nonassociative learning mechanisms on the responsiveness of prefrontal and accumbal dopamine transmission to food stimuli in rats fed ad libitum. J. Neurosci. 1997;17:851–861. doi: 10.1523/JNEUROSCI.17-02-00851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERRIDGE K.C. Food reward: brain substrates of wanting and liking. Neurosci. Biobehav. Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- COLOMBO G., AGABIO R., DIAZ G., LOBINA C., REALI R., GESSA G.L. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci. 1998;63:L113–L117. doi: 10.1016/s0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- CRAWLEY J.N., CORWIN R.L., ROBINSON J.K., FELDER C.C., DEVANE W.A., AXELROD J. Anandamide, an endogenous ligand of the cannabinoid receptor, induces hypomotility and hypothermia in vivo in rodents. Pharmacol. Biochem. Behav. 1993;46:967–972. doi: 10.1016/0091-3057(93)90230-q. [DOI] [PubMed] [Google Scholar]
- DEVANE W.A., HAUNS L., BREUER A., PERTWEE R.G., STEVENSON L.A., GRIFFIN G., GIBSON D., MANDELBAUM A., ETINGER A., MECHOULAM R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., GOPARAJU S.K., WANG L., LIU J., BATKAI S., JARAI Z., FEZZA F., MIURA G.I., PALMITER R.D., SUGIURA T., KUNOS G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- FRIDE E., GINZBURG Y., BREUER A., BISOGNO T., DI MARZO V., MECHOULAM R. Critical role of the endogenous cannabinoid system in mouse pup suckling and growth. Eur. J. Pharmacol. 2001;419:207–214. doi: 10.1016/s0014-2999(01)00953-0. [DOI] [PubMed] [Google Scholar]
- GARDNER E.L., PAREDES W., SMITH D., DONNER A., MILLING C., COHEN D.H., MORRISON D. Facilitation of brain stimulation reward by Δ9-tetrahydrocannabinol. Psychopharmacol. (Berl.) 1988;96:142–144. doi: 10.1007/BF02431546. [DOI] [PubMed] [Google Scholar]
- HAO S., AVRAHAM Y., MECHOULAM R., BERRY E.M. Low dose anandamide affects food intake, cognitive function, neurotransmitter and corticosterone levels in diet-restricted mice. Eur. J. Pharmacol. 2000;392:147–156. doi: 10.1016/s0014-2999(00)00059-5. [DOI] [PubMed] [Google Scholar]
- MAILLEUX P., VANDERHAEGHEN J.J. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- MALONE D.T., TAYLOR D.A. Modulation by fluoxetine of striatal dopamine release following Δ9-tetrahydrocannabinol: a microdialysis study in conscious rats. Br. J. Pharmacol. 1999;128:21–26. doi: 10.1038/sj.bjp.0702753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARK G.P., SMITH S.E., RADA P.V., HOEBEL B.G. An appetitively conditioned taste elicits a preferential increase in mesolimbic dopamine release. Pharmacol. Biochem. Behav. 1994;48:651–660. doi: 10.1016/0091-3057(94)90327-1. [DOI] [PubMed] [Google Scholar]
- NAVARRO M., FERNANDEZ-RUIZ J.J., DE MIGUEL R., HERNANDEZ M.L., CEBEIRA M., RAMOS J.A. An acute dose of δ9-tetrahydrocannabinol affects behavioral and neurochemical indices of mesolimbic dopaminergic activity. Behav. Brain Res. 1993;57:37–46. doi: 10.1016/0166-4328(93)90059-y. [DOI] [PubMed] [Google Scholar]
- PAXINOS G.P., WATSON C. Sydney: Academic press; 1986. The rat brain in stereotaxicco-ordinates. [Google Scholar]
- RINALDI-CARMONA M., BARTH F., HEAULME M., SHIRE D., CALANDRA B., CONGY C., MARTINEZ S., MARAUNI J., NELIAT G., CAPUT D., FERRARA P., SOUBRIE P., BRELIERE J.C., LE FUR G. SR 141716A, a potent and selective antagonist of the brain cannabinoid receptor. Fed. Eur. Bioch. Soc. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- ROMERO J., WENGER T., DE MIGUEL R., RAMOS J.A., FERNANDEZ-RUIZ J.J. Cannabinoid receptor binding did not vary in several hypothalamic nuclei after hypothalamic deafferentation. Life Sci. 1998;63:351–356. doi: 10.1016/s0024-3205(98)00283-5. [DOI] [PubMed] [Google Scholar]
- SIMIAND J., KEANE M., KEANE P.E., SOUBRIE P. SR 141716, a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behav. Pharmacol. 1998;9:179–181. [PubMed] [Google Scholar]
- TAYLOR D.A., JAMSHIDI N. The effect of Δ9-tetrahydrocannabinol and SR 141716 on the appetite of rats. Br. J. Pharmacol. 1999;128:205. [Google Scholar]
- TORELLI G.F., MEGUID M.M., MIYATA G., FETISSOV S.O., CARTER J.L., KIM H.J., MUSCARITOLI M., FANELLI F.R. VMN hypothalamic dopamine and serotonin in anorectic septic rats. Shock. 2000;13:204–208. doi: 10.1097/00024382-200003000-00006. [DOI] [PubMed] [Google Scholar]
- WILLIAMS C.M., KIRKHAM T.C. Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacol. (Berl.) 1999;143:315–317. doi: 10.1007/s002130050953. [DOI] [PubMed] [Google Scholar]


